Abstract
This study aimed at determining the relationship between baseline cystatin C levels and coronavirus disease 2019 (COVID-19) and investigating the potential prognostic value of serum cystatin C in adult patients with COVID-19. 481 patients with COVID-19 were consecutively included in this study from January 2, 2020, and followed up to April 15, 2020. All clinical and laboratory data of COVID-19 patients with definite outcomes were reviewed. For every measure, COVID-19 patients were grouped into quartiles according to the baseline levels of serum cystatin C. The highest cystatin C level was significantly related to more severe inflammatory conditions, worse organ dysfunction, and worse outcomes among patients with COVID-19 (P values < 0.05). In the adjusted logistic regression analyses, the highest cystatin C level and ln-transformed cystatin C levels were independently associated with the risks of developing critically ill COVID-19 and all-cause death either in overall patients or in patients without chronic kidney disease (P values < 0.05). As a potential inflammatory marker, increasing baseline levels of serum cystatin C might independently predict adverse outcomes for COVID-19 patients. Serum cystatin C could be routinely monitored during hospitalization, which showed clinical importance in prognosticating for adult patients with COVID-19.
1. Introduction
At the end of 2019, serial clinical cases of unknown etiological pneumonia were reported in Wuhan, a provincial capital city of Hubei, China [1, 2]. Within weeks of the early investigations, the pathogenic organism was identified as a novel coronavirus (2019-nCoV), subsequently named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. This formidable SARS-CoV-2 is similar to other pathogens in the coronavirus family, with rapid transmission from human to human [4]. A public health alert is now upgraded to an international emergency owning to the outbreak and pandemic of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, imposing a huge burden all over the world [5].
COVID-19 has been revealed a wide clinical range from asymptomatic infection to critical pneumonia with acute respiratory distress syndrome (ARDS) [6–8]; even so, the prevalence of multiple organ dysfunction and the mortality were still high among patients with COVID-19 [9, 10]. The exacerbation of COVID-19 was largely accompanied by violent cytokine storm due to overactive inflammation and immune response [11]. Cystatin C is a cysteine protease inhibitor secreted from human cells to blood circulation and directly interacts with human coronaviruses [12]. Apart from the indication of renal function [13], serum cystatin C originated from human alveolar macrophages might also associate with lung inflammation and respiratory dysfunction [14, 15]. Furthermore, cystatin C was suggested as an independent predictive factor of high mortality among the elderly population [16, 17]. As yet, little was known of the relationship between circulating cystatin C levels and disease severity or all-cause death in COVID-19.
In this hospital-based cohort study, all laboratory-confirmed patients with COVID-19 were recruited from a single center, and their clinical data were retrospectively analyzed to determine the relevance of serum cystatin C levels to the risks of critical illness and subsequent mortality among COVID-19 patients.
2. Materials and Methods
2.1. Study Subjects
In this retrospective cohort study, 481 consecutive adult subjects were confirmed as COVID-19, when admitted to the Central Hospital of Wuhan from January 2, 2020, and followed up to April 15, 2020. The diagnosis of COVID-19 was based on the World Health Organization interim guidance [18]. Throat swab specimens at admission were gathered from any suspected patients. Real-time reverse transcription-polymerase chain reaction was utilized to confirm SARS-CoV-2 infection in accordance with the manufacturer's protocol (Beijing Genomics Institution and Geneodx biotechnology Co., Ltd.). The verification of virus clearance prior to hospital discharge was required for each confirmed case via repeated tests of SARS-CoV-2. Only SARS-CoV-2-infected patients identified by throat swab samples were included in the current study. Any patient was excluded with the following conditions: a history of craniocerebral surgery or carcinomas; any recent life-threatening organic disease within 3 months; died on admission; and transferred to other hospitals. All data were anonymous, and oral informed consents were obtained from all participants due to the emergency of COVID-19 outbreaks. The institutional Ethics Committees approved this study, which followed the Declaration of Helsinki.
2.2. Data Collection
A trained physician team reviewed all clinical parameters from case report forms, nursing records, laboratory results, and radiological features. Patient electronic medical records provided in-hospital information on demographic characteristics, comorbidities, clinical manifestations, laboratory findings, therapy, and outcomes. Clinical laboratory markers, such as arterial blood lactate, PaO2/FiO2 ratio, white blood cell count, lymphocyte count, neutrophil/lymphocyte ratio, platelet count, hemoglobin, C-reactive protein, procalcitonin, cystatin C, blood urea nitrogen, creatinine, total bilirubin, alanine aminotransferase, aspartate aminotransferase, fibrinogen, D-dimer, lactate dehydrogenase, and creatine kinase, were measured and collected at admission. As stated by the testing Project Manual and reagent directions of the hospital, the normal reference interval of cystatin C was 0.6-1.55 mg/L. Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were summarized within 24 h after admission. ARDS was diagnosed in line with the Berlin definition [19]. The severity of COVID-19 was classified according to the Handbook of COVID-19 Prevention and Treatment [20]. Other data on therapeutic management were also obtained during hospitalization. Patient outcomes were monitored up to their death or discharge.
2.3. Statistical Analysis
Baseline continuous variables were shown as median (interquartile range, IQR), while categorical variables as n (%). To present and compare the baseline characteristics, all patients were divided into quartiles according to the levels of serum cystatin C. For continuous variables, a generalized liner regression model was applied to obtaining the P value of the trend test. For categorical variables, chi-squared test or Fisher's exact test was utilized. Differences in cystatin C levels between groups were determined by the Mann–Whitney U test. To estimate the association of baseline cystatin C with the risks of critical illness and all-cause mortality, logistic regression models were used in terms of the following confounding factors: age, sex, chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and chronic kidney disease (CKD). Both cystatin C and blood creatinine may represent renal function, and patients with acute kidney injury (AKI) are more likely to develop critical illness or death. Besides, a high APACHE II score may reflect renal organ failure and associate with high mortality in critically ill patients. For these reasons, blood creatinine levels and APACHE II scores were also considered in the regression models. The Kaplan-Meier methods were used for survival curve plotting. The R software (The R Foundation, http://www.r-project.org, version 3.6.1) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) were utilized for analyzing all statistics. A two-sided level of P < 0.05 was defined as statistically significant.
3. Results
3.1. Clinical Characteristics Associated with Serum Cystatin C Levels
The baseline clinical parameters of all 481 subjects with COVID-19 were summarized according to the quartile levels of serum cystatin C in Table 1. Compared to the lower cystatin C levels (<1.36 mg/L), the highest cystatin C level (≥1.36 mg/L) was associated with older age, higher prevalence among male patients, and higher incidence of the coexistent conditions, such as chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and CKD (all P values for trend < 0.05, Table 1). The highest cystatin C level was also found in significant association with increased lactate levels, decreased PaO2:FiO2 ratio, and higher incidence of ARDS (all P values for trend < 0.05, Table 1). The occurrence of critically ill COVID-19 was more prevalent in the highest cystatin C group, with APACHE II and SOFA scores ascending (all P values for trend < 0.05, Table 1).
Table 1.
Baseline clinical features of patients with COVID-19, according to quartiles of cystatin C levels.
| Characteristic | Quartile of serum cystatin C, mg/L | P trend | |||
|---|---|---|---|---|---|
| Quartile 1 (n = 119) <0.90 |
Quartile 2 (n = 116) 0.90-1.08 |
Quartile 3 (n = 124) 1.08-1.36 |
Quartile 4 (n = 122) ≥1.36 |
||
| Age, median (IQR), y | 45 (34-59) | 53 (37-64) | 62 (49-71) | 68 (59-77) | <0.001 |
| Male, no. (%) | 37 (31%) | 43 (37%) | 69 (56%) | 78 (64%) | <0.001 |
| Comorbidities, no. (%) | |||||
| Chronic pulmonary disease | 6 (5%) | 4 (3%) | 10 (8%) | 17 (14%) | 0.01 |
| Hypertension | 24 (20%) | 39 (34%) | 41 (33%) | 80 (66%) | <0.001 |
| Diabetes | 15 (13%) | 17 (15%) | 15 (12%) | 44 (36%) | <0.001 |
| Cardiovascular disease | 12 (10%) | 8 (7%) | 16 (13%) | 25 (20%) | 0.01 |
| Cerebrovascular disease | 1 (1%) | 5 (4%) | 7 (6%) | 29 (24%) | <0.001 |
| Chronic kidney disease | 0 | 0 | 1 (1%) | 25 (20%) | <0.001 |
| Blood gas and severity | |||||
| PaO2:FiO2, median (IQR) | 420 (296-521) | 385 (271-520) | 322 (240-485) | 274 (180-406) | <0.001 |
| Lactate, mmol/L, median (IQR) | 1.2 (0.8-1.8) | 1.2 (0.8-1.9) | 1.4 (0.9-2.0) | 1.5 (0.9-2.3) | 0.02 |
| ARDS, no. (%) | 30 (25%) | 38 (33%) | 58 (47%) | 69 (57%) | <0.001 |
| Severity | |||||
| General, no. (%) | 82 (69%) | 75 (65%) | 56 (45%) | 32 (26%) | |
| Severe, no. (%) | 27 (23%) | 29 (25%) | 48 (39%) | 28 (23%) | |
| Critically ill, no. (%) | 10 (8%) | 12 (10%) | 20 (16%) | 62 (51%) | <0.001 |
| SOFA score, median (IQR) | 1 (0-6) | 1 (0-7) | 2 (0-7) | 3 (0-10) | <0.001 |
| APACHE II score, median (IQR) | 2 (0-16) | 2 (0-20) | 3 (0-15) | 7 (0-21) | <0.001 |
| Admission laboratory markers, median (IQR) | |||||
| White blood cell count, ×109/L | 4.6 (3.5-5.7) | 4.8 (4.1-6.0) | 5.0 (4.0-6.6) | 5.4 (4.1-7.7) | <0.001 |
| Lymphocyte count, ×109/L | 1.1 (0.7-1.4) | 1.1 (0.8-1.4) | 1.0 (0.7-1.3) | 0.8 (0.5-1.2) | 0.11 |
| Neutrophil/lymphocyte ratio | 2.6 (1.7-4.1) | 3.1 (1.7-5.2) | 3.6 (2.2-6.2) | 4.9 (2.6-9.5) | <0.001 |
| Platelet count, ×109/L | 175 (147-228) | 186 (151-245) | 186 (139-232) | 162 (118-224) | 0.75 |
| Hemoglobin, g/L | 128 (120-140) | 127 (118-138) | 130 (119-142) | 124 (110-136) | 0.11 |
| C-reactive protein, mg/dL | 1.1 (0.3-3.2) | 1.8 (0.5-4.6) | 2.2 (0.7-5.5) | 3.9 (1.4-7.1) | <0.001 |
| Procalcitonin, ng/mL | 0.05 (0.04-0.06) | 0.05 (0.04-0.08) | 0.06 (0.04-0.10) | 0.12 (0.05-0.31) | <0.001 |
| Blood urea nitrogen, mmol/L | 3.6 (3.0-4.8) | 3.7 (3.1-4.3) | 4.3 (3.5-5.3) | 6.3 (4.6-8.9) | <0.001 |
| Creatinine, μmol/L | 56 (48-71) | 57 (49-70) | 70 (53-80) | 88 (71-114) | <0.001 |
| Total bilirubin, mmol/L | 8.2 (6.2-10.5) | 8.4 (6.9-11.7) | 9.9 (7.5-12.8) | 8.8 (6.0-11.9) | 0.008 |
| Alanine aminotransferase, U/L | 18 (13-28) | 22 (13-32) | 25 (15-43) | 19 (14-32) | 0.04 |
| Aspartate aminotransferase, U/L | 20 (17-29) | 23 (18-34) | 25 (19-41) | 26 (18-37) | 0.005 |
| Fibrinogen, g/L | 2.8 (2.4-3.2) | 3.0 (2.5-3.4) | 3.1 (2.6-3.5) | 3.3 (2.6-3.8) | 0.07 |
| D-dimer, mg/L | 0.40 (0.23-0.90) | 0.49 (0.28-1.03) | 0.69 (0.32-2.24) | 0.91 (0.50-2.39) | 0.03 |
| Lactate dehydrogenase, U/L | 182 (140-232) | 192 (152-258) | 207 (173-256) | 218 (171-296) | <0.001 |
| Creatine kinase, U/L | 79 (52-147) | 72 (45-119) | 77 (53-124) | 88 (60-153) | 0.90 |
| Creatine kinase-MB, U/L | 8 (5-12) | 7 (6-11) | 7 (6-10) | 9 (6-12) | 0.57 |
| Pharmacotherapy, no. (%) | |||||
| Quinolones | 75 (63%) | 80 (69%) | 87 (70%) | 83 (68%) | 0.66 |
| Cephalosporins | 52 (44%) | 48 (41%) | 63 (51%) | 69 (57%) | 0.08 |
| Ribavirin | 105 (88%) | 101 (87%) | 110 (89%) | 100 (82%) | 0.39 |
| Oseltamivir | 26 (22%) | 27 (23%) | 20 (16%) | 44 (36%) | 0.003 |
| Arbidol | 35 (29%) | 32 (28%) | 35 (28%) | 25 (20%) | 0.39 |
| Glucocorticoid therapy | 68 (57%) | 64 (55%) | 86 (69%) | 64 (52%) | 0.04 |
| Intravenous immunoglobulin | 72 (61%) | 64 (55%) | 63 (51%) | 54 (44%) | 0.08 |
| Noninvasive ventilation, no. (%) | 13 (11%) | 11 (9%) | 17 (14%) | 35 (29%) | <0.001 |
| Outcomes | |||||
| Hospital stays for survivors, median (IQR), d | 21 (15-31) | 24 (17-31) | 27 (17-37) | 28 (19-42) | 0.008 |
| Hospital mortality, no. (%) | 2 (2%) | 8 (7%) | 9 (7%) | 40 (33%) | <0.001 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; ARDS: acute respiratory distress syndrome; COVID-19: coronavirus disease 2019; FiO2: a fraction of inspired oxygen; IQR: interquartile range; PaO2: partial pressure of oxygen; SOFA: Sequential Organ Failure Assessment.
The highest cystatin C level was significantly associated with higher admission levels of white blood cell count, C-reactive protein, and procalcitonin, as well as greater neutrophil/lymphocyte ratio (all P values for trend < 0.05, Table 1). Besides, statistical differences were detected in the baseline levels of blood urea nitrogen, creatinine, total bilirubin, alanine aminotransferase, aspartate aminotransferase, D-dimer, and lactate dehydrogenase (all P values for trend < 0.05, Table 1). Except for total bilirubin and alanine aminotransferase, other biomarkers had significantly higher levels in the highest cystatin C group.
Patients in the highest cystatin C group were more likely to be treated with Oseltamivir and noninvasive ventilation, but less likely to receive glucocorticoid therapy (all P values for trend < 0.05, Table 1). Longer in-hospital stays for survivors were observed in the highest cystatin C group, while the mortality rate in this group (33%) was significantly higher (all P values for trend < 0.05, Table 1).
Similarly, there were significant differences in the levels of cystatin C between different subgroups, as shown in Table 2. A higher admission level of serum cystatin C was seen in males, elderly patients (over 65 years old), patients with each comorbidity, and nonsurvivors (all P values < 0.05, Table 2). In addition, increased baseline levels of serum cystatin C were significantly related to higher levels of blood creatinine, higher APACHE II scores, and critical illness in COVID-19 (all P values < 0.05, Table 2).
Table 2.
Association between cystatin C levels and clinical characters in COVID-19 patients.
| Group | Number | Cystatin C levels, mg/L median (IQR) | P value |
|---|---|---|---|
| Sex | |||
| Male | 227 | 1.20 (0.98-1.49) | <0.001 |
| Female | 254 | 1.00 (0.84-1.23) | |
| Age, y | |||
| ≥65 | 173 | 1.29 (1.05-1.62) | <0.001 |
| <65 | 308 | 1.00 (0.84-1.21) | |
| Comorbidity | |||
| With chronic pulmonary disease | 37 | 1.27 (1.05-1.75) | <0.001 |
| Without chronic pulmonary disease | 444 | 1.07 (0.89-1.33) | |
| With hypertension | 184 | 1.29 (1.02-1.66) | <0.001 |
| Without hypertension | 297 | 1.01 (0.85-1.23) | |
| With diabetes | 91 | 1.33 (0.98-1.83) | <0.001 |
| Without diabetes | 390 | 1.06 (0.89-1.30) | |
| With chronic kidney disease | 26 | 3.22 (1.88-7.40) | <0.001 |
| Without chronic kidney disease | 455 | 1.07 (0.89-1.31) | |
| With cardiovascular disease | 61 | 1.19 (1.02-1.64) | 0.006 |
| Without cardiovascular disease | 420 | 1.07 (0.89-1.33) | |
| With cerebrovascular disease | 42 | 1.68 (1.30-2.28) | <0.001 |
| Without cerebrovascular disease | 439 | 1.06 (0.89-1.31) | |
| Level of blood creatinine, μmol/L | |||
| ≥97 | 55 | 2.08 (1.46-3.48) | <0.001 |
| <97 | 426 | 1.05 (0.88-1.27) | |
| Admission APACHE II score | |||
| ≥8 | 83 | 1.57 (1.31-2.41) | <0.001 |
| <8 | 398 | 1.03 (0.88-1.24) | |
| Severity of COVID-19 | |||
| Critical illness | 104 | 1.49 (1.12-2.27) | <0.001 |
| Noncritical illness | 377 | 1.03 (0.88-1.23) | |
| Outcome | |||
| Survivors | 422 | 1.05 (0.88-1.29) | <0.001 |
| Nonsurvivors | 59 | 1.62 (1.32-2.41) |
APACHE II: Acute Physiology and Chronic Health Evaluation II; COVID-19: coronavirus disease 2019; IQR: interquartile range.
3.2. Association of Cystatin C with Mortality and Critical Illness in all Subjects
Several potential risk factors of disease severity and all-cause death for COVID-19 were adjusted in the logistic regression models, including age, sex, chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, CKD, blood creatinine levels, and APACHE II scores. As presented in Table 3, either the highest cystatin C level (≥1.36 mg/L) or ln-transformed cystatin C levels were independently related to the elevated risks of developing critical illness and mortality in all COVID-19 patients, after adjusting for the above confounders (all P values < 0.05).
Table 3.
Association of cystatin C with mortality and critical illness in all patients with COVID-19.
| Cystatin C levels, mg/L | Quartile 1 (<0.90) |
Quartile 2 (0.90-1.08) |
Quartile 3 (1.08-1.36) |
Quartile 4 (≥1.36) |
ln-transformed Cystatin C, mg/L∗ |
|---|---|---|---|---|---|
| No. of death/total | 2/119 | 8/116 | 9/124 | 40/122 | |
| Crude model | 1 (ref.) |
4.33 (0.90, 20.85) 0.0674 |
4.58 (0.97, 21.64) 0.0549 |
28.54 (6.71, 121.37) <0.0001 |
7.04 (3.86, 12.85) <0.0001 |
| Model 1 | 1 (ref.) |
3.55 (0.72, 17.51) 0.1202 |
2.26 (0.46, 11.06) 0.3147 |
9.98 (2.24, 44.54) 0.0026 |
4.23 (2.34, 7.65) <0.0001 |
| Model 2 | 1 (ref.) |
3.56 (0.70, 18.08) 0.1254 |
2.22 (0.44, 11.21) 0.3325 |
7.80 (1.64, 37.02) 0.0097 |
3.62 (1.61, 8.16) 0.0019 |
| Model 3 | 1 (ref.) |
7.66 (0.89, 66.19) 0.0642 |
4.69 (0.55, 40.01) 0.1580 |
15.54 (1.90, 126.80) 0.0104 |
5.64 (1.92, 16.57) 0.0017 |
| No. of critical/total | 10/119 | 12/116 | 20/124 | 62/122 | |
| Crude model | 1 (ref.) |
1.26 (0.52, 3.04) 0.6101 |
2.10 (0.94, 4.69) 0.0716 |
11.26 (5.38, 23.57) <0.0001 |
23.73 (10.66, 52.83) <0.0001 |
| Model 1 | 1 (ref.) |
1.08 (0.44, 2.64) 0.8682 |
1.33 (0.58, 3.10) 0.5015 |
5.93 (2.66, 13.21) <0.0001 |
13.91 (6.11, 31.68) <0.0001 |
| Model 2 | 1 (ref.) |
1.04 (0.42, 2.58) 0.9289 |
1.33 (0.56, 3.13) 0.5138 |
3.57 (1.51, 8.46) 0.0039 |
8.83 (3.52, 22.15) <0.0001 |
| Model 3 | 1 (ref.) |
1.20 (0.47, 3.06) 0.7005 |
1.41 (0.58, 3.42) 0.4473 |
2.86 (1.13, 7.28) 0.0272 |
5.84 (1.91, 17.84) 0.0019 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; COVID-19: coronavirus disease 2019. Data are shown as odds ratios (OR) and 95% confidence intervals (CI). ∗Modeled as per unit increase of ln-transformed cystatin C. Model 1 adjusted for age and sex. Model 2 adjusted for model 1 plus chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and chronic kidney disease. Model 3 adjusted for model 1 plus model 2 plus blood creatinine and APACHE II score.
The nonlinear association of ln-transformed cystatin C levels with critical illness and mortality in COVID-19 was then visually described in Figure 1. The prevalence of critically ill COVID-19 ascended with increasing ln-transformed cystatin C levels, and a gradual tendency of the gentle curve was observed around ln-transformed cystatin C levels of more than 1.50 mg/L (Figure 1(a)). Admission levels of ln-transformed cystatin C were also positively associated with the mortality among COVID-19 patients, which indicated that higher ln-transformed cystatin C levels might imply higher death rates during hospitalization of patients with COVID-19 (Figure 1(b)). Subsequent analysis via the Kaplan-Meier methods revealed that overall COVID-19 patients with a baseline cystatin C level ≥ 1.36 mg/L had significantly worse outcomes, compared to those with cystatin C levels < 1.36 mg/L (P value < 0.0001, Figure 2(a)).
Figure 1.
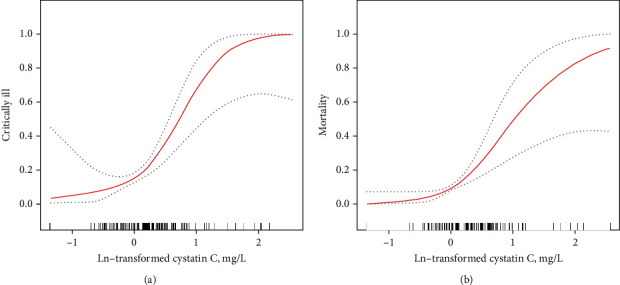
The nonlinear relationship between baseline ln-transformed cystatin C levels and the prevalence of critical illness (a) and mortality (b) in all COVID-19 patients. The generalized additive model was used for presenting the smoothing splines, adjusted for age, sex, blood creatinine levels, APACHE II scores, and coexistent conditions (such as chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and chronic kidney disease). The red lines suggested the risks of critical illness (a) and mortality (b), respectively. The blue dot lines suggested 95% confidence intervals.
Figure 2.
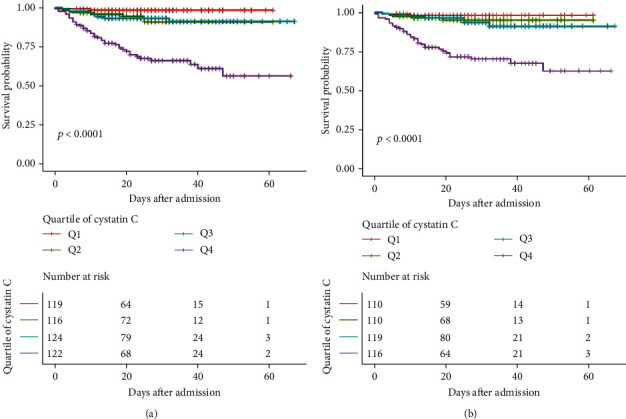
The Kaplan-Meier curves categorized by the quartiles of baseline serum cystatin C levels. (a) In overall COVID-19 patients, serum cystatin C on admission was grouped into the levels of <0.90 mg/L (Q1), 0.90-1.08 mg/L (Q2), 1.08-1.36 mg/L (Q3), and ≥1.36 mg/L (Q4). (b) In COVID-19 patients without chronic kidney disease, serum cystatin C on admission was grouped into the levels of <0.89 mg/L (Q1), 0.89-1.06 mg/L (Q2), 1.06-1.31 mg/L (Q3), and ≥1.31 mg/L (Q4).
3.3. Association of Cystatin C with Mortality and Critical Illness in Subjects without CKD
To minimize the impact of coexisting CKD on the progression of COVID-19, CKD patients were excluded in the following analyses. 455 subjects without CKD were then categorized by the quartiles of admission serum cystatin C levels (<0.89 mg/L; 0.89-1.06 mg/L; 1.06-1.31 mg/L; and ≥1.31 mg/L). The logistic regression models in Table 4 adjusted for age, sex, chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, blood creatinine levels, and APACHE II scores. The highest cystatin C level (≥1.31 mg/L) was an independent risk factor for critically ill COVID-19 and all-cause death in patients without CKD, after adjustment for the underlying confounders (all P values < 0.05, Table 4). ln-transformed cystatin C levels were also independently associated with the risks for critical illness and mortality among COVID-19 patients without CKD (all P values < 0.05, Table 4). Additionally, the Kaplan-Meier analysis further suggested that the worst outcome was observed in the baseline cystatin C levels ≥ 1.31 mg/L (P value < 0.0001, Figure 2(b)).
Table 4.
Association of cystatin C with mortality and critical illness in COVID-19 patients without chronic kidney disease.
| Cystatin C levels, mg/L | Quartile 1 (<0.89) |
Quartile 2 (0.89-1.06) |
Quartile 3 (1.06-1.31) |
Quartile 4 (≥1.31) |
ln-transformed Cystatin C, mg/L∗ |
|---|---|---|---|---|---|
| No. of death/total | 2/110 | 5/110 | 7/119 | 33/116 | |
| Crude model | 1 (ref.) |
2.57 (0.49, 13.55) 0.2652 |
3.37 (0.69, 16.61) 0.1346 |
21.47 (5.01, 92.04) <0.0001 |
17.69 (6.58, 47.52) <0.0001 |
| Model 1 | 1 (ref.) |
2.22 (0.41, 12.01) 0.3560 |
1.63 (0.32, 8.37) 0.5569 |
7.67 (1.70, 34.69) 0.0081 |
5.95 (2.01, 17.63) 0.0013 |
| Model 2 | 1 (ref.) |
2.40 (0.43, 13.56) 0.3204 |
1.85 (0.35, 9.83) 0.4689 |
8.40 (1.75, 40.19) 0.0077 |
5.73 (1.79, 18.32) 0.0032 |
| Model 3 | 1 (ref.) |
5.25 (0.56, 49.57) 0.1474 |
3.87 (0.43, 34.58) 0.2259 |
16.77 (1.99, 141.06) 0.0095 |
6.61 (1.64, 26.70) 0.0080 |
| No. of critical/total | 10/110 | 9/110 | 16/119 | 47/116 | |
| Crude model | 1 (ref.) |
0.89 (0.35, 2.29) 0.8104 |
1.55 (0.67, 3.59) 0.3022 |
6.81 (3.22, 14.40) <0.0001 |
18.78 (7.82, 45.08) <0.0001 |
| Model 1 | 1 (ref.) |
0.79 (0.30, 2.05) 0.6220 |
0.97 (0.40, 2.34) 0.9518 |
3.60 (1.59, 8.11) 0.0020 |
10.07 (3.90, 26.05) <0.0001 |
| Model 2 | 1 (ref.) |
0.77 (0.29, 2.02) 0.5918 |
1.02 (0.42, 2.47) 0.9704 |
3.51 (1.51, 8.16) 0.0036 |
8.89 (3.28, 24.13) <0.0001 |
| Model 3 | 1 (ref.) |
0.88 (0.32, 2.37) 0.7969 |
1.06 (0.42, 2.66) 0.8987 |
2.96 (1.20, 7.35) 0.0190 |
6.37 (2.03, 19.93) 0.0015 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; COVID-19: coronavirus disease 2019. Data are shown as odds ratios (OR) and 95% confidence intervals (CI). ∗Modeled as per unit increase of ln-transformed cystatin C. Model 1 adjusted for age and sex. Model 2 adjusted for model 1 plus chronic pulmonary disease, hypertension, diabetes, cardiovascular disease, and cerebrovascular disease. Model 3 adjusted for model 1 plus model 2 plus blood creatinine and APACHE II score.
4. Discussion
To the best of our knowledge, this study is the first to report the relationship between baseline levels of cystatin C and COVID-19 outcomes, and to highlight the prognostic values of monitoring serum cystatin C on hospitalized patients with SARS-CoV-2 infection. Firstly, clinical features of COVID-19 patients were compared under the quartile classification of baseline cystatin C levels (less than 0.90, 0.90 to 1.08, 1.08 to 1.36, and more than 1.36 mg/L, respectively). We found that the highest cystatin C level on admission was strongly associated with more severe inflammatory status, worse organ dysfunction, and worse outcomes among patients with COVID-19. Secondly, the highest cystatin C level and ln-transformed cystatin C levels were identified in the adjusted analyses as independent risk factors for critical illness and death from all causes in overall patients with COVID-19. Thirdly, even after CKD patients were excluded in the logistic regression models, the highest cystatin C level and ln-transformed cystatin C levels were still independently associated with the risks of developing critically ill COVID-19 and subsequent mortality. Therefore, circulating cystatin C could serve as a potential inflammatory target for preventing COVID-19 from the likely progression of critical illness and in-hospital death, not just representing kidney function.
In the pathological process of infection and inflammation, the crucial functions of cystatin C need to be emphasized [12]. After abundant secretion into various human body fluids, cystatin C extracellularly suppresses the activation of cysteine proteases that are utilized by invading pathogens into the host [21–23]. Accordingly, recombinant human cystatin C was reported to play an inhibitory role during the growth period of human coronaviruses [24]. The interference of cystatin family with replicating human coronavirus was also found in the host lung cells [25]. Besides, cytokines from inflammatory cells can modulate the expressions of cystatin C gene [26], while cystatin C will, in turn, fundamentally influence immune regulation to maintain homeostasis in the host immune system [12].
Cystatin C is easily detectable in the biological fluids and acts as a reliable marker, independently of age, sex, and muscle mass [27–29]. Previous studies not only confirmed the role of cystatin C in indicating organ dysfunction but also clarified that serum cystatin C levels might associate with inflammatory phase and adverse outcomes in various diseases [30–33]. These findings accorded with our observations of cystatin C levels and COVID-19. Of note, a multicenter cohort study of pediatric subjects infected by human immunodeficiency viruses (HIV) demonstrated that cystatin C levels were strongly related to kidney function and inflammatory state [34]. Positive relevance of elevating cystatin C levels to plasma HIV RNA status was also observed in adult patients with HIV infection whose antiretroviral therapy was randomly interrupted [35]. This underlying interaction between circulating cystatin C and viral infection may provide insight into our understanding of pathophysiological events in COVID-19.
A serious deterioration in COVID-19 was mainly characterized by the evolution of ARDS [36]. In the inflammatory course of ARDS due to SARS-CoV-2, lymphocytes infiltrated into lung tissues, while the host immune system was excessively activated and aggravated tissue injury [36]. In part, our findings might account for this phenomenon. First, the incidence rates of ARDS and critically ill COVID-19 were obviously higher among patients with the highest cystatin C level, who were also accompanied by elevated lactate levels, but a reduced PaO2:FiO2 ratio. Second, the highest cystatin C level was significantly related to the increased levels of inflammatory indexes, including white blood cell count, C-reactive protein, procalcitonin, neutrophil/lymphocyte ratio, and D-dimer. In addition to acute lung tissue damage, renal dysfunction was reported in COVID-19 patients as well [7, 9]. If kidney disease occurred at admission or AKI progressed during hospitalization, COVID-19-related death rate was ascended dramatically [37]. In the current study, we found positive association of a higher cystatin C level with increased values of blood creatinine and APACHE II score. However, the logistic regression models adjusting for these confounding factors further revealed that elevated levels of serum cystatin C were independently associated with the risks for critical illness and death in COVID-19. We thus reckon that circulating cystatin C may function as a potential biomarker of systemic inflammation during the exacerbation of COVID-19, rather than only reflecting renal function in patients infected by SARS-CoV-2.
Generally, the process of COVID-19 involved the overactivation of inflammatory and immune responses in severely ill cases, which eventually resulted in the high mortality for SARS-CoV-2-infected patients [38, 39]. Our results are meaningful. The highest admission level of serum cystatin C was strongly associated with more severe inflammation, worse organ dysfunction, and worse outcomes in COVID-19. Moreover, increasing baseline levels of cystatin C were independently related to the risks of occurring critical illness and in-hospital death either in overall COVID-19 patients or in COVID-19 patients without CKD. In other words, higher cystatin C levels on admission might predict significantly worse outcomes for adult patients with COVID-19. Yet, the underlying mechanism is still unclear. Our observations should be further verified with more basic and clinical studies.
Limitations should be considered in this study. Firstly, the causal relationship between serum cystatin C and COVID-19 outcomes could not be concluded, due to the nature of an observational research. Secondly, the likelihood of other residual or unnoticeable confounders could not be excluded. Thirdly, we did not investigate whether the dynamic changes of cystatin C were associated with long-term outcomes, because of partial missing data on follow-up.
5. Conclusions
In this hospital-based study of 481 adult patients confirmed as COVID-19, we revealed that baseline serum cystatin C might serve as a potential inflammatory biomarker, independently predicting poor outcomes for SARS-CoV-2 infection. Monitoring cystatin C during in-hospital stays may thus be of clinical importance in the prognosis for adult patients with COVID-19.
Acknowledgments
This study was supported by the Health and Family Planning Commission of Wuhan Municipality, grant number WX18A02.
Contributor Information
Liangkai Chen, Email: clk@hust.edu.cn.
Junhui Yang, Email: youngjunhui@msn.com.
Li Yu, Email: yuli196410@sina.com.
Data Availability
The datasets generated and/or analyzed in this study are not publicly available because of the respect to and the protection of patient privacy but are available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Dan Chen, Wenwu Sun, Jia Li, and Bohua Wei contributed equally to this work.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. The New England Journal of Medicine. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake M. A. What we know so far: COVID-19 current clinical knowledge and research. Clinical Medicine (London, England) 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabaan A. A., al-Ahmed S. H., Haque S., et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Le Infezioni in Medicina. 2020;28(2):174–184. [PubMed] [Google Scholar]
- 5.Columbus C., Brust K. B., Arroliga A. C. 2019 novel coronavirus: an emerging global threat. Baylor University Medical Center Proceedings. 2020;33(2):209–212. doi: 10.1080/08998280.2020.1731272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Y., Yu X., du X., et al. Epidemiological and clinical characteristics of 26 asymptomatic SARS-CoV-2 carriers. The Journal of Infectious Diseases. 2020;221(12):1940–1947. doi: 10.1093/infdis/jiaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):p. 1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G., Zangrillo A., Zanella A., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):p. 1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jose R. J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. The Lancet Respiratory Medicine. 2020;8(6):e46–e47. doi: 10.1016/s2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zi M., Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunology Letters. 2018;196:80–90. doi: 10.1016/j.imlet.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernando S., Polkinghorne K. R. Cystatin C: not just a marker of kidney function. Brazilian Journal of Nephrology. 2020;42(1):6–7. doi: 10.1590/2175-8239-jbn-2019-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telo S., Kuluöztürk M., Deveci F., Kırkıl G., Öner Ö., Kaman D. Serum cystatin C levels in COPD: potential diagnostic value and relation between respiratory functions. Journal of Medical Biochemistry. 2018;37(4):434–440. doi: 10.1515/jomb-2017-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman H. A., Jr., Reilly J. J., Jr., Yee R., Grubb A. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. The American Review of Respiratory Disease. 1990;141(3):698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak M. G., Sarnak M. J., Katz R., et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England Journal of Medicine. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 17.Ingelfinger J. R., Marsden P. A. Estimated GFR and risk of death--is cystatin C useful? The New England Journal of Medicine. 2013;369(10):974–975. doi: 10.1056/NEJMe1308505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. World Health Organization; 2020. Published January 2020. [Google Scholar]
- 19.The ARDS Definition Task Force, Ranieri V. M., Rubenfeld G. D., Thompson B. T., Ferguson N. D., Caldwell E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 20.Hongliu C., Yu C., Zuobing C., et al. In: Handbook of COVID-19 Prevention and Treatment in Compiled According to Clinical Experience. Tingbo L., editor. The First Affiliated Hospital, Zhejiang University School of Medicine; 2020. [Google Scholar]
- 21.Tavera C. Tissue and biological fluid distribution of cysteine proteinases inhibitor: rat cystatin C. Biological Chemistry Hoppe-Seyler. 1990;371:187–192. [PubMed] [Google Scholar]
- 22.Bobek L. A., Levine M. J. Cystatins--inhibitors of cysteine proteinases. Critical Reviews in Oral Biology and Medicine. 2016;3(4):307–332. doi: 10.1177/10454411920030040101. [DOI] [PubMed] [Google Scholar]
- 23.Turk B., Turk D., Salvesen G. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Current Pharmaceutical Design. 2002;8(18):1623–1637. doi: 10.2174/1381612023394124. [DOI] [PubMed] [Google Scholar]
- 24.Collins A. R., Grubb A. Inhibitory effects of recombinant human cystatin C on human coronaviruses. Antimicrobial Agents and Chemotherapy. 1991;35(11):2444–2446. doi: 10.1128/AAC.35.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins A. R., Grubb A. Cystatin D, a natural salivary cysteine protease inhibitor, inhibits coronavirus replication at its physiologic concentration. Oral Microbiology and Immunology. 1998;13(1):59–61. doi: 10.1111/j.1399-302X.1998.tb00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T., Jiang H., Konno T., et al. Bunina bodies in motor and non-motor neurons revisited: a pathological study of an ALS patient after long-term survival on a respirator. Neuropathology. 2014;34(4):392–397. doi: 10.1111/neup.12105. [DOI] [PubMed] [Google Scholar]
- 27.Finney H., Newman D. J., Thakkar H., Fell J. M., Price C. P. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Archives of Disease in Childhood. 2000;82(1):71–75. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finney H., Newman D. J., Price C. P. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Annals of Clinical Biochemistry. 2000;37(1):49–59. doi: 10.1258/0004563001901524. [DOI] [PubMed] [Google Scholar]
- 29.Vinge E., Lindergård B., Nilsson–Ehle P., Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scandinavian Journal of Clinical and Laboratory Investigation. 2009;59(8):587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 30.Andrade López A. C., Bande Fernández J. J., Díaz Corte C. Cystatin C in estimation of renal function in liver transplantation candidates. Medicina Clinica. 2019 doi: 10.1016/j.medcli.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Mao W., Liu S., Wang K., et al. Cystatin C in evaluating renal function in ureteral calculi hydronephrosis in adults. Kidney & Blood Pressure Research. 2020;45(1):109–121. doi: 10.1159/000504441. [DOI] [PubMed] [Google Scholar]
- 32.Rokadia H. K., Agarwal S. Serum cystatin C and emphysema: results from the National Health and Nutrition Examination Survey (NHANES) Lung. 2012;190(3):283–290. doi: 10.1007/s00408-012-9374-z. [DOI] [PubMed] [Google Scholar]
- 33.Hu G., Wu Y., Zhou Y., Yu Y., Liang W., Ran P. Cystatin C as a predictor of in-hospital mortality after exacerbation of COPD. Respiratory Care. 2016;61(7):950–957. doi: 10.4187/respcare.04034. [DOI] [PubMed] [Google Scholar]
- 34.Deyà-Martínez À., Fortuny C., Soler-Palacín P., et al. Cystatin C: a marker for inflammation and renal function among HIV-infected children and adolescents. The Pediatric Infectious Disease Journal. 2016;35(2):196–200. doi: 10.1097/INF.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 35.Mocroft A., Wyatt C., Szczech L., et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C. AIDS. 2009;23(1):71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tay M. Z., Poh C. M., Rénia L., MacAry P. A., Ng L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conti P., Ronconi G., Caraffa A. L., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in this study are not publicly available because of the respect to and the protection of patient privacy but are available from the corresponding authors on reasonable request.


