Abstract
Tick-borne encephalitis is an important human arbovirus neuroinfection spread across the Northern Eurasia. Inhibitors of tick-borne encephalitis virus (TBEV) strain Absettarov, presumably targeting E protein n-octyl-β-d-glucoside (β-OG) pocket, were reported earlier. In this work, these inhibitors were tested in vitro against seven strains representing three main TBEV subtypes. The most potent compound, 2-[(2-methyl-1-oxido-5,6,7,8-tetrahydroquinazolin-4-yl)amino]-phenol, showed EC50 values lower than 22 µM against all the tested strains. Nevertheless, EC50 values for virus samples of certain strains demonstrated a substantial variation, which appeared to be consistent with the presence of E protein not only in infectious virions, but also in non-infectious and immature virus particles, protein aggregates, and membrane complexes.
Keywords: Tick-borne encephalitis virus, flaviviruses, antivirals, broad spectrum antiviral activity, 4-aminopyrimidine N-oxides, envelope protein
Introduction
Tick-borne encephalitis (TBE) is an important human arbovirus neuroinfection spread across the Northern Eurasia.1,2 It is caused by tick-borne encephalitis virus (TBEV) from the Flavivirus genus. Despite the availability of several vaccines, TBE incidence is high among unvaccinated people; therefore, a significant threat exists for travelers going to popular destinations in Europe and Siberia.1,3 The risk of TBEV infection among unvaccinated travelers to highly endemic regions is estimated to be 1/10,000.4 There are no developed, approved, and widely accepted methods of post-onset TBE therapy, and directly acting small molecule drugs are required as a therapeutic option and post-tick bite prophylaxis.
TBEV is divided into three subtypes (European, Eu; Siberian, Sib; Far-Eastern, FE),5 and two phylogenetically separate groups (Baikalian and Buryat-Mongol).6 Recently, a new Himalayan subtype was also proposed.7TBEV-Eu is the most common in the continental Europe. TBEV-Sib is prevalent on the Russian territory.6TBEV-FE circulates in China, Japan, Mongolia, and the Russian Far-East.5 All TBEV subtypes co-circulate in Russia and in the Baltics.6,8 Subtypes are rather closely related genetically, and the variation of polyprotein sequences between subtypes is about 2–7%.5,6,9 Clinical manifestations vary from asymptomatic to encephalitis and death for all the TBEV subtypes.1
Certain small molecule TBEV inhibitors were recently suggested and tested against Eu strains Neudoerfl and Hypr10,11 or Absettarov.12–14 We reported several series of potent TBEV reproduction inhibitors15,16 identified with the help of molecular docking against the n-octyl-β-d-glucoside (β-OG) binding pocket17 in the homology model of TBEV envelope protein ectodomain sE, which plays a pivotal role in the process of pH-driven membrane fusion between viral particles and host cell vesicles during endocytosis and viral entry.18 Among them, 4-aminopyrimidine N-oxides have shown a good efficiency against strain Absettarov coupled with a low cytotoxicity. In time-of-addition studies, we showed that the envelope protein E is the putative target for them.16 Given the ability to inhibit reproduction of multiple TBEV variants belonging to different phylogenetic lineages is crucial for a good antiviral agent, a thorough characterization of the compounds’ anti-TBEV activity spectrum is required for the further development.
In the present work, we demonstrated an antiviral activity of 4-aminopyrimidine N-oxides against a panel of strains belonging to all main TBEV subtypes. Compounds varied in their antiviral activity against strains Absettarov and 256, which did not differ in E protein amino acid sequence. We observed variation of the ratio of infectious virions versus decoy E protein containing particles, including non-infectious and immature virus particles, E protein aggregates and membrane complexes, in the virus sample. These non-infection particles could influence observed activity values of small molecules targeting envelope proteins.
Materials and methods
Sequence analysis
E protein sequences were obtained from GenBank via Pubmed Protein interface (access date 26.10.2015) using search string “(tick-borne encephalitis) AND ((E protein) OR (envelope protein))” and lower sequence length limit of 240 amino acid residues. TBEV strain DV936k sequence (GenBank accession no. GU125722.1) was updated and uploaded to GenBank later (GenBank accession no. GU125722.2). Multiple sequence alignment was performed via Clustal Omega web service19,20 with default parameters (Supplementary alignment file). Sequences lacking residues 46 to 285 were excluded and substitutions in the pocket region compared to the TBEV strain Absettarov were analyzed using an in-house Python 3.5 script.
Cells and viruses
Porcine embryo kidney (PEK) cell line was maintained at 37°C in media 199 on Hanks’ balanced salt solution and Earle’s balanced salt solution (2:1, v:v, FSBSI “Chumakov FSC R&D IBP RAS”, Russia) supplemented with 5% fetal bovine serum (FBS, Invitrogen).
Vero cell line was maintained at 37°C in DMEM media with L-glutamine (FSBSI “Chumakov FSC R&D IBP RAS”, Russia) supplemented with 10% fetal bovine serum (FBS, Invitrogen).
TBEV strains used in the work are presented in Table 1.21,22 TBEV strains from laboratory collection were multiplied in PEK and/or Vero cells. Cultural fluids were collected 72 h post infection (cytopathic effect over 75% of cell monolayer), aliquoted, and stored at −70°C. Each strain sample was titrated by plaque assay in PEK cells at least three times to determine geometrical mean titer of infectious virions and by qPCR at least three times to determine the mean concentration of RNA-containing particles.
Table 1.
TBEV strains used in the study.
| TBEV strain | Region and year of isolation | Origin of isolation | Passagesa | GenBank accession № |
|---|---|---|---|---|
| FE | ||||
| 205KGG | Khabarovskiy krai, Russia, 1973 | I. persulcatus | MxM1P3 | GU121964 |
| DV936k | Primorskiy krai, Russia, 1975 | I. persulcatus | M3P2 | GU125722 |
| Eu | ||||
| Absettarov | Leningrad region, Russia, 1951 | blood of a TBE patient | MxM5V1 | KU885457 |
| 256 | Belarus, 1940 | I. ricinus | MxM2P1 | AF091014 |
| Sib | ||||
| Vasilchenko | Novosibirsk region, Russia, 1961 | blood of a TBE patient | MxM2V1 | L40361 |
| EK-328 | Estonia, 1971 | I. persulcatus | M6P1M5P1 | DQ486861 |
| Lesopark11 | Novosibirsk, Russia, 1986 | I. persulcatus | MxM2P3 | KJ701416 |
| TV08-T2546 | Republic of Tuva, Russia, 2008 | I. persulcatus | M2V1 | KU052690 |
aM – passages in mouse brain (Mx – passages performed by strain authors before the viruses were obtained in the laboratory); V – passages in Vero cells; P – passages in PEK cells.
4-Aminopyrimidine N-oxides
The compounds were synthesized from commercially available starting materials by previously described methods16,23,24 and stored as solutions in DMSO at −20°C.
Plaque assay
Ten-fold dilutions of virus suspension in medium 199 made on Earle’s balanced salt solution (FSBSI “Chumakov FSC R&D IBP RAS”, Russia) were added to PEK cell monolayers in two replicates and incubated in a CO2-incubator for 1 h at 37°C. Then cells were overlaid with 1.26% methylcellulose (Sigma) prepared in media 199 on Hanks’ balanced salt solution and Earle’s balanced salt solution (2:1, v:v) containing 2% FBS. After incubation at 37°C in a CO2-incubator for six days, cells were fixed with 96% ethanol. Plaques were stained with 0.4% gentian violet. Plaques were counted and the virus titer was calculated as log10PFU/mL. Each strain was titrated in at least three biological replicates to determine the mean concentration of infectious virions.
Plaque reduction assay
Two-fold dilutions of studied compounds with concentrations starting from 50 µM were prepared in medium 199 made on Earle's balanced salt solution. Equal volumes of virus suspension, containing 20–35 PFU, were added to compound dilution (1:1, v:v). The control virus was added to the same sequential concentrations of DMSO, as in compounds dilutions. Virus-compound mixtures were incubated at 37°C in a CO2-incubator for 1 h and then added to the cells, seeded on 24-well plates, in two replicates and incubated at 37°C in a CO2-incubator for 1 h. Then the cells were overlaid with methylcellulose and incubated for six days, and plaques were stained as described for the plaque assay. EC50 values were calculated according to Reed-and-Muench method.25 At least two biological replicates were performed to determine each compound activity.
ELISA
The content of E protein in virus samples was assessed by enzyme-linked immunosorbent assay (ELISA) using commercial kit D1154 VectorTBE-antigen (Vector-Best, Russia) according to the manufacturer’s instructions.
The standard curve was calculated for purified standard antigen prepared from TBEV strain Sofjin26 (ST1, Figure S1), obtained by Dr. V. N. Lyapustin as described elsewhere.27
To determine the amount of E protein in the standard ST1, two-fold dilutions of bovine serum albumin (BSA, Combithek) with mass range from 1 to 0.01 mg/mL were mixed with 3×Sample buffer (SDS-mercaptoethanol) and resolved in 12% SDS-PAGE at MiniPROTEAN cell (Bio-Rad). Gel was fixed in water solution containing 10% of acetic acid and 50% of ethanol, stained by Coomassie blue (Figure S2), and scanned. Optical density of protein bands was estimated in OneDscan (DSP Inc., USA). A calibration curve of bands’ optical density from protein concentration was built based on BSA dilutions, and was used to determine the amount of total E protein content in the standard sample ST1. The procedure was repeated in three independent experiments.
To obtain a standard calibration curve for E protein concentration from optical density of samples, series of two-fold ST1 dilutions was measured in ELISA with VectorTBE-antigen (Vector-Best, Russia) according to the manufacturer’s instructions (Figure S1).
The standard calibration curve was used to estimate the amount of E protein in the virus samples by their optical density measured in ELISA. Optical density of six two-fold dilutions of each sample was studied in at least two biological replicates.
The amount of E protein from infectious particles and genome-containing particles (GCP) was estimated assuming average molecular mass of TBEV E protein of 53 kDa and 180 protein molecules per viral particle.
qRT-PCR
qRT-PCR procedure was performed as described earlier28,29 with some changes. Sabin1 strain of poliovirus type 1 was used as an internal control and was added to the samples prior to RNA extraction. Reverse transcription was performed with M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s protocol with primers Pow-TBE-3′: 5′-AGCGGGTGTTTTTCCGAGTC-3′ for TBEV and PVR1: 5′-CGAACGTGATCCTGAGTGTT-3′ for Poliovirus. PCR was performed with primers for TBEV (R-TBE: 5′-ACACATCACCTCCTTGTCAGACT-3′, F-TBE: 5′-GGGCGGTTCTTGTTCTCC-3′) and probe TBE: 5′-(FAM*)-TGAGCCACCATCACCCAGACACA-(BHQ1*)-3′ targeted on 3′NTR of TBEV genome and by using standards with a known concentration of TBEV RNA.29 Quantitative real-time PCR was carried out on DNA Engine analyzer (BioRad) using RT-PCR kit (Syntol, Russia). For qRT-PCR internal control PVR1, PVL1: 5′-GGCAGACGAGAAATACCCAT-3′ and probe PVP: 5′-(R6G*)-TTGATTCATGAATTTCCTTCATTGGCA-(BHQ1)-3′ were used. Each virus sample was examined in at least three biological replicates.
Statistical analysis
Mann–Whitney two-tailed U-test was applied to compare strain 256 sample’s characteristics to the other strain samples. The threshold of statistical significance was set at p ≤ 0.05. Analysis was performed with an in-house Python 3.5 script.
Results
Selection of TBEV strains
To investigate the spectrum of anti-TBEV activity of 4-aminopyrimidine N-oxides, we selected seven TBEV strains representing all three main TBEV subtypes from the laboratory collection. These strains were isolated in various regions of Russia and the Baltics and had a different passage history (Table 1). Strain sequencing and characterization have been presented previously.21,22
Our previous studies showed that 4-aminopyrimidine N-oxides would most likely interact with the ectodomain of the virus envelope protein E. Thus, we compared the protein E sequences of the selected TBEV strains. Variable amino acid positions are presented in Table 2 and in Figure S3. Strain 256 did not contain amino acid substitutions in the E protein compared to strain Absettarov, thus allowing direct comparison of the compounds’ activity against the strains with identical E proteins.
Table 2.
Comparison of the sE protein sequences of the TBEV strains used in activity spectrum study. All variable residues are shown.
| SubType | Strain | Identity to absettarov E, % |
Residue number |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 41 | 47 | 81 | 88 | 115 | 119 | 120 | 175 | 178 | 206 | 216 | 218 | 228 | 234 | 267 | 277 | 279 | 306 | 313 | 315 | 317 | 331 | 349 | ||||
| Eu | Absettarov | М | A | T | G | A | A | A | T | E | V | H | D | K | N | A | E | T | M | T | K | A | T | S | ||
| 256 | 100 | |||||||||||||||||||||||||
| Sib | Vasilchenko | 96.4 | S | S | T | V | D | L | R | Q | S | D | A | A | T | F | ||||||||||
| TV08-T2546 | 96.9 | L | S | A | S | T | D | L | E | H | S | D | T | |||||||||||||
| Lesopark11 | 97.2 | S | S | T | D | L | R | H | S | D | A | T | ||||||||||||||
| EK-328 | 96.9 | S | S | T | N | D | L | R | H | S | D | R | T | |||||||||||||
| FE | 205KGG | 96.9 | S | S | T | S | D | S | R | S | D | T | T | A | ||||||||||||
| DV 936k | 97.4 | S | S | T | S | D | S | S | D | I | A | |||||||||||||||
Antiviral activity spectrum
The spectrum of anti-TBEV activity of nine 4-aminpyrimidine N-oxides, which had shown EC50 < 50 µM against strain Absettarov in our previous study in the focus reduction assay in PEK cells,16 was assessed against selected strains in the plaque reduction assay in the same cells against 20–35 PFU. The EC50 values are shown in Table 3.
Table 3.
Anti-TBEV activity spectrum of 4-aminopyrimidine N-oxide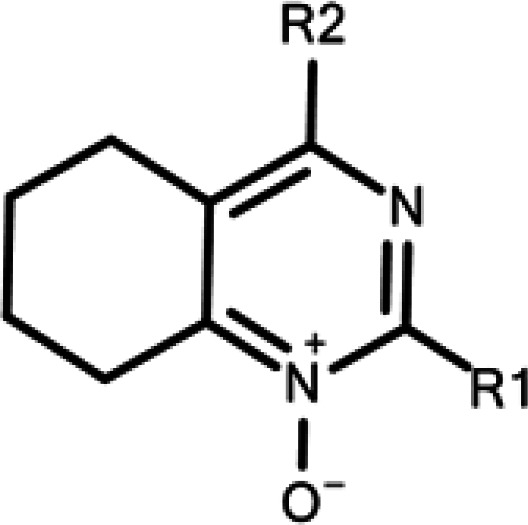
| Code | R1 | R2 |
Subtype/Strain/EC50 (mean ± SD, µM) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Eu |
Sib |
FE |
||||||||
| Absettarov | 256 | Vasilchenko | TV08-T2546 | Lesopark11 | EK-328 | 205KGG | DV 936k | |||
| 7a | Me | NHBu | 31 ± 5 | >50 | >50 | 18 ± 4 | >50 | >50 | >50 | 18 ± 3 |
| 7c | t-Bu | NHCH2Ph | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 7o | Me | NH(2-OH-C6H4) | 8 ± 3 | 21 ± 2 | 3.4 ± 0.2 | 4.0 ± 0.4 | 6.5 ± 0.1 | 12 ± 1 | 7.0 ± 0.2 | 7.4 ± 0.3 |
| 7t | Et | NH(CH2)2(1-Ad) | 35 ± 2 | >50 | >50 | 25 ± 3 | >50 | >50 | >50 | >50 |
| 7u | t-Bu | NH(CH2)2(1-Ad) | 6 ± 2 | >50 | 13 ± 2 | 7 ± 2 | 6.0 ± 1.5 | 9.7 ± 0.7 | 6.9 ± 0.8 | 6.5 ± 0.6 |
| 7w | t-Bu | NH(CH2)2(2-Ad) | 6 ± 3 | >50 | 16 ± 2 | 11.4 ± 0.9 | 4.4 ± 0.2 | 7.5 ± 0.6 | 8.3 ± 0.4 | 5 ± 2 |
| 7y | Me | NHCH(1-Ad)Ph | 8 ± 3 | >50 | 16 ± 2 | 15 ± 2 | 23 ± 4 | 15.0 ± 0.2 | 10.9 ± 0.5 | 14.7 ± 0.1 |
| 7z | t-Bu | NHCH(1-Ad)Ph | 4 ± 1 | 26 ± 2 | 4.3 ± 0.3 | 3.3 ± 0.4 | 9 ± 1 | 10.1 ± 0.9 | 4.1 ± 0.3 | 4.3 ± 0.1 |
| 7ab | t-Bu |
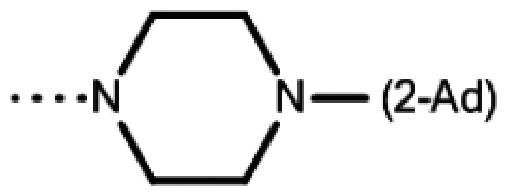
|
23 ± 6 | >50 | >50 | 29 ± 1 | 35 ± 5 | 39 ± 2 | 37 ± 1 | 12 ± 2 |
The majority of the studied compounds showed a broad spectrum of activity, inhibiting the reproduction of strains belonging to all main TBEV subtypes. Compound 7c, which poorly suppressed reproduction of strain Absettarov in the previous study,16 was inactive against other strains in the similar conditions. The general trend was that compounds with the highest anti-Absettarov activity showed the most consistent viral reproduction inhibition profile in the panel. The same was true for the strain TV08-T2546, which belongs to the Sib subtype and passed only three passages in the laboratory (Table 1). Strains Absettarov and TV08-T2546 may serve as a good model of TBEV infection for initial scaffold identification in vitro, as a high activity against them can be used as a predictor of broad-spectrum anti-TBEV activity. However, this assumption requires further investigation with different compound series.
All strains except 256 bore differences in the amino acid sequence of protein E; however, inhibitory activity of the most potent compounds against these strains was at the same level. Compounds 7o and 7z, with EC50 values against strain Absettarov of 8 and 4 µM, respectively, inhibited the reproduction of all the selected strains. Only these two compounds appeared to inhibit reproduction of the strain 256 in the studied concentration range. Strain 256 belongs to Eu subtype along with the strain Absettarov and has the same E protein amino acid sequence. According to our data, there was no obvious correlation between antiviral activity and amino acid substitutions in E protein; moreover, difference in antiviral activity could not be explained by amino acid variations in β-OG pocket (see ‘E protein sequence variation’ below). As time-of-addition assay have indicated that the compounds most likely interact with the virus envelope protein E,16 we hypothesized that the amount of decoy E protein in non-infectious virus particles and immature virions covered by E protein, together with E protein aggregates and membrane complexes, in virus sample may influence apparent compounds activity. To assess it, we measured the total concentration of E protein in virus samples and estimated E protein amount from infectious and virus genome-containing particles (GCP) in these samples (see ‘Quantification of E protein in virus samples’ below).
E protein sequence variation
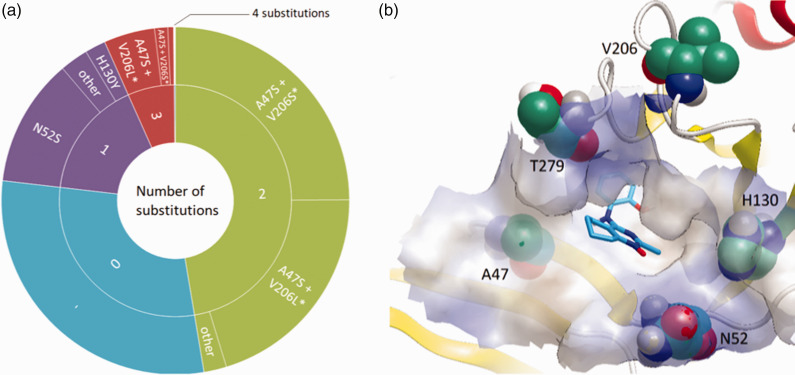
Our analysis of the TBEV strain Absettarov envelope protein soluble ectodomain (sE) homology model16 revealed that the following 34 residues should be considered as comprising the pocket: 46–55, 128–132, 140, 142, 199, 201, 204–206, 211, 212, 214, 273–275, 278–280, 282, 284, 285. To assess the variability of β-OG pocket, we analyzed full and partial TBEV E sequences from GenBank (Supplementary alignment file) containing residues 46 to 285 (E protein numbering), which comprised the whole pocket (Figure S4). The sequence of strain Absettarov E protein was used as a reference. Of 570 retrieved sequences, 168 (30%) did not contain amino acid substitutions in the pocket region, and six were identical to strain Absettarov E protein residues 1 to 395, comprising the whole soluble ectodomain sE. The remaining 402 sequences had no more than four substitutions in a limited number of positions at the pocket periphery (Figure 1).
Figure 1.
(a) Distribution of amino acid substitutions in the β-OG pocket of TBEV E protein sequences obtained from GenBank in comparison with the one of strain Absettarov. *Combination also occurs in triple substitutions; (b) Location of the most frequently substituted residues in the E protein β-OG pocket (represented by surface) of TBEV strain Absettarov model together with binding mode of compound 7o predicted by docking.16 Compound 7o does not form directed interactions with these residues. Picture was created in VIDA 4.3.0.4.30
The most common (258 sequences, 45%) was the double substitution A47S + V206S/L on the pocket periphery (Figure 1(a)). In rare cases, it was accompanied by another one, with the most common combination A47S + V206L + T279A met in 11 sequences (2%). The most common single substitution was N52S, observed in 68 sequences (12%). Only one sequence with four substitutions in the pocket was found (GenBank accession no. AIB53033).
Quantification of E protein in virus samples
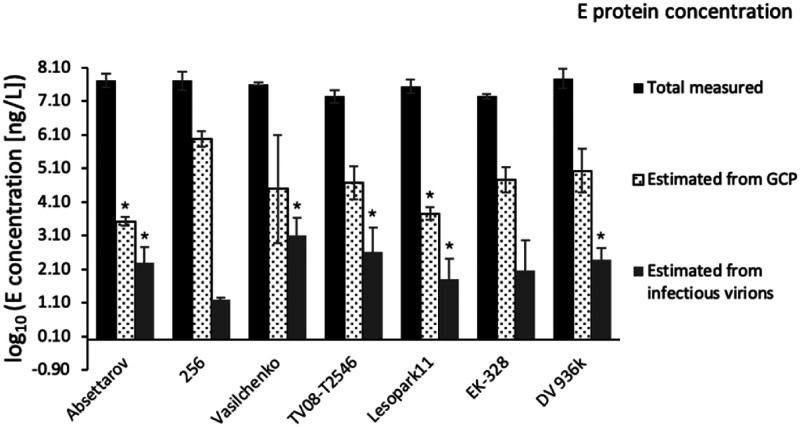
We determined the concentration of infectious virions in plaque assay in PEK cells (Table 4) and calculated E protein amount associated with them (Table 4, Table S1). For the calculation of the total amount of E protein associated with GCP, which include mature, immature, partially mature, and defective virus particles, we used qRT-PCR (Table 4). The concentration of GCP for all strains was higher than the concentration of infectious virions measured in plaque assay. The highest GCP to PFU ratio was observed for strain 256, while the lowest – for strain Absettarov (Figure 2). Since these strains have identical E protein amino acid sequences, it can be assumed that an excessive amount of GCP could affect the binding of antiviral compounds to infectious virions of strain 256.
Table 4.
Quantitative characterization of TBEV strain samples. Values are presented as mean ± ½ 95% CI. *Corresponds to significant difference with strain 256 with p ≤ 0.05.
| Strain |
Concentration of virus particles (infectious or RNA-containing) |
Total measured E protein concentration(mg/L) | ||
|---|---|---|---|---|
| log10(PFU/mL) | log10(GCP/mL) | |||
| 256 | 6.0 ± 0.1 | 10.8 ± 0.2 | 51 ± 34 | |
| Absettarov | 7.1 ± 0.5* | 8.3 ± 0.1* | 54 ± 25 | |
| Vasilchenko | 7.9 ± 0.5* | 9 ± 2 | 40 ± 4 | |
| TV08-T2546 | 7.4 ± 0.7* | 9.5 ± 0.5 | 18 ± 8 | |
| Lesopark11 | 6.6 ± 0.6* | 8.6 ± 0.2* | 35 ± 16 | |
| EK-328 | 6.8 ± 0.9 | 9.6 ± 0.4 | 18 ± 3 | |
| DV 936k | 7.2 ± 0.4* | 9.8 ± 0.7 | 61 ± 40 | |
Figure 2.
Log10 concentration of E protein in the strain samples – measured in ELISA and estimated from GCP or PFU concentrations, assuming average molecular mass of TBEV E protein of 53 kDa and 180 protein molecules per virus particle (Table S1). *Corresponds to significant difference with strain 256 with p ≤ 0.05 .
Virus samples may contain not only infectious and genome containing particles, but also E protein aggregates, membrane complexes and virus-like particles covered with E protein, but lacking RNA.31–34 We determined the total concentration of the E protein in our virus samples using a commercial ELISA kit. The main assumption was that the sensitivity of ELISA kit is comparable for virions and decoy E protein, and does not depend on the virus subtype.
Concentration of total measured E protein in all strain samples was comparable or lower than in strain 256, while concentration of infectious virions was higher than in strain 256 (Table 4). These data indicated that the amount of decoy E protein per infectious virion in strain 256 exceeded these amounts for the other strains.
Discussion
The main goal of our study was to determine the spectrum of antiviral activity of the 4-aminopyrimidine N-oxides, which previously showed high activity against TBEV strain Absettarov. Based on the results, the most active compounds showed good antiviral activity against all selected TBEV strains representing three main subtypes. It gave the prospect that they have a broad spectrum of antiviral activity and would effectively inhibit all strains of TBEV.
Initially 4-aminopyrimidine N-oxides were selected as a potential TBEV inhibitor based on a docking study of their ability to interact with E protein’s β-OG pocket (Figure S5). Based on time-of-addition assay, we also confirmed that the compounds would most likely interact with the E protein16 and the soluble ectodomain is the only easily accessible component of it.35
The amino acid composition of mosquito-borne flavivirus E protein β-OG pocket was shown to influence different aspects of the flavivirus life cycle, including pH requirements for fusion, virus in vivo properties, such as neurovirulence, neuroinvasiveness, etc.36–39 Therefore, we conducted a substitution analysis of the residues that should be considered as comprising the pocket for the strains selected in our work and obtained in GenBank in comparison with strain Absettarov’s ones. All the selected strains, as well the strains presented in the GenBank, did not have substitutions directly inside the pocket, indicating its high conservation. Substitutions appear in a very limited number of positions, which are located only on the periphery of the β-OG pocket (Figure 1).
Nevertheless, comparing the inhibitory effects of the compounds against strains 256 and Absettarov, both belonging to Eu subtype and expressing E proteins with the identical amino acid sequences, we observed substantial differences in their activity, which needed additional investigation. The possible explanations were that the inhibitors could have a target other than E protein or the replication kinetics of the strains may account for difference in inhibitory activity of the compounds. However, our time-of-addition assay16 had indicated that compounds most likely interact with the E protein and affected fusion and early stages of virus reproduction cycle, thus we proposed a hypothesis that this differential inhibitory effect can be explained by difference in decoy E protein content in viral strain samples. Cells infected with flaviviruses can produce not only fully functional mature infectious virions, but also defective virions and virus-like particles lacking RNA.31–34 Virions also greatly vary in the degree of maturation (e.g. completeness of furin cleavage of prM into M and E protein conformational switch during the virion exocytosis) that may affect their infectivity. The presence of E protein aggregates and membrane-bound structures produced during cell infection or virion degradation upon storage is also anticipated. Virus-like particles without genome were shown to possess antigenic structure similar to infectious virions and thus to bind neutralizing antibodies.40 Therefore, decoy E proteins in non-infectious virus particles and immature virions, E protein aggregates and membrane complexes, may in principle bind small molecules. The reproduction capability of different virus particles differs, and for a virus sample characterized by a high content of decoy E protein, molecules of the inhibitor could be wasted due to binding with it. Thus, EC50 values of TBEV reproduction inhibitors targeting E proteins may be influenced by the ratio between the amounts of E protein from infectious virions and E protein from decoy particles.
The ratio between the total measured concentration of E protein in the sample and estimated E protein concentration from infectious particles represents how many decoy E protein molecules may compete with target E proteins from infectious virions for binding to inhibitor (Figure 2). The greater this ratio, the higher is the probability of the inhibitor to bind E protein molecule that does not take part in the infection process. A high content of decoy E protein explains the low activity of 4-aminopyrimidine N-oxides against the strain 256 compared to the one for strain Absettarov: to inhibit formation of the same number of plaques, a greater amount of compound is required in the case of the strain 256 due to compounds binding to decoy E protein.
The content of decoy E protein most likely depends on the intrinsic properties of the strain (e.g. accuracy of the viral polymerase or properties of the viral proteins responsible for the virion assembly), the method of virus sample preparation (multiplicity of infection, cell line, degree of the cytopathic effect on the moment of virus harvesting), and storage conditions. Nevertheless, the extent to which these factors influence the ratio between infections virions and decoy particles, as well as the difference in the ability to bind small molecules between different decoy particles is a matter of further research. Apparently, the ratio between the total amount of E protein and the one from infectious virions could be a critical factor for in vitro experiments based on the infectivity measurement, influencing the observed activity values of small molecule antivirals targeting the envelope proteins.
Conclusions
On the example of 4-aminopyrimidine N-oxides, we demonstrated that small molecule compounds exhibit a broad range of anti-TBEV activity. Compound 7o with seven days cytotoxicity (CC50) of 340 µM16 showed the EC50 values lower than 22 µM against all strains, including strain 256 with a high content of decoy E protein, and could be considered as the most perspective compound for further structure optimization and structure-activity relationship exploration.
Our results showed that the outcome of activity measurement of a small molecule targeting the envelope protein of a TBEV strain could be influenced by the ratio of the infectious virions and decoy E protein in the virus sample. This ratio varied between the samples of the studied strains and could misrepresent the results of infectivity measurement in vitro assays. Testing potential reproduction inhibitors against TBEV strains with high content of E protein out of infectious particles could be an important step in the study of the antiviral activity of compounds binding to the envelope proteins. This fact should be considered during the selection and characterization of virus preparation for assessment of the antiviral compounds and comparison of their activity.
Supplemental Material
Supplemental material, sj-pdf-1-avc-10.1177_2040206620943462 for Spectrum of antiviral activity of 4-aminopyrimidine N-oxides against a broad panel of tick-borne encephalitis virus strains by Evgenia V Dueva, Ksenia K Tuchynskaya, Liubov I Kozlovskaya, Dmitry I Osolodkin, Kseniya N Sedenkova, Elena B Averina, Vladimir A Palyulin and Galina G Karganova in Antiviral Chemistry and Chemotherapy
Acknowledgements
The authors thank Dr. Alexander Litov for qPCR standards preparation. Free academic license for OpenEye software was kindly provided by OpenEye Scientific Software Inc. Free academic license for Instant JChem, used for structure database management, was kindly provided by ChemAxon (Instant JChem 18.3.0, 2018, ChemAxon http://www.chemaxon.com)).
Authors’ contributions
GGK, EVD, KKT, LIK, DIO designed the study; EVD performed sequence analysis, cell-based assays and experimental data interpretation and analysis; KKT performed protein and RNA content measurements; KNS, EBA provided the compounds; GGK, LIK, DIO, KNS, EBA and VAP supervised the study; EVD, DIO, LIK wrote the manuscript draft; all the authors provided feedback.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Russian Foundation for Basic Research, grants no. 15–04-08365 and 18–03-00651. BSL-3 facilities, virus and cell collection were supported by the State research funding of FSBSI “Chumakov FSC R&D IBP RAS”.
ORCID iDs
Evgenia V Dueva https://orcid.org/0000-0001-8399-5944
Dmitry I Osolodkin https://orcid.org/0000-0002-0462-2945
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Ruzek D, Avsic Zupanc T, Borde J, et al. Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Res 2019; 164: 23–51. [DOI] [PubMed] [Google Scholar]
- 2.Dobler G, Gniel D, Petermann R, et al. Epidemiology and distribution of tick-borne encephalitis. Wien Med Wochenschr 2012; 162: 230–238. [DOI] [PubMed] [Google Scholar]
- 3.Amicizia D, Domnich A, Panatto D, et al. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother 2013; 9: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunze U. and ISW-TBE. Tick-borne encephalitis (TBE): an underestimated risk … still: report of the 14th annual meeting of the International Scientific Working Group on Tick-Borne Encephalitis (ISW-TBE). Ticks Tick Borne Dis 2012; 3: 197–201. [DOI] [PubMed] [Google Scholar]
- 5.Simmonds P, Becher P, Collett MS, et al. Family flaviviridae. In: King AMQ, Adams MJ, Carstens EB, et al. (eds) Virus taxonomy: classification and nomenclature of viruses: ninth report of the international committee on taxonomy of viruses. Amsterdam: Elsevier Academic Press, 2012, pp. 1003–1020.
- 6.Demina TV, Dzhioev YP, Verkhozina MM, et al. Genotyping and characterization of the geographical distribution of tick-borne encephalitis virus variants with a set of molecular probes. J Med Virol 2010; 82: 965–976. [DOI] [PubMed] [Google Scholar]
- 7.Dai X, Shang G, Lu S, et al. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg Microbes Infect 2018; 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golovljova I, Vene S, Sjolander KB, et al. Characterization of tick-borne encephalitis virus from Estonia. J Med Virol 2004; 74: 580–588. [DOI] [PubMed] [Google Scholar]
- 9.Lindenbach B, Murray C, Thiel H-J, et al. Fields virology. 6th ed. In: Fields B, Knipe D and Howley P et al. (eds) Flaviviridae. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013, pp. 712–746.
- 10.Eyer L, Valdes JJ, Gil VA, et al. Nucleoside inhibitors of tick-borne encephalitis virus. Antimicrob Agents Chemother 2015; 59: 5483–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyer L, Smidkova M, Nencka R, et al. Structure-activity relationships of nucleoside analogues for inhibition of tick-borne encephalitis virus. Antiviral Res 2016; 133: 119–129. [DOI] [PubMed] [Google Scholar]
- 12.Orlov AA, Drenichev MS, Oslovsky VE, et al. New tools in nucleoside toolbox of tick-borne encephalitis virus reproduction inhibitors. Bioorg Med Chem Lett 2017; 27: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 13.Orlov AA, Chistov AA, Kozlovskaya LI, et al. Rigid amphipathic nucleosides suppress reproduction of the tick-borne encephalitis virus. Med Chem Commun 2016; 7: 495–499. [Google Scholar]
- 14.Kozlovskaya LI, Golinets AD, Eletskaya AA, et al. Selective inhibition of enterovirus a species members' reproduction by Furano[2,3-d]pyrimidine nucleosides revealed by antiviral activity profiling against (+)ssRNA viruses. ChemisrySelect 2018; 3: 2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osolodkin DI, Kozlovskaya LI, Dueva EV, et al. Inhibitors of tick-borne flavivirus reproduction from structure-based virtual screening. ACS Med Chem Lett 2013; 4: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedenkova KN, Dueva EV, Averina EB, et al. Synthesis and assessment of 4-aminotetrahydroquinazoline derivatives as tick-borne encephalitis virus reproduction inhibitors. Org Biomol Chem 2015; 13: 3406–3415. [DOI] [PubMed] [Google Scholar]
- 17.Modis Y, Ogata S, Clements D, et al. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 2003; 100: 6986–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiasny K, Fritz R, Pangerl K, et al. Molecular mechanisms of flavivirus membrane fusion. Amino Acids 2011; 41: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 19.Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed 8 July 2020).
- 21.Chernokhaeva LL, Rogova YV, Vorovitch MF, et al. Protective immunity spectrum induced by immunization with a vaccine from the TBEV strain Sofjin. Vaccine 2016; 34: 2354–2361. [DOI] [PubMed] [Google Scholar]
- 22.Kozlovskaya LI, Osolodkin DI, Shevtsova AS, et al. GAG-binding variants of tick-borne encephalitis virus. Virology 2010; 398: 262–272. [DOI] [PubMed] [Google Scholar]
- 23.Sedenkova KN, Averina EB, Grishin YK, et al. Three-component heterocyclization of gem-bromofluorocyclopropanes with NOBF4: access to 4-fluoropyrimidine N-oxides. J Org Chem 2012; 77: 9893–9899. [DOI] [PubMed] [Google Scholar]
- 24.Sedenkova KN, Averina EB, Grishin YK, et al. Nitronium salts as novel reagents for the heterocyclization of gem-bromofluorocyclopropanes into pyrimidine derivatives. Tetrahedron Lett 2015; 56: 4927–4930. [Google Scholar]
- 25.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 1938; 27: 493–497. [Google Scholar]
- 26.Tuchinskaya K, Volok V, Illarionova V, et al. Development of a method for assessing the structural heterogeneity of a population of different strains of tick-borne encephalitis virus. Patogenez 2018; 16: 108–111. [Google Scholar]
- 27.Ljapustin VN, Vorovich MF. Method of obtaining virion antigen of tick-borne encephalitis virus. Russian Federation Patent. RU2402606. 2010.
- 28.Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol 2003; 27: 136–145. [DOI] [PubMed] [Google Scholar]
- 29.Romanova L, Gmyl AP, Dzhivanian TI, et al. Microevolution of tick-borne encephalitis virus in course of host alternation. Virology 2007; 362: 75–84. [DOI] [PubMed] [Google Scholar]
- 30.VIDA 4.3.0.4: OpenEye Scientific Software, Santa Fe, NM, www.eyesopen.com (accessed 8 July 2020).
- 31.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Partial maturation: an immune-evasion strategy of dengue virus? Trends Microbiol 2011; 19: 248–254. [DOI] [PubMed] [Google Scholar]
- 32.Pierson TC, Diamond MS. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2012; 2: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allison SL, Tao YJ, O'Riordain G, et al. Two distinct size classes of immature and mature subviral particles from tick-borne encephalitis virus. J Virol 2003; 77: 11357–11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Füzik T, Formanová P, R ůžek D, et al. Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat Commun 2018; 9: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Ge P, Yu X, et al. Cryo-EM structure of the mature dengue virus at 3.5-A resolution. Nat Struct Mol Biol 2013; 20: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butrapet S, Childers T, Moss KJ, et al. Amino acid changes within the E protein hinge region that affect dengue virus type 2 infectivity and fusion. Virology 2011; 413: 118–127. [DOI] [PubMed] [Google Scholar]
- 37.Lee E, Weir RC, Dalgarno L. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology 1997; 232: 281–290. [DOI] [PubMed] [Google Scholar]
- 38.Monath TP, Arroyo J, Levenbook I, et al. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J Virol 2002; 76: 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurrelbrink RJ, McMinn PC. Attenuation of Murray Valley encephalitis virus by site-directed mutagenesis of the hinge and putative receptor-binding regions of the envelope protein. J Virol 2001; 75: 7692–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metz SW, Thomas A, White L, et al. Dengue virus-like particles mimic the antigenic properties of the infectious dengue virus envelope. Virol J 2018; 15: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-avc-10.1177_2040206620943462 for Spectrum of antiviral activity of 4-aminopyrimidine N-oxides against a broad panel of tick-borne encephalitis virus strains by Evgenia V Dueva, Ksenia K Tuchynskaya, Liubov I Kozlovskaya, Dmitry I Osolodkin, Kseniya N Sedenkova, Elena B Averina, Vladimir A Palyulin and Galina G Karganova in Antiviral Chemistry and Chemotherapy