Abstract
A stronger association for low and high density lipoprotein particle (LDL-P and HDL-P) versus cholesterol concentrations (LDL-C and HDL-C) in predicting coronary heart disease (CHD) has been noted. We evaluate the role of these factors and extent of particle-cholesterol discordance in those with diabetes (DM) and metabolic syndrome (MetS) for event prediction. In the Multi-Ethnic Study of Atherosclerosis, we examined discordance of LDL and HDL (defined as a subject’s difference between baseline particle and cholesterol percentiles), LDL-C, LDL-P, HDL-C, and HDL-P in relation to incident CHD and cardiovascular disease (CVD) events in subjects with DM, MetS (without DM), or neither condition using Cox regression. Among 6,417 subjects with 10-year follow-up, those with MetS (N=1596) and DM (N=838) had significantly greater LDL and HDL discordance compared to those without these conditions. In discordance models, only LDL discordance [per standard deviation (SD)] within the MetS group was positively associated with CHD events [adjusted hazard ratio (HR) =1.22, 95% confidence interval (CI):1.01–1.48, p<0.05]. In models with individual particle/cholesterol variables (per SD), within the DM group, HDL-P was inversely (HR=0.71, 95% CI: 0.52–0.96, p<0.05) and LDL-C positively (HR=1.47, 95% CI: 1.07–2.03, p<0.05) associated with CHD. In those with MetS, only LDL-P was positively associated with CHD (HR=1.34, 95% CI: 1.00–1.78, p<0.05). Similar findings were also seen for CVD. LDL discordance and higher LDL-P in MetS, and higher LDL-C and lower HDL-P in DM, predict CHD and CVD, supporting a potential role for examining lipoprotein particles and discordances in persons with MetS and DM.
Keywords: Lipoproteins, coronary disease, cardiovascular disease, diabetes mellitus
Current guidelines recommend statin therapy in patients with diabetes (DM) for prevention of cardiovascular disease (CVD).1 Low density lipoprotein-cholesterol concentration (LDL-C) has been the primary focus of treatment, but many with well-controlled LDL-C levels still have considerable residual CVD risk.2 While HDL cholesterol (HDL-C) is inversely associated with coronary heart disease (CHD),3 it does not fully capture HDL-related CVD risk.4,5 Both LDL and HDL particles range in size, densities, and composition. While HDL particle number and size has been correlated to total HDL-C, the association is complex. HDL particle concentration (HDL-P), but not HDL cholesterol content is inversely associated with CHD and subclinical disease,4 and other studies have shown stronger associations of LDL particle concentration (LDL-P) than LDL-C with both subclinical disease 6 and CVD events.7 Further, studies show in those with low LDL-C but discordantly high LDL-P, or high LDL-C but discordantly low LDL-P, the risk of CVD events is related to LDL-P, but not LDL-C.8,9 There is, however, a lack of information on the relation of LDL-P and HDL-P on CVD risk prediction in DM and metabolic syndrome (MetS) patients. We hypothesize that the extent of discordance between LDL and HDL particle and cholesterol concentration at the patient-level is related to CVD risk in MetS and DM.
Methods
The design of the Multiethnic Study of Atherosclerosis (MESA), a National Institutes of Health-sponsored prospective epidemiologic study of the prevalence, risk factors, and subclinical disease predictors of CVD has been previously published.10 Briefly, 6,814 multiethnic participants aged 45–84, were recruited from six U.S. communities in 2000–2002 and were absent of known CVD. Recruitment was based on lists of residents, dwellings, telephone exchanges, lists of Medicare beneficiaries, and referrals by participants. The present study included 6,417 subjects with lipid concentration/particle and required covariates for CVD and CHD event analysis. IRB approval was obtained from all MESA Field Centers.
Age, sex, race/ethnicity, and risk factor information were collected at the baseline MESA examination (2000–2002). Smoking was categorized as being either a former smoker (smoked ≥100 cigarettes in life-time) or current (smoked cigarette in last 30 days). Family history of CHD was defined as a history of “heart attack” in parents, siblings, or child. Blood was drawn after a 12-hour fast and stored at −70°C. Lipids and glucose were measured at a central laboratory. Lipids were assayed on thawed ethylenediaminetetraacetic acid plasma using Centers for Disease Control Prevention/NHLBI standards. HDL-C was measured using the cholesterol oxidase method (Roche Diagnostics, Indianapolis) after precipitation of non–HDL-C with magnesium/ dextran (c.v. 2.9%). LDL-C was calculated using the Friedewald equation.11 Plasma lipoprotein particle concentrations were measured at LipoScience, Inc. by NMR spectroscopy. HDL-P and LDL-P (coefficient of variation <4%) are the sums of the particle concentrations of their respective subclasses, quantified from particle size using the amplitudes of their lipid methyl group NMR signals, and mean particle sizes are the weighted average of related subclasses.12
DM was defined as a fasting glucose ≥7.0 mmol/l (126 mg/dl), or if on insulin or oral DM medications. In those without DM, MetS was defined with ≥3 of the following: 1) waist circumference >88cm (35 in) for women and >102 cm (40 in) for men, 2) HDL-C <1.0 mmol/l (40 mg/dl) for men or <1.3 mmol/l (50 mg/dl) for women, 3) fasting triglycerides ≥1.7 mmol/l (150 mg/dl), 4) blood pressure (BP) ≥130mmHg systolic or ≥85mmHg diastolic, or on treatment, or 5) fasting glucose of 5.6–7.0 mmol/l (100–125 mg/dl), based on the American Heart Association/National Heart, Lung, and Blood Institute definition.13 Those not defined as having DM or MetS were categorized into the neither disease group.
Incident CHD (myocardial infarction, CHD death, resuscitated cardiac arrest, definite angina or probable angina followed by revascularization) and CVD events (CHD, fatal or nonfatal stroke, or other atherosclerotic CVD death) were ascertained and adjudicated for MESA as previously described.14 Follow-up time for those experiencing events was defined from the baseline examination date to the date of the first qualifying event. Those without an event were followed to death (from non-CVD causes), last follow-up, or the end of the study, after which they were censored.
Analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina). For patients with DM, MetS without DM (MetS), and neither disease group we compared baseline laboratory values and cardiovascular risk factors using the chi-square test for categorical variables and the Student’s t-test or ANOVA for continuous variables. Percentile distributions of LDL-P, LDL-C, HDL-P, and HDL-C were calculated from the study sample. LDL and HDL discordance was defined as a subject’s difference between respective baseline lipoprotein particle and cholesterol percentiles (e.g. LDL-P% – LDL-C%). The Student’s t-test was used to calculate significance among groups (MetS, DM, or neither condition) for mean LDL and HDL discordance. Incident CHD and CVD event rates were calculated by quartile of discordance for both LDL and HDL discordance among the three groups.
Cox proportional hazards regression provided hazard ratios (HRs) and corresponding 95% confidence intervals for CHD and CVD events. HDL-C, HDL-P, LDL-C, and LDL-P were each separately modeled with adjustments for baseline age, sex, race/ethnicity, family history, systolic BP, BP medication, smoking, body mass index, and statin use in each of the three groups and among all participants. Three separate models were conducted for all participants together and then for each of the three groups using the above covariate adjustments. Model 1 examined the continuous HDL discordance variable, adjusted for LDL-C and LDL-P. Model 2 examined the continuous LDL discordance variable, adjusted for HDL-C and HDL-P. Model 3 examined the variables of HDL-C, HDL-P, LDL-C, and LDL-P separately, but in the same model. All three models reflect adjustments for both particle and cholesterol concentration variables similar to prior studies.4 All hazard ratios were reported per standard deviation (SD) to allow for direct comparison. Interaction between each LDL or HDL discordance, particle, and cholesterol variable with group variables were evaluated for significance. Interactions with sex and race/ethnicity were also examined. Sensitivity analyses were conducted for Models 1–3 among all study groups, excluding patients with a history of statin use or hormone therapy at baseline, and, separately, with the additional adjustment for excessive alcohol use (>14 drinks/week) and exercise (minutes/week).
Analysis involving dichotomous discordance (as compared to continuous discordance analysis) as studied by others 9 involving categorizing individuals as < or ≥ median levels of LDL-C. Discordance was defined as LDL-C ≥ median and LDL-P < median level, or vice versa. This was also done for discordance groups between HDL-C and HDL-P. Risk factor-adjusted Cox regression was also used for also used for discordantly high cholesterol/low particle groups vs high cholesterol/high particle groups..
Results
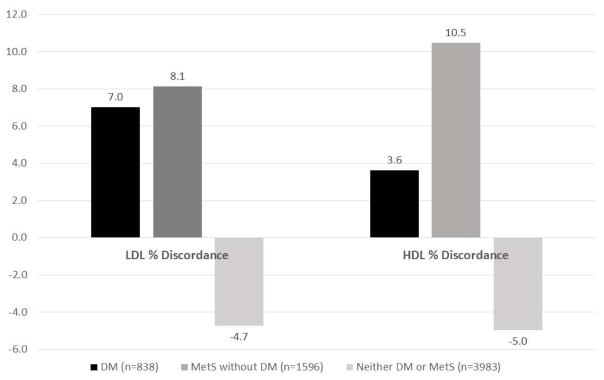
Table 1 shows significant differences in baseline covariates among the three study groups (Table 1). Both HDL-C and HDL-P were higher in participants without MetS or DM as compared to neither disease (p<0.001). LDL-P was lower in those with neither disease as compared to both disease groups (p<0.001). Mean (SD) LDL discordance (LDL-P percentile – LDL-C percentile) among groups were 7.0 (21.3) for DM, 8.1 (19.3) for MetS alone, and −4.7 (19.4) for those with neither disease. LDL discordance differed across all groups (p<0.001), except comparing DM and MetS (p=0.20) (Figure 1). Mean HDL discordance (HDL-P percentile – HDL-C percentile) among groups were 3.6 (22.7) for DM, 10.5 (19.4) for MetS alone, and −5.0 (19.5) for those with neither disease (p<0.001 between groups).
Table 1.
Baseline Characteristics of study subjects with and without Diabetes or Metabolic Syndrome by Study Group: Multi-Ethnic Study of Atherosclerosis
| Characteristic | MetS without DM (N=1596) | DM (N=838) | Neither DM nor MetS (N=3983) |
|---|---|---|---|
| Mean ± SD or N (%) | |||
| Age (years)* | 63.1 ± 10.0 | 64.7 ± 9.6 | 61.2 ± 10.3 |
| Male | 656 (41.1 %) | 438 (52.3 %) | 1957 (49.1 %) |
| Female | 940 (58.9 %) | 400 (47.7 %) | 2026 (50.1 %) |
| White | 644 (25.9 %) | 157 (6.3 %) | 1683 (67.8 %) |
| Black | 150 (19.4 %) | 102 (13.2 %) | 522 (67.4 %) |
| Hispanic | 407 (23.0 %) | 325 (18.4 %) | 1034 (58.6 %) |
| Chinese American | 395 (28.4 %) | 254 (18.2 %) | 744 (53.4 %) |
| Systolic BP (mm Hg)* | 133.6 ± 20.6 | 133.0 ± 22.2 | 122.2 ± 20.6 |
| BP Medication* | 760 (47.6 %) | 472 (56.3 %) | 862 (21.6 %) |
| BMI (kg/m2)* | 30.8 ± 5.1 | 30.3 ± 5.8 | 26.7 ± 4.9 |
| Cholesterol (mg/dL)* | 195.8 ± 35.5 | 188.0 ± 37.5 | 193.8 ± 33.6 |
| Triglycerides (mg/dL)* | 172.5 ± 68.6 | 144.4 ± 73.6 | 102.9 ± 49.0 |
| Smoker | 790 (49.6 %) | 422 (50.4 %) | 1954 (49.2 %) |
| Family History of CHD† | 688 (43.1 %) | 320 (38.2 %) | 1549 (38.9 %) |
| Statin Use* | 259 (16.2 %) | 208 (24.8 %) | 470 (11.8 %) |
| HDL-C (mg/dl)* | 43.2 ± 10.2 | 46.8 ± 13.2 | 55.2 ± 15.1 |
| HDL-P (μmol/l)* | 32.6 ± 6.4 | 32.7 ± 6.3 | 34.8 ± 6.7 |
| LDL-C (mg/dl)* | 118.1s 32.3 | 112.3 ± 33.4 | 118.0 ± 30.5 |
| LDL-P (μmol/l)* | 1354.3 ± 354.9 | 1264.8 ± 344.9 | 1199.2 ± 313.3 |
p<0.001,
p<0.01.
Denotes statistical significance between the three study groups (DM, MetS without DM, and Neither Disease)
Abbreviations: DM, Diabetes; MetS, Metabolic Syndrome; BP, Blood Pressure; BMI, Body Mass Index; CHD, Coronary Heart Disease; HDL-C, high-density lipoprotein cholesterol; HDL-P, high-density lipoprotein particle; LDL-C, low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle.
Figure 1. Percent Discordance of Particle vs Cholesterol Concentration for LDL and HDL.
LDL % discordance and HDL % discordance is defined as a subject’s difference between respective baseline lipoprotein particle and cholesterol percentiles. For LDL % discordance: p<0.001 among groups, except for DM vs MetS (p=0.20). For HDL % discordance: P<0.001 among groups. Among all participants, HDL % discordance ranged from −96.9 to 93.1, while LDL % discordance ranged from −86.7 to 78.7. Abbreviations: LDL, low-density lipoprotein; HDL, high-density lipoprotein; DM, Diabetes; MetS, Metabolic Syndrome.
Among 6,417 subjects, 462 subjects experienced CHD events and 659 subjects CVD events over an average 10-year follow-up. HDL discordance (Model 1) was not predictive among the three study groups for either CHD or CVD (Table 2); however, in the entire sample, higher levels of HDL discordance were associated with decreased CHD (HR=0.90, 95% CI: 0.81–1.00, p<0.05) and CVD (HR=0.90, 95% CI: 0.83–0.99, p<0.05) events. Similar results were seen in sensitivity analyses in those without prior statin or hormone therapy use for CHD (HR=0.86, 95% CI: 0.76–0.98, p<0.05) and CVD (HR=0.88, 95% CI: 0.79–0.98, p<0.05); additionally, HDL discordance was associated with decreased CHD and CVD within the DM group. LDL discordance (Model 2) was positively associated with CHD and CVD in the MetS group only, but attenuated in sensitivity analyses excluding those with statin or hormone therapy use. When adjusting for the standard baseline covariates, interaction terms of HDL and LDL discordance variables with group, sex, race/ethnicity variables were found to be insignificant.
Table 2.
Adjusted Hazard Ratio (95% Confidence Limits) for the Likelihood of Coronary Heart Disease and Cardiovascular Events by Study Groups
| Hazard ratio (95% confidence interval) per standard deviation | |||
|---|---|---|---|
| Variable | MetS (without DM) (N= 1596) | DM (N= 838) | Neither DM nor MetS (N=3983) |
| CHD Events | 139 | 105 | 218 |
| ‡ Model 1: HDL Discordance | 0.84 (0.68–1.04) | 0.88 (0.72–1.08) | 0.94 (0.80–1.10) |
| § Model 2: LDL Discordance | 1.21 (1.01–1.47)* | 0.88 (0.71–1.10) | 0.87 (0.74–1.02) |
| || Model 3: | |||
| HDL-C | 1.12 (0.73–1.73) | 1.00 (0.70–1.43) | 1.14 (0.90–1.45) |
| HDL-P | 0.86 (0.63–1.17) | 0.71 (0.52–0.96)* | 0.82 (0.66–1.03) |
| LDL-C | 0.80 (0.59–1.08) | 1.47 (1.07–2.03)* | 1.27 (1.01–1.59)* |
| LDL-P | 1.34 (1.01–1.78)* | 0.82 (0.58–1.17) | 0.93 (0.71–1.22) |
| CVD Events | 200 | 152 | 307 |
| ‡ Model 1: HDL Discordance | 0.95 (0.80–1.13) | 0.90 (0.77–1.06) | 0.88 (0.77–1.01) |
| § Model 2: LDL Discordance | 1.26 (1.07–1.47) † | 0.88 (0.74–1.06) | 0.91 (0.79–1.04) |
| || Model 3: | |||
| HDL-C | 1.05 (0.74–1.48) | 0.98 (0.73–1.32) | 1.12 (0.92–1.37) |
| HDL-P | 0.97 (0.76–1.25) | 0.75 (0.58–0.97)* | 0.80 (0.66–0.96)* |
| LDL-C | 0.77 (0.60–1.00) | 1.41 (1.08–1.84)* | 1.17 (0.96–1.42) |
| LDL-P | 1.39 (1.09–1.75) † | 0.76 (0.57–1.02) | 0.99 (0.79–1.24) |
p < 0.05,
p<0.01
All models were adjustments for age, sex, race/ethnicity, family history, systolic blood pressure, use of blood pressure medication, smoking, body mass index, statin use.
Model 1 additionally adjusted for LDL-C and LDL-P.
Model 2 additionally adjusted for HDL-C and HDL-P
Model 3 include simultaneous adjustment with all four lipid variables
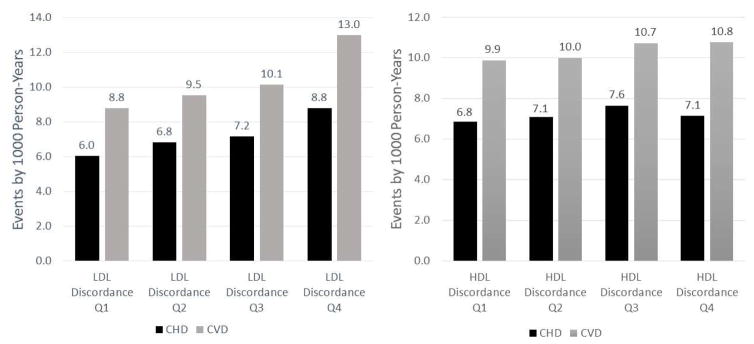
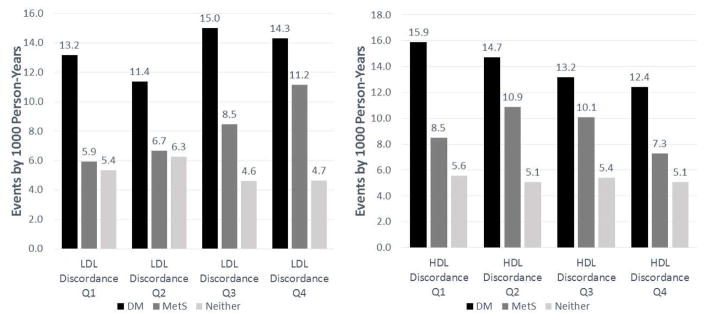
Figure 2 shows with each increasing quartile of LDL discordance there is a graded increase CHD and CVD event rates; this association with CHD was strongest in those with MetS (Figure 3), but is less clear within the DM or neither disease groups. In the overall sample there was no association between HDL discordance quartiles and CHD and CVD events (Figure 2). However, when evaluated by group, those with DM had lower event rates with higher levels of HDL discordance (Figure 3).
Figure 2. CHD and CVD Events by LDL and HDL Discordance Quartiles.
LDL discordance and HDL discordance is defined as a subject’s difference between respective baseline lipoprotein particle and cholesterol percentiles. Increasing levels of quartile discordance is denoted moving from Q1 to Q4. Abbreviations: CHD, Coronary Heart Disease; CVD, Cardiovascular Disease; LDL-C, low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle; HDL-C, high-density lipoprotein cholesterol; HDL-P, high-density lipoprotein particle; Q, Quartile. LDL Discordance Ranges: Q1 (-86.7,-12.3); Q2 (-12.3, 0.0); Q3 (0.0, 11.9), Q4 (11.9, 78.7). HDL Discordance Ranges: Q1 (-96.9,-11.9); Q2 (-11.9, 0.4); Q3 (0.4, 12.3), Q4 (12.3, 93.1)
Figure 3. Disease Group CHD Events by LDL and HDL Discordance Quartiles.
LDL discordance and HDL discordance is defined as a subject’s difference between respective baseline lipoprotein particle and cholesterol percentiles. Increasing levels of quartile discordance is denoted moving from Q1 to Q4. Abbreviations: CHD, Coronary Heart Disease; CVD, Cardiovascular Disease; HDL-C, high-density lipoprotein cholesterol; HDL-P, high-density lipoprotein particle; LDL-C, low-density lipoprotein cholesterol; LDL-P, low-density lipoprotein particle; Q, Quartile. LDL Discordance Ranges: Q1 (-86.7,-12.3); Q2 (-12.3, 0.0); Q3 (0.0, 11.9), Q4 (11.9, 78.7). HDL Discordance Ranges: Q1 (-96.9,-11.9); Q2 (-11.9, 0.4); Q3 (0.4, 12.3), Q4 (12.3, 93.1)
When evaluating lipid parameters separately in all subjects (not reported in Table 2), both LDL-C (HR=1.17, 95% CI: 1.06–1.28, P<0.01) and LDL-P (HR=1.16, 95% CI: 1.05–1.28, P<0.01) were shown to be associated with CHD. Similar results were seen for CVD events. In the overall sample HDL-C was not found to be significantly associated with CHD events, but was modestly negatively associated with CVD events. HDL-P was more strongly negatively associated with CHD (HR=0.82, 95% CI: 0.73–0.91, P<0.001) and CVD (HR=0.84, 95% CI: 0.76–0.92, P<0.001) events.
When evaluating lipid parameters separately (not reported in Table 2), in those with neither DM nor MetS, LDL-C was predictive of CHD and CVD. Within the DM group, LDL-C predicted CHD and trended towards significance for CVD. LDL-C was not found to be significantly associated with CHD or CVD in the MetS group. Only in those with neither condition was LDL-P was predictive of CHD (HR=1.17, 95% CI: 1.00–1.36, p<0.05) and CVD (HR=1.16, 95% CI: 1.02–1.33, p<0.01). HDL-P was significantly protective of CHD (HR=0.72, 95% CI: 0.56–0.92, P<0.01) and CVD (HR=0.77, 95% CI: 0.63–0.95, p<0.05) events in DM. HDL-C did not predict CHD or CVD in any group.
When adjusted for baseline covariates and each lipoprotein particle and cholesterol variable in the same model (Model 3), among all participants, HDL-P was the only variable that was significantly associated with CHD (HR=0.79, 95% CI: 0.68–0.92, p<0.01) and CVD (HR=0.82, 95% CI: 0.72–0.93, p<0.01). Within the DM group, HDL-P was negatively associated with CHD and CVD events (Table 2). HDL-C did not predict CHD or CVD events in any of the groups. Sensitivity analyses showed similar findings for HDL-P and HDL-C for CHD and CVD. LDL-C was positively associated with CHD and CVD events in the DM group (Table 2). Adjusting for the other lipid measures, LDL-P remained associated with CHD (HR=1.34, 95% CI: 1.00–1.78, p<0.05) and CVD (HR=1.39, 95% CI: 1.09–1.75, p<0.01) in the MetS group, but LDL-P did not predict CHD or CVD (despite a trend) for those with DM, the neither disease group, or the overall sample (Table 2). Similarly, LDL-P was not found to be significant within these groups in sensitivity analyses. All interaction terms between particle and cholesterol variables with group, sex, race/ethnicity variables were found to be insignificant. Additional adjustment for excessive alcohol use and exercise did not materially affect the results.
Using a dichotomous discordance measure, above-median LDL-C, but below-median LDL-P trended towards overestimating CHD risk (Adjusted HRs 0.30–0.82 among study groups) and CVD (Adjusted HRs 0.52–0.88 among study groups) as compared to particle/ cholesterol concordant groups. For those with above-median HDL-C, but below-median HDL-P, underestimation of CVD risk was present among the whole sample (HR: 1.46, 95% CI: 1.11–1.92, p<0.01) and those with neither DM nor MetS (HR: 1.70, 95% CI: 1.22–2.37, p<0.01) as compared to particle/cholesterol concordant groups. Similar trends were seen with above-median HDL-C discordance in MetS and DM groups.
Discussion
We show LDL and HDL positive discordance indicated by particle greater than concentration percentile in those with MetS and DM, but a negative discordance in those without these conditions. Further, in MetS we show a greater magnitude of LDL particle to cholesterol concentration discordance predicts events. We show that LDL-P is predictive of CHD and CVD in those with MetS and HDL-P is protective of CHD and CVD in those with DM, even when adjusting for each other, HDL-C, and LDL-C. Our study is the first major prospective evaluation of these effects in those with MetS and DM in relation to future CHD and CVD events. Our findings indicate that LDL and HDL concentrations alone may not adequately capture CVD risk in those with MetS or DM. We show trends indicating overestimation of risk with discordantly high LDL-C to low LDL-P and underestimation of risk with discordantly high HDL-C to low HDL-P. This may reflect difficulty assessing CVD risk in rigid cholesterol to particle discordance groups based on subjective cut-offs. Rather, LDL-P and HDL-P may play a useful role in risk assessment specifically for patients with MetS and DM where individual discordance is greatest.
While others have shown inverse relations between HDL-P with CHD and CVD,4,7 we found this association to be primarily present in those with DM, but not in those with or without MetS. The Multiple Risk Factor Intervention Trial showed in a case-control study HDL-P (particularly medium sized) to predict CHD death in MetS.15 This may be in part due to less robust adjustments or categorization of HDL-P into quartiles as compared to our continuous analysis. There were also other more important predictors of risk in those with MetS, (age, sex, family history, and systolic blood pressure) which may have prevented HDL-P to emerge as independently predictive of risk. Nonetheless, for those with DM, we show this association with CHD and CVD was not attenuated with the additional adjustments of LDL-P, LDL-C, and HDL-C and that HDL-C was not associated with either CHD or CVD.
Our data show HDL-C is inferior to HDL-P for prediction of clinical events among those with DM. While HDL-C is correlated to HDL-P, the relation of HDL-C with CVD is complex, influenced by atherogenic lipoproteins, inflammation, and insulin resistance making it a poorer marker in those with DM.16 In fact, in those with DM, HDL-P but not HDL-C is associated with cholesterol efflux17 and that prediction of CHD by HDL-C may be explained by markers associated with MetS.18 This was not the case for HDL-P, which was also predictive of CHD and remained predictive after additional adjustments for apolipoprotein B and triglycerides. Given this, it should be no surprise that in those with DM, HDL-C may reflect metabolic risk and not add to event prediction and possibly why recent clinical trials that increased HDL-C but had minimal effects on HDL-P,19 failed to show CVD protection.20,21
Persons with DM carry significant residual risk that inadequately explained by LDL-C.2 We continue to show the LDL-C plays an important role in predicting CHD and CVD events in those with DM. However, in those with MetS, we show LDL-C may underestimate CHD and CVD risk when compared to LDL-P and the magnitude of discrepancy between particle and cholesterol concentration in itself is predictive of CHD and CVD events. This indicates that simply having low LDL-C (as commonly measured in clinical practice), either from therapy or naturally, can underestimate LDL’s predictive power for clinical events. While we did not show LDL discordance to predict CVD in the DM group, this could be related to a bias of high intensity statin treatment attenuating the relationship of LDL with events. In addition, the continued dominant relation of LDL-C (in addition to risk factors of age, sex, and systolic blood pressure) with CHD and CVD events in DM may have made it more difficult for LDL discordance or particle number to emerge as independently predictive. Nonetheless, LDL-P was clearly found to be a better predictor for clinical events in those with MetS. Markers of overall LDL-P (as in this study) rather than size have been shown to be a more important determinant for CVD events.6,22
Strengths of MESA include its ethnic diversity and community-based recruitment; however with any non-randomized design there is the possibility of bias from unmeasured cofounders. Not all variables that are known to predict CVD events (such as Apo A-I and apo B) could be included given their unavailability in the MESA dataset. Also, while statin use was adjusted for and sensitivity analyses preformed, we could not adjust for changes in statin use and their possible effects on CVD events.
As recent guidelines have suggested aggressive statin use in DM for preventing CVD,1 the extent to which statin use affects the relationship between CVD and lipid profile discordance is also of interest. It is clear that statin treatment changes the cholesterol and triglyceride content of LDL particles.23 Studies using different statins have shown that the percentage decrease in LDL-C exceeds the percentage reduction in LDL-P,24,25 which is concerning given the closer relation of LDL-P with CVD events.8 In addition, secondary analysis of the JUPITER study has shown that in non-diabetic patients, potent rosuvastatin treatment leads to increase in HDL-P and HDL-C. However, HDL-P, not HDL-C, was shown to be inversely associated with CVD when adjusting for each other and other CVD risk factors.26
There has been increasing evidence documenting the value of HDL-P and LDL-P in predicting CVD and as a marker for successful lipid-lowering therapy. The American Association of Clinical Endocrinologists recently specified a LDL-P target of <1200 nmol/L for moderate CVD risk and LDL-P target of <1000 nmol/L for high CVD risk,27 supported by evidence that high-risk patients who achieve LDL-P<1000 nmol/L as compared to a LDL-C<100 mg/dL had a greater reduction in CVD events.28
Our study shows in persons with MetS greater LDL discordance is associated with future CVD. We show that HDL-P, not HDL-C, is inversely related to CVD. While this study and others have shown the benefit of examining lipoprotein particles in patients free of baseline CVD, our results further support a potential role for examining lipoprotein particles and their magnitude of discordance with cholesterol concentration for risk assessment and evaluation of therapeutic goals in patients with MetS and DM.
Acknowledgments
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dr. Budoff reports research grants from General Electric, Drs. Cromwell and Otvos report employment by Liposcience and Dr. Rosenblit reports speakers bureau participation with Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012;14:1–10. doi: 10.1007/s11883-011-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirovsky DR, Fedirko V, Cui Y, Sazonov V, Barter P. Prospective studies on the relationship between high-density lipoprotein cholesterol and cardiovascular risk: a systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16:404–423. doi: 10.1097/HJR.0b013e32832c8891. [DOI] [PubMed] [Google Scholar]
- 4.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:508–516. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208:305–316. doi: 10.1016/j.atherosclerosis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. doi: 10.1016/j.jacl.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergeer M, Holleboom AG, Kastelein JJ, Kuivenhoven JA. The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J Lipid Res. 2010;51:2058–2073. doi: 10.1194/jlr.R001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan HC, Tai ES, Sviridov D, Nestel PJ, Ng C, Chan E, Teo Y, Wai DC. Relationships between cholesterol efflux and high-density lipoprotein particles in patients with type 2 diabetes mellitus. Journal of Clinical Lipidology. 2011;5:467–473. doi: 10.1016/j.jacl.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 18.El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Krauss RM, Wojnooski K, Orr J, Geaney JC, Pinto CA, Liu Y, Wagner JA, Luk JM, Johnson-Levonas AO, Anderson MS, Dansky HM. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012;53:540–547. doi: 10.1194/jlr.M018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 21.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 22.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 23.Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia : preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler Thromb Vasc Biol. 2000;20:189–197. doi: 10.1161/01.atv.20.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Miller M, Dolinar C, Cromwell W, Otvos JD. Effectiveness of high doses of simvastatin as monotherapy in mixed hyperlipidemia. Am J Cardiol. 2001;87:232–234. A239. doi: 10.1016/s0002-9149(00)01327-8. [DOI] [PubMed] [Google Scholar]
- 25.Blake GJ, Albert MA, Rifai N, Ridker PM. Effect of pravastatin on LDL particle concentration as determined by NMR spectroscopy: a substudy of a randomized placebo controlled trial. Eur Heart J. 2003;24:1843–1847. doi: 10.1016/j.ehj.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE, Davidson MH. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement--executive summary. Endocr Pract. 2013;19:536–557. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toth PP, Grabner M, Punekar RS, Quimbo RA, Cziraky MJ, Jacobson TA. Cardiovascular risk in patients achieving low-density lipoprotein cholesterol and particle targets. Atherosclerosis. 2014;235:585–591. doi: 10.1016/j.atherosclerosis.2014.05.914. [DOI] [PubMed] [Google Scholar]