Abstract
Purpose.
It can be challenging to differentiate pseudoprogression from progression. We assessed the ability of dynamic contrast enhanced T1 MRI (DCE-MRI) perfusion to identify pseudoprogression in melanoma brain metastases.
Methods.
Patients with melanoma brain metastases who underwent immunotherapy and DCE-MRI were identified. Enhancing lesions ≥ five mm in diameter on DCE-MRI and that were new or increased in size between a week from beginning the treatment, and a month after completing the treatment were included in the analysis. The 90th percentiles of rVp and rKtrans and the presence or absence of hemorrhage were recorded. Histopathology served as the reference standard for pseudoprogression. If not available, pseudoprogression was defined as neurological and radiographic stability or improvement without any new treatment for ≥ 2 months.
Results.
Forty-four patients were identified; 64% received ipilimumab monotherapy for a median duration of 9 weeks (range, 1-138). Sixty-four lesions in 44 patients were included in the study. Of these, nine lesions in eight patients were determined to be pseudoprogression and seven lesions were previously irradiated. Forty-four progression lesions and 8 pseudoprogression lesions were hemorrhagic. Median lesion volume for pseudoprogression and progression were not significantly different, at 2.3 cm3 and 3.2 cm3, respectively (p=0.82). The rVp90 was smaller in pseudoprogression versus progression, at 2.2 and 5.3, respectively (p=0.02), and remained significant after false discovery rate adjustment (p=0.04).
Conclusions.
Pseudoprogression exhibited significantly lower rVp90 on DCE-MRI compared with progression. This knowledge can be useful for managing growing lesions in patients with melanoma brain metastases who are receiving immunotherapy.
Keywords: DCE-MRI, pseudoprogression, melanoma, brain metastases, immune checkpoint inhibitor
Introduction
Melanoma patients face poor prognosis and higher mortality risk, especially when multiple brain metastases are present.1,2 Immune checkpoint inhibition of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) and Programmed Death-1 Receptor (PD-1) has become the standard of care for metastatic melanoma.3 However, evaluation of its clinical efficacy remains challenging due to treatment induced pseudoprogression.
Several treatment response criteria for determining treatment response versus failure have been proposed, including Response Evaluation Criteria in Solid Tumors (RECIST), immune-related RECIST (irRECIST), immune-related response criteria (irRC), and Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM). These rely on changes in the size of the enhancing lesion pre- and post-treatment.4–6 However, during the course of immunotherapy, the use of these criteria is often complicated by an initial increase in enhancing size before treatment response, which may occur as a result of activation of the body’s own immune system.4,7
There is an urgent need for prompt, accurate diagnosis of pseudoprogression to make informed clinical treatment decisions. Current response criteria require confirmation such as additional follow up and/or histopathology at re-resection, which may result in delayed or inappropriate treatments. MRI perfusion allows for the quantification of lesion vascularity and hemodynamics. Several studies have shown that MRI perfusion can be useful for determining pseudoprogression in glioblastomas.8–12 However, its utility has not been established for melanoma brain metastases. We hypothesized that pseudoprogression occurring in melanoma brain metastases will demonstrate lower T1-weighted dynamic contrast enhanced MRI (DCE-MRI) perfusion parameters than those due to true progression.
Materials and Methods
Study design and participants
We undertook a single institution, Health Insurance Portability and Accountability compliant, retrospective study approved by the local Institutional Review Board under a waiver of informed consent. From institutional and departmental databases, we identified adult patients (18 years or older) who had malignant melanoma and brain metastases, received immune checkpoint inhibition, and underwent MRI perfusion from January 2011 to June 2016. Chart reviews were performed by a neuro-oncologist with six years of clinical experience with brain tumor patients. BRAF V600E/K mutation data of the patients’ original tumors were collected when available. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Lesion selection and diagnosis
All enhancing lesions that were five mm or more in diameter and that were new or increased in size >1 week after beginning of immunotherapy and <1 month after completion of immunotherapy were included in the analysis. According to the recently proposed RANO-BM criteria, up to five lesions were measured in each patient.5 When histopathology was available, pseudoprogression was defined as necrotizing treatment effects without tumor. When histopathology was unavailable, pseudoprogression was defined as neurological and radiographic stability or improvement without any new treatment for at least two months.12,13
DCE-MRI protocol and analysis
MRI was acquired on 1.5T and 3T magnets (Discovery 450/450w and 750/750w, GE Healthcare, Milwaukee, WI). A dynamic bolus of gadobutrol (Gadavist, Bayer Healthcare, Wayne, NJ) was administered at 0.1 mmol/kg body weight via by power injector at 2-3 mL/s followed by a 40 mL saline flush. DCE-MRI of the brain was acquired with a 3D T1-weighted spoiled gradient recalled sequence (SPGR) sequence with time-to-repetition=4-5ms; time-to-echo 1-2ms; slice thickness=5mm; flip angle=25°; field-of-view=24cm; matrix=128x128; and temporal resolution=5-6s. The T1 weighted gradient echo sequence is sensitive to the low concentrations of contrast that permeate through the capillary walls and is used to measure parameters that reflect permeability. The sequence has excellent spatial resolution and relative insensitivity to hemorrhage-related susceptibility artifacts compared to dynamic susceptibility contrast (DSC) perfusion.
Standard MRI sequences including T1-weighted images with and without contrast, and T2-weighted, FLAIR, diffusion-weighted, and susceptibility weighted (SWI) images were also obtained. DCE-MRI data and matching contrast T1-weighted images were transferred to an off-line workstation and processed using commercial software (NordicIce 2.3.14; Nordic NeuroLab, Bergen, Norway). Image analysis included noise and motion correction preprocessing, followed by a semi-automated arterial input function to model the input function curve and the concentration-time curve.14 The two-compartment pharmacokinetic model proposed by Tofts was applied to calculate plasma volume (Vp) and time-dependent leakage constant (Ktrans) parametric maps.15
A trained operator with one year of experience in DCE-MRI, while blinded to the lesion diagnosis, performed all perfusion postprocessing and measurements under the direct supervision of a board-certified radiologist with a certificate of added qualification in neuroradiology and over sixteen years of experience. A volume-of-interest (V OI) was manually drawn around the entire enhancing lesion including any hemorrhage, and then transferred to the Vp and Ktrans maps. The measurements were binned into histograms and then normalized to the normal contralateral brain parenchyma to derive Vp and Ktrans ratios (rVp and rKtrans). The normal regions-of-interest (ROI) were selected using a fixed 4-5mm diameter ROI at the mid slice through the lesion. Based on literature suggesting optimal sensitivity and specificity,12,16 the 90th percentiles of normalized rVp (rVp90) and rKtrans (rKtrans90) were recorded. An experienced neuroradiologist also examined the MRI scans to record the presence or absence of hemorrhage using susceptibility weighted images (SWI), and when present if the lesion consisted of ≥90% hemorrhage.
Statistical analysis
Univariate analysis was performed using the Wilcoxon rank-sum test to determine the relationship between DCE-MRI parameters in the pseudoprogression and progression groups and the Monte Carlo estimate for the exact Kruskal-Wallis test to assess the relationship between DCE-MRI parameter and type of immunotherapy courses. The Hochberg and Benjamini false discovery rate (FDR) procedure was used to adjust for simultaneous multiple testing. Area under the curves (AUC) were plotted to determine the discrimination power of DCE-MRI parameters by progression status and were compared using the Delong’s test. The relationship between previous irradiation and progression status was analyzed using Fisher’s exact test.
The median overall survival (OS) was determined using the Kaplan-Meier method, calculated from the MRI-DCE scan date that determined pseudoprogression vs. progression to the date of death or follow up. For patients who had multiple lesions, the scan date of the first lesion was used in OS analysis. The OS in two groups were compared using the log-rank test. Statistical significance was set at p<0.5. SAS release 9.4 (SAS Institute, Carry NC) and R version 3.5.2 package ROCR and pROC were used for analyses.
Results
Patient characteristics
As summarized in Figure 1, a total of 44 patients with melanoma brain metastases met all study inclusion criteria. Patient characteristics are listed in Table 1. The median age of patients was 64 years (range, 32-86) and 34% were women (n=15). The most common immunotherapy course at the time of brain lesion enlargement was ipilimumab monotherapy (64%, n=28), followed by PD-1 inhibitor monotherapy (pembrolizumab or nivolumab, 27%, n=12). Other immunotherapy courses were nivolumab combined with ipilimumab (2%, n=l) or lirilumab (2%, n=l), and monotherapy with pidilizumab (2%, n=l) or flanvotumab (2%, n=l). The median duration of immunotherapy course was 9 weeks (range, 1-138). A total of 64 brain lesions were measured, with a median of 1 lesion per patient (range, 1-5); 32 patients had 1 measurable lesion and 12 patients had > 1 measurable lesions.
Figure 1.
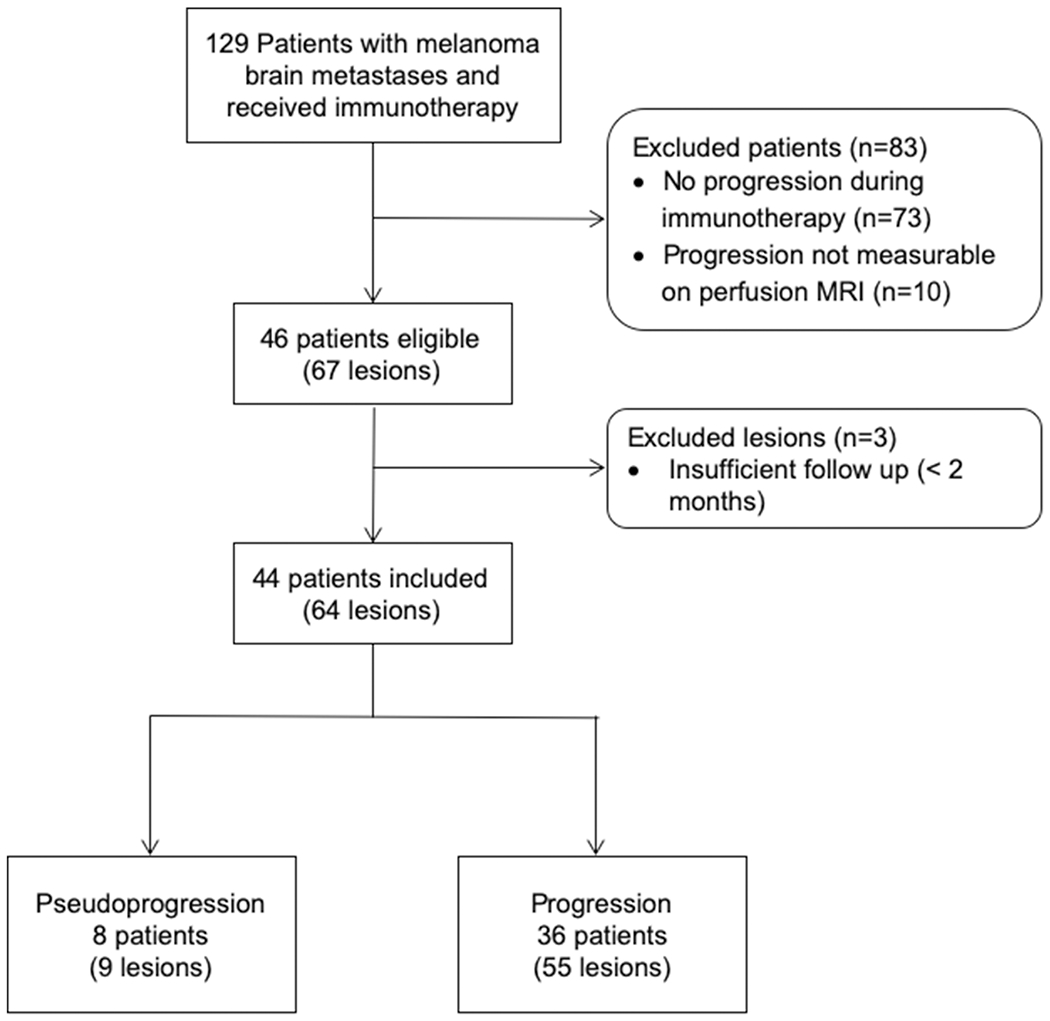
Eligibility and inclusion of patients .Total of 129 patients with melanoma brain metastases who received immunotherapy were screened for this retrospective study. Patients were excluded for no observed progression while receiving immunotherapy (n=73), non-measurable progression on DCE-MRI perfusion (n=10). Out of 67 lesions from 46 patients who were eligible, 3 lesions were excluded from this study as they were felt to be pseudoprogression, however, there was no histopathological confirmation nor sufficient follow up duration of 2 months. Finally, 64 lesions from 44 patients were included in the study, which comprised of 8 pseudoprogression lesions from 9 patients, and 55 progression lesions from 36 patients.
Table 1.
Patient demographics and lesion characteristics
| All | Pseudoprogression | Progression | |
|---|---|---|---|
| Patients, n | 44 | 8 | 36 |
| Median age (range) | 64 (32–86) | 52 (38–67) | 66 (32–86) |
| Women, n (%) | 15 (34%) | 4 (50%) | 11 (31%) |
| Immunotherapy: n (%) | |||
| Ipilimumab monotherapy | 28 (64%) | 4 (50%) | 24 (67%) |
| Pembrolizumab monotherapy | 9 (20%) | 2 (25%) | 9 (19%) |
| Nivolumab monotherapy | 3 (7%) | 1 (13%) | 2 (6%) |
| Other | 4 (9%) | 1 (13%) | 3 (8%) |
| Median immunotherapy duration, weeks (range) | 9 (0–138) | 9 (4–138) | 9 (0–67) |
| Lesions, n | 64 | 9 | 55 |
| Median # per patient, (range) | 1 (1–5) | 1 (1–2) | 1 (1–5) |
| Tissue diagnosis, n (%) | 14 (22%) | 1 (11%) | 13 (24%) |
| New lesions, n (%) | 20 (31%) | 1 (11%) | 19 (35%) |
| Previously irradiated, n (%) | 20 (31%) | 8 (89%) | 11 (20%) |
| Stereotactic radiosurgery | 14 | 7 | 7 |
| Whole brain radiotherapy | 5 | 0 | 5 |
| Hypofractionated radiation | 1 | 1 | 0 |
| Median days from radiation (range) | 65 (12–826) | 121 (38–826) | 32 (12–89) |
| Median days from immunotherapy (range) | 49 (5–762) | 55 (37–361) | 48 (5–762) |
| Hemorrhagic, n (%) | 52 (81%) | 8 (89%) | 44 (80%) |
Pseudoprogression
Nine lesions in 8 patients were determined to be pseudoprogression (including by tissue diagnosis in 1 lesion). The median age of patients with pseudoprogression was 52 years (range, 38-67), and 50% were women (n=4). Immunotherapy courses included ipilimumab monotherapy (n=4, 50%), PD-1 inhibitor monotherapy (pembrolizumab or nivolumab, n=3, 38%), and pidilizumab monotherapy (n=l, 13%). Seven patients had only 1 measurable lesion and one patient had 2 measurable lesions.
Pseudoprogression was noted on MRI-DCE at a median of 1.8 months (range 1.2-12.0) after starting immunotherapy. One pseudoprogression lesion (11%) presented as a new enhancing lesion. Eight pseudoprogression lesions (89%) had previously been irradiated; 7 received stereotactic radiosurgery (SRS) at 2100 cGy, and 1 received hypoffactionated radiotherapy at 600 cGy x5 fractions. In lesions previously irradiated, pseudoprogression was noted on DCE-MRI at a median of 4.0 months (range 1.3-27.5) after the completion of radiotherapy. Eight lesions (89%) were found to be hemorrhagic, with 7 (78%) consisting of ≥90% hemorrhage.
One patient had a lesion with pathologic confirmation of pseudoprogression, which was also a new lesion on MRI rather than a change in previously noted lesions. This was also the only lesion that had not been irradiated previously. The other 7 patients remained neurologically stable for a median of 10.3 months (range 2.1-50.7) from the time of MRI-DCE without change in treatment.
Progression
Fifty-five lesions in 36 patients were determined to be progression (including by tissue diagnosis in 13 lesions). Median age was 66 years (range, 32-86), and 31% were women (n=11). Progression was noted on MRI-DCE at a median of 1.6 months (range 0.2-25.4) after starting immunotherapy. Immunotherapy courses consisted of ipilimumab monotherapy (n=24, 67%), PD-1 inhibitor monotherapy (pembrolizumab or nivolumab, n=9, 25%), nivolumab with ipilimumab (n=1, 3%) or lirilumab (n=1, 3%), and flanvotumab monotherapy (n=1, 3%). Twenty-five patients had only 1 measurable lesion and eleven patients had 2-5 lesions. Nineteen out of 55 lesions (35%) were new and 11 lesions (20%) had previously been irradiated. Of the irradiated lesions, 7 lesions underwent SRS at 1800 cGy (n=3) or 2100 cGy (n=4), and the other 5 lesions were treated with whole brain radiotherapy with total dose of 3750 cGy divided in 15 fractions. In lesions previously irradiated, DCE-MRI was performed at a median of 32 days (range, 1289) after completion of radiation therapy. Forty-four lesions (80%) were hemorrhagic, with more than half of these lesions (n=25, 57%) consisting of >90% hemorrhage.
Comparison of lesion volume and DCE-MRI parameters between the two groups
The median lesion volume was not significantly different between the pseudoprogression group versus the progression group, at 2.3 cm3 (range, 0.5-22.6) versus 3.2 cm3 (range, 0.4-59.0), respectively (p=0.82). The median lesion volume for lesions with >90% hemorrhage was significantly greater than that of the ≤90% hemorrhagic and non-hemorrhagic lesions, at 4.6 cm3 (range, 0.6-59) and 2.6 cm3 (range 0.4-26.4), respectively (p=0.009) in 55 lesions with progression.
As for DCE-MRI parameters (Table 2), the median rVp90 was smaller in pseudoprogression at 2.2 (range, 1.0-9.4) than in progression at 5.3 (range, 1.9-17.4), (p=0.02). Results remained significant after false discovery rate adjustment (p=0.04). The median rKtrans90 was also smaller in pseudoprogression at 4.0 (range, 2.3-12.3) than in progression at 6.8 (range, 1.3-37.4), (p=0.047); however, this did not remain significant after false discovery rate adjustment (p=0.06). There was no difference in rVp90 (p=0.09) nor rKtrans90 (p=0.20) between the different immune checkpoint inhibitors in 55 lesions with POD.
Table 2.
DCE-MRI perfusion measurements
| Pseudoprogression | Progression | p-value | FDR adjusted p-value | |
|---|---|---|---|---|
| Median Lesion volume, cm3 (range) | 2.3 (0.5–22.6) | 3.2 (0.4–59.0) | 0.82 | 0.82 |
| Median rVp90 (range) | 2.2 (1.0–9.4) | 5.3 (1.9–17.4) | 0.02 | 0.04 |
| Median rKtrans90 (range) | 4.0 (2.3–12.3) | 6.8 (1.3–37.4) | 0.047 | 0.06 |
rVP90 = 90th percentile plasma volume ratio; rKtrans90 = 90th percentile time-dependent leakage constant; FDR = false discovery rate
There were more previously irradiated lesions in the pseudoprogression group (89%) than in the progression group (20%) (p=0.001). Although there seemed to be a difference in proportion of BRAF V600E/K mutation in pseudoprogression (67%) and in progression (42%), the difference in the distribution of pseudoprogression and progression occurrences between BRAF V600E/K mutated melanomas and other melanoma metastases was not statistically significant (p=0.28 by Fisher’s exact test).
An rVp90 threshold of ≥3.2 had 66.7% specificity and 85.5% sensitivity for predicting progression – where 33.3% of progressions would be misclassified while 14.5% of pseudoprogressions would be misclassified. An rKtrans90 threshold of ≥5.1 had 66.7% specificity and 69.1% sensitivity – where 33.3% of progressions would be misclassified while 30.9% of pseudoprogressions would be misclassified. Representative AUCs are shown in Figure 2, with statistical insignificant results between rVp90 at 0.754 and rKtrans90 at 0.709 (p=0.21 by Delong’s test). The AUC of lesion volume was 0.525.
Figure 2.
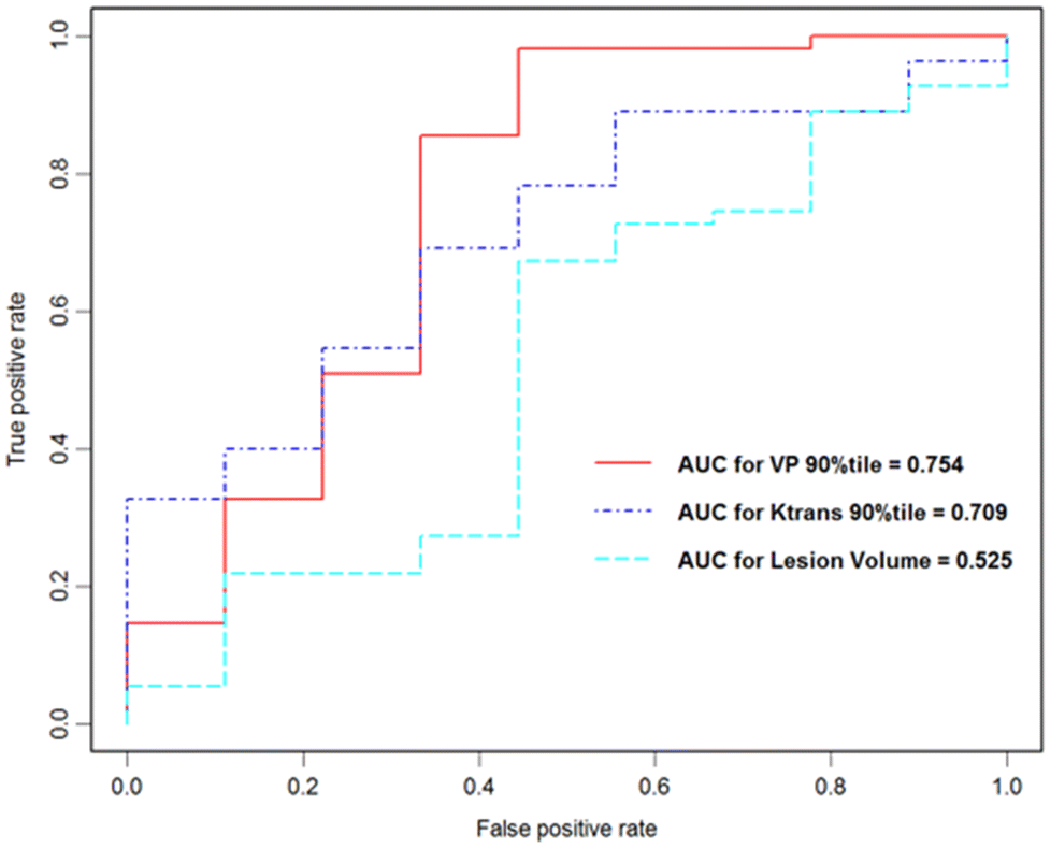
Area under the ROC curves for DCE-MRI perfusion versus lesion volume
DCE-MRI derived rKtrans90 and rVp90 measurements performed significantly better than lesion volume in determining tumor progression (p=0.047 and p=0.02, respectively).
Examples of progression and pseudoprogression DCE-MRI perfusion scans are demonstrated in Figure 3.
Figure 3.
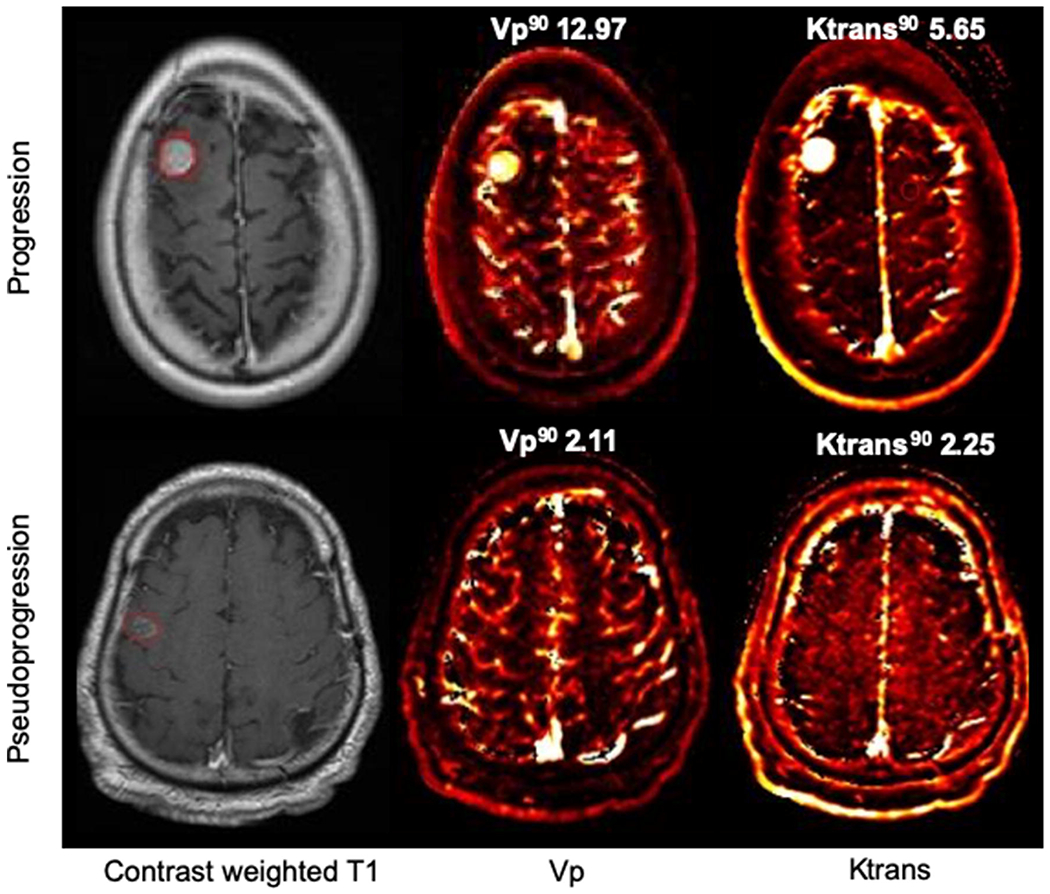
DCE-MRI perfusion scans
In progression (top row), T1 contrast enhancing lesion has corresponding elevated Vp90 and Ktrans90 on DCE-MRI perfusion scan. In pseudoprogression (bottom row), the contrast enhancing area has lower corresponding Vp90 and Ktrans90 values.
Exploratory survival analysis
During the follow up period, 35 patients (80%) died and 9 were alive (20%). The median follow up time for survivors was 58 months (range, 13-86). The median OS from the MRI-DCE scan date in the pseudoprogression group (n=8) was 23.1 months (95% confidence interval 14.37- not reached), and in the progression group (n=36) was 12 months (95% confidence interval, 7-29 months). There was no statistically significant difference in OS between the two groups (p=0.20) during the follow-up period.
Discussion
This study evaluated if DCE-MRI perfusion parameters could distinguish between pseudoprogression and progression in immunotherapy-treated melanoma brain metastases. We found that both pseudoprogression and progression lesions were statistically similar in volume, suggesting that size may be an insufficient measure for distinguishing between these two entities. Pseudoprogression lesions had significantly lower plasma volume compared with progression lesions. Our results confirmed our study hypothesis.
In patients with brain melanoma metastases, immune checkpoint inhibition is becoming the standard of care. As such, challenges in interpreting radiographic response in systemic lesions as well as brain metastases are important to address. Favorable treatment responses to immunotherapy have been shown to occur after an initial worsening of imaging results, which would otherwise have been deemed as progression by conventional measurement criteria.4 Immunotherapy has also been associated with increased delayed radiation necrosis in irradiated lesions compared to lesions treated with cytotoxic or targeted therapy.17 Our results suggest that DCE-MRI perfusion scans may facilitate early, accurate diagnosis of pseudoprogression, enabling more informed decisions to continue effective treatment.
Immune checkpoint inhibitors are increasingly being studied in various cancers. Thus, it is necessary to develop a method to differentiate pseudoprogression from true disease progression to guide effective treatment in both primary and metastatic brain tumors.3 Previously DCE-MRI perfusion have been shown able to distinguish pseudoprogression from true progression in glioblastomas, where pseudoprogression is a familiar entity occurring after radiation and chemotherapy in glioblastomas.12,18 As in our study, pseudoprogression lesions demonstrated lower perfusion parameters than progression lesions.
Several potential limitations are associated with this study. First, this was a retrospective study with a relatively small number of subjects. However, our results show significant differences between the pseudoprogression and progression lesions and suggest that DCE-MRI may be a useful diagnostic tool. Second, not all lesions were histopathologically confirmed. However, since lesions without sufficient clinical follow up durations were excluded from the study, we remain confident in the lesion diagnoses. This also mimics usual clinical practice, when most patients are followed and pathologic confirmation is often not obtained due to neurosurgical risks and feasibility. Follow up also avoids the inherent referral bias in preferentially sending patients thought likely to have tumor progression to repeat surgery, since surgery provides both diagnostic data and therapeutic benefit. The immunotherapy-specific RANO working committee recommends following patients radiographically for three months after the initial radiographic evidence of progressive disease, even when patients develop new lesions. These recommendations should be followed if patients do not have substantial neurologic decline to avoid prematurely declaring progression of disease in patients with pseudoprogression.18 Third, the majority of pseudoprogression lesions were also previously irradiated; therefore, there is also probably a component of radiation-induced treatment effect in addition to the immunotherapy effect. Separating the effects of immunotherapy and radiation therapy is difficult given the complementary roles of both treatments in routine clinical practice. In addition, pseudoprogressions were likely attributable for both immunotherapy and radiation, and it is difficult to differentiate due to the retrospective design of this study. Moreover, most of the pseudoprogresssion lesions were previously treated with radiotherapy, and we are unable to differentiate the attribution between radiation effect alone from immunotherapy effect alone, however the combination likely contributed for the overall pseudoprogression. Fourth, hemorrhage is common in some brain metastases including melanoma. 19 Although hemorrhage is a major confounder in T2*-weighted dynamic susceptibility contrast (DSC)-MRI perfusion techniques, the T1-weighted DCE-MRI perfusion is relatively resilient to susceptibility artifacts induced by hemorrhage and therefore able to yield accurate measurements. Despite the number of mostly hemorrhagic lesions containing >90% hemorrhage, our measurements in no case were compromised by technical artifact caused by the hemorrhage. Lastly, the exploratory survival analysis was limited with the small number of events.
We found lower perfusion parameters in pseudoprogression, suggesting that DCE-MRI derived capillary permeability (Ktrans) and plasma volume measurements can be helpful in the diagnosis of pseudoprogression in melanoma patients with brain metastases after being treated with immunotherapy. This trend of perfusion parameters is may also be seen in immunotherapy-related pseudoprogression in brain metastases from other cancers and primary brain tumors. Additional research examining perfusion changes in the setting of immunotherapy will improve our understanding and facilitate better informed treatment decisions.
Acknowledgments
Funding
Research reported in this publication was partially supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA020449 and Core Grant P30 CA008748. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Conflict of Interest
Alexander N Shoushtari is on the advisory board for Bristol-Myers Squibb, Immunocore, Castle Bioscences, and has institutional research support from Bristol-Myers Squibb, Immunocore, AstraZeneca, and Xcovery. Robert J Young consults for Agios, Puma, NordicNeuroLab, Icon, and has a grant funding from Agios. All other authors have no conflict of interests related to this study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Previous Presentations
This study was presented orally at the 5th Quadrennial Meeting of the World Federation of Neuro-Oncology Societies held in Zurich, Switzerland in May 2017.
References
- 1.Gupta G, Robertson AG, MacKie RM. Cerebral metastases of cutaneous melanoma. British journal of cancer. 1997;76(2):256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30(6):515–520. [DOI] [PubMed] [Google Scholar]
- 3.Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33(31):3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–278. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 7.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8): 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR American journal of neuroradiology. 2015;36(5):877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young RJ, Gupta A, Shah AD, et al. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clinical imaging. 2013;37(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, Baradaran H, Delgado D, et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro-oncology. 2017;19(1): 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Martinez-Lage M, Sakai Y, et al. Differentiating Tumor Progression from Pseudoprogression in Patients with Glioblastomas Using Diffusion Tensor Imaging and Dynamic Susceptibility Contrast MRI. AJNR American journal of neuroradiology. 2016;37(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas AA, Arevalo-Perez J, Kaley T, et al. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol. 2015;125(1):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 14.Jung SC, Yeom JA, Kim JH, et al. Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR American journal of neuroradiology. 2014;35(6): 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging : JMRI. 1999;10(3):223–232. [DOI] [PubMed] [Google Scholar]
- 16.Arevalo-Perez J, Thomas AA. T1-Weighted Dynamic Contrast-Enhanced MRI as a Noninvasive Biomarker of Epidermal Growth Factor Receptor vIII Status. AJNR Am J Neuroradiol. 2015;36(12):2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016; 125(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 18.Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruitt AA. Medical management of patients with brain tumors. Continuum (Minneap Minn). 2015;21(2 Neuro-oncology) :314–331. [DOI] [PubMed] [Google Scholar]


