Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.1
Keywords: pathology competencies, organ system pathology, hematopathology, leukocytosis, eosinophilia, neutrophilia, monocytosis, basophilia
Primary Objective
Objective HWC1.5: Neutrophilia. Describe the common causes for neutrophilia, lymphocytosis, monocytosis, eosinophilia, and basophilia.
Competency 2: Organ system Pathology; Topic HWC: Hematopathology—White Cell Disorders; Learning Goal 1: Development of White Blood Cells and Nonneoplastic Causes of Neutropenia.
Patient Presentation
A 26-year-old Caucasian male presents for evaluation of symptoms which include rhinorrhea, sinusitis, epiphora (watering eyes), pharyngitis, and fatigue. He reported that he recently adopted a dog from the animal shelter and is concerned that animal dander may be the reason for his symptoms. However, the patient was confused as to why he is experiencing symptoms from animal dander because he did not experience these allergies until approximately a week after adopting the dog.
Diagnostic Findings
The patient’s vital signs are blood pressure of 125/80 mm Hg, a heart rate of 72 beats per minute, a respiratory rate of 18 breaths per minute, and a temperature of 98.9 °F. Physical examination shows dry skin, small, raised bumps on the patient’s chest and red-brown patches on the chest and legs behind the knees. The physician ordered an initial diagnostic workup including a complete blood count (CBC), differential (Table 1), IgE antibodies (Table 2), and a percutaneous allergy skin test (Table 3).
Table 1.
Complete Blood Count.
| CBC | Patient | Reference range |
|---|---|---|
| WBC count | 13 × 103/uL | 4.5-11.0 × 103/uL |
| RBC count | 5.0 × 106/uL | 4.5-5.5 × 106/uL |
| Hemoglobin | 15.2 g/dL | 13.5-17.5 g/dL |
| Hematocrit | 42.0% | 35.0%-45.0% |
| MCV | 88.0 fL | 78.0-100.0 fL |
| MCH | 29.0 pg | 26.0-34.0 pg |
| MCHC | 33.0 g/dL | 31.0-37.0 g/dL |
| RDW | 12.0% | 11.0%-14.0% |
| Platelet count | 300 × 10^3/dL | 150-450 ×10^3/uL |
| MPV | 7.0 fL | 6.0-10.0 fL |
| Neutrophil, % | 55 | 50-70 |
| Lymphocytes, % | 22 | 20-30 |
| Monocyte, % | 10 | 3-11.0 |
| Eosinophil, % | 11 | 0.0-6.0 |
| Basophil, % | 2 | 0.0-1.0 |
Abbreviations: CBC, complete blood count; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin content; MCV, mean corpuscular volume; MPV, mean platelet volume; RBC, red blood cell; RDW, red cell distribution width; WBC, white blood cell.
Table 2.
IgE Antibody.
| Immunoglobulin E antibody value (RIST) | 610 U/mL | Reference range: 4.2-595 U/mL |
Abbreviation: RIST, radioimmunosorbent test.
Table 3.
Percutaneous Allergy Skin Test.
| Allergen | Patient wheal size (mm) | Result |
|---|---|---|
| Positive control (histamine) | 3.5 mm | Positive |
| Negative control (saline) | 0 mm | Negative |
| Pollen | 0 mm | Negative |
| Animal dander (dog) | 3.2 mm | Positive |
| Dust mites | 0 mm | Negative |
Questions/Discussion Points
Interpret the Patient’s Complete Blood Cell Count
The patient has an elevated white blood cell count. Specifically, the white blood cell differential shows elevated eosinophils (11%). Elevated eosinophil counts could be due to an exaggerated immune response to an allergen, infection, or inflammation among other conditions such as a parasitic infection or certain drug reactions seen with leukocytosis. The red blood cell indices such as the red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red cell distribution width are unremarkable and the slide review correspondingly shows normochromic and normocytic red blood cells. The platelet count and mean platelet volume are also within the reference range and the slide shows that platelets were normal in number and morphology. Both the red blood cell and platelet indices are normal which indicates that they are not affected by the allergic reaction.
Given the Eosinophilia Presented in the CBC and Peripheral Smear, What Conditions Would Be Considered in the Differential Diagnosis?
Eosinophilia can be seen in conditions such as skin disease, a parasitic disease, hypersensitivity reactions, transplant rejection, myeloproliferative disorders, and asthma.2 Formed in the bone marrow, eosinophils undergo the same early maturation as the most common leukocyte, neutrophils. As the cell matures, specific refractive granules containing peroxidase, arginine, lysine, phospholipids, and major basic protein form, and these play an important role in combating parasitic invaders. Eosinophils spend approximately 1 to 8 hours in the blood in transit to tissues to perform their functions. Eosinophils are considered a phagocyte and some chemotactic factors that eosinophils respond to are bacterial products and antigen–antibody complexes, such as a possible response to an allergen in this case. Phagocytosis by eosinophils is entirely antibody-dependent but may be seen in collagen-vascular diseases, neoplastic disorders, and some immune deficiency states.3
In the case of parasitic invaders, the most common cause of eosinophilia is due to helminth infections from resource-limited countries, refugees/immigrants, and travelers.4 These include Strongyloides, Trichinella, Toxocara canis, and Schistosoma. Echinococcus granulosus is a tapeworm that enters the body of patients who have had contact with dog feces that may carry the parasite.5
What Is Most Likely the Differential Diagnosis?
The eosinophilia presented in the differential and peripheral smear (Figure 1) is consistent with an allergic immune response. Activation of the immune system and mast cell degranulation is thought to be the mechanism for eosinophilia-producing conditions. An eosinophil’s role in allergic reactions is due to modulation of the inflammatory response seen in response to an antigen or allergen.6 The peripheral smear shows an abundance of eosinophils which contain round and abundant pale orange to dark orange granules, 2-3 nuclear lobes connected by thin filaments without visible chromatin or nucleoli.7 A figure of an eosinophil is presented in Figure 1.
Figure 1.
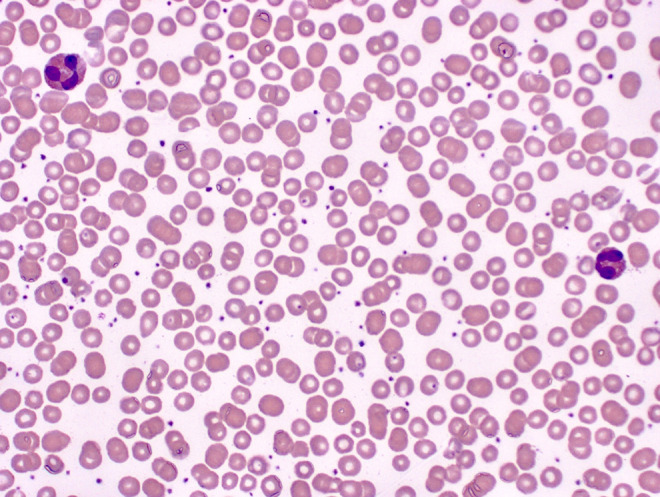
Blood: two eosinophils. Note the orange granules, and nuclear lobes connected by filaments (500× magnification).
Does the Patient’s Percutaneous Allergy Skin Test Support the Likely Diagnosis of Allergic Rhinitis?
Yes, the patients allergic in vivo response to dog dander were positive, which showed inflammation and redness in the area the site of injection. The positive skin test signified that the patient had an immune response to dog dander but not the other allergens tested.
Does the Patient’s IgE Antibody Levels Support the Likely Diagnosis of Allergic Rhinitis?
Yes. Increased levels of different types of immunoglobulin antibodies can indicate an immune response. In this case, IgE antibody directly correlates with the patients’ allergic response to dog dander. Total IgE is measured using a radioimmunosorbent test (RIST) and is not specific for test allergens. However, coupled with the percutaneous allergy skin test, it can be deduced that the patient was having an allergic response to a specific allergen, such as dog dander which is the cause of the patient’s high IgE levels.
Another test, the ImmunoCAP as well as the radioallergosorbent test (RAST) can be used to evaluate levels of IgE specific for a particular allergen. The patient’s serum reacts with an allergen-coated matrix and the amount of anti-IgE antibodies bound to the matrix is measured for specific allergens, either by fluorescence (ImmunoCAP)8 or by radioactivity (RAST).9 However, this test may take a significant amount of time to obtain results compared to the other diagnostic tests that were available in this study.
What Conditions Cause an Increase in Other Types of White Blood Cells? How Do These Cells Appear Morphologically?
An increase above reference range in other white blood cells can be present in patients with different conditions in response to infection, inflammation, chronic disease, and more. White blood cell disorders are critical to understanding full clinical pictures for patients and can indicate a plethora of pathology.
Neutrophilia is most commonly seen in infections and also seen in conditions related to stress response, malignancies, sepsis, surgery, physical conditions (heat, cold, shock), and inflammatory response. Neutrophils originate in the bone marrow, have a life span of 5 to 90 hours, and are activated by endothelial cell surface receptors which recruit more neutrophils to the infection site through cell surface receptors which essentially lead to phagocytosis.2 Neutrophils have an abundance of granules, a pale pink cream-colored or colorless cytoplasm, and 2 to 5 lobes (typically 3) connected by thin filaments without visible chromatin or visible nucleoli.7 These cells also can have significant alterations in peripheral smears that correlate with a patient’s various disease states. Included in these alterations are Döhle bodies, toxic granulation, toxic vacuolization, hypo segmentation, hyper segmentation, intracellular and extracellular bacteria, platelet satellitism, and granules that can determine very rare diseases such as Chediak-Higashi granules seen in Chediak-Higashi Syndrome.2 A neutrophil with no significant alteration is presented in Figure 2, and a neutrophil with toxic granulation is presented in Figure 3.
Figure 2.
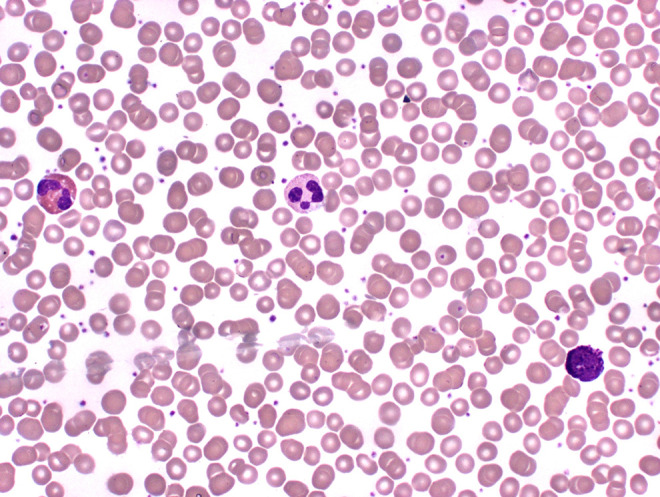
Blood: (from left to right) eosinophil, neutrophil, basophil (500× magnification).
Figure 3.
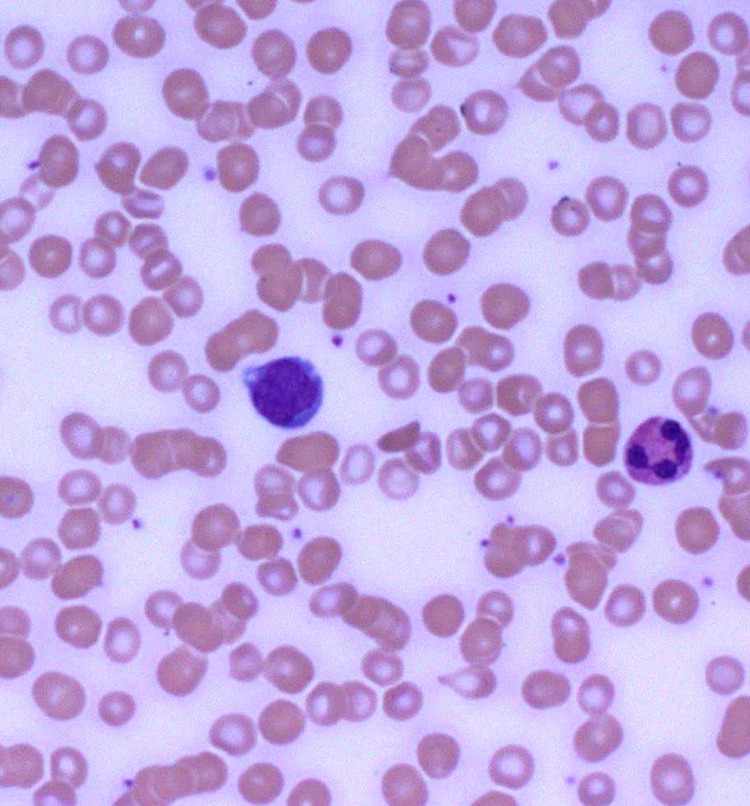
Blood: (from left to right) monocyte, neutrophil with toxic granulation (500× magnification).
Monocytosis can be seen in conditions related to malignancies, chronic infections (eg, tuberculosis), leukemias with a strong monocytic component, and bone marrow failure.2 Morphologically, monocytes are often the largest of the leukocytes found in a differential and contain many fine ground glass appearing granules, a variable-shaped nucleus usually horseshoe or kidney-shaped with “brainlike” convolutions. The cytoplasm may contain numerous vacuoles, and the chromatin usually appears lacy with no visible nucleoli.7 A monocyte is presented in Figures 3 and 4.
Figure 4.
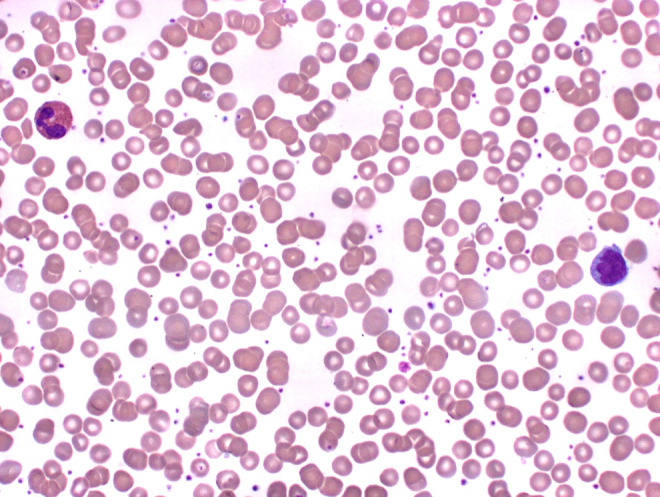
Blood: (from left to right) eosinophil, monocyte (500× magnification).
Basophilia is rare but can be seen in conditions related to myeloproliferative disorders, hypersensitivity reactions, ulcerative colitis, and chronic inflammatory conditions.2 Morphologically, basophils contain deep purple to black irregular-shaped granules that overlies a multilobed nucleus. These granules are separated into 2 types: specific granules that contain substances such as heparin, histamine, heparan sulfate, and leukotrienes; primary granules that contain lysosomal acid hydrolases.6 A basophil is presented in Figure 2.
Lymphocytosis can be seen in conditions of viral infections such as EBV, CMV, hepatitis viruses, HIV, and autoimmune disorders. Lymphocytes are derived from 2 locations and not solely the bone marrow. The primary lymphoid organs are the sites from which lymphocytes arise and consists of the bone marrow (like the majority of leukocytes) and the thymus. The secondary lymphoid organs are the spleen, lymph nodes, Peyer’s patches or the gastrointestinal tract, and the tonsils. Functionally, the lymphocytes main goal is immunological: realizing what is foreign, forming antibodies, and securing immunity. Lymphocytes have 2 subpopulations—B lymphocytes and T lymphocytes—however, on a peripheral smear for a differential, they appear morphologically similar. B lymphocytes derive from bone marrow stem cells and comprise approximately 10% to 20% of the total lymphocyte population. T lymphocytes also arise from stem cells that migrate to the thymus and comprise 60% to 80% of the total lymphocyte population. The life span of lymphocytes varies, with long-lived lymphocytes producing cytokines and short-lived lymphocytes producing antibodies. Speculation has it that some lymphocytes may live 4 years.2 Morphologically, lymphocytes have an intensely staining, slightly indented, spherical nucleus with a cytoplasm that appears as a very thin, pale blue rim surrounding the nucleus.6 A lymphocyte is presented in Figure 5.
Figure 5.
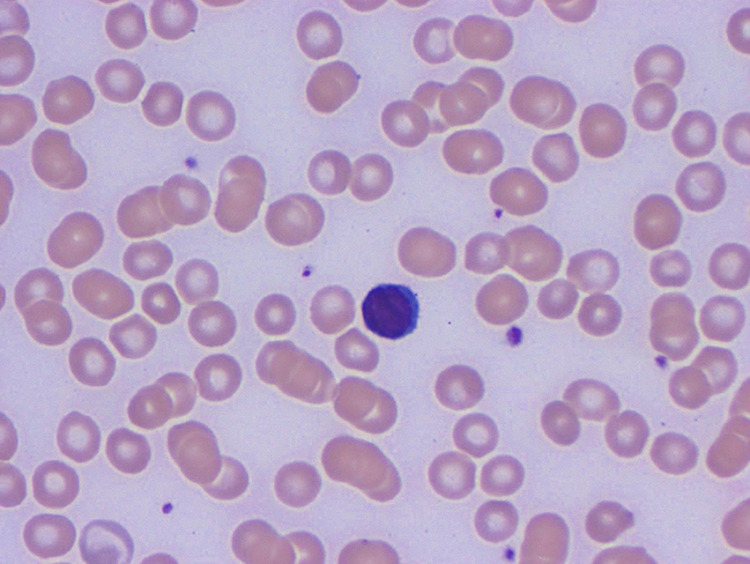
Blood: lymphocyte (1000× magnification).
What Is the Patient’s Diagnosis, Treatment, and Likely Outcome?
Once the results of the diagnostic tests are finalized, the physician determined that the patient’s diagnosis is allergic rhinitis, commonly known as “Hay fever.” The culmination of the diagnostic tests including the complete blood count and differential which showed leukocytosis and eosinophilia, the increased IgE antibody using RIST, and the positive percutaneous skin test to dog dander is what led to this diagnosis. The most basic and self-evident method of prevention of allergic reactions is allergen avoidance6; therefore, the patient may want to look into returning the dog due to his hypersensitivity reaction. Unfortunately, a particular individual’s genetic composition is what determines their susceptibility to allergens and should avoid such allergens due to the chance of anaphylaxis. However, if the patient does not want to part with the dog, allergen immunotherapy is an option which involves subcutaneous injections of gradually increasing quantities of specific allergens to an allergic patient until a dose is reached that will raise the patient’s tolerance to the allergen over time, thereby minimizing symptomatic expression of the disease.10 The patient opts out of this option and is treated with an antihistamine and makes a full recovery as seen by his follow-up diagnostic tests 2 weeks following this encounter, which showed a normal CBC and differential. Allergic individuals have a higher level of circulating IgE than nonallergic individuals; therefore, the patient’s IgE levels are expected to be on the high end of the reference range.9
Teaching Points
Eosinophils play very important roles in the immune system including destroying foreign substances and inflammation regulation, along with other kinds of white blood cells.
Eosinophils have brilliant and noticeable pink-orange granules that contain histamine. When histamine is released from eosinophils, this chemical stimulates allergic reactions such as what the patient with allergic rhinitis was exhibiting, noticeably so rhinorrhea and itchy eyes.3
Allergic rhinitis is classified as a type I hypersensitivity reaction which is defined as IgE mediated. In these types of reactions, a patient is exposed to an allergen (antigen), and when first exposed to the antigen, the patient may not experience symptoms because the cascade of biochemical events is not yet activated until a second exposure.2 This explains why the patient did not immediately experience symptoms when he adopted the dog and was exposed to animal dander. Rather, it took approximately a week after the exposure for an exaggerated immune response to develop to the antigen.
Testing for a hypersensitivity immune response can be conducted in numerous ways. Mentioned in this case study was an RIST to test for total IgE level, a percutaneous allergy skin test for specific allergens, and the ImmunoCap and RAST which uses fluorescence and radioactivity, respectively, to measure for specific allergens.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The article processing fee for this article was funded by an Open Access Award given by the Society of ‘67, which supports the mission of the Association of Pathology Chairs to produce the next generation of outstanding investigators and educational scholars in the field of pathology. This award helps to promote the publication of high-quality original scholarship in Academic Pathology by authors at an early stage of academic development.
ORCID iD: Stacy Beal  https://orcid.org/0000-0003-0862-4240
https://orcid.org/0000-0003-0862-4240
References
- 1. Knollmann Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017:4 doi:10.1177/2374289517715040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciesla B. Abnormalities of white blood cells: quantitative, qualitative, and the lipid storage diseases In: Ciesla B, ed. Hematology in Practice. 2nd ed F.A. Davis; 2012:144–146. [Google Scholar]
- 3. Lotspeich-Steininger CA, Stiene-Martin EA, Koepke J. Nonmalignant reactive disorders of phagocytes In: Clinical Hematology. Principles, Procedures, Correlations. The Phagocytic Leukocytes – Morphology, Kinetics, and Function. Lippincott Company; 1992:298;341–342. [Google Scholar]
- 4. O’Connell EM, Nutman TB. Eosinophilia in infectious diseases. Immunol Allergy Clin North Am. 2015;35:493–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cystic echinococcosis. In parasites-echinococcosis. Centers for disease control and prevention. 2020. Accessed September 2, 2019 https://www.cdc.gov/parasites/echinococcosis/
- 6. Ross MH, Pawlina W. Blood In: Michael HR, Wojciech P, ed. Histology: a Text and Atlas: With Correlated Cell and Molecular Biology.7th ed Wolters Kluwer Health; 2016:280–288. [Google Scholar]
- 7. Rodak BF, Carr JH. Clinical Hematology Atlas. 4th ed Elsevier Saunders; 2013. [Google Scholar]
- 8. Popescu FD, Vieru M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J Methodol. 2018;8:17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rittenhouse-Olson K, Nardin ED. Hypersensitivity reactions In: Kate Rittenhouse O, Ernesto DN, ed. Contemporary Clinical Immunology and Serology. 1st ed. Pearson; 2013:138–143. [Google Scholar]
- 10. Huggins J, Looney John R. Allergen immunotherapy. Am Fam Physician. 2004;70:689–696. [PubMed] [Google Scholar]


