Abstract
Background
A number of circulating plasma biomarkers have been shown to predict survival in patients with idiopathic pulmonary fibrosis (IPF), but most were identified before the use of antifibrotic (AF) therapy in this population. Because pirfenidone and nintedanib have been shown to slow IPF progression and may prolong survival, the role of such biomarkers in AF-treated patients is unclear.
Research Question
To determine whether plasma concentration of cancer antigen 125 (CA-125), C-X-C motif chemokine 13 (CXCL13), matrix metalloproteinase 7 (MMP7), surfactant protein D (SP-D), chitinase-3-like protein-1 (YKL-40), vascular cell adhesion protein-1 (VCAM-1), and osteopontin (OPN) is associated with differential transplant-free survival (TFS) in AF-exposed and nonexposed patients with IPF.
Study Design and Methods
A pooled, multicenter, propensity-matched analysis of IPF patients with and without AF exposure was performed. Optimal thresholds for biomarker dichotomization were identified in each group using iterative Cox regression. Longitudinal biomarker change was assessed in a subset of patients using linear mixed regression modeling. A clinical-molecular signature of IPF TFS was then derived and validated in an independent IPF cohort.
Results
Three hundred twenty-five patients were assessed, of which 68 AF-exposed and 172 nonexposed patients were included after propensity matching. CA-125, CXCL13, MMP7, YKL-40, and OPN predicted differential TFS in AF-exposed patients but at higher thresholds than in AF-nonexposed individuals. Plasma biomarker level generally increased over time in nonexposed patients but remained unchanged in AF-exposed patients. A clinical-molecular signature predicted decreased TFS in AF-exposed patients (hazard ratio [HR], 5.91; 95% CI, 2.25-15.5; P < .001) and maintained this association in an independent AF-exposed cohort (HR, 3.97; 95% CI, 1.62-9.72; P = .003).
Interpretation
Most plasma biomarkers assessed predicted differential TFS in AF-exposed patients with IPF, but at higher thresholds than in nonexposed patients. A clinical-molecular signature of IPF TFS may provide a reliable predictor of outcome risk in AF-treated patients but requires additional research for optimization and validation.
Key Words: antifibrotic, biomarker, idiopathic pulmonary fibrosis, interstitial lung disease, survival
Abbreviations: AF, antifibrotic; CA-125, cancer antigen 125; CMS, clinical-molecular signature; CXCL13, C-X-C motif chemokine 13; Dlco, diffusing capacity of the lungs for carbon monoxide; GAP, gender, age, and physiology; HR, hazard ratio; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; MMP7, matrix metalloproteinase 7; OPN, osteopontin; RS, risk score; SP-D, surfactant protein D; TFS, transplant-free survival; UC-Davis, University of California at Davis; UChicago, University of Chicago; VCAM-1, vascular cell adhesion protein-1; YKL-40, chitinase-3-like protein-1
FOR EDITORIAL COMMENT, SEE PAGE 1321
A number of circulating plasma biomarkers have been shown to predict survival in patients with idiopathic pulmonary fibrosis (IPF), including cancer antigen 125 (CA-125)`, C-X-C motif chemokine 13 (CXCL13), matrix metalloproteinase 7 (MMP7), surfactant protein D (SP-D), chitinase-3-like protein-1 (YKL-40), vascular cell adhesion protein-1 (VCAM-1), and osteopontin (OPN), with increased concentration associated with reduced survival.1, 2, 3, 4, 5, 6, 7 Longitudinal change in CA-125 is also a strong predictor of outcome, with rising concentration predictive of increased mortality risk.2 In addition to outcome prediction, a composite index of MMP7, SP-D, and OPN also may differentiate IPF from other idiopathic interstitial pneumonias, with higher levels predictive of IPF.8
Although informative, most of these studies were performed before the approval of antifibrotic (AF) therapy for the treatment of IPF. Pirfenidone and nintedanib both have been shown to slow IPF progression in prospective clinical trials,9, 10, 11 and post hoc analyses of pooled clinical trial datasets suggest that they also improve survival.12 13 Accordingly, AF treatment is recommended for most patients with IPF.14 Because biomarkers of IPF survival reflect molecular changes of fibrogenesis, what effect AF therapy may have on previously reported survival associations and longitudinal change in biomarker concentration remains unclear.
Here we conduct a pooled, multi-center, propensity-matched investigation of IPF patients with and without AF exposure to determine whether plasma concentration of CA-125, CXCL13, MMP7, SP-D, YKL-40, VCAM-1, and OPN is associated with differential survival in these treatment groups. We then assessed whether longitudinal change in plasma biomarker concentration varied by AF exposure groups. We hypothesized that AF exposure modulates survival association of these biomarkers and longitudinal change in their concentration. Finally, we derived a clinical molecular signature of IPF survival in AF-treated patients and validated this signature in an independent cohort of AF-exposed patients.
Methods
Study Populations
This study was conducted at the University of California at Davis (UC-Davis) and University of Chicago (UChicago) and was approved by the institutional review board at each institution as part of a protocol to study clinical and biologic markers of disease outcomes in patients with interstitial lung disease (UC-Davis #875917 and UChicago #14163). Consecutive consenting patients with a diagnosis of IPF according to international consensus guidelines15 who provided a research blood sample were eligible for study inclusion. Patients from UC-Davis were enrolled from May 2016 to January 2019, and those from UChicago were enrolled from May 2010 to June 2017.
Pertinent clinical data were extracted from the electronic medical record, including baseline demographics, lung function, treatment exposure dates, and outcome data. AF exposure was defined as (1) pirfenidone or nintedanib initiation after research blood draw; (2) 6 or more months of total AF exposure; and (3) AF exposure for 50% or more of the follow-up period. Patients who failed to meet this definition (n = 10) and those exposed to AF therapy at the time of blood draw (n = 37) were excluded. Patients without longitudinal follow-up data were excluded, as were those treated 3 months or more with azathioprine, mycophenolate mofetil, or prednisone ≥20 mg daily.
Plasma Processing and Biomarker Measurement
Peripheral venous blood was collected in 10-mL ethylene-diamine tetra-acetic acid-containing tubes and kept on ice until processing, at which time plasma was isolated, aliquoted, and stored at -80°C. Plasma concentration for CA-125, CXCL13, MMP7, SPD-D, YKL-40, OPN, and VCAM-1 were determined at UC-Davis, using a Luminex magnetic bead-based custom premixed multiplex assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Briefly, plasma samples were diluted 1:2 in calibrator diluent provided in the assay kit, and 50 μL was used for the assay procedure. All washes were performed using the Bio-Rad Bio-Plex Pro II Wash Station (Bio-Rad), and the final readings were performed on a Bio-Rad Bioplex 200. Biomarker values above and below the limits of detection were imputed to the highest and lowest detectable levels, respectively, with less than 1% of data imputed. Plasma biomarkers were measured in duplicate in a subset of patients (n = 62) to ensure assay consistency, with very high correlation observed (e-Fig 1).
Statistical Analysis
Continuous variables are reported as means with SD when normally distributed or median with interquartile range when non-normally distributed. The Student t test and Mann-Whitney U-test were used to compare normally and non-normally distributed continuous variables, respectively. Categorical variables are presented as count and percentage and compared using a χ2 test. The primary end point assessed was 3-year transplant-free survival (TFS) from time of blood draw. Vital status was assessed by review of the medical record and follow-up phone calls to patients and family members.
Propensity matching was performed using a multivariable logistic regression model with AF exposure as the dependent variable. Covariates included in the model were center, race, smoking status, and gender, age, physiology (GAP) score.16 Radius matching was performed with common support defined as propensity score distance of 0.001 or less. Patients with propensity score outside the range of common support were excluded.
Optimal thresholds for biomarker dichotomization were identified separately in AF-exposed and nonexposed patients using iterative Cox proportional hazards regression. All possible biomarker thresholds were explored to identify the threshold with the maximal TFS association biomarker based on Wald P value. Longitudinal change in biomarker level in AF-exposed and nonexposed individuals was assessed in a subset of patients from UC-Davis (n = 56), using linear mixed effects regression. Patients with serial blood samples collected before and after AF initiation (n = 5) were considered as independent observations. Exploratory analysis using restricted maximum likelihood was performed to establish an appropriate variance-covariance correlation structure and assess for inclusion of random intercept and slope terms for each biomarker based on Bayesian information criteria. The final mixed-effects models were fit using maximum likelihood estimation and were adjusted for race, smoking status, and baseline GAP score.
A clinical-molecular signature (CMS) of TFS in AF-treated patients was derived by identifying relevant variables associated with differential TFS in a multivariable Cox regression model. Variables assessed for CMS development included all dichotomized biomarkers (using AF-treated thresholds) along with age, sex, race, smoking status, and percent predicted FVC and diffusing capacity of the lungs for carbon monoxide (DLCO). Final model variables were selected using forward stepwise regression with entry criteria (P < .1). Final model beta coefficients were used to generate a weighted clinical molecular risk score, which was then categorized into “low-risk” and “high-risk” CMS strata. The final CMS stratification threshold was chosen based on the threshold with highest corresponding Harrel’s C-statistic. TFS is plotted using the Kaplan-Meier estimator. All Cox models were checked to ensure the proportional hazard assumption was met. Because this was a sample size of convenience, no formal power calculation was performed. All statistical analyses were performed using Stata (StataCorp, 2019, Release 16). Statistical significance was set at P < .05.
Results
Cohort Characteristics
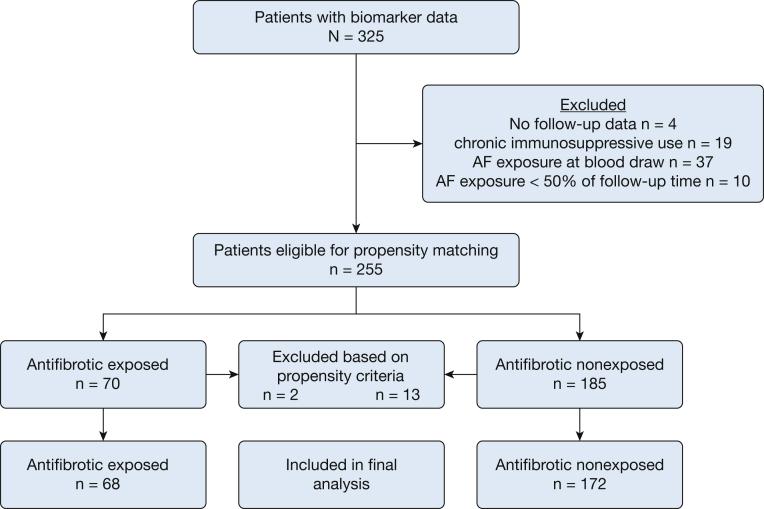
Of 325 patients with available biomarker data, 255 were advanced for propensity matching after exclusion criteria were applied, including 70 patients with AF exposure and 185 patients without. Sixty-eight AF-exposed patients and 172 nonexposed patients met propensity score matching criteria and were included in the final analysis (Fig 1). Groups were globally balanced based on an R-index17 of 0 and covariate variance ratios ranging from 0.87 to 1.36 (e-Table 1). When comparing cohort characteristics, groups were similar regarding age, sex, race, smoking status, and lung function.
Figure 1.
Consort/STROBE diagram.
Among AF-exposed patients, 39 (57.4%) were treated with pirfenidone, 20 (29.4%) were treated with nintedanib, and nine (13.2%) were treated nonconcurrently with both medications (Table 1). The median time from blood draw to AF initiation was 44 days (interquartile range [IQR], 0-112 days). Median treatment duration was 16.3 (IQR, 10-22.5) months and was similar between individual treatment groups. Median follow-up time was 21.1 (IQR, 11.2-31.4) months and was similar between groups. A higher proportion of deaths occurred in the nonexposed group compared with the AF-exposed group (41.1% vs 25%, respectively; P = .03). Baseline median biomarker concentration was similar between groups except for VCAM-1, which was lower in the AF-treated group.
Table 1.
Baseline Characteristics, Outcomes, Treatment, and Biomarker Level
| Baseline Characteristics | AF Nonexposed (n = 172) | AF Exposed (n = 68) | P |
|---|---|---|---|
| Age, mean (±SD) | 72.1 (8.9) | 72.1 (8.5) | .97 |
| Male sex, No. (%) | 129 (75) | 53 (77.9) | .63 |
| White race, No. (%) | 153 (89) | 62 (91.2) | .61 |
| Ever smoker, No. (%) | 118 (68.6) | 40 (58.8) | .15 |
| FVC, % predicted; mean (±SD) | 68.5 (19.3) | 67.5 (15) | .71 |
| DLCO, % predicted; mean (±SD) | 48.7 (20.3) | 50.4 (17.5) | .55 |
| Treatment | |||
| Pirfenidone, No. (%) | … | 39 (57.4) | … |
| Exposure months, median (IQR) | … | 17.3 (13.1-22.7) | … |
| Nintedanib, No. (%) | … | 20 (29.4) | … |
| Exposure months, median (IQR) | … | 14 (9.8-21.6) | … |
| Pirfenidone and nintedanib,a No. (%) | … | 9 (13.2) | … |
| Combined exposure months, median (IQR) | … | 10 (7.3-25.2) | … |
| Outcomes | |||
| Death, No. (%) | 72 (41.1) | 18 (25) | .03 |
| Transplant, No. (%) | 7 (4) | 2 (2.8) | .67 |
| Follow-up months, median (IQR) | 20.6 (8.4-32.3) | 22.8 (17.4-27) | .17 |
| Biomarkers | |||
| CA-125, pg/mL; median (IQR) | 40.6 (24.0-68.4) | 39.2 (18.4-59.3) | .4 |
| CXCL13, pg/mL; median (IQR) | 119.9 (76.7-186.8) | 109.9 (72.8-158.7) | .25 |
| MMP7, ng/mL; median (IQR) | 3.0 (1.8-4.7) | 3.4 (2.1-5.6) | .12 |
| SP-D, ng/mL; median (IQR) | 39.7 (25.9-53.5) | 34.4 (24.3-47.0) | .09 |
| YKL-40, ng/mL; median (IQR) | 54.9 (33.9-94.8) | 50.1 (29.2-107.7) | .89 |
| VCAM-1, ng/mL; median (IQR) | 944 (707-1283) | 797 (556-1023) | .006 |
| Osteopontin, ng/mL; median (IQR) | 33.0 (23.5-44.9) | 31.1 (21.2-42.7) | .12 |
AF = antifibrotic; CA-125 = cancer antigen 125; CXCL13 = C-X-C motif chemokine 13; Dlco = diffusing capacity of the lungs for carbon monoxide; IQR = interquartile range; MMP7 = matrix metalloproteinase 7; SP-D = surfactant protein D; VCAM-1 = vascular cell adhesion protein-1; YKL-40 = chitinase-3-like protein-1.
Nonconcurrent.
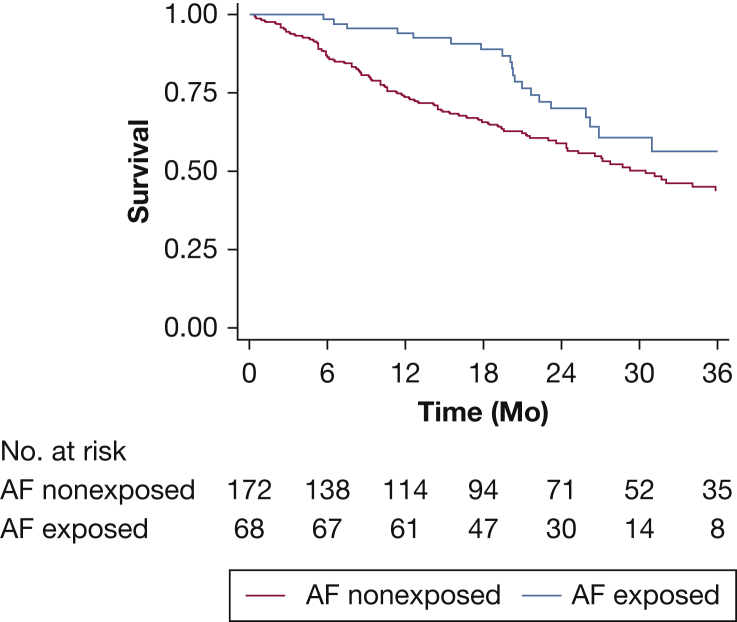
AF Exposure Survival Analysis
TFS was better among AF-exposed patients compared with nonexposed patients (Plogrank = .02) (Fig 2), with similar survival curves observed among each AF exposure group (e-Fig 2). AF exposure was associated with improved TFS in unadjusted analysis (HR, 0.57; 95% CI, 0.35-0.93; P = .03) and after adjustment for GAP score (HR, 0.55; 95% CI, 0.34-0.91; P =.02).
Figure 2.
Transplant-free survival in patients with idiopathic pulmonary fibrosis stratified by antifibrotic exposure.
Baseline Biomarker Survival Analysis
Among patients without AF exposure, increased CXCL13, MMP7, SP-D, VCAM-1, and OPN predicted reduced TFS (Table 2), with the strongest effect size observed for SP-D (HR, 2.69; 95% CI, 1.48-4.88). Suggestive association between TFS and YKL-40 concentration was also observed at P < .1. Among AF-exposed patients, increased concentrations of CA-125, CXCL13, MMP7, YKL-40, and OPN predicted reduced TFS, with YKL-40 demonstrating the strongest effect size (HR, 4.9; 95% CI, 2.01-11.96). Although CXCL13, MMP7, and OPN concentrations were associated with differential TFS in each treatment group, the optimal threshold for outcome prediction was 23% to 50% higher among AF-exposed individuals, with MMP7 having the highest relative difference in threshold (Table 2). Results were similar after multivariable adjustment, but with less precise effect estimates (e-Table 2).
Table 2.
Biomarker Survival Association Stratified by Antifibrotic Exposure
| Biomarker | AF Nonexposed (n = 172) |
AF Exposed (n = 68) |
||||
|---|---|---|---|---|---|---|
| Optimal Threshold | HR | 95% CI | Optimal Threshold | HR | 95% CI | |
| CA-125 | 225 pg/mL | 2 | 0.73-5.5 | 100 pg/mL | 4.27a | 1.23-14.8 |
| CXCL13 | 140 pg/mL | 1.74a | 1.12-2.7 | 209 pg/mL | 3.48a | 1.25-9.66 |
| MMP7 | 3.4 ng/mL | 1.65a | 1.06-2.58 | 5.1 ng/mL | 3.87b | 1.58-9.47 |
| SP-D | 30 ng/mL | 2.69b | 1.48-4.88 | 33 ng/mL | 1.25 | 0.51-3.06 |
| YKL-40 | 85 ng/mL | 1.58 | 0.98-2.54 | 101 ng/mL | 4.9b | 2.01-11.96 |
| VCAM-1 | 1,600 ng/mL | 2.26b | 1.32-3.87 | 1,520 ng/mL | 2.81 | 0.82-9.65 |
| OPN | 47 ng/mL | 1.72a | 1.04-2.84 | 58 ng/mL | 3.37a | 1.11-10.2 |
A Clinical-Molecular Signature of IPF Survival in AF-Exposed Patients
Variables constituting the final selected model were CA-125, MMP7, YKL-40, OPN, age, and percent predicted FVC (Table 3). Beta coefficients for these variables were used to generate a CMS-risk score (CMS-RS) based on the following equation:
| Eq. 1 |
Table 3.
Cox Regression Model Biomarker Beta Coefficients, Clinical Molecular Risk Score, and Clinical Molecular Signature Classification
| Biomarker | HR | Beta Coefficient | 95% CI | P | |
|---|---|---|---|---|---|
| CA-125 | 12.2 | 2.502 | 0.89 | 4.11 | .002 |
| MMP7 | 4.63 | 1.532 | 0.38 | 2.68 | .009 |
| YKL-40 | 6.59 | 1.886 | 0.82 | 2.96 | .001 |
| OPN | 4.98 | 1.605 | 0.25 | 2.96 | .02 |
| Age (1-unit increase) | 0.92 | −0.080 | −0.15 | −0.01 | .03 |
| FVC % predicted (1-unit increase) | 0.97 | −0.035 | −0.07 | 0.00 | .06 |
| CMS risk score (CMS-RS) = 11−(2.502 × CA125 “high”) + (1.532 × MMP7 “high”) + (1.886 × YKL-40 “high”) + (1.605 × OPN “high”) − (0.08 × age) − (0.0.035 × FVC [% predicted]). | |||||
| CMS-RS | Clinical Molecular Signature Classification | ||||
| 0-3.99 | Low risk | ||||
| 4.0-9.0 | High risk | ||||
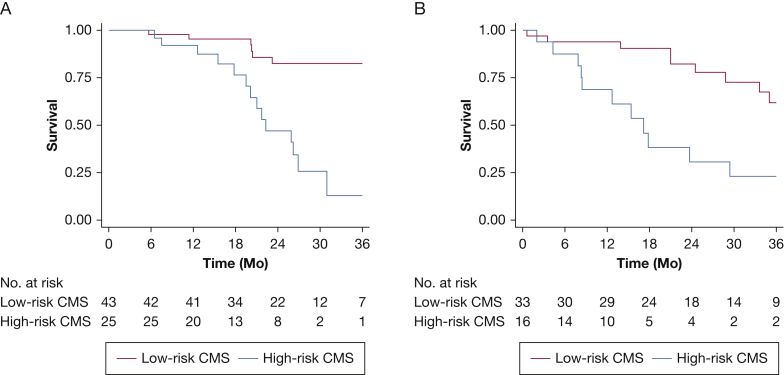
Based on the CMS-RS, patients were classified into “low-risk” and “high-risk” CMS groups (Table 3). TFS was significantly worse in those with a high-risk CMS compared with low-risk CMS (P < .001) (Fig 3A). Those with a high-risk CMS had a sixfold increase in mortality risk (HR, 5.91; 95% CI, 2.25-15.5; P < .001), which was maintained after adjustment for sex, race, smoking history, and percent predicted DLCO (HR, 5.35; 95% CI, 1.98-14.41; P = .001). The C-statistic for TFS risk discrimination was 0.68 for the CMS, compared with 0.61 for the GAP index. The CMS consistently predicted differential TFS in patients followed up at UC-Davis (n = 45; HR, 4.32; 95% CI, 1.36-14.45; P = .01), and UChicago (n = 23; HR, 7.13; 95% CI, 1.38-36.94; P = .02). The CMS showed sensitivity of 56% and specificity of 86% in discriminating survivors vs nonsurvivors during the follow-up period, with positive and negative predictive values of 0.77 and 0.7, respectively.
Figure 3.
Transplant-free survival in antifibrotic-treated patients with idiopathic pulmonary fibrosis stratified by clinical-molecular signature classification in derivation (A) and validation (B) cohorts.
Clinical-Molecular Signature Validation
The CMS was applied to an independent cohort of AF-exposed patients (n = 49) excluded from the initial analysis because of inclusion/exclusion criteria. This included 37 excluded because of AF exposure at the time of blood draw, 10 excluded because of AF exposure less than 50% of the follow-up period, and two excluded through propensity matching. TFS was significantly worse in those with a high-risk CMS compared with low-risk CMS (P = .001) (Fig 3B). Those with a high-risk CMS had a fourfold increase in mortality risk (HR, 3.97; 95% CI, 1.62-9.72; P = .003), which persisted after multivariable adjustment (HR, 3.88; 95% CI, 1.42-10.59; P = .008). The C-statistic for TFS risk discrimination was 0.68 for the CMS compared with 0.64 for the GAP index. The CMS showed sensitivity of 62% and specificity of 76% in discriminating survivors vs nonsurvivors during the follow-up period, with positive and negative predictive values of 0.57 and 0.79, respectively.
Longitudinal Change in Biomarker Concentration by Treatment Group
Among patients without AF exposure (n = 23), a significant annual increase in CA-125 (25.2 pg/mL; 95% CI, 7.08-43.32; P = .006), SP-D (12.96 ng/mL; 95% CI, 6.36-19.56; P < .001), VCAM-1 (313.68 ng/mL; 95% CI, 101.88-525.48; P = .004), and OPN (21.0; 95% CI, 10.92-30.96; P < .001) was observed (Table 4). Annual increases in CXCL13 and YKL-40 were also observed in nonexposed patients, but these did not reach statistical significance. All biomarkers except MMP7 decreased over 1 year in AF-exposed patients (n = 33), but none reached statistical significance. The effect of individual AF therapies on longitudinal change in biomarkers could not be assessed given the small sample size.
Table 4.
Annual Change in Plasma Biomarkers and FVC in Antifibrotic Exposed and Nonexposed Individuals
| Biomarker | Antifibrotic Nonexposed (n = 23) |
Antifibrotic Exposed (n = 33) |
||||||
|---|---|---|---|---|---|---|---|---|
| Change | 95% CI | P | Change | 95% CI | P | |||
| CA-125 (pg/mL) | 3.78 | 0.56 | 6.98 | .02 | −0.82 | −4.92 | 3.26 | .69 |
| CXCL13 (pg/mL) | 18.80 | 1.66 | 35.96 | .03 | −10.70 | −32.74 | 11.34 | .34 |
| MMP7 (ng/mL) | 0.02 | −0.14 | 0.18 | .79 | −0.04 | −0.24 | 0.16 | .68 |
| SP-D (ng/mL) | 1.82 | 0.66 | 2.96 | .002 | 0.26 | −1.20 | 1.74 | .73 |
| YKL-40 (ng/mL) | 3.78 | −1.26 | 8.80 | .14 | −4.68 | −11.20 | 1.72 | .15 |
| VCAM-1 (ng/mL) | 35.80 | 2.32 | 69.40 | .04 | 4.62 | −38.18 | 47.40 | .83 |
| Osteopontin (ng/mL) | 2.82 | 1.20 | 4.42 | .001 | −0.60 | −2.58 | 1.56 | .62 |
| Lung function | ||||||||
| FVC, mL | −27.74 | −54.20 | −1.30 | .005 | 8.80 | −25.32 | 43.10 | .33 |
| DLCO, mL/min/mm Hg | −0.31 | −0.55 | -0.07 | .01 | 0.14 | −0.17 | 0.45 | .38 |
Models adjusted for race, smoking history, and baseline GAP score. See Table 1 legend for expansions of abbreviations.
Discussion
In this investigation, we assessed biomarkers of TFS in IPF patients with and without AF exposure. We found that increased concentrations of CA-125, CXCL13, MMP7, YKL-40, and OPN predicted TFS in patients exposed to AF therapy but that thresholds were higher among AF-exposed patients compared with nonexposed patients. We then derived a CMS that predicted differential TFS in AF-exposed patients and validated this CMS in an independent cohort of AF-exposed patients. We then showed that biomarker concentration increased over time among patients without AF exposure but did not change in patients exposed to AF therapy. Taken together, these findings suggest that AF exposure may modulate relevant IPF biomarkers, supporting the need for validation of prior biomarker findings in AF-exposed patients.
To our knowledge, this study is among the first to assess the influence of AF therapy on IPF biomarkers as they relate to survival. Neighbors and colleagues recently assessed these and other biomarkers as predictors of FVC decline in patients participating in the pirfenidone clinical trials and found that only CCL18 concentration reliably predicted FVC decline across two independent cohorts.18 A suggestive association was observed with CXCL13, but this did not reach statistical significance. The observation that few biomarkers of IPF survival predict FVC decline parallels that observed with clinical prediction models19 and underscores the need for additional novel clinical and molecular predictors of lung function decline.
We also found that longitudinal change in biomarker concentration may be modulated by AF exposure, because these individuals showed little change in biomarker concentration over time. This stands in contrast to patients without AF exposure, in whom rising concentrations were observed for all biomarkers except MMP7. Interestingly, biomarker concentration may track with changes in lung function, because FVC and DLCO declined in those without AF exposure as biomarker concentrations rose. The significance of our findings remains unclear given the small subgroup of patients for whom longitudinal data were available. Neighbors and colleagues showed a similar suggestion of longitudinal modulation of biomarker level by pirfenidone exposure, albeit with inconsistent results across the two cohorts assessed.18 Maher and colleagues20 did not find any measurable effect of nintedanib exposure on longitudinal change in biomarkers of collagen synthesis, which have been shown to predict IPF progression when measured longitudinally and in cross-section.21,22 Our longitudinal analysis was limited by a small number of patients and could have been influenced by individual AF therapies, which could not be assessed given the small sample size for each.
Our preliminary CMS of IPF survival performed well in AF-exposed patients and showed consistent, albeit less discriminatory results in an independent validation cohort. Like the GAP index, this composite CMS takes advantage of cumulative risk explanation by multiple variables simultaneously that is missed when modeling individual biomarkers in isolation. Neighbors and colleagues pursued a similar approach and found a composite score of periostin, CCL18 ,and CXCL14 level predicted differential FVC decline in a derivation cohort but did not perform better than CCL18 alone in a validation cohort. TFS could not be assessed as an independent outcome in the Neighbors study, given the low prevalence of death and lung transplantation over the 52-week trial period. We believe our results demonstrate proof of concept and highlight the great potential for a composite clinical molecular signature to one day guide the management of this patient population. A similarly derived CMS that discriminates those with and without short-term FVC decline could allow for conservative management of some patients and enrichment of clinical trial datasets with patients likely to experience disease progression during the trial follow-up period.
Our findings may have implications for prior observations. Maher and colleagues2 showed that rising concentration of CA-125, a marker of epithelial injury, predicted increased mortality risk in a large, multi-center IPF cohort from the United Kingdom.2 We found that mean CA-125 concentration increased by 25.2 pg/mL (95% CI, 7.07-43.32) over 1 year in patients without AF exposure but remained stable in those exposed to AF therapy. Such modulation may influence attempts to risk stratify patients treated with AF therapy with longitudinal change in CA-125. Another study by White and colleagues8 showed that composite measure of MMP7, SP-D, and OPN concentration demonstrated good test performance in differentiating IPF from other forms of non-IPF ILD.8 Our findings suggest that AF exposure at the time of application may influence test performance. These biomarkers also appear to predict survival in non-IPF forms of ILD,23 so this issue will be compounded by increasing use of AF therapy in patients with non-IPF ILDs after recent clinical trials demonstrated that nintedanib and pirfenidone slow FVC decline in patients with progressive fibrosing ILD24 and progressive unclassifiable ILD,25 respectively.
We pursued a propensity-matched approach for this analysis in an attempt to balance cohorts drawn from different centers and over different timeframes. Such approach is among the preferred methods for assessment of treatment effect in observational26 data and should be considered when using historical ILD cohorts to assess treatment effect and compare biomarker prediction between treatment groups. This approach minimizes bias introduced by covariates on treatment effect and also may help account for variability in biomarker measures between treatment groups.27
This study had several limitations. First, despite efforts to robustly assess treatment effect in observational data by our propensity-matched approach, unmeasured variables may have influenced our propensity estimation, and our findings demonstrate association rather than causation. Prospective validation of these results will be needed. Next, although patients were recruited prospectively at each center and longitudinal blood samples were obtained at regular intervals, no standardized protocol for clinical data and blood sample acquisition was instituted. Next, despite our multicenter approach, sample sizes were relatively small after biomarker dicotomization, which likely left several of our analyses underpowered, especially with longitudinal modeling. Follow-up time was also asymmetric between institutions, leading us to censor survival at 36 months. This did not appear to influence our results, because findings were similar when using all available follow-up time (e-Table 3). Next, we chose to aggregate AF medications into a single dichotomous variable to increase power. Inherent differences in pirfenidone and nintedanib as they relate to biomarker modulation may have been missed by this approach and should be addressed individually in larger cohorts. Finally, protein degradation and batch effects may have influenced our results to an unknown extent.
Conclusion
With the increasing use of AF therapy in patients with IPF and high likelihood that AF therapy will soon be used to treat non-IPF forms of progressive ILD, our findings suggest that prior biomarker research should be validated in patients receiving this standard of care therapy. The last decade saw a rapid expansion of our understanding of IPF through biomarker data. The next decade has great potential to use these and other biomarkers to identify composite clinical-molecular signatures that inform outcome risk and treatment response.
Acknowledgments
Author contributions: J. M. O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Clinical data acquisition: A. A., S. A., N. S., C. T. L., W. B., J. V. P., M. E. S., I. N., J. M. O. Biologic sample processing: A. L., S. F. M., A. S., A. H., J. M. O. Study design: A. A., S. A., A. H., M. E. S., I. N., J. M. O. Data analysis: A. A., J. M. O. Interpretation of results: A. A., S. A., A. L., A. H., A. S., M. E. S., I. N., J. M. O. Manuscript preparation and review: A. A., S. A., N. S., C. T. L., W. B., J. V. P., A. L., S. F. M., A. H., M. E. S., I. N., J. M. O. All authors reviewed, revised, and approved the manuscript for submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. A. reports personal fees from Boehringer Ingelheim outside submitted work. M. E. S. reports grant funding from Boehringer Ingelheim, Galapagos, and Novartis and honoraria and consulting fees from Boehringer Ingelheim unrelated to the submitted work. I. N. reports grants from the NHLBI, honoraria for advisory boards with Boehringer Ingelheim, InterMune, Genentech and Anthera outside the submitted work. He has also received speaking honoraria from GSK and receives consulting fees for Immuneworks outside the submitted work. He also has study contracts with the NIH, Stromedix, Sanofi, and BI for the conduct of clinical trials in IPF. J. M. O. reports grants from the NHLBI and personal fees from Boehringer Ingelheim and Genentech outside submitted work. None declared (S. A., A. L., W. S. B., C. T. L., J. V. P., N. S., S. F. M., A. H., A. S.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Adegunsoye and Alqalyoobi contributed equally to this manuscript.
FUNDING/SUPPORT:National Heart, Lung, and Blood Institute (K23HL146942 [A. A.] and K23HL138190 [J. M. O.]) and American College of Chest Physicians Foundation Award (J. M. O.).
Supplementary Data
References
- 1.Korthagen N.M., van Moorsel C.H., Barlo N.P. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med. 2011;105(1):106–113. doi: 10.1016/j.rmed.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Maher T.M., Oballa E., Simpson J.K. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5(12):946–955. doi: 10.1016/S2213-2600(17)30430-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosas I.O., Richards T.J., Konishi K. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J.W., Do K.H., Jang S.J. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143(5):1422–1429. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 5.Tzouvelekis A., Herazo-Maya J.D., Slade M. Validation of the prognostic value of MMP-7 in idiopathic pulmonary fibrosis. Respirology. 2017;22(3):486–493. doi: 10.1111/resp.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuga L.J., Tedrow J.R., Pandit K.V. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189(8):966–974. doi: 10.1164/rccm.201309-1592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards T.J., Kaminski N., Baribaud F. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White E.S., Xia M., Murray S. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194(10):1242–1251. doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richeldi L., du Bois R.M., Raghu G. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 10.King T.E., Jr., Bradford W.Z., Castro-Bernardini S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 11.Noble P.W., Albera C., Bradford W.Z. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 12.Nathan S.D., Albera C., Bradford W.Z. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41. doi: 10.1016/S2213-2600(16)30326-5. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster L., Crestani B., Hernandez P. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res. 2019;6(1) doi: 10.1136/bmjresp-2018-000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu G., Rochwerg B., Zhang Y. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis—an update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 15.Raghu G., Remy-Jardin M., Myers J.L. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 16.Ley B., Ryerson C.J., Vittinghoff E. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rubin D.B. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Services & Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 18.Neighbors M., Cabanski C.R., Ramalingam T.R. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir Med. 2018;6(8):615–626. doi: 10.1016/S2213-2600(18)30185-1. [DOI] [PubMed] [Google Scholar]
- 19.Ley B., Bradford W.Z., Vittinghoff E. Predictors of mortality poorly predict common measures of disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;194(6):711–718. doi: 10.1164/rccm.201508-1546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maher T.M., Stowasser S., Nishioka Y. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir Med. 2019;7(9):771–779. doi: 10.1016/S2213-2600(19)30255-3. [DOI] [PubMed] [Google Scholar]
- 21.Organ L.A., Duggan A.R., Oballa E. Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir Res. 2019;20(1):148. doi: 10.1186/s12931-019-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins R.G., Simpson J.K., Saini G. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3(6):462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 23.Alqalyoobi S., Adegunsoye A., Linderholm A. Circulating plasma biomarkers of progressive interstitial lung disease. Am J Respir Crit Care Med. 2020;201(2):250–253. doi: 10.1164/rccm.201907-1343LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty K.R., Wells A.U., Cottin V. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2020;382(8):780. doi: 10.1056/NEJMc1917224. [DOI] [PubMed] [Google Scholar]
- 25.Maher T.M., Corte T.J., Fischer A. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 26.Stuart E.A. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuan P.F. Propensity score method for partially matched omics studies. Cancer Inform. 2014;13(Suppl 7):1–10. doi: 10.4137/CIN.S16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.