PREFACE
The American College of Cardiology (ACC) has a long history of developing documents (e.g., decision pathways, health policy statements, appropriate use criteria) to provide members with guidance on both clinical and nonclinical topics relevant to cardiovascular (CV) care. In most circumstances, these documents have been created to complement clinical practice guidelines and to inform clinicians about areas where evidence may be new and evolving or where sufficient data may be more limited. In spite of this, numerous care gaps continue to exist, highlighting the need for more streamlined and efficient processes to implement best practices in service to improved patient care.
Central to the ACC’s strategic plan is the generation of “actionable knowledge”–a concept that places emphasis on making clinical information easier to consume, share, integrate, and update. To this end, the ACC has evolved from developing isolated documents to the development of integrated “solution sets.” Solution sets are groups of closely related activities, policy, mobile applications, decision support, and other tools necessary to transform care and/or improve heart health. Solution sets address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care. They use both established and emerging methods to disseminate information for CV conditions and their related management. The success of the solution sets rests firmly on their ability to have a measurable impact on the delivery of care. Because solution sets reflect current evidence and ongoing gaps in care, the associated content will be refined over time to best match changing evidence and member needs.
Expert consensus decision pathways (ECDPs) represent a key component of solution sets. The methodology for ECDPs is grounded in assembling a group of clinical experts to develop content that addresses key questions facing our members across a range of high-value clinical topics (1). This content is used to inform the development of various tools that accelerate real time use of clinical policy at the point of care. They are not intended to provide a single correct answer; rather, they encourage clinicians to ask questions and consider important factors as they define a treatment plan for their patients. Whenever appropriate, ECDPs seek to provide unified articulation of clinical practice guidelines, appropriate use criteria, and other related ACC clinical policy. In some cases, covered topics will be addressed in subsequent clinical practice guidelines as the evidence base evolves. In other cases, these will serve as stand-alone policy.
Ty J. Gluckman, MD, FACC
Chair, ACC Solution Set Oversight Committee
1. INTRODUCTION
Despite major therapeutic advances leading to improved outcomes over the past 2 decades, CV disease remains the leading cause of morbidity and mortality in patients with type 2 diabetes (T2D) (2–4). Over this time, the prevalence of T2D has increased, while the excess risk of adverse CV events in patients with T2D (compared with patients without diabetes) has remained largely unchanged (5,6). Accordingly, the development of treatment strategies to improve CV outcomes in this vulnerable patient population remains a major priority. Diabetes is typically thought of as a disease of elevated blood glucose (7). Although large clinical trials have consistently demonstrated an improvement in microvascular outcomes in patients with T2D with intensive versus conservative glucose control, similar results have not been demonstrated for CV outcomes in patients with T2D, despite the clinically important differences in hemoglobin A1c (HbA1c) achieved between treatment groups in glucose-lowering trials (8–11). The opportunities for improving clinical outcomes in patients with T2D and CV disease have recently expanded.
Many sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1RAs) have been demonstrated to significantly reduce the risk of major adverse cardiovascular events (MACE) (12–19). SGLT2 inhibitors also substantially diminish the risks of heart failure (HF) hospitalization and progression of diabetic kidney disease (DKD). Although the exact mechanisms of CV and renal benefits remain uncertain, they appear to exceed the direct glucose-lowering effects of these agents and may be related to additional mechanisms of action of each class of medications (20,21). Data proving that SGLT2 inhibitors and GLP-1RAs improve outcomes in patients with T2D and CV disease have triggered a major paradigm shift beyond glucose control to a broader strategy of comprehensive CV risk reduction (2,22,23). The potential of these compounds has also stimulated re-examination of the traditional roles of various medical specialties in the management of T2D, compelling CV specialists to adopt a more active role in prescribing drugs that may previously have been seen primarily as glucose-lowering therapies. This evolving role has created a need for novel clinical care delivery models that are collaborative, interprofessional, and multidisciplinary in their approach to managing this high-risk patient group with multiple comorbidities. The purpose of this ECDP is to update the 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease (ASCVD) (24) with data from emerging studies, and continue to provide succinct, practical guidance on the use of specific agents for reducing CV risk in patients with T2D.
1.1. A Focus on Comprehensive CV Risk Reduction in T2D
Although the primary focus of patients, clinicians, and healthcare systems should be the prevention of T2D (25), a significant proportion of patients cared for by CV clinicians have known T2D, undiagnosed diabetes, or prediabetes (26). Because most morbidity and mortality in T2D comes from CV events (27), the CV specialist has a key role in optimizing these patients’ care and is well-positioned to address 3 key areas in the management of patients with T2D:
Screening for T2D in their patients with or at high risk of CV disease;
Aggressively treating CV risk factors; and
Incorporating newer glucose-lowering agents with evidence for improving CV outcomes into routine practice.
Data from the NCDR PINNACLE registry from 2008 through 2009 show that only 13% of outpatients in the United States with coronary artery disease cared for primarily by cardiologists are screened for T2D (28). While the proportion screened is likely to have improved in the decade since that report was published, there remains a need for improvement in comprehensive CV risk factor control among patients with T2D (29,30), as current care delivery is often fragmented, episodic, and focused on treating acute events. Comprehensive CV risk factor control reduces events and improves survival in patients with T2D (31,32). This includes encouraging a healthy diet, regular physical activity, weight loss, smoking cessation, assiduous control of blood pressure (33), lowering of atherogenic blood lipids (34,35), and use of antiplatelet agents in accordance with current treatment guidelines (2,35,36). Only a minority of patients with diabetes achieve these key benchmarks (37). Beyond these core recommendations, CV specialists should be aware of the strong clinical evidence regarding specific glucose-lowering therapies proven to lower CV risk. Given that patients with T2D and CV disease frequently follow up with their CV specialists, a firm understanding of the efficacy and safety profiles and net clinical benefits of these agents is important. Such encounters are an ideal time to review the patient’s overall management and consider the initiation of these novel agents to favorably impact patient care and outcomes.
2. METHODS
The ACC created the Heart House Roundtables, a structured format of interactive discussion among a broad group of stakeholders, to address high-value topics and issues that clinicians and patients face daily, such as the treatment of CV disease in patients with T2D (38). The planning committee for the Managing CV Disease Risk in Diabetes roundtable was led by Mikhail Kosiborod, MD, FACC, and Larry Sperling, MD, FACC. To accommodate the multiple perspectives concerning new therapeutic options for patients with T2D, the roundtable included several experts in diverse medical specialties, such as cardiology, family medicine, internal medicine, and endocrinology, and included physicians, nurses, advanced practice providers, and pharmacists. Recognizing the significant impact of recently available CV outcomes trial data, discussions focused on the real-world challenges faced in working toward comanaging T2D and CV disease for improved patient outcomes. As a result, the ACC saw an opportunity to provide guidance to fill the current gap between CV clinicians and diabetes care providers who jointly manage patients with T2D and ASCVD, HF, and/or DKD. To support this effort, a writing committee of multidisciplinary experts was convened in 2017 to develop an ECDP providing guidance on the use of antidiabetic agents proven to reduce CV risk in patients with T2D (24). For this update, the writing committee convened in late 2019 via conference call attended only by writing committee members and ACC staff. Differences were resolved by consensus among the group, and no portions of the ECDP required administrative decision overrides. The work of the writing committee was supported only by the ACC and did not have any commercial support. Writing committee members were all unpaid volunteers.
The ACC and the Solution Set Oversight Committee (SSOC) recognize the importance of avoiding real or perceived relationships with industry (RWI) or other entities that may affect clinical policy. The ACC maintains a database that tracks all relevant relationships for ACC members and persons who participate in ACC activities, including those involved in the development of ECDPs. ECDPs follow ACC RWI Policy in determining what constitutes a relevant relationship, with additional vetting by the SSOC.
ECDP writing groups must be chaired or co-chaired by an individual with no relevant RWI. While vice chairs and writing group members may have relevant RWI, this must constitute less than 50% of the writing group. Relevant disclosures for the writing group, external reviewers, and SSOC members can be found in Appendixes 1 and 2. Participants are discouraged from acquiring relevant RWI throughout the writing process.
3. ASSUMPTIONS AND DEFINITIONS
To facilitate interpretation of the recommendations provided in this ECDP, specific assumptions were made by the writing committee as specified in Section 3.1.
3.1. General Clinical Assumptions
The principal focus of this effort, including ECDP considerations, applies to patients with T2D and CV disease or who are at high risk for CV disease.
The writing committee endorses the evidence-based approaches to CV disease risk reduction recommended in the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (33), the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol (34), and the 2019 ACC/AHA Guidelines on the Primary Prevention of Cardiovascular Disease (39).
The writing committee endorses the evidence-based approaches to diabetes management outlined in the American Diabetes Association (ADA) Standards of Medical Care in Diabetes: Chapter 10. Cardiovascular Disease and Risk Management (2).
The writing committee endorses the evidence-based approaches to HF therapy and management enumerated in the 2013 ACCF/AHA Guideline for the Management of Heart Failure, the 2016 ACC/AHA/HFSA Focused Update on the New Pharmacological Therapy for Heart Failure: an Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure, and the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction (40–42). It is important to note that the 2013 and 2017 HF guidelines as well as the 2017 ECDP do not include major trials that are described in this ECDP because of the timing of those publications.
Optimal patient care decisions should properly reflect the patient’s preferences and priorities as well as those of the managing clinician.
This ECDP is not intended to supersede good clinical judgement. The treating clinician should seek input as needed from relevant experts (e.g., pharmacists, cardiologists, endocrinologists).
This ECDP is based on the best data currently available. New information is being generated rapidly (e.g., CV outcomes trials of additional agents and including other patient populations), and as these data become available, they will impact the considerations made here. Clinicians should be careful to incorporate relevant information published after this ECDP.
A background effort aimed at comprehensive CV risk reduction is essential, using the full complement of diet, exercise, and lifestyle recommendations, as well as CV risk factor modification and other preventive medical therapies described in the ADA Standards of Care and/or the applicable AHA/ACC guidelines or ACC ECDPs.
Although implementing relevant portions of these recommendations in the acute inpatient setting may be reasonable, this ECDP is primarily focused on management in the outpatient ambulatory setting.
3.2. Definitions
Atherosclerotic cardiovascular disease (ASCVD):
a history of an acute coronary syndrome or myocardial infarction (MI), stable or unstable angina, coronary heart disease with or without revascularization, other arterial revascularization, stroke, or peripheral artery disease assumed to be atherosclerotic in origin. This definition is intended to be consistent with that used in the 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk (34).
Cardiovascular (CV) disease includes ASCVD, HF, and CV-related death.
Diabetic kidney disease (DKD):
a clinical diagnosis marked by a decrease in estimated glomerular filtration rate (eGFR), the presence of albuminuria, or both in a patient with diabetes. This definition is intended to be consistent with those used in the ADA Standards of Medical Care for Diabetes and the clinical trials referenced throughout this ECDP (19,43).
Heart failure (HF):
defined per criteria outlined in the 2013 ACCF/AHA Guideline for the Management of Heart Failure and the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction (42,44). An HF event, including hospitalization, is defined by the criteria outlined by the 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials (45).
Heart failure with reduced ejection fraction (HFrEF):
clinical diagnosis of HF and left ventricular ejection fraction ≤40% (42,46).
High risk for ASCVD:
patients with end organ damage such as left ventricular hypertrophy, retinopathy, or multiple risk factors (e.g., age, hypertension, smoking, obesity, dyslipidemia)
Major adverse cardiovascular event (MACE):
either a “3-point MACE” composite endpoint of nonfatal myocardial infarction (MI), nonfatal stroke, or CV death, or a “4-point MACE” composite endpoint of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, or CV death.
4. PATHWAY SUMMARY GRAPHIC
Figure 1 provides an overview of what is covered in the ECDP. See each section for more detailed considerations and guidance.
FIGURE 1. Summary Graphic.
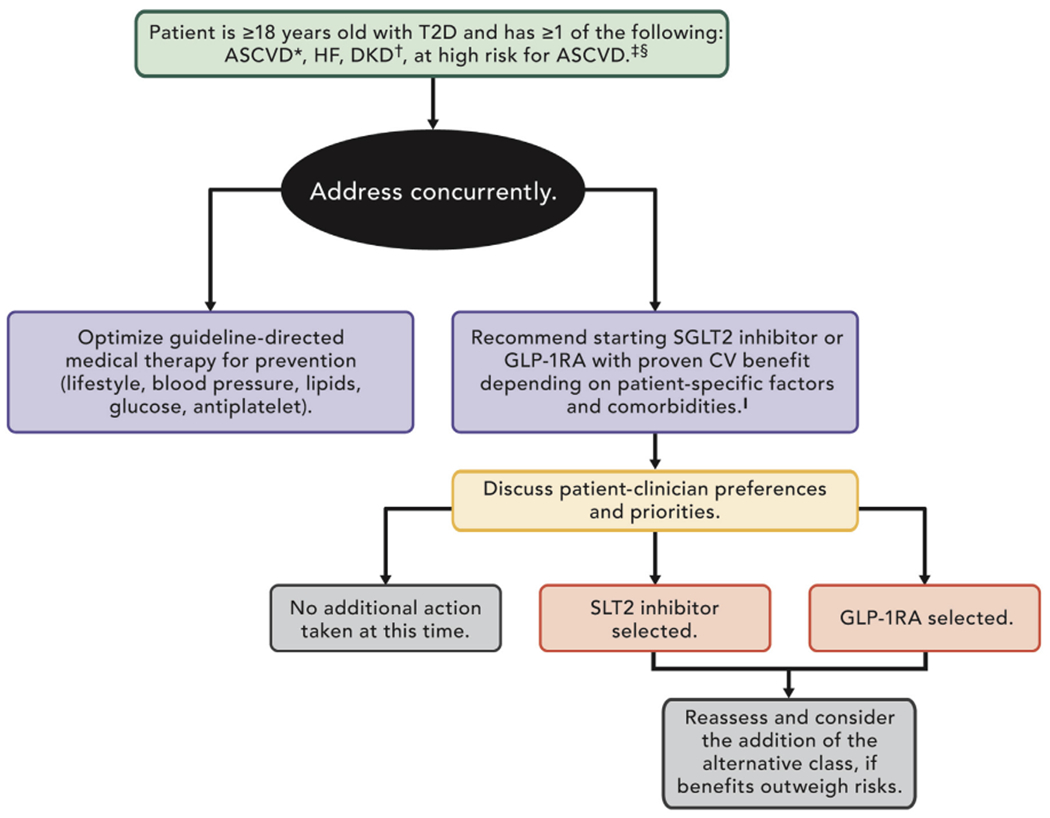
*ASCVD is defined as a history of an acute coronary syndrome or MI, stable or unstable angina, coronary heart disease with or without revascularization, other arterial revascularization, stroke, or peripheral artery disease assumed to be atherosclerotic in origin.
†DKD is a clinical diagnosis marked by reduced eGFR, the presence of albuminuria, or both. Consider an SGLT2 inhibitor when your patient has established ASCVD, HF, DKD or is at high risk for ASCVD.
‡Consider a GLP-1RA when your patient has established ASCVD or is at high risk for ASCVD.
§Patients at high risk for ASCVD include those with end organ damage such as left ventricular hypertrophy or retinopathy or with multiple CV risk factors (e.g., age, hypertension, smoking, dyslipidemia, obesity).
∥Most patients enrolled in the relevant trials were on metformin at baseline as glucose-iowering therapy.
ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; GLP-1RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; MI = myocardial infarction; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes
5. DESCRIPTION AND RATIONALE
CV specialists should be aware of the evidence supporting the use of specific SGLT2 inhibitors and GLP-1RAs to reduce risk in patients with T2D and established CV disease.
5.1. SGLT2 Inhibitors
SGLT2 inhibitors have emerged as important new oral therapies for patients with T2D. Large, randomized controlled trials in patients with T2D have demonstrated that many of these agents reduce MACE in patients with established ASCVD and/or DKD, and reduce the risk of HF hospitalizations (see Table 1).
TABLE 1.
Summary of the Published SGLT2 Inhibitor CV and Renal Outcomes Trials
| EMPA-REG OUTCOME (12) | CANVAS/CANVAS-R (16) | DECLARE-TIMI 58 (17) | CREDENCE (19) | DAPA-HF* (47) | |
|---|---|---|---|---|---|
| Patients enrolled, n | 7,020 | 10,142 | 17,160 | 4,401 | 4,744 |
| Drug | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin | Dapagliflozin |
| Dose | 10 or 25 mg PO daily | 100 or 300 mg PO daily | 10 mg PO daily | 100 mg PO daily | 10 mg PO daily |
| Median duration of follow-up (years) | 3.1 | 2.4 | 4.2 | 2.6 | 1.5 |
| Mean baseline HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.3 | * |
| Mean duration of diabetes (years) | N/A† | 13.5 | 11.0 | 15.8 | * |
| Baseline statin use (%) | 77 | 75 | 75 | 69 | n/a |
| Baseline prevalence of CV disease/HF (%) | 99 | 72 | 41 | 50 | Not reported |
| Baseline prevalence of HF (%) | 10 | 14 | 10 | 15 | 100* |
| MACE outcome, HR (95% CI)‡ | 0.86 (0.74-0.99) | 0.86 (0.75-0.97) | 0.93 (0.84-1.03) | 0.80 (0.67-0.95) | Not reported |
| Hospitalization for HF or CV death, HR (95% CI)§ | 0.66 (0.55-0.79) | 0.78 (0.67-0.91) | 0.83 (0.73-0.95) | 0.69 (0.57-0.83) | 0.75 (0.65-0.85) |
| CV death, HR (95% CI) | 0.62 (0.49-0.77) | 0.87 (0.72-1.06) | 0.98 (0.82-1.17) | 0.78 (0.61-1.00) | 0.82 (0.69-0.98) |
| Fatal or nonfatal MI, HR (95% CI) | 0.87 (0.70-1.09) | 0.89 (0.73-1.09) | 0.89 (0.77-1.01) | Not reported | Not reported |
| Fatal or nonfatal stroke, HR (95% CI) | 1.18 (0.89-1.56) | 0.87 (0.69-1.09) | 1.01 (0.84-1.21) | Not reported | Not reported |
| All-cause mortality, HR (95% CI) | 0.68 (0.57-0.82) | 0.87 (0.74-1.01) | 0.93 (0.82-1.04) | 0.83 (0.681.02) | 0.83 (0.71-0.97) |
| HF hospitalization, HR (95% CI) | 0.65 (0.50-0.85) | 0.67 (0.52-0.87) | 0.73 (0.61-0.88) | 0.61 (0.47-0.80) | 0.70 (0.59-0.83) |
| Renal composite endpoint,∥ HR (95% CI) | 0.54 (0.40-0.75) | 0.60 (0.47-0.77) | 0.53 (0.43-0.66) | 0.70 (0.59-0.82) | 0.71 (0.44-1.16) |
58.2% of patients enrolled in DAPA-HF did not have diabetes mellitus. All patients enrolled in DAPA-HF had HFrEF.
Mean duration of diabetes was not provided for EMPA-REG OUTCOME, but 57% of patients enrolled had diabetes for more than 10 years.
This outcome was the primary outcome for CANVAS and EMPA-REG OUTCOME and was a dual primary outcome for DECLARE-TIMI 58. It was a secondary outcome for CREDENCE. It consists of 3-point MACE, a composite of nonfatal MI, nonfatal stroke, and CV death. The p value for superiority for the primary endpoint for empagliflozin (all doses) vs. placebo was 0.04, and the p value for superiority for the primary endpoint for canagliflozin (all doses) vs. placebo was 0.02. The p values for the other comparisons are available in the primary EMPA-REG OUTCOME report but were not published in the CANVAS/CANVAS-R report. DECLARE-TIMI 58 had dual primary endpoints of MACE and hospitalization for HF or CV death. MACE was not assessed in DAPA-HF, which was an HF outcome trial.
Hospitalization for HF or CV death was a dual primary endpoint of the DECLARE-TIMI 58 trial. In EMPA-REG OUTCOME, this endpoint excluded fatal stroke events. The primary endpoint of DAPA-HF was worsening HF (urgent HF visit or hospitalization for HF) or CV death. Dapagliflozin, 10 mg daily was associated with a 26% reduction in the primary endpoint (HR: 0.74; 95% CI: 0.65 to 0.85).
A renal endpoint reported in a recent meta-analysis was a composite of sustained doubling of serum creatinine or a 40% decline in eGFR, end-stage kidney disease, or death of renal cause (49). The DAPA-HF renal composite endpoint was defined as a sustained decline in eGFR of 50% or greater, end-stage kidney disease, renal transplantation, renal death, and death from any cause.
CANVAS/CANVAS-R = Canagliflozin CV Assessment Study/A Study of the Effects of Canagliflozin (JNJ-28431754) on Renal Endpoints in Adult Participants With T2D; CI = confidence interval; CREDENCE = Evaluation of the Effects of Canagliflozin on Renal and CV Outcomes in Participants With Diabetic Nephropathy; CV = cardiovascular; DAPA-HF = Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening HF or CV Death in Patients With Chronic HF; DECLARE-TIMI 58 = Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of CV Events-Thrombolysis In Myocardial Infarction 58; eGFR = estimated glomerular filtration rate; EMPA-REG OUTCOME= (Empagliflozin) CV Outcome Event Trial in T2D Patients; HbA1c = hemoglobin A1c; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; MACE = major adverse cardiovascular event; MI = myocardial infarction; PO = “per os,” by mouth; SGLT2 = sodium-glucose cotransporter-2.
These benefits may be similar for agents within this class, although there are differences that seem likely to reflect the patient populations enrolled in the trials (48–50). The benefit of reducing HF hospitalizations in these trials reflected primarily prevention of symptomatic HF in T2D patients at high risk, as ~90% did not have HF at baseline (and those who did were not well-characterized). The benefits of an SGLT2 inhibitor in treating established HF were demonstrated in the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening HF or CV Death in Patients With Chronic HF) trial, in which dapagliflozin significantly reduced the risk of CV death or worsening HF, and improved HF-related symptoms in ~4,800 patients with HFrEF. Of note, more than half of patients in this trial did not have T2D, and there was no difference in the treatment benefit of dapagliflozin across the subgroups of patients with or without T2D. Beneficial effects of dapagliflozin on symptoms, functional status, and quality of life in patients with HFrEF were also seen in the DEFINE-HF (Dapagliflozin Effect on Symptoms and Biomarkers in Patients With HF) trial (51). Additional trials in both HFrEF and heart failure with preserved ejection fraction (HFpEF) are ongoing with various agents. Furthermore, consistent reductions in the secondary outcome of risk of kidney disease progression were seen with all agents in the CV outcomes trials (although the number of “hard” renal events was small). The CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and CV Outcomes in Participants With Diabetic Nephropathy) trial–the first dedicated renal outcome trial of the SGLT2 inhibitor class–reported that canagliflozin significantly reduced the risk of DKD progression, including development of end-stage kidney disease and initiation of dialysis. Patients in the CREDENCE trial were enrolled with an eGFR as low as 30 ml/min/1.73 m2 and continued to be treated with canagliflozin even if their eGFR was below that threshold. Benefits and adverse effects in the group with the lowest eGFR were consistent with those in the remainder of the cohort (19).
5.1.1. SGLT2 Inhibitors: Mechanism of Action
SGLT2 is a sodium-glucose cotransporter in the proximal tubule of the nephron that is responsible for approximately 90% of urinary glucose reabsorption. Inhibition of SGLT2 results in glucose lowering through induction of glucosuria. This effect is more pronounced in the setting of hyperglycemia, where significant amounts of glucose are filtered into the urine. Glucosuria diminishes significantly as blood glucose normalizes (9). In addition, as eGFR decreases, the effects of SGLT2 on blood glucose are smaller. The risk of hypoglycemia for patients taking an SGLT2 inhibitor is extremely low unless such an agent is used concomitantly with insulin or insulin secretagogues (such as sulfonylureas and glinides). Beyond their effect on blood glucose, SGLT2 inhibitors also cause diuretic and natriuretic effects, promote weight loss, and lower systolic blood pressure (52). Interestingly, changes in traditional risk factors such as elevated HbA1C and lipids do not seem to be the key determinants of the beneficial effects of SGLT2 inhibitors on CV and renal outcomes (20,21). Although the mechanisms of SGLT2 inhibitor benefit have not been fully elucidated, a number of putative mechanisms have been proposed, including reductions in preload and afterload through diuresis, alterations in myocardial metabolism, and prevention of myocardial fibrosis, among others (53).
5.1.2. SGLT2 Inhibitors and ASCVD Events
The EMPA-REG OUTCOME (Empagliflozin CV Outcome Event Trial in T2D Patients) trial (12) showed a 14% relative risk reduction in the primary endpoint of 3-point MACE (hazard ratio [HR]: 0.86; 95% confidence interval [CI]: 0.74 to 0.99) compared with placebo. This reduction in the primary outcome and the observed 32% reduction in all-cause mortality (HR: 0.68; 95% CI: 0.57 to 0.82) were driven predominantly by a 38% reduction in CV death (HR: 0.62; 95% CI: 0.49 to 0.77) (54). The effects of empagliflozin on fatal or nonfatal MI were more modest, with confidence intervals that overlapped 1.0 (HR: 0.87; 95% CI: 0.70 to 1.09), and there was no significant difference in fatal or nonfatal stroke, with confidence interval limits also broadly overlapping 1.0 (HR: 1.18; 95% CI: 0.89 to 1.56). Importantly, the secondary endpoint of HF hospitalization was reduced by 35% (HR: 0.65; 95% CI: 0.50 to 0.85). Separation in the cumulative event curves suggested an early benefit of the compound (55) and was consistent across patient subgroups with or without prevalent HF at study entry (56). Empagliflozin is specifically approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of CV death in adults with T2D and established CV disease (57).
Two large CV outcomes trials have assessed the impact of canagliflozin on MACE; the CANVAS (Canagliflozin CV Assessment Study) and CANVAS-R (Study of the Effects of Canagliflozin [JNJ-28431754] on Renal Endpoints in Adult Participants With T2D) trials (13) enrolled 4,330 and 5,812 patients, respectively, 72% of whom had established ASCVD. Study participants were randomized to placebo or canagliflozin (100 or 300 mg in CANVAS, and 100 mg with an optional increase to 300 mg in CANVAS-R). Results from CANVAS and CANVAS-R are mostly consistent with those of EMPA-REG OUTCOME. Analyses of the effects of canagliflozin versus placebo on the secondary endpoints of CV and all-cause death were directionally consistent with the primary endpoint (16,58). As with EMPA-REG OUTCOME, no difference in outcomes was seen between SGLT2 inhibitor doses. The combined analysis of the 2 CANVAS trials demonstrated a 14% relative reduction in the primary endpoint of triple MACE (HR: 0.86; 95% CI: 0.75 to 0.97 from 31.5 to 26.9 events per 1,000 person-years) compared with placebo (16,58). Although CANVAS was underpowered for the individual components of the primary outcome and thus none were statistically significant on their own, the point estimates for each component were consistently in favor of SGLT2 inhibitor therapy-CV death (HR: 0.87; 95% CI: 0.72 to 1.06); fatal or nonfatal MI (HR: 0.89; 95% CI: 0.73 to 1.09), and fatal or nonfatal stroke (HR: 0.87; 95% CI: 0.69 to 1.09)-as was the point estimate for reduction in all-cause mortality (HR: 0.87; 95% CI: 0.74 to 1.01).
Three-point MACE was a prespecified secondary outcome of the CREDENCE trial (19), which studied patients with established DKD (see Table 1). In CREDENCE, patients randomized to canagliflozin 100 mg daily experienced a 20% relative risk reduction in the composite MACE endpoint of CV death, MI, or stroke (HR: 0.80; 95% CI: 0.67 to 0.95). A qualitatively similar, although not statistically significant, 17% reduction was seen in all-cause mortality (HR: 0.83; 95% CI: 0.68 to 1.02). Canagliflozin is now approved by the FDA to reduce the risk of MACE in patients with established CV disease, to prevent hospitalizations for HF in patients with DKD and albuminuria, and to reduce the risk of progression of diabetic nephropathy.
The DECLARE-TIMI 58 (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of CV Events-Thrombolysis In Myocardial Infarction 58) (17,60) is the largest SGLT2 inhibitor trial to date. More than half of the trial participants did not have established ASCVD; the overwhelming majority also had normal kidney function and no significant albuminuria. MACE was 1 of 2 primary endpoints, along with the composite of CV death or hospitalization for HF. In DECLARE-TIMI 58, patients randomized to receive dapagliflozin 10 mg compared with placebo had a nonstatistically significant 7% relative risk reduction in MACE (HR: 0.93; 95% CI: 0.84 to 1.03). Again, this was quite close to the 7% nonsignificant reduction seen in all-cause mortality (HR: 0.93; 95% CI: 0.82 to 1.04). Whether the smaller treatment effect of dapagliflozin 10 mg on reducing MACE seen in DECLARE-TIMI 58 reflects the much lower-risk patient cohort (as compared with EMPA-REG and CANVAS), a true drug-specific effect, or a combination of both, is not known. Importantly, dapagliflozin significantly reduced the risk of the second dual primary endpoint-composite of CV death or hospitalization for HF (HR: 0.83; 95% CI: 0.73 to 0.95). The 10-mg dose of dapagliflozin is now approved by the FDA to reduce the risk of HF in patients with T2D who have established or are at high risk for ASCVD. The results of the VERTIS-CV trial (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease) were presented at the American Diabetes Association Virtual Scientific Sessions on June 16, 2020. The risk of the primary endpoint of CV death, nonfatal MI, or stroke was similar in the ertugliflozin and placebo groups (HR 0.97%, 95% CI 0.85-1.11), and ertugliflozin reduced the rate of hospitalization for heart failure (59). A prospective CV outcomes trial of SGLT2 inhibitor ertugliflozin (60) and the SGLT2 and SGLT1 inhibitor sotagliflozin (61) is currently underway.
5.1.3. SGLT2 Inhibitors in Patients With and Without Established ASCVD
A recently published meta-analysis of data from CANVAS, CREDENCE, DECLARE-TIMI 58, and EMPA-REG OUTCOME reported a 12% reduction in MACE (HR: 0.88; 95% CI: 0.82 to 0.94) with no statistically significant interaction based on primary versus secondary prevention (P interaction = 0.252) (62). Note that these observations do not apply to the effect of SGLT2 inhibitors on the risk of hospitalization for HF or progression of DKD, which are outlined in the following text.
5.1.4. SGLT2 Inhibitors and HF Events
HF is increasingly common and is a source of considerable morbidity and mortality for patients with diabetes. All of the published randomized trials, as well as several observational studies of claims databases and registries, have demonstrated substantial benefits for an SGLT2 inhibitor in the prevention of hospitalization for HF and in the composite of hospitalization for HF and CV death.
The effects of SGLT2 inhibitors on HF hospitalization appear remarkably consistent across the class. In the EMPA-REG OUTCOME trial, CV death or hospitalization for HF was an exploratory secondary outcome. Patients randomized to empagliflozin had a 34% reduction in this endpoint (HR: 0.66; 95% CI: 0.55 to 0.79) (12). The individual effects on HF hospitalization alone (HR: 0.65; 95% CI: 0.50 to 0.85) were similar. In the CANVAS program, a 33% reduction in HF hospitalization was seen (HR: 0.67; 95% CI: 0.52 to 0.87). In CREDENCE, patients randomized to canagliflozin experienced a 39% relative risk reduction in HF hospitalization (HR: 0.61; 95% CI: 0.47 to 0.80) (48). The composite of CV death or hospitalization for HF was one of the dual primary endpoints in DECLARE-TIMI 58, in which patients randomized to receive dapagliflozin had a 17% relative risk reduction in that dual primary endpoint (HR: 0.83; 95% CI: 0.73 to 0.95) compared with placebo. This reduction was driven by a 27% reduction in HF hospitalization (HR: 0.73; 95% CI: 0.61 to 0.88) (60). This observation was consistent regardless of whether patients had a history of established HF or ASCVD at the time of trial enrollment.
Importantly, in the CV outcome trials of patients with T2D, ~90% of patients did not have HF at baseline; moreover, those who did were not well-characterized in terms of ejection fraction, natriuretic peptides, symptom burden, or adequacy of guideline-directed optimal medical therapy for HF. Therefore, while the effects of SGLT2 inhibitors on prevention of HF were clear and consistent, whether they would also be effective in the treatment of patients with established HF (including those with and without T2D) was unclear. The recent DAPA-HF trial was specifically designed to address these knowledge gaps. DAPA-HF enrolled patients with HFrEF on contemporary HF therapy, more than half of whom did not have diabetes, and demonstrated a 26% relative reduction in the risk of CV death or worsening of HF (HR: 0.74; 95% CI: 0.65 to 0.85), as well as independent reduction in CV death (HR: 0.82; 95% CI: 0.69 to 0.98) and reduced HF-related symptom burden. Importantly, these results were consistent regardless of presence or absence of T2D (47), and dapagliflozin is now approved for treatment of HF in patients with and without T2D (63). In the DEFINE-HF trial–a smaller multicenter randomized trial of patients with HFrEF (with and without T2D) in the United States–dapagliflozin also significantly improved HF-related symptoms, functional status, and quality of life after just 12 weeks of treatment, although there was no significant difference in mean N-terminal pro-B-type natriuretic peptide, the study’s coprimary endpoint (51). Indeed, the role of SGLT2 inhibitors in both the prevention and treatment of HFrEF appears poised to expand. Multiple ongoing trials will further elucidate the optimal role of SGLT2 inhibitors in patients with HFrEF and HFpEF.
5.1.5. SGLT2 Inhibitors and Renal Events
In patients with T2D, canagliflozin, dapagliflozin, and empagliflozin have demonstrated favorable effects on kidney function (13,16,60,64,65). CREDENCE was the first trial of patients with established DKD and macroalbuminuria specifically powered to evaluate the effects of canagliflozin on a primary renal outcome. Patients randomized to canagliflozin 100 mg had a 30% relative risk reduction in the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine, or renal or CV death (HR: 0.70; 95% CI: 0.59 to 0.82) when compared with placebo (19). Similar results were seen in prespecified secondary analyses of CANVAS (HR: 0.60; 95% CI: 0.47 to 0.77), DECLARE-TIMI 58 (HR: 0.53; 95% CI: 0.43 to 0.66), and EMPA REG OUTCOME (HR: 0.54; 95% CI: 0.40 to 0.75) (12,16,17,19,48) (see Table 1). Mechanisms to explain these observations may include tubuloglo-merular feedback, reduction in glomerular hypertension, containment of hyperfiltration injury, and effects on sodium-hydrogen exchange.
5.1.6. SGLT2 Inhibitors: Safety Concerns
The contraindications and potential safety concerns of SGLT2 inhibitors are included in Table 2.
TABLE 2.
Doses, Indications, Dose Modifications, Contraindications, Cautions, and Adverse Effects of SGLT2 Inhibitors With Demonstrated CV Benefit
| Canagliflozin | Dapagliflozin | Empagliflozin | |
|---|---|---|---|
| Recommended doses for CV benefit* | ■ 100 mg PO daily | ■ 10 mg PO daily | ■ 10 mg PO daily |
| Indications | ■ Improve glycemic control in adults with T2D as an adjunct to diet and exercise ■ Reduce risk of MI, stroke, or CV death in adults with T2D and CV disease ■ Reduce the risk of end-stage kidney disease, doubling of serum creatinine, CV death, and hospitalization for HF in patients with T2D and diabetic nephropathy with albuminuria |
■ Improve glycemic control in adults with T2D as an adjunct to diet and exercise ■ Reduce the risk of hospitalization for HF in adults with T2D and established CV disease or multiple CV risk factors ■ Reduce the risk of CV death and hospitalization for HF in adults with HFrEF |
■ Improve glycemic control in adults with T2D as an adjunct to diet and exercise ■ Reduce risk of CV death in adults with T2D and established CV disease |
| Dose modifications | ■ eGFR 30 to 59 ml/min/1.73 m2: max dose 100 mg daily ■ eGFR <30 ml/min/1.73 m2: use is not recommended for glycemic control |
■ eGFR <45 ml/min/1.73 m2: use is not recommended for glycemic control ■ eGFR <30 mL/min/1.73 m2: use is contraindicated. |
■ eGFR <45 mL/min/1.73 m2: use is not recommended. |
| Contraindications | ■ History of serious hypersensitivity reaction to drug ■ Pregnancy or breastfeeding ■ On dialysis ■ eGFR <30 mL/min/1.73 m2 (dapagliflozin) ■ ESRD (dapagliflozin and empagliflozin) ■ Severe renal impairment (empagliflozin) |
||
| Cautions | ■ Discontinue at least 3 days before a planned surgery to prevent postoperative ketoacidosis. ■ If HbA1c well-controlled at baseline, or known history of frequent hypoglycemic events, wean or stop sulfonylurea or glinide and consider reducing total daily insulin dose by ~20% when starting therapy. ■ May contribute to intravascular volume contraction; consider stopping or reducing diuretic dose if applicable. ■ Use with caution in patients with prior amputation, severe peripheral neuropathy, severe peripheral vascular disease, or active diabetic foot ulcers or soft tissue infections. ■ Possible increased risk of bone fractures (canagliflozin) |
||
| Adverse effects to monitor | ■ Genital fungal infections ■ Urinary tract infections ■ Euglycemic diabetic ketoacidosis ■ Lower limb ulcerations and soft tissue infections |
||
Because there is no evidence of a graded dose response regarding CV and renal effects, SGLT2 inhibitors with CV benefit should be initiated at the Lowest dose tested in CV and renal outcomes trials. Those doses are listed here. No further dose titration is needed for CV or renal risk reduction. However, dose increases may provide further glucose reduction benefits if indicated.
CV = cardiovascular; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; HbA1c = hemoglobin A1c; HF= heart failure; PO = “per os,” by mouth; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes.
An increased risk for genital mycotic infections (mostly candida vaginitis in women, balanitis in men) has been seen with all SGLT2 inhibitors (16,52,66,67). Perineal hygiene should be discussed with all individuals placed on these agents. Although these infections are usually not serious and tend to resolve with a brief course of antifungal agents, careful education and monitoring should take place in patients considered to be at high risk of infectious complications, including the immunocompromised (16,52). Although there have been spontaneous postmarketing reports of pyelonephritis and urosepsis requiring hospitalization in patients receiving SGLT2 inhibitors, large clinical trials have shown no difference in the rates of any urinary tract infections between SGLT2 inhibitors and placebo. Rare postmarketing reports of necrotizing fasciitis of the perineum led the FDA to request a warning be added to SGLT2 inhibitor prescribing instructions; whether these very rare but serious infections are causally related to SGLT2 inhibitor use remains unclear (68), and no necrotizing fasciitis safety signal was seen in DECLARE (60).
Patients taking SGLT2 inhibitors who develop diabetic ketoacidosis may do so in the absence of significant hyperglycemia–often called “euglycemic diabetic ketoacidosis”–although moderate hyperglycemia is common in these patients. This risk has been shown to be relatively low in the large randomized controlled trials of patients with T2D, particularly in those not requiring insulin therapy (69). Patients with signs or symptoms of ketoacidosis, such as dyspnea, nausea, vomiting, and abdominal pain, should be instructed to discontinue SGLT2 inhibitors and seek immediate medical attention (52). Providers should be aware of precipitating factors (e.g., insulin cessation, prednisone administration, dehydration, hyperglycemia) and prevention strategies, which have been reviewed recently (70). Patients should be encouraged to discuss prevention strategies with their diabetes care provider. Canagliflozin was associated with increased risk for lower limb amputation in CANVAS (6.3 versus 3.4 amputations per 1,000 patient-years of observation after a median follow-up of 126 weeks; p < 0.001) (13,2), prompting the FDA to add a box warning to the canagliflozin prescribing information in May 2017 (71). In CREDENCE, canagliflozin did not have a significantly higher rate of amputation compared with placebo (12.3 versus 11.2 events/1,000 patient-years, respectively, HR: 1.11; 95% CI: 0.79 to 1.56). This was despite a higher rate of amputations in CREDENCE compared with CANVAS due to a higher-risk patient population. However, the increased scrutiny given to foot exams in CREDENCE may mitigate the generalizability of that result. A numerical excess of amputations in the phase III trials with ertugliflozin (0.1% [n = 1] with placebo versus 0.5% [n = 8] with the 15 mg dose) is reported in the prescribing information. This risk has not been observed with dapagliflozin (in either DECLARE-TIMI 58 or DAPA-HF trials) or with empagliflozin in the post-hoc analyses of EMPA-REG OUTCOME (72–74). The clinical importance of any possible increase in amputation risk remains unclear, but caution is suggested in those with a history of peripheral artery disease, severe peripheral neuropathy, lower extremity diabetic ulcers, or soft tissue infections. All patients taking SGLT2 inhibitors should be getting regular foot exams. Bone fractures (including from low-trauma events) were observed to be more common among those treated with canagliflozin than with placebo in CANVAS, but not in the CANVAS-R or CREDENCE trials, or in any of the large trials with empagliflozin or dapagliflozin (75). Last, given a diuretic and antihypertensive effect, SGLT2 inhibitors may increase the risk of volume depletion and hypotension; in large randomized control trials, this risk was slightly higher with canagliflozin than with placebo but was not increased with empagliflozin or dapagliflozin (even in patients with HFrEF, nearly all of whom were treated with loop diuretics) (47). However, it is prudent to educate patients about signs and symptoms of dehydration, which may be more of a concern outside the clinical trial setting. Although there were early potential concerns about acute kidney injury with SGLT2 inhibitors, these risks have not been observed in large randomized control trials to date; in fact, in several trials of SGLT2 inhibitors, the risk of acute kidney injury was significantly lower when compared with placebo (19,47). SGLT2 inhibitors should be discontinued in the context of acute kidney injury. Large outcome trials in patients with chronic kidney disease, regardless of T2D status, are ongoing, and 1 study, the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial, was stopped early for evidence of efficacy in patients with chronic kidney disease (76).
5.2. GLP-1RAs
Specific agents in the GLP-1RA class have also demonstrated benefits for CV event prevention in patients with T2D, particularly among patients with established ASCVD. Albiglutide, dulaglutide, liraglutide, and injectable semaglutide have been shown to reduce MACE (see Table 3).
TABLE 3.
Summary of the GLP-1RA CV Outcomes Trials
| ELIXA (77) | LEADER (14) | SUSTAIN-6* (15) | EXSCEL (78) | REWIND (16) | PIONEER-6 (79) | |
|---|---|---|---|---|---|---|
| Patients enrolled | 6,068 | 9,340 | 3,297 | 14,752 | 9,901 | 3183 |
| Drug | Lixisenatide | Liraglutide | Semaglutide SQ | Exenatide QW | Dulaglutide | Semaglutide oral |
| Dose | 10 mcg or 20 mcg per day | 1.8 mg or max tolerated dose per day | 0.5 mg or 1 mg per week | 2 mg per week | 1.5 mg per week | 14 mg or max tolerated dose per day |
| Median follow-up (years) | 2.1 | 3.8 | 2.1 | 3.2 | 5.4 | 1.3 |
| Baseline HbA1c | 7.7 | 8.7 | 8.7 | 8.0 | 7.2 | 8.2 |
| Mean duration of diabetes (years) | 9.3 | 12.8 | 13.9 | 12.0 | 9.5 | 14.9 |
| Baseline statin use (%) | 93 | 72 | 73 | 74 | 66 | 85 |
| Baseline prevalence of ASCVD†/HF (%) | 100 | 81 | 72 | 73 | 31 | 85 |
| Baseline prevalence of HF (%) | 22 | 18 | 24 | 16 | 9 | NR |
| Primary outcome, HR (95% CI)‡ | 4-point MACE 1.02 (0.89-1.17) | 3-point MACE 0.87 (0.78-0.97) | 3-point MACE 0.74 (0.58-0.95) | 3-point MACE 0.91 (0.83-1.00) | 3-point MACE 0.88 (0.79-0.99) | 3-point MACE 0.79 (0.57-1.11) |
| CV death, HR (95% CI) | 0.98 (0.78-1.22) | 0.78 (0.66-0.93) | 0.98 (0.65-1.48) | 0.88 (0.76-1.02) | 0.91 (0.78-1.06) | 0.49 (0.27-0.92) |
| Fatal or nonfatal MI, HR (95% CI)§ | 1.03 (0.87-1.22) | 0.86 (0.73-1.00) | 0.74 (0.51-1.08) | 0.97 (0.85-1.10) | 0.96 (0.79-1.15) | 1.18 (0.73-1.90) |
| Fatal or nonfatal stroke, HR (95% CI)§ | 1.12 (0.79-1.58) | 0.86 (0.71-1.06) | 0.61 (0.38-0.99) | 0.85 (0.70-1.03) | 0.76 (0.62-0.94) | 0.74 (0.35-1.57) |
| All-cause mortality, HR (95% CI) | 0.94 (0.78-1.13) | 0.85 (0.74-0.97) | 1.05 (0.74-1.50) | 0.86 (0.77-0.97) | 0.90 (0.80-1.01) | 0.51 (0.31-0.84) |
| HF hospitalization, HR (95% CI)∥ | 0.96 (0.75-1.23) | 0.87 (0.73-1.05) | 0.86 (0.48-1.55) | 0.94 (0.78-1.13) | 0.93 (0.77-1.12)∥ | 1.11 (0.77-1.61) |
| Renal composite outcome¶ | 0.84 (0.68-1.02) | 0.78 (0.67-0.92) | 0.64 (0.46-0.88) | 0.88 (0.76-1.01) | 0.85 (0.77-0.93) | 0.64 (0.46-0.88) |
As noted in the text, SUSTAIN-6 was designed and powered as a noninferiority trial. Testing for superiority for the primary CV outcome was not prespecified.
SUSTAIN-6 reported that 72.2% of patients had established CV disease with or without chronic kidney disease, and 10.7% had chronic kidney disease without ASCVD.
Three-point MACE is a composite of CV death, MI, or stroke. The 4-point MACE used in the ELIXA trial was a composite of CV death, MI, stroke, or hospitalization for unstable angina.
The risk estimates and 95% CIs for ELIXA, SUSTAIN-6, and PIONEER 6 are for nonfatal MI (excluding fatal MI) or nonfatal stroke (excluding fatal stroke). The effect estimates for the composite endpoints of fatal or nonfatal MI and fatal or nonfatal stroke were not available in the primary manuscripts.
Urgent HF visit or hospitalization for HF.
The renal composite outcome reported in a recent meta-analysis was a composite of the development of macroalbuminuria, doubling of serum creatinine, a ≥40% decline in eGFR, development of end-stage kidney disease, or death due to renal causes (81). For SUSTAIN-6, the renal composite was persistent macroalbuminuria, persistent doubling of serum creatinine with an eGFR <45 ml/min/1.73 m2 or need for continuous renal replacement therapy.
CI = confidence interval; CV = cardiovascular; ELIXA = Evaluation of CV Outcomes in Patients With T2D After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide); EXSCEL = Exenatide Study of CV Event Lowering Trial; GLP-1RA = glucagon-like peptide-1 receptor agonists; HbA1c = hemoglobin A1c; HF = heart failure; HR = hazard ratio; LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results; MACE = major adverse cardiovascular event; MI = myocardial infarction; NR = not reported; PIONEER-6 = A Trial Investigating the CV Safety of Oral Semaglutide in Subjects With T2D; QW = once weekly; REWIND = Researching Cardiovascular Events With a Weekly Incretin in Diabetes; SQ = subcutaneous; SUSTAIN-6 = Trial to Evaluate CV and Other Long-term Outcomes With Semaglutide in Subjects With T2D.
Exenatide once weekly and oral semaglutide showed numerically favorable but not statistically significant results for 3-point MACE when compared with placebo (HR for exenatide: 0.91; 95% CI: 0.83 to 1.00 and HR for oral semaglutide: 0.79; 95% CI: 0.57 to 1.11) (77,78). Lixisenatide did not lower risk for CV events after an acute coronary syndrome compared with placebo (79). The potential for clinically relevant heterogeneity within the class exists, leaving dulaglutide, liraglutide, and injectable semaglutide the currently preferred agents (albiglutide is no longer available in the United States) (80).
5.2.1. GLP-1RAs: Mechanisms of Action
GLP-1 is a peptide hormone released from the distal ileum and colon after oral nutrient intake (81). Following administration of a GLP-1RA, supraphysiological concentrations of GLP-1 reduce glucose by increasing glucose-dependent insulin secretion from beta cells in the pancreas, by decreasing glucagon secretion, as well as by delaying gastric emptying, which leads to satiety (81). GLP-1RAs also have beneficial effects on important determinants of CV risk, including weight loss, blood pressure, and triglyceride reduction as well as anti-inflammatory effects.
5.2.2. GLP-1RAs: CV Benefits
Most GLP-1RA CV outcomes trials (see Table 3) used a 3-point MACE outcome of CV death, nonfatal MI, or nonfatal stroke. Inclusion criteria varied across trials. The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results) trial randomized 9,340 patients with established ASCVD (81% of the total) or older patients with ASCVD risk factors (19% of the total) to either liraglutide or placebo (14). The 3-point MACE composite was reduced by 13% (HR: 0.87; 95% CI: 0.78 to 0.97) with liraglutide versus placebo. All components of the composite contributed to a reduction in 3-point MACE, and all-cause mortality was reduced by 15% (HR: 0.85; 95% CI: 0.74 to 0.97). The reduction in all-cause mortality was driven by a reduction in CV death. No statistically significant reduction in HF events was noted (HR: 0.87; 95% CI: 0.73 to 1.05).
The SUSTAIN-6 (Trial to Evaluate CV and Other Long-term Outcomes With Semaglutide in Subjects With T2D) enrolled 3,297 patients using the same trial inclusion criteria and the same primary composite endpoint as LEADER (15). Semaglutide given subcutaneously reduced 3-point MACE by 26% (HR: 0.74; 95% CI: 0.58 to 0.95), with consistent effects for the key components of nonfatal stroke (HR: 0.61; 95% CI: 0.38 to 0.99) and nonfatal MI (HR: 0.74; 95% CI: 0.51 to 1.08). No reduction in all-cause mortality (HR: 1.05; 95% CI: 0.74 to 1.50), CV mortality (HR: 0.98; 95% CI: 0.65 to 1.48), or HF hospitalization (HR: 1.11; 95% CI: 0.77 to 1.61) was observed.
In the REWIND (Researching CV Events With a Weekly Incretin in Diabetes) (18) trial–which enrolled 9,901 patients, most of whom did not have a prior ASCVD event–dulaglutide reduced the risk of 3-point MACE by 12% (HR: 0.88; 95% CI: 0.79 to 0.99). These results were consistent across the subgroups of patients with and without known ASCVD and were driven by a 24% reduction in the risk of stroke (HR: 0.76; 95% CI: 0.62 to 0.94). To date, liraglutide, semaglutide SC, and dulaglutide are approved by the FDA to reduce the risk of MACE in adults with T2D and established CV disease, with dulaglutide being the only agent also approved for CV disease reduction in patients without established ASCVD (57).
Other trials, including PINOEER-6 (A Trial Investigating the CV Safety of Oral Semaglutide in Subjects With T2D), EXSCEL (The Exenatide Study of CV Event Lowering), and ELIXA (Evaluation of CV Outcomes in Patients With T2D After Acute Coronary Syndrome During Treatment With AVE0010 [Lixisenatide]) are summarized in Table 3. A recent meta-analysis suggests that this class of medications may offer modest reductions in the risk of hospitalization for HF, although this appears to be driven by the results from the CV outcome trial for albiglutide, rather than being a consistent effect for all medications in this class (80,82).
5.2.3. GLP-1RA in Patients With and Without Established ASCVD
A meta-analysis of the data from ELIXA, EXSCEL, LEADER, and SUSTAIN-6 reported a 12% (HR: 0.88; 95% CI: 0.84 to 0.94) relative reduction in the risk of MACE across those trials. However, the benefit appeared to be confined to those with established ASCVD (HR: 0.87; 95% CI: 0.82 to 0.92) and was not seen in those with CV risk factors but no established ASCVD (HR: 1.03; 95% CI: 0.87 to 1.23; P-heterogeneity 0.028) (50). However, the HARMONY-OUTCOMES (Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on MACE in Subjects With T2D), PIONEER-6, and REWIND trials were not included in this prior meta-analysis. In REWIND, the dulaglutide point estimate for MACE in primary prevention was identical to that for secondary prevention (HR: 0.87; 95% CI: 0.74 to 1.02 for both), and dulaglutide is currently the only GLP-1RA approved for CV disease risk reduction in patients both with and without established ASCVD (83). A subsequent meta-analysis that included these more recent data from HARMONY-OUTCOMES, PIONEER-6 and REWIND reported a risk of 3-point MACE of HR 0.86 (95% CI: 0.79 to 0.94) among those with established CV disease and 0.95 (95% CI: 0.83 to 1.08) among those without (p interaction = 0.22) (80).
5.2.4. GLP-1RAs and Renal Events
Although it has yet to be confirmed in a randomized trial with a primary renal outcome, existing studies suggest that some of the GLP-1RAs may provide modest renal benefits (see Table 3). A meta-analysis of ELIXA, EXSCEL, LEADER, and SUSTAIN-6 showed a 17% reduction in a composite renal outcome of development of macroalbuminuria, doubling of serum creatinine or decline in eGFR $40%, development of end-stage kidney disease, or death due to kidney disease (HR: 0.83; 95% CI: 0.78 to 0.89) (80). That same meta-analysis reported that while GLP-1RAs reduced the risk of adverse kidney outcomes when considering a broad composite endpoint, the benefits appeared to be driven by reductions in proteinuria. No significant improvements were seen for eGFR, in contrast to what has been observed for SGLT2 inhibitors (80). The FLOW (A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease) trial will test the effects of injectable semaglutide versus placebo on a composite renal outcome of persistent eGFR decline ≥50%, end-stage renal disease, renal death, or death from CV disease in patients with T2D and chronic kidney disease (84).
5.2.5. GLP-1RAs and Weight
Weight loss, ranging from 2% to 4% of total body weight for dulaglutide, exenatide, and liraglutide, and 4 to 6 kg (85) for semaglutide at standard glucose-lowering doses, can be expected with use of a GLP-1RA (18,86,87). GLP-1RAs appear to modestly lower blood pressure. Compared with placebo, use of liraglutide produced a 20% reduction in the occurrence of confirmed hypoglycemia and a 31% reduction in severe hypoglycemia (14). These observations of lower rates of hypoglycemia among those randomly assigned to receive an active GLP-1RA are consistent across the class.
5.2.6. GLP-1RAs: Safety Concerns
The contraindications and potential safety concerns of GLP-1RAs are included in Table 4.
TABLE 4.
Doses, Indications, Dose Modifications, Contraindications, Cautions, and Adverse Effects of GLP-1RAs With Demonstrated CV Benefit
| Dulaglutide | Exenatide QW | Liraglutide | Lixisenatide | Semaglutide SC | Semaglutide PO | |
|---|---|---|---|---|---|---|
| Recommended doses for CV benefit | ■ Initiate 0.75 mg SC per week ■ Titrate slowly to 1.5 mg or maximally tolerated dose based on prescribing information. |
■ 2 mg SC per week | ■ Initiate 0.6 mg SC daily. ■ Titrate slowly to 1.8 mg or maximally tolerated dose based on prescribing information. |
■ 10 mcg SC daily ■ Titrate as tolerated to 20 mcg daily based on prescribing information. |
■ Initiate 0.25 mg SC per week. ■ Titrate slowly to 1 mg once weekly or maximally tolerated dose based on prescribing information. |
■ Initiate 3 mg PO per day for the first 30 days. ■ Titrate slowly to 14 mg daily or maximally tolerated dose based on prescribing information. |
| Indications | ■ Improve glycemic control in adults with T2D. ■ Reduce MACE for people with T2D with and without established CV disease. |
■ Improve glycemic control in adults with T2D. | ■ Improve glycemic control in adults with T2D. ■ Reduce risk of MI, CVA, or CV death in adults with T2D and CV disease. |
■ Improve glycemic control in adults with T2D. | ■ Improve glycemic control in adults with T2D. ■ Reduce risk of MI, CVA, or CV death in adults with T2D and CV disease. |
■ Improve glycemic control in adults with T2D. |
| Dose modifications | ■ Up-titrate slowly to reduce nausea and vomiting. ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. ■ No dose adjustment necessary with renal or hepatic impairment; data in end-stage renal disease are limited. |
■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. ■ eGFR <45 mL/min/1.73 m2: Use is not recommended. |
■ Up-titrate slowly to reduce nausea and vomiting. ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. ■ No dose adjustment is necessary with renal or hepatic impairment. |
■ Up-titrate slowly to reduce nausea and vomiting. ■ Discontinue if pancreatitis is suspected, and do not restart if pancreatitis is confirmed . ■ eGFR ≥30 mL/min/1.73 m2: No dosage adjustment is required. ■ eGFR 15 to 29 mL/min/1.73 m2: Use caution and monitor renal function. ■ eGFR <15 mL/min/1.73 m2: Use is not recommended. |
■ Up-titrate slowly to reduce nausea and vomiting. ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. ■ No dose adjustment is necessary with renal or hepatic impairment. |
■ Up-titrate slowly to reduce nausea and vomiting. ■ Discontinue if pancreatitis is suspected and do not restart if pancreatitis is confirmed. ■ No dose adjustment is necessary with renal or hepatic impairment. |
| Contraindications | ■ History of serious hypersensitivity reaction to drug ■ Pregnancy or breast feeding ■ Severe renal impairment or end-stage renal failure (exenatide, lixisenatide) ■ Personal or family history of medullary thyroid cancer ■ Personal or family history of MEN2 |
|||||
| Cautions | ■ Hypoglycemia risk increased with insulin, sulfonylureas, or glinides. ■ May delay gastric emptying; not recommended in patients with clinically meaningful gastroparesis. This effect is usually transient with longer-acting GLP-1Ras. ■ Care should be taken in patients with prior gastric surgery, including bariatric surgery. ■ Diabetic retinopathy complications were reported with semaglutide (injectable), although it is unclear if this is a direct effect of the drug or due to other factors such as rapid improvement in blood glucose control. |
|||||
| Adverse effects to monitor | ■ Nausea, vomiting, diarrhea, headache, weakness,or dizziness ■ Hypoglycemia when given with insulin, sulfonylureas, or glinides. ■ Weight loss ■ Injection site reactions |
|||||
CV = cardiovascular; CVA = cerebrovascular accident; eGFR = estimated glomerular filtration rate; GLP-1RA = glucagon-like peptide-1 receptor agonist; MACE = major adverse cardiovascular events; MEN2 = multiple endocrine neoplasia, type 2; MI = myocardial infarction; PO = “per os,” by mouth; QW = once weekly; SC = subcutaneous; T2D = type 2 diabetes.
The most frequently reported side effects of GLP-1RAs are nausea and vomiting (60). These gastrointestinal symptoms are usually transient for longer-acting GLP-1RAs and can be mitigated by escalating the dose gradually (88) and educating patients to reduce meal size. GLP-1RAs may also increase the risk of gallbladder disease, including acute cholecystitis (14,15). Caution should be used in patients with prior gastric surgery (89,90). GLP-1RAs can lead to modest elevations in heart rate, although the clinical relevance of these effects is unclear (83,91,92). GLP-1RAs are unlikely to cause hypoglycemia on their own, but they may increase the risk of hypoglycemia when used in combination with insulin or insulin secretagogues–most commonly sulfonylureas (52). Although postmarketing case reports have suggested possible associations between GLP-1RAs and acute pancreatitis, none of the large trials has demonstrated any increase in the risk of pancreatitis (14); that being said, patients at high pancreatitis risk were generally excluded from the trials. These agents should be discontinued if pancreatitis occurs. The FDA and the European Medicines Agency have not identified a link between this class of drugs and either pancreatitis or pancreatic cancer (88). In the SUSTAIN-6 trial, diabetic retinopathy complications were reported with injectable semaglutide, although it is unclear if this is a direct effect of the drug or due to other factors such as rapid improvement in blood glucose control. Therefore, patients should be advised to undergo appropriate, guideline-recommended eye examinations before starting therapy if an examination has not been completed within the last 12 months (75). This is currently being studied prospectively in the FOCUS (Semaglutide Compared to Placebo Affects Diabetic Eye Disease in People with Type 2 Diabetes) trial (NCT03811561).
5.3. Considerations for Optimal Therapy Initiation and Treatment Individualization
The CV benefits of many SGLT2 inhibitors and GLP-1RAs appear robust, creating new options to improve the CV outcomes of patients with T2D and CV disease. There are several circumstances in which clinicians might consider starting 1 of these agents with demonstrated CV benefit (see Table 5).
TABLE 5.
Opportunities to Initiate an SGLT2 inhibitor or a GLP-1RA With Demonstrated CV or Renal Benefit in Patients With T2D*
| ■ In a patient with T2D and ASCVD (SGLT2 inhibitor or GLP-1RA) |
| ■ At the time of diagnosis of clinical ASCVD (SGLT2 inhibitor or GLP-1RA), DKD, and/or HF (SGLT2 inhibitor)† in a patient with T2D on a drug regimen that does not include an SGLT2 inhibitor or GLP-1RA with CV benefit |
| ■ At the time of diagnosis of T2D in a patient with clinical ASCVD (SGLT2 inhibitor or GLP-1RA), DKD, and/or HF (SGLT2 inhibitor†‡ |
| ■ At hospital discharge (with close outpatient follow-up) after admission for an ASCVD (SGLT2 inhibitor or GLP-1RA) or HF (SGLT2 inhibitor) event§ |
| ■ In a patient with T2D and diabetic kidney disease (SGLT2 inhibitor, alternatively GLP-1RA for eGFR <30 ml/min/1.73 m2)‡ |
| ■ In patients determined to be at high risk of ASCVD∥ (SGLT2 inhibitor or GLP-1RA) or HF (SGLT2 inhibitor†‡ |
At the time of hospital discharge or in the outpatient setting. Increased vigilance regarding hypoglycemia surveillance is warranted, especially if on background insulin, sulfonylurea, or glinide therapy.
A minority of patients included in the CANVAS, LEADER, SUSTAIN-6, and EXSCEL trials and a majority of patients in the REWIND trial could be characterized as high-risk primary prevention patients. These patients did not have established ASCVD but did have prespecified ASCVD risk factors.
Use clinical judgement when initiating an SGLT2 inhibitor in a patient who will be starting or up-titrating an ACE inhibitor or ARB if the patienťs renal function is impaired.
Hospitalized patients were not included in most of the CV outcome trials discussed here. There is a lack of practical and safety data regarding in-hospital addition of SGLT2 inhibitors or GLP-1RAs to a patienťs regimen.
Consider for patients at very high risk of ASCVD to include patients with end-organ damage such as left ventricular hypertrophy or retinopathy or with multiple CV risk factors (e.g., age, hypertension, smoking, dyslipidemia, obesity).
ACE = angiotensin-converting enzyme; ARB = angiotensin reception blocker; ASCVD = atherosclerotic cardiovascular disease; CANVAS = Canagliflozin Cardiovascular Assessment Study; CV = cardiovascular; DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; EXSCEL = Exenatide Study of Cardiovascular Event Lowering Trial; GLP-1RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; REWIND = Researching CV Events With a Weekly Incretin in Diabetes; SGLT2 = sodium-glucose cotransporter-2; SUSTAIN-6 = Trial to Evaluate CV and Other Long-term Outcomes With Semaglutide in Subjects With T2D;T2D = type 2 diabetes.
We recommend initiating a patient-clinician discussion about the use of an SGLT2 inhibitor and/or a GLP-1RA with demonstrated CV benefit at the time of a clinical follow-up visit for patients with T2D who have or who are at very high risk for clinical ASCVD, HF, and/or DKD.
Because of the evidence outlined in this ECDP, an SGLT2 inhibitor with demonstrated CV benefit is recommended for patients with T2D and HF, especially HFrEF, or who are at high risk of developing HF, DKD, clinically evident ASCVD, or any combination of these conditions. A new diagnosis of T2D in a patient with clinical ASCVD, DKD, and/or HFrEF or a new diagnosis of clinical ASCVD, DKD, and/or HFrEF in a patient with T2D offers the opportunity to begin a patient-clinician discussion about starting an SGLT2 inhibitor proven to improve CV outcomes.
A GLP-1RA with demonstrated CV benefit is recommended for patients with established or at very high risk for ASCVD. Alternatively, or in conjunction with a patient-clinician discussion, consider discussing these medications with the clinician caring for the patient’s blood glucose control. Furthermore, a new diagnosis of T2D in a patient with clinical ASCVD (or at very high risk for ASCVD) or a new diagnosis of clinical ASCVD in a patient with T2D offers the opportunity to begin a patient-clinician discussion about starting a GLP-1RA proven to improve CV outcomes.
Patients with T2D may become eligible for initiation of these therapies if they are subsequently hospitalized or diagnosed with ASCVD, HF, and/or DKD (57). It is important to note that hospitalized patients were not included in most of the CV outcome trials discussed within this ECDP, and hospital inpatient formularies may not include these agents (93). However, outpatient adherence to therapy after an acute CV event can be favorably influenced by initiation of medications at discharge. These factors must be weighed if contemplating in-hospital addition of SGLT2 inhibitors or GLP-1RAs. Because T2D is common among patients with ASCVD, DKD, and/or HF, CV specialists should consider periodic screening for T2D in these patients by measuring HbA1c at guideline-recommended intervals (e.g., annually in patients with prediabetes). Patients with ASCVD or at high risk of ASCVD and/or HF should consider initiation of an SGLT2 inhibitor or GLP-1RA with demonstrated CV benefit irrespective of HbA1c levels (2). Whether these should be initiated with metformin is an active discussion topic that is addressed later in this ECDP.
Although canagliflozin, dapagliflozin, and empagliflozin have differences in their FDA-approved CV indications, they appear to have broadly similar CV and renal benefits. The choice of an individual agent should be made after appropriate patient-clinician discussion of benefits and potential risks. Because there is no evidence of a graded dose response vis-à-vis CV and renal effects, SGLT2 inhibitors with CV benefit should be initiated at the lowest dose tested in CV and renal outcomes trials (e.g., 100 mg for canagliflozin, 10 mg for dapagliflozin, 10 mg for empagliflozin). No further dose titration is needed for CV or renal risk reduction, although doses may be increased by the clinician managing the patient’s glucose and cardiologists should make patients aware that this may happen for non-CV disease/renal risk reduction reasons.
Among the GLP-1RAs, data support the use of dulaglutide, liraglutide, or injectable semaglutide as having demonstrated CV benefit to reduce the risk of MACE. In accordance with randomized controlled trials, a GLP-1RA with demonstrated CV benefit should be initiated at the lowest dose and up-titrated stepwise to the doses used in the trials or the otherwise maximal tolerated dose. Prior to initiating T2D therapies aimed at CV disease risk reduction, a detailed patient-clinician risk discussion is recommended (94). This discussion should review risks, potential benefits, and different treatment options. Specifically, potential side effects, drug-drug interactions, and safety issues should be explained clearly, patient preference and other concerns elicited, and cost discussed, because SGLT2 inhibitors and GLP-1RAs can be expensive and out-of-pocket costs could be considerable for many patients (95).
5.3.1. Should I Recommend an SGLT2 inhibitor or a GLP-1RA for My Patient?
Because many SGLT2 inhibitors and GLP-1RAs have been demonstrated to have CV benefit in patients with T2D, patient-clinician discussions regarding use of these agents must include discussion of which specific agent is most appropriate (see Table 5). As noted, patient preferences and medical history can help guide that decision. The SGLT2 inhibitors with demonstrated CV benefit reduce MACE, incident HF, HF hospitalization, and CV death for patients with established HFrEF and also reduce progression of DKD, but increase the risk of genital mycotic infections, polyuria, and potential volume depletion in the context of hyperglycemia, and possible additional risks of rare events as previously outlined. Use clinical judgement when initiating an SGLT2 inhibitor in a patient who will be starting or up-titrating an angiotensin-converting enzyme (ACE) inhibitor or angiotensin reception blocker (ARB) if the patient’s renal function is impaired. GLP-1RAs with demonstrated CV benefit reduce MACE and progression of macroalbuminuria but are associated with transient nausea and vomiting, especially when initiating therapy or up-titrating doses, and with possible additional risks of rare events as previously outlined. Both classes of agents have nonglycemic benefits in systolic blood pressure and weight and have a low risk of hypoglycemia on their own or when used with metformin and other oral glucose-lowering medications (except for insulin secretagogues). Notably, the SGLT2 inhibitor dapagliflozin was used safely even in patients without diabetes in the DAPA-HF trial (47). Differences in the route of administration (oral for SGLT2 inhibitors, subcutaneous or oral for GLP-1RA) may influence patient and clinician decision making; however, the injectable GLP-1RAs are given with a small needle and pen device to ease administration and patient acceptance. The first oral GLP-1RA, semaglutide, has now been approved by the FDA for improving glycemic control in patients with T2D (96). Cost should also be considered, as insurance coverage for these agents can vary significantly. The clinical importance of any possible increase in the amputation risk remains unclear, but caution is suggested when starting a SGLT2 inhibitor in those with a history of peripheral artery disease, severe peripheral neuropathy, lower extremity diabetic ulcers, or soft tissue infections. For patients with active proliferative retinopathy (especially if HbA1c is high and significant rapid reduction is expected), consider a GLP-1RA alternative to semaglutide SQ. Furthermore, the use of GLP-1RAs in patients with active gallbladder disease or a history of pancreatitis has not been studied, so caution is suggested when using a GLP-1RA in these patient populations.
Figures 2 and 3 provide guidance for managing CV disease risk in patients with T2D using a SGLT2 inhibitors and GLP-1RAs.
FIGURE 2. Using an SGLT2 inhibitor to Manage ASCVD, HF, and DKD Risk.
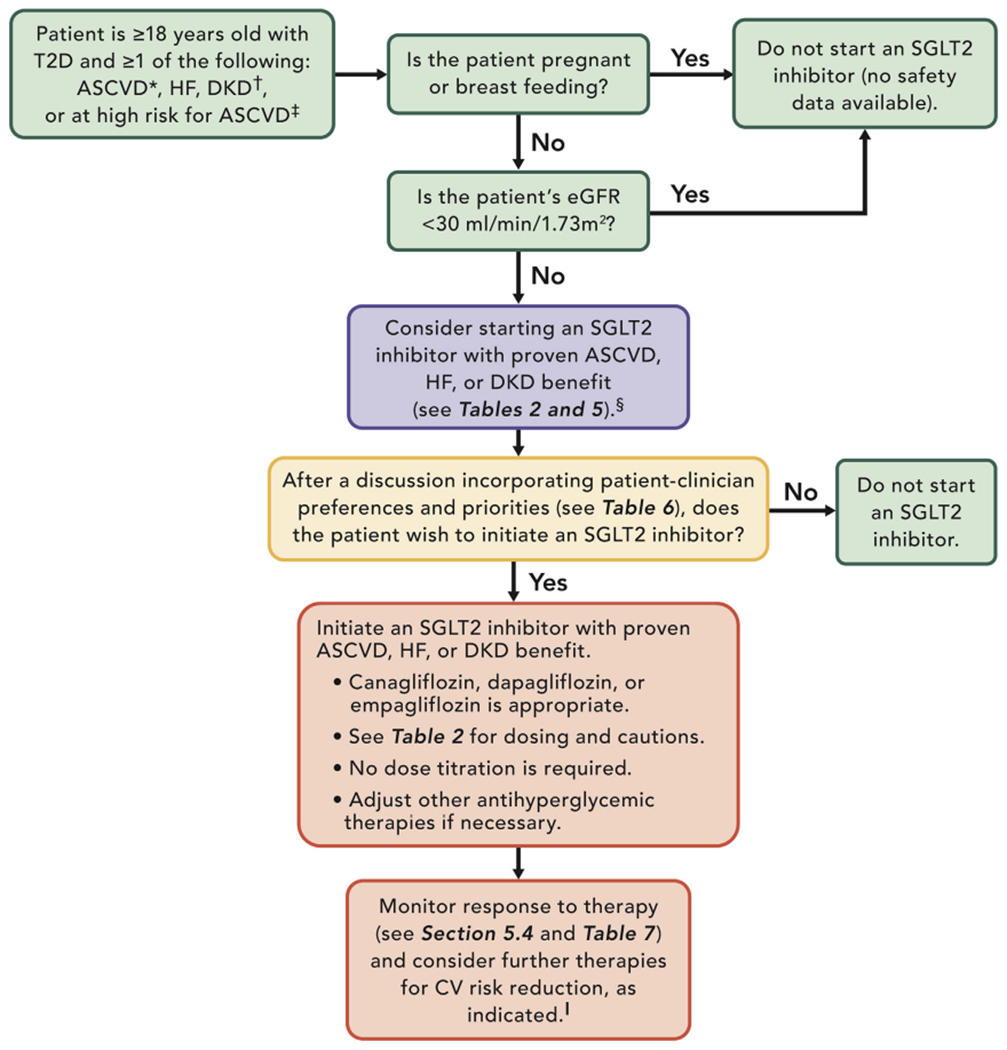
*ASCVD is defined as a history of an acute coronary syndrome or MI, stable or unstable angina, coronary heart disease with or without revascularization, other arterial revascularization, stroke, or peripheral artery disease assumed to be atherosclerotic in origin.
†DKD is a clinical diagnosis marked by reduced eGFR, the presence of albuminuria, or both.
‡Patients at high risk for ASCVD include those with end organ damage such as left ventricular hypertrophy or retinopathy or with multiple CV risk factors (e.g., age, hypertension, smoking, dyslipidemia, obesity).
§Most patients enrolled in the relevant trials were on metformin at baseline as glucose-lowering therapy.
∥This may include the addition of a GLP-1RA in the appropriate patient (see Section 5.3.3).
ASCVD = atherosclerotic cardiovascular disease; CV = cardiovascular; DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; GLP-1RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; MI = myocardial infarction; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes
FIGURE 3. Using a GLP-1RA to Manage ASCVD Risk.
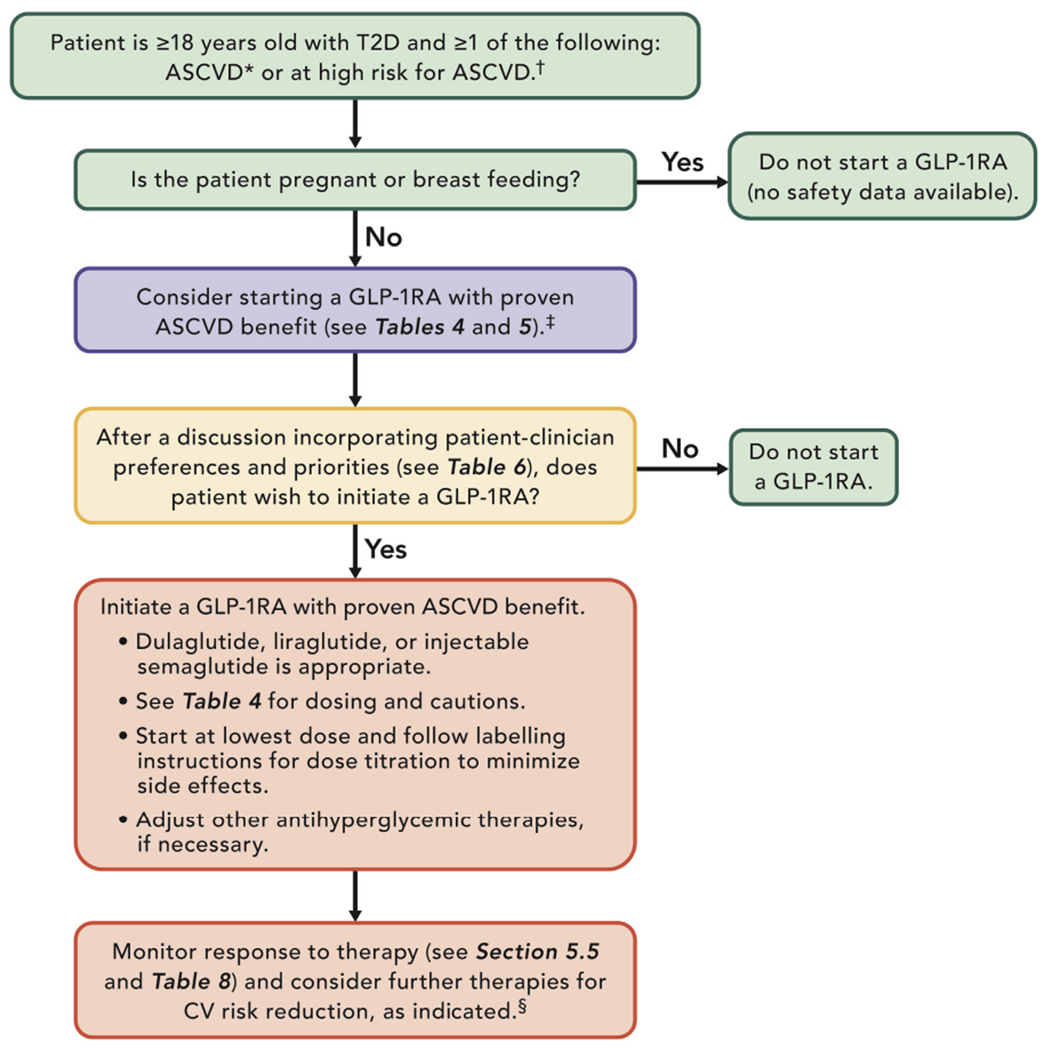
*ASCVD is defined as a history of an acute coronary syndrome or MI, stable or unstable angina, coronary heart disease with or without revascularization, other arterial revascularization, stroke, or peripheral artery disease assumed to be atherosclerotic in origin.
†Patients at high risk for ASCVD include patients with end organ damage such as left ventricular hypertrophy or retinopathy or with multiple CV risk factors (e.g., age, hypertension, smoking, dyslipidemia, obesity).
‡Most patients enrolled in the relevant trials were on metformin at baseline as glucose-lowering therapy.
§This may include the addition of an SGLT2 inhibitor in the appropriate patient (see Section 5.3.3).
ASCVD = atherosclerotic cardiovascular disease; GLP-1RA = glucagon-like peptide-1 receptor agonist; MI = myocardial infarction; SGLT2 = sodium-glucose cotransporter-2; T2D = type 2 diabetes
Table 6 outlines patient and clinician preferences and priorities to consider when selecting 1 of these therapies. Tables 7 and 8 provide an overview of considerations for initiating and monitoring an SGLT2 inhibitor and a GLP-1RA.
TABLE 6.
Patient and Clinician Preferences and Priorities for Considering SGLT2 Inhibitors With Demonstrated CV Benefit Versus GLP-1RAs With Demonstrated CV Benefit
| Preference or Priority | Consider Using an SGLT2 Inhibitor First When Patient and Clinician Priorities Include: | Consider Using a GLP-1RA First When Patient and Clinician Priorities Include: |
|---|---|---|
| MACE prevention | +++ | +++ |
| HF prevention | +++ | |
| Weight loss | + | +++ |
| Renal disease progression prevention | +++ | + |
| Mode of administration | Oral | Subcutaneous |
| Considerations that may prompt use of an alternative class | ■ Severely reduced kidney function*,† ■ History of prior amputation, severe peripheral arterial disease, or active diabetic foot ulcers (caution with canagliflozin) ■ History of recurrent genital candidiasis ■ History of diabetic ketoacidosis ■ History of fracture (caution with canagliflozin) ■ The patient is considering pregnancy ■ The patient is breast feeding |
■ Persistent nausea, despite appropriate dietary education and low doses ■ History of gastroparesis ■ Active gallbladder disease ■ History of MEN2 or medullary thyroid cancer ■ History of proliferative retinopathy (caution with semaglutide or dulaglutide) ■ The patient is considering pregnancy ■ The patient is breast feeding |
eGFR <45 ml/min/1.73 m2 is currently a caution due to a decrease in glycemic efficacy (not due to safety), but ongoing studies are testing whether SGLT2 inhibitors offer renal benefits in these patients. The FDA label for canagliflozin allows use of canagliflozin to an eGFR of 30 ml/min/1.73m2 specifically for patients with DKD.
Use clinical judgement when initiating an SGLT2 inhibitor in a patient who will be starting or up-titrating an ACE inhibitor or ARB if the patienťs renal function is impaired
ACE = angiotensin-converting enzyme; ARB = angiotensin reception blocker; CV = cardiovascular; DKD = diabetic kidney disease; eGFR = estimated glomerular filtration rate; FDA = Food and Drug Administration; GLP-1RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; MACE = major adverse cardiovascular event; MEN2 = multiple endocrine neoplasia type 2; SGLT2 = sodium-glucose cotransporter-2.
TABLE 7.
Considerations for Drug Initiation and Monitoring in Patients Starting an SGLT2 Inhibitor With Demonstrated CV Benefit
| ■ If HbA1c is well-controlled at baseline, or known history of frequent hypoglycemic events, wean or stop sulfonylurea and consider reducing total daily insulin dose by ~20% when starting therapy. |
| ■ Educate patients regarding potential for genital mycotic infections and importance of genital hygiene. |
| ■ Avoid hypovolemia. May need to reduce diuretic dose if the patient has symptoms of dehydration. Educate patients regarding symptoms of dehydration (lightheadedness, orthostasis, weakness) and to hold medication if low oral intake. |
| ■ Instruct patients to more closely monitor glucose at home for the first 4 weeks of therapy (especially if on insulin, sulfonylurea, and/or glinides). Consider discontinuing any sulfonylurea or glinide. For patients taking insulin, consider modestly reducing total daily insulin dose (by up to 20%). |
| ■ Educate patients regarding symptoms of diabetic ketoacidosis (nausea, vomiting, abdominal pain, weakness) and that diabetic ketoacidosis can occur even if blood glucose readings are in the 150-250 mg/dL range. If patient experiences diabetic ketoacidosis-like symptoms, he/she should be instructed to seek urgent medical attention. |
| ■ Educate patients regarding foot care, especially in patients with diabetic neuropathy. Ask patients to report any foot wounds immediately. |
CV = cardiovascular; HbA1c = hemoglobin A1c; SGLT2 = sodium-glucose cotransporter-2.
TABLE 8.
Considerations for Drug Initiation and Monitoring in Patients Starting a GLP-1RA With Demonstrated CV Benefit
| ■ If HbA1c is well-controlled at baseline or known history of frequent hypoglycemic events, wean or stop sulfonylurea and consider reducing total daily insulin dose by ~20% when starting therapy. |
| ■ Instruct patients to more closely monitor glucose at home for the first 4 weeks of therapy. Consider discontinuing any sulfonylurea or glinide. For patients taking insulin, consider modestly reducing total daily insulin dose (by up to 20%). |
| ■ Discontinue DPP-4 inhibitor before starting. |
| ■ To mitigate nausea, recommend small portion sizes for meals, start at the Lowest dose, and up-titrate as tolerated toward the goal doses used in CV outcome trials. |
| ■ Advise patients to undergo appropriate, guideline-recommended eye examinations before starting therapy if not done within the last 12 months. |
| ■ Discuss potential risk of diabetic retinopathy complications (for dulaglutide or injectable semaglutide). |
| ■ Avoid in patients with diabetic gastroparesis or active gallbladder disease. |
CV = cardiovascular; DPP4 = dipeptidyl peptidase-4; GLP-1RA = glucagon-like peptide-1 receptor agonist; HbA1c = hemoglobin A1c.
5.3.2. Do Patients Need to Be on Metformin Before Initiating an SGLT2 Inhibitor or a GLP-1RA? Can an SGLT2 inhibitor and/ or a GLP-1RA Be Used for CV Protection in Patients With Well-Controlled HbA1c?
Although the pivotal trials that showed evidence of CV benefit for many SGLT2 inhibitors and GLP-1RAs enrolled a high proportion (approximately 75%) of patients who were treated with metformin at baseline (12,14,16), a substantial minority of patients were not receiving metformin. This ECDP is focused on the cardioprotective effects of SGLT2 inhibitors and GLP-1RAs, rather than their glucose-lowering effects, and there has been no evidence to suggest that the cardioprotective effects vary according to whether patients were taking metformin at baseline. In the EMPA-REG OUTCOME and LEADER trials, no evidence was found to suggest that the effects of either empagliflozin or liraglutide were modified by baseline medication use, including metformin. Perhaps the strongest evidence that the CV effects of these agents are independent of both HbA1c and background antidiabetic agent use come from the DAPA-HF trial, in which most patients did not have T2D and were not on glucose-lowering therapies at baseline and yet still experienced an identical reduction in CV death or worsening HF (49). Current ADA guidelines continue to recommend metformin as first-line therapy for glucose-lowering in patients with T2D (97). In contrast, the most recent European Society of Cardiology/European Association for the Study of Diabetes guidelines now recommend starting with an SGLT2 inhibitor or GLP-1RA before metformin in newly diagnosed T2D patients who are treatment naïve and either have established CV disease or are at very high CV disease risk (22). We expect that most patients with T2D and CV disease will continue to be treated with metformin along with an SGLT2 inhibitor and/or GLP-1RA with proven CV benefit. Accordingly, decisions regarding initiation of an SGLT2 inhibitor (for CV or kidney risk reduction) or a GLP-1RA (for CV risk reduction) should not be contingent on HbA1c levels. Nevertheless, if an SGLT2 or GLP-1RA is added to the regimen of a patient with well-controlled T2D, dose adjustment of background medications may be required to avoid hypoglycemia in the context of insulin, sulfonylurea, or glinide therapy, particularly in patients at or near glycemic goals (see Sections 5.4 and 5.5). Full efforts to achieve glycemic and blood pressure targets and to adhere to lipid, antiplatelet, antithrombotic, and smoking cessation guidelines should continue after an SGLT2 inhibitor or GLP-1RA is added.
5.3.3. Should SGLT2 Inhibitors and GLP-1RAs Be Used Concomitantly?
To date, no trials have studied the CV outcome effects of concomitant use of both an SGLT2 inhibitor and a GLP-1RA with demonstrated CV benefit. DURATION-8 (Phase 3 28-Week Study With 24-Week and 52-Week Extension Phases to Evaluate Efficacy and Safety of Exenatide Once Weekly and Dapagliflozin Versus Exenatide and Dapagliflozin Matching Placebo) demonstrated greater reductions in blood pressure and body weight in patients randomly allocated to the combination of dapagliflozin and exenatide than to either agent alone (97). Combination therapy with both an SGLT2 inhibitor and a GLP-1RA for glycemic management also accords with current T2D management guidelines (22,75). In randomized, placebo-controlled trials, dulaglutide, liraglutide, and semaglutide have shown an additive glucose-lowering benefit over placebo in patients treated with background SGLT2 inhibitors, suggesting some independence of effect (98–100). Therefore, it appears reasonable to use both an SGLT2 inhibitor and a GLP-1RA, with demonstrated CV benefit, concomitantly, if clinically indicated, even though such combination therapy has not been studied for CV risk reduction. Note that the out-of-pocket cost of using both classes of drugs may be very high for some patients.
5.4. What to Monitor When Prescribing an SGLT2 Inhibitor
Patients starting an SGLT2 inhibitor should be informed about the higher risk of genital mycotic infections, and that this risk could be lowered with careful attention to personal hygiene of the perineum. Topical antifungal agents can be used for initial treatment if mycotic infections occur, although in practice, effective treatment of the infection may require temporary discontinuation of the SGLT2 inhibitor. Oral antifungals can be used but require close attention to corrected QT interval (QTc) duration in patients who are also taking certain antiar-rhythmic agents or other QTc-prolonging drugs.
Patients should also be informed about the potential risk of hyperglycemic or euglycemic diabetic ketoacidosis, taught prevention strategies, and advised to seek immediate care if they develop symptoms potentially associated with diabetic ketoacidosis (e.g., nausea, vomiting, abdominal pain, generalized weakness). Home monitoring with urine ketone test strips may be a reasonable choice in some higher-risk patients. To avoid precipitating diabetic ketoacidosis, avoid initial reductions in total daily insulin dose of >20%. Patients on a complex insulin regimen or with a history of labile blood glucose should have an SGLT2 inhibitor initiated in collaboration with the clinician caring for the patient’s diabetes. Conversely, patients requiring only oral glucose-lowering medications are at lower risk of euglycemic diabetic ketoacidosis. Approximately 5% to 10% of adult-onset diabetes is late-onset type 1 (101). These patients have an increased risk of diabetic ketoacidosis, and there are no CV outcomes trial data for patients with type 1 diabetes.
Patients taking insulin or an insulin secretagogue (i.e., a sulfonylurea or glinide) should be advised of the risk of hypoglycemic events when adding an SGLT2 inhibitor for CV benefit, especially if HbA1c is already well-controlled at baseline. In these patients, discontinuing or weaning the sulfonylurea or glinide or modestly reducing total daily insulin dose by up to 20% could reduce the risk of hypoglycemia. These dose adjustments of insulin or sulfonylureas should be considered a reasonable starting point, but any adjustments should be based on clinical judgment and should be tailored specifically to each patient’s needs and requirements. Complex insulin regimens or “brittle” diabetes should be carefully managed in coordination with the patient’s diabetes care provider. These patients should be advised to self-monitor blood glucose levels closely during the first 3 to 4 weeks after initiating SGLT2 inhibitors. In contrast, the risk of hypoglycemia is not significantly increased with the addition of SGLT2 inhibitors in patients who are not taking either insulin or an insulin secretagogue, although it is possible that dose adjustments of other agents may occasionally be needed.
Patients should additionally be advised that a diuretic effect may be observed with SGLT2 inhibitors and potentially additive natriuretic effects when SGLT2 inhibitors are administered with loop diuretics (102). Patients should be advised to monitor for signs of volume depletion such as orthostatic lightheadedness and to contact their clinician if these occur. For patients on concomitant loop diuretics starting an SGLT2 inhibitor, decreasing the diuretic dose may be warranted if these symptoms occur. Therapy with SGLT2 inhibitors may cause a modest initial decrease in eGFR. However, longer-term nephroprotective effects have been consistently observed in large clinical trials, and no increase in acute kidney injury (and in some cases, significantly lower risk of acute kidney injury) was seen in SGLT2 inhibitor trials, so this should not hinder use of these agents. Monitoring renal function in the first few weeks of therapy is reasonable, particularly in patients with impaired renal function at baseline. Consider alternatives to canagliflozin when prescribing an SGLT2 inhibitor to patients with a history of prior amputations, severe peripheral neuropathy, severe peripheral artery disease, or active lower-extremity soft tissue ulcers or infections (16,19). All patients should be getting regular foot exams in accordance with ADA Standards of Medical Care for Diabetes (103).
5.5. What to Monitor When Prescribing a GLP-1RA
The strategy to reduce hypoglycemic events with a GLP-1RA is the same as that for SGLT2 inhibitors. Patients initiating a GLP-1RA should be informed that transient nausea is a relatively common side effect. Nausea and vomiting can be minimized by starting with the lowest dose, up-titrating gradually according to the label recommendations, ceasing uptitration when the nausea becomes uncomfortable, and eating smaller portions. A low-fat diet can also help. This nausea does not imply gastrointestinal pathology and is usually self-limited in patients treated with longer-acting GLP-1RAs. However, GLP-1RAs should be used with caution in patients who have had problems with clinically significant gastroparesis. If treatment is suspended, reinitiation should again be at the lowest dose, with gradual up-titration to avoid recurrent nausea and vomiting. GLP-1RA should not be coadministered with DPP4 inhibitors given that both work through GLP-1 signaling and have not been studied for use together. An increased risk of diabetic retinopathy complications has been noted with semaglutide, predominantly in patients with a prior history of proliferative retinopathy. Therefore, these patients should have regular eye examinations, as recommended by the current guidelines (57).
5.5.1. Systems Factors in Caring for Patients With T2D and CV Disease
Challenges to utilization of and adherence to evidence-based and guideline-recommended therapies remain (37,104). CV specialists have recognized preventing morbid CV outcomes as central to their clinical mission and have typically taken ownership of therapies that are effective in preventing such outcomes. Because of their effects on MACE, specific SGLT2 inhibitors and GLP-1RAs are the newest examples of therapies that support this goal. However, some CV specialists may be reluctant to use them, perhaps because these agents were originally approved for glucose reduction, or due to incomplete knowledge of their benefits and/or risks, lack of familiarity with their use and monitoring, or systems factors that discourage CV specialists from using them. One potential approach to optimizing their use would be employing what might be called the “consultative” approach, in which the discussion of these agents is encouraged in conversations or communication with the clinician caring for the patient’s diabetes and/or with the patient. This approach requires clear, open communication and does not require the CV specialist to or preclude them from initiating and monitoring these medications. An alternative might be a more comprehensive “team” approach, such as that which has been implemented for patients with other chronic diseases, such as human immunodeficiency virus, or organ transplantation. Members of the care team for patients with diabetes include primary care physicians, endocrinologists, cardiologists, podiatrists, ophthalmologists, pharmacists, nurses, advanced practice providers, and dietitians. With both approaches, the key elements are patient-centered care, shared decision making, and integration across disciplines and patient care roles. Given the data supporting comprehensive CV risk reduction in patients with T2D, CV clinicians should be both champions and change agents as strong advocates for our patients, recognizing unmet needs in healthcare delivery, and extending our comfort zone in implementing the use of new evidence-based therapies that reduce CV event rates.
5.6. Unresolved Questions
Several important clinical questions regarding the use of SGLT2 inhibitors and GLP-1RAs remain unanswered:
What are the benefits and risks of using both classes of medications simultaneously? Current guidelines do suggest the use of both classes of medications in some patient groups, but whether combination therapy leads to further improvements in outcome is unknown (22).
Should an SGLT2 inhibitor or a GLP-1RA be the initial therapy in drug-naive patients with T2D and ASCVD?
What is the role for these medications in patients who do not have DKD or established ASCVD but are at high risk? Here again, the data are incomplete, although we and others recommend their use in patients with a high burden of risk factors for CV disease (22).
Finally, an important challenge facing CV medicine in general is how to prioritize, sequence, and to reduce the risk of major CV events in this population by choosing among an array of novel therapies, including icosapent ethyl, proprotein convertase subtilisin/kexin type 9 inhibitors, antiplatelet and antithrombotic medications, anti-inflammatory therapies, and the classes of medications discussed in this ECDP.
The writing committee emphasizes the importance of these drugs to CV specialists on the basis of their effects on CV risk reduction rather than a direct effect through glucose lowering. However, increased vigilance to avoid hypoglycemia in patients with HbA1c near or below target levels at SGLT2 inhibitor or GLP-1RA initiation is warranted, especially if the patient’s existing T2D therapies include sulfonylureas, glinides, or insulin (see Sections 5.4, and 5.5). Ongoing trials will seek to address the role of an SGLT2 inhibitor and a GLP-1RA for CV event reduction in a wide array of populations, including those with chronic kidney disease and HFrEF and HFpEF (with and without T2D).
6. DISCUSSIONS AND IMPLICATION OF PATHWAY
The paradigm of how the CV specialist should approach the care of patients with T2D is changing, and that change is reflected in this ECDP. Previously, CV specialists focused on risk factor optimization in patients with diabetes. Medications used for glycemic control were not adjusted by CV specialists, in part because they were not expected to demonstrate direct CV benefit. However, the recent development of SGLT2 inhibitors and GLP-1RAs has, for the first time, demonstrated that specific treatments developed for glucose lowering can directly improve CV outcomes. In large, well-conducted, randomized clinical trials, specific medications in these 2 classes have been proven to reduce rates of acute MI, stroke, and CV death in patients with T2D (most with established ASCVD). SGLT2 inhibitors also have strong data supporting an HF benefit, even in patients without T2D, and improvement in renal outcomes. These benefits appear to be independent of their effects on HbA1c. Thus, CV specialists now need to incorporate these agents into their care of patients with T2D, and coordinate care with the primary diabetes care providers, to optimize clinical outcomes in patients with diabetes.
This ECDP provides a practical guide to CV specialists for the initiation and monitoring of SGLT2 inhibitors and GLP-1RAs with the express goal of reducing CV risk. This ECDP and associated treatment algorithms should be used in concert with established risk factor modification guidelines for the prevention of MACE in patients with T2D, including guidelines on lipids (34,35), blood pressure (33), and antiplatelet therapy (36). This ECDP should also be applied in the context of guideline-directed diabetes care (75). Although intended to facilitate clinical decision making, the information provided in this ECDP should complement, rather than supersede, good clinical judgement. The treatment of patients with T2D and CV disease is increasingly complex. It involves physicians and advanced practice providers across a wide array of specialties, including primary care, endocrinology, cardiology, nephrology, podiatry, and ophthalmology. It also involves associated providers such as nurses, pharmacists, and dieticians. Ultimately, the main goals of care for these high-risk patients should be improving survival and quality of life. Achieving these important goals requires a team-based approach to achieve optimal outcomes. If used appropriately, the SGLT2 inhibitors and GLP-1RAs discussed in this ECDP should significantly reduce CV morbidity and mortality in these patients. The writing committee has highlighted the potential benefits and risks associated with these novel therapies and has sought to provide a context for the rational use of these medications. Further evidence is still emerging, and other CV outcomes trials are currently underway. As such, this area of care for affected patients is likely to continue evolving rapidly. We anticipate that the algorithms proposed here will change as new evidence emerges but that the overarching goal of improving CV outcomes in patients with T2D and clinical ASCVD will remain consistent.
APPENDIX 1. AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (RELEVANT)-2020 ACC EXPERT CONSENSUS DECISION PATHWAY ON NOVEL THERAPIES FOR CARDIOVASCULAR RISK REDUCTION IN PATIENTS WITH TYPE 2 DIABETES
To avoid actual, potential, or perceived conflicts of interest that may arise as a result of industry relationships or personal interests among the writing committee, all members of the writing committee, as well as peer reviewers of the document, are asked to disclose all current healthcare-related relationships, including those existing 12 months before initiation of the writing effort. The ACC Solution Set Oversight Committee reviews these disclosures to determine what companies make products (on market or in development) that pertain to the document under development. Based on this information, a writing committee is formed to include a majority of members with no relevant relationships with industry (RWI), led by a chair with no relevant RWI. RWI is reviewed on all conference calls and updated as changes occur. Author RWI pertinent to this document is disclosed in the table below and peer reviewer information is disclosed in Appendix 2. Additionally, to ensure complete transparency, authors’ comprehensive disclosure information-including RWI not pertinent to this document–is available online. Disclosure information for the ACC Solution Set Oversight Committee is also available online, as is the ACC disclosure policy for document development.
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Sandeep R. Das (Co-Chair) | UT Southwestern Medical Center–Associate Professor of Medicine | None | None | None | None | None | None |
| Brendan M. Everett (Co-Chair) | Brigham and Women’s Hospital/Harvard Medical School–Assistant Professor of Medicine | None | None | None | None | None | None |
| Kim K. Birtcher | University of Houston College of Pharmacy–Clinical Professor | None | None | None | None | None | None |
| Jenifer M. Brown | Brigham and Women’s Hospital–Fellow in Cardiology | None | None | None | None | None | None |
| James L. Januzzi, Jr. | Massachusetts General Hospital/Harvard Medical School–Professor of Medicine | None | None | None | ■ Boehringer Ingelheim (DSMB)* ■ Janssen Pharmaceuticals (DSMB)* |
None | None |
| Rita R. Kalyani | Johns Hopkins University School of Medicine–Associate Professor of Medicine | None | None | None | None | None | None |
| Mikhail Kosiborod | Saint Luke’s Mid America Heart Institute and University of Missouri-Kansas City–Professor of Medicine | ■ AstraZeneca* ■ Boehringer Ingelheim* ■ Eli Lilly* ■ Janssen Pharmaceuticals* ■ Merck (Diabetes)* ■ Novo Nordisk* ■ Sanofi-Aventis* |
None | None | ■ AstraZeneca* ■ Boehringer Ingelheim* |
■ AstraZeneca* | None |
| Melissa L. Magwire | Saint Luke’s Health System, Cardiometabolic Center Alliance–Program Director | ■ Boehringer Ingelheim ■ Novo Nordisk* |
None | None | None | None | None |
| Pamela B. Morris | Medical University of South Carolina–Director, Seinsheimer Cardiovascular Health Program; Co-Director, Women’s Heart Care | None | None | None | None | None | None |
| Joshua Neumiller | Washington State University–Vice Chair; Allen I. White Distinguished Associate Professor, Pharmacotherapy | None | None | None | None | None | None |
| Laurence S. Sperling | Emory Heart Disease Prevention Center-Professor of Medicine (Cardiology); Emory University-Director, Professor of Global Health, Hubert Department of Global Health, Rollins School of Public Health | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. According to the ACC, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document or makes a competing drug or device addressed in the document; or c) the person or a member of the person’s household has a reasonable potential for financial, professional, or other personal gain or loss as a result of the issues/content addressed in the document.
Significant relationship.
ACC = American College of Cardiology; DSMB = Data Safety Monitoring Board; UT = University of Texas.
APPENDIX 2. PEER REVIEWER INFORMATION-2020 EXPERT CONSENSUS DECISION PATHWAY ON NOVEL THERAPIES FOR CARDIOVASCULAR RISK REDUCTION IN PATIENTS WITH TYPE 2 DIABETES
This table represents the individuals, organizations, and groups that peer reviewed this document. A list of corresponding comprehensive healthcare-related disclosures for each reviewer is available online.
| Reviewer | Representation | Employment |
|---|---|---|
| Vanita Aroda | American Diabetes Association | Brigham and Women’s Hospital, Harvard Medical School–Director, Diabetes Clinical Research, Division of Endocrinology, Diabetes and Hypertension, Department of Medicine |
| George L. Bakris | American Diabetes Association | University of Chicago Medicine–Professor of Medicine; Director, Hypertensive Diseases Unit |
| Nicole M. Bhave | Solution Set Oversight Committee | University of Michigan–Assistant Professor of Cardiovascular Medicine |
| Roger S. Blumenthal |
2019 Prevention Guideline Co-Chair | Johns Hopkins Hospital–Pollin Professor of Cardiology |
| John B. Buse | American Diabetes Association | University of North Carolina School of Medicine–Director, Diabetes Care |
| Matthew A. Cavender | ACC Expert | University of North Carolina, Chapel Hill–Assistant Professor of Medicine, Interventional Cardiology |
| Deborah S. Croy | ACC Expert | Bland County Medical Clinic, Virginia–Adult Nurse Practitioner |
| Prakash C. Deedwania | ACC Diabetes and Cardiometabolic Work Group | University of California San Francisco–Chief of Cardiology |
| Gregory J. Dehmer | Solution Set Oversight Committee | Carilion Clinic, Cardiology and Carilion Cardiovascular Institute–Medical Director, Quality and Outcomes; Virginia Tech Carilion School of Medicine |
| Boris Draznin | American Diabetes Association | University of Colorado Denver, School of Medicine–Director, Adult Diabetes Program, Celeste and Jack Grynberg Professor of Medicine, Division of Endocrinology, Metabolism and Diabetes |
| James M. Falko | National Lipid Association | University of Colorado Lone Tree Medical Center–Clinical Professor of Medicine |
| Jennifer Green | American Diabetes Association | Duke University Medical Center–Professor of Medicine, Department of Medicine, Division of Endocrinology |
| Martha Gulati | Official Lead Reviewer, Solution Set Oversight Committee | University of Arizona College of Medicine–Chief of Cardiology |
| Stuart T. Haines | American Pharmacists Association | University of Mississippi School of Pharmacy–Professor and Director, Pharmacy Professional Development |
| Joshua J. Joseph | American Heart Association | The Ohio State University Wexner Medical Center–Assistant Professor of Medicine, Division of Endocrinology, Diabetes and Metabolism; Investigator, Diabetes and Metabolism Research Center; Affiliated Faculty, Translational Data Analytics Institute |
| Cynthia A. Lamendola | Preventive Cardiovascular Nurses Association | Stanford University School of Medicine–Nurse Practitioner and Clinical Research Nurse Coordinator |
| Shahar Lavi | Official BOG Peer Reviewer | Western University London Health Sciences Centre–Associate Professor; Director of Cardiovascular Interventional Research, Division of Cardiology, Department of Medicine |
| Joanna Mitri | Joslin Diabetes Center | Harvard Medical School–Research Associate, Section on Clinical, Behavioral, and Outcomes Research Lipid Clinic, Adult Diabetes Section; Joslin Diabetes Center Clinical Instructor |
| Mary H. Parker | American Society of Health-System Pharmacists | 1F/1D/1H Ambulatory Care Clinics–Clinic Coordinator; Durham VA HealthCare System–Clinical Pharmacy Specialist |
| Jane Reusch | American Diabetes Association | University of Colorado-Anschutz Medical Campus–Professor, Division of Endocrinology, Metabolism and Diabetes; Director, Diabetes Research and Personalized Medicine to Transform Care; Associate Director, Center for Women’s Health Research; Co-Director, University of Colorado NIH Diabetes Research Center, Departments of Medicine, Integrative Physiology, and Bioengineering; Rocky Mountain Regional VAMC–Staff Physician and Merit Investigator |
| Guillermo E. Umpierrez | American Association of Clinical Endocrinologists | Emory University–Professor of Medicine; Grady Health System–Section Head, Diabetes and Endocrinology |
| Deborah Wexler | American Diabetes Association | Massachusetts General Hospital–Associate Clinical Chief, MGH Diabetes Unit; Clinical Director, MGH Diabetes Center |
| Veronica Wilbur | American Association of Nurse Practitioners | West Chester University, West Chester Pennsylvania–Assistant Professor; Next Century Medical Care, Wilmington Delaware–Family Nurse Practitioner |
| David E. Winchester | Solution Set Oversight Committee (SSOC) | University of Florida College of Medicine–Associate Professor of Medicine; Malcom Randall VAMC–Staff Cardiologist; Assistant Cardiology Fellowship Program Director, Quality and Research; Co-Director, Advanced Fellowship for Cardiovascular Imaging; Co-Director, University of Florida Health Cardiac Imaging Group |
| Nathan D. Wong | ACC Expert | University of California, Irvine–Professor and Director, UCI Heart Disease Prevention Program |
| Eugene Yang | ACC Prevention Council | University of Washington School of Medicine–Clinical Professor of Medicine, Division of Cardiology, Carl and Renee Behnke Endowed Professorship for Asian Health; UW Medicine Eastside Specialty Center–Medical Director; Co-Director, Cardiovascular Wellness and Prevention Program |
ACC = American College of Cardiology; BOG = Board of Governors; MGH = Massachusetts General Hospital; NIH = National Institutes of Health; VA = Veterans Administration; VAMC = Veterans Administration Medical Center.
APPENDIX 3. ABBREVIATIONS
- ACC
American College of Cardiology
- ADA
American Diabetes Association
- AHA
American Heart Association
- ASCVD
atherosclerotic cardiovascular disease
- CV
cardiovascular
- DKD
diabetic kidney disease
- ECDP
Expert Consensus Decision Pathway
- eGFR
estimated glomerular filtration rate
- FDA
U.S. Food and Drug Administration
- GLP-1RA
glucagon-like peptide-1 receptor agonist
- HbAlc
hemoglobin Alc HF = heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HFSA
Heart Failure Society of America
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- SGLT2
sodium-glucose cotransporter-2
- T2D
type 2 diabetes
Footnotes
Copies: This document is available on the World Wide Web site of the American College of Cardiology (www.acc.org). For copies of this document, please contact Elsevier Inc. Reprint Department via fax (212) 633-3820 or e-mail (reprints@elsevier.com).
REFERENCES
- 1.Januzzi JL Jr., Ahmad T, Binder LG, et al. 2019 methodology for creating expert consensus decision pathways: a report of the American College of Cardiology. J Am Coll Cardiol. 2019;74:1138–50. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S111–34. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41: 917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawshani A, Rawshani A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;377:300–1. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA. 2019;321: 1867–8. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Introduction: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S1–2. [DOI] [PubMed] [Google Scholar]
- 8.King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel A, MacMahon S, Chalmers J, et al. , for the ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 11.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 14.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 16.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 17.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. [DOI] [PubMed] [Google Scholar]
- 19.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 20.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? isights from a medication analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:356–63. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Woodward M, Perkovic V, et al. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. J Am Coll Cardiol HF. 2020;8:57–66. [DOI] [PubMed] [Google Scholar]
- 22.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43: 487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperling LS, Mechanick JI, Neeland IJ, et al. The CardioMetabolic Health Alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–67. [DOI] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 28.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andary R, Fan W, Wong ND. Control of cardiovascular risk factors among US adults with type 2 diabetes with and without cardiovascular disease. Am J Cardiol. 2019;124:522–7. [DOI] [PubMed] [Google Scholar]
- 30.Fan W, Song Y, Inzucchi SE, et al. Composite cardiovascular risk factor target achievement and its predictors in US adults with diabetes: The Diabetes Collaborative Registry. Diabetes Obes Metab. 2019;21: 1121–7. [DOI] [PubMed] [Google Scholar]
- 31.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. [DOI] [PubMed] [Google Scholar]
- 32.Bittner V, Bertolet M, Barraza Felix R, et al. Comprehensive cardiovascular risk factor control improves survival: the BARI 2D trial. J Am Coll Cardiol. 2015;66:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guidelines for the prevention, detection, evaluation and management of high blood pressure in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-Cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785–822. [DOI] [PubMed] [Google Scholar]
- 36.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–471. [DOI] [PubMed] [Google Scholar]
- 37.Arnold SV, de Lemos JA, Rosenson RS, et al. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140:618–20. [DOI] [PubMed] [Google Scholar]
- 38.Morris PB, Kovacs RJ, Allen LA, et al. 2019 methodology for heart house roundtables: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1116–37. [DOI] [PubMed] [Google Scholar]
- 39.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guidelines on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/ AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 41.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on the new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–88. [DOI] [PubMed] [Google Scholar]
- 42.Yancy CW, Januzzi JL Jr., Allen LA, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71:201–30. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S135–51. [DOI] [PubMed] [Google Scholar]
- 44.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 128:1810–52. [DOI] [PubMed] [Google Scholar]
- 45.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 46.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 47.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 48.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. [DOI] [PubMed] [Google Scholar]
- 49.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–27. [DOI] [PubMed] [Google Scholar]
- 50.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agnosists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus Circulation 2019;139:2022–31. [DOI] [PubMed] [Google Scholar]
- 51.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects of biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF Trial. Circulation. 2019;140: 1463–76. [DOI] [PubMed] [Google Scholar]
- 52.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–17. [DOI] [PubMed] [Google Scholar]
- 54.Fitchett D, Inzucchi SE, Lachin JM, et al. Cardiovascular mortality reduction with empagliflozin in patients with type 2 diabetes and cardiovascular disease. J Am Coll Cardiol. 2018;71:364–7. [DOI] [PubMed] [Google Scholar]
- 55.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37: 1526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2018;39:363–70. [DOI] [PubMed] [Google Scholar]
- 57.American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38:10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumbhani DJ. Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial - VERTIS CV. American College of Cardiology website, June 16, 2020. Available at: https://www.acc.org/latest-in-cardiology/clinical-trials/2020/06/16/11/24/vertis#references-for-article. Accessed June 24, 2020. [Google Scholar]
- 60.ClinicalTrials.gov. Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease, the VERTIS CV study (MK-8835–004). Available at: https://clinicaltrials.gov/ct2/show/NCT01986881. Accessed August 1, 2018. [Google Scholar]
- 61.ClinicalTrials.gov. Effect of sotagliflozin on cardiovascular and renal events in patients with type 2 diabetes and moderate renal impairment who are at cardiovascular risk (SCORED). Available at: https://clinicaltrials.gov/ct2/show/NCT03315143. Accessed August 1, 2018. [Google Scholar]
- 62.Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.AstraZeneca. Farxiga (dapagliflozin) [package insert], https://www.azpicentral.com/farxiga/farxiga.pdf#page=1 Revised May 2020 Accessed May 6, 2020. [Google Scholar]
- 64.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5: 610–21. [DOI] [PubMed] [Google Scholar]
- 65.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17. [DOI] [PubMed] [Google Scholar]
- 66.Li D, Wang T, Shen S, et al. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:348–55. [DOI] [PubMed] [Google Scholar]
- 67.Jabbour S, Seufert J, Scheen A, et al. Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang JY, Wang T, Pate V, et al. Real-world evidence on sodium-glucose cotransporter-2 inhibitor use and risk of Fournier's gangrene. BMJ Open Diabetes Res Care. 2020;8:e000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erondu N, Desai M, Ways K, et al. Diabetic ketoacidosis and related events in canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38:1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42. [DOI] [PubMed] [Google Scholar]
- 71.Kaptoge S, Di Angelantonio E, Pennells L, et al. Emerging Risk Factors Collaboration, C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inzucchi SE, Iliev H, Pfarr E, et al. Empagliflozin and assessment of lower-limb amputation in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:e4–5. [DOI] [PubMed] [Google Scholar]
- 73.Verma S, Mazer CD, Al-Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–7. [DOI] [PubMed] [Google Scholar]
- 74.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol. 2017;5:680–1. [DOI] [PubMed] [Google Scholar]
- 75.American Diabetes Association. Introduction: standards of medical care in diabetes - 2019. Diabetes Care. 2019;42:S1–2. [DOI] [PubMed] [Google Scholar]
- 76.AstraZeneca. Farxiga phase III DAPA-CKD trial will be stopped early after overwhelming efficacy in patients with chronic kidney disease. Available at: https://www.astrazeneca.se/media/pressmeddelanden/svenska_pressmeddelanden/2020/farxiga-phase-iii-dapa-ckd-trial-will-be-stopped-early-after-ov.html. Accessed May 13, 2020. [Google Scholar]
- 77.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. [DOI] [PubMed] [Google Scholar]
- 79.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. [DOI] [PubMed] [Google Scholar]
- 80.Kristensen SL, Rorth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7: 776–85. [DOI] [PubMed] [Google Scholar]
- 81.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Har-monyOutcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- 83.Eli Lilly and Company. Trulicity (dulaglutide) [package insert]. U.S. Food and Drug Administration website, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s007s008lbl.pdf. Revised January 2017 Accessed April 27, 2020. [Google Scholar]
- 84.ClinicalTrials.gov. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). Available at: https://clinicaltrials.gov/ct2/show/NCT03819153 Accessed February 10, 2020. [Google Scholar]
- 85.Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45:409–18. [DOI] [PubMed] [Google Scholar]
- 86.Davidson JA. Incretin-based therapies: focus on effects beyond glycemic control alone. Diabetes Ther. 2013;4:221–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nauck MA, Meier JJ, Cavender MA, et al. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70. [DOI] [PubMed] [Google Scholar]
- 88.Nauck M Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miras AD, Perez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:549–59. [DOI] [PubMed] [Google Scholar]
- 90.Bailey CJ. Treating T2DM and obesity with bariatric surgery and GLP1 agents. Nat Rev Endocrinol. 2019;15: 504–6. [DOI] [PubMed] [Google Scholar]
- 91.Nordisk Novo. Victoza (liraglutide) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf. . [package insert]. Revised August 2017 Accessed May 13, 2020. [Google Scholar]
- 92.Nordisk Novo. Ozempic (semaglutide) https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf [package insert]. Revised December 2017 Accessed May 13, 2020. [Google Scholar]
- 93.Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22:713–22. [DOI] [PubMed] [Google Scholar]
- 94.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avorn J The psychology of clinical decision making - implications for medication use. N Engl J Med. 2018; 378:689–91. [DOI] [PubMed] [Google Scholar]
- 96.Professional Practice Committee. Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018; 41:S3. [DOI] [PubMed] [Google Scholar]
- 97.Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4: 1004–16. [DOI] [PubMed] [Google Scholar]
- 98.Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6: 370–81. [DOI] [PubMed] [Google Scholar]
- 99.Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2019;7:356–67. [DOI] [PubMed] [Google Scholar]
- 101.Harris MI, Robbins DC. Prevalence of adult-onset IDDM in the U.S. population. Diabetes Care. 1994;17: 1337–40. [DOI] [PubMed] [Google Scholar]
- 102.Wilcox CS, Shen W, Boulton DW, et al. Interaction between the sodium-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Professional Practice Committee. Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020; 43:S3. [DOI] [PubMed] [Google Scholar]
- 104.Hirsh BJ, Smilowitz NR, Rosenson RS, et al. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015;66:184–92. [DOI] [PubMed] [Google Scholar]


