Abstract
The RNS System is not approved in patients under 18, although a critical need for novel treatment modalities in this vulnerable population persist. We present two pediatric patients with drug‐resistant epilepsy secondary to Lennox‐Gastaut Syndrome (LGS) and autism spectrum disorder (ASD) treated with the RNS System. Both patients have experienced 75–99% clinical seizure reductions in >1 year of follow‐up. We illustrate that children with diffuse onset, multifocal epilepsy, including frontal and thalamic circuits thought to exist in the generation of LGS seizures, can be treated with responsive neurostimulation safely and effectively, targeting thalamic networks, and avoiding palliative disconnections and resections.
Introduction
Neuromodulation is utilized in patients with drug‐resistant epilepsy (DRE), especially when resective surgery is not possible or seizures are multifocal. 1 The RNS System (NeuroPace, Mountain View, CA, USA) is able to deliver responsive electrical stimulation once epileptiform activity is detected, thus impeding seizure propagation. Long‐term data have shown that RNS provides sustained seizure reduction with continued improvement with each year of treatment including favorable safety and efficacy results. 2 RNS as a closed‐loop system requires less electrical stimulation compared to the open‐loop DBS system, thus providing higher efficacy, lower power consumption, longer battery lifespan and ultimately fewer surgeries being performed in this vulnerable population. However, the current RNS System is only approved in patients ≥18 years and only approved for treatment of ≤2 seizure foci. We have used RNS in children (off‐label) 3 , 4 , 5 and adults, and have found no difference in safety or efficacy in our early experience. To reach a dramatically underserved population, children with MR‐negative epilepsy, 6 we have broadened the scope of RNS utilization. A recent study has shown reproducible evidence of a cortically driven process within the epileptic network of LGS. This study strongly supports the hypothesis that the mesial prefrontal cortex and centromedian nucleus of the thalamus are two key nodes in the network of LGS. 7 We present two cases of responsive thalamic stimulation in children with LGS and ASD, in an effort to provide targeted but widespread neuromodulatory seizure control via the centromedian nucleus of the thalamus (CMT). 8
Methods
Patient‐1: A 16‐year‐old girl with DRE was diagnosed with infantile spasms at age 3 months. She began to have myoclonic and generalized tonic‐clonic (GTC) seizures in clusters upon awakening, from age 9 months. The patient’s developmental history was notable for moderate ASD. There were no genetic etiologies identified. Her epilepsy was MRI‐negative (Fig. 1A), and with video‐electroencephalographic (vEEG) demonstrating slow spike and wave discharges consistent with LGS (Fig. 1B), underwent an intracranial monitoring procedure with subdural grid and strip electrodes at age 7 at another institution, followed by a small frontal cortical resection, the histopathology of which was unrevealing of any malformation of cortical development. The patient underwent Vagus Nerve Stimulator (VNS) insertion at age 11 without any reduction in seizure frequency or severity. Upon presentation to our center, she continued to suffer from atonic, myoclonic, and GTC seizures, having tried and failed multiple antiseizure medication trials.
Figure 1.
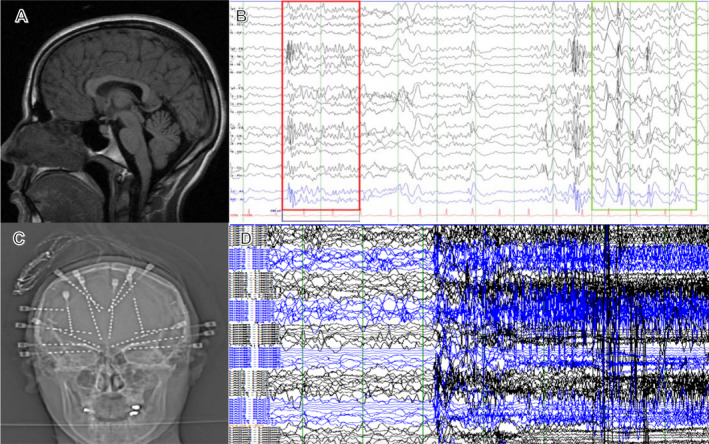
(A) MRI T1‐sagittal view demonstrating mild callosal dysgenesis but no focal abnormality; (B) Scalp EEG showing LGS pattern of underlying paroxysmal fast activity (red rectangle) and slow spike and wave (green rectangle); (C) AP X‐ray of the skull displaying relative mirror image stereotactic EEG electrode placement, with investigation of frontal, temporal, and insular networks; (D) sEEG showing generalized seizure pattern (black‐left hemisphere; blue‐right hemisphere).
We placed bilateral stereo‐EEG (sEEG) electrodes to lateralize or regionalize her seizure onsets (Fig. 1C). Monitoring of frontal and temporal lobes showed multifocal and generalized epileptiform activity (Fig. 1D) and mostly diffuse seizure onsets with right orbitofrontal lead‐in predominance. An RNS System with bilateral CMT leads and bilateral fronto‐polar depth leads was implanted post targeting (Fig. 2A–D). 9 The neurostimulator can be connected to two leads: right fronto‐polar and left CMT electrodes were connected, and the patient saw a decrease in her seizure frequency by >50% (multiple seizures/day to one seizure every 4 days). After 18 months, to further improve seizure control, we turned off the right frontal lead to determine if that stimulation was contributing to her therapy. With the right fronto‐polar lead inactivated, her seizure frequency remained static. Thus, at neurostimulator battery replacement, we disconnected the right fronto‐polar lead, and connected the right CMT depth electrode. At latest follow‐up, 26 months after initial implantation and 8 months after lead modification, the patient has achieved: 70–90% improvement in drop attacks, 100% improvement in myoclonus, 100% improvement in GTCs with only a few short seizures per month. The patient had tried 17 medications before being on a regimen of clobazam, rufinamide, brivaracetam, and diazepam prior to surgery. Currently, the patient is on clobazam, rufinamide, brivaracetam, and cannabidiol with decreased doses for all medications. RNS system parameters are as follows (bilaterally): current 0.7 mA, pulse width 160 μsec, charge density 0.7 μC/Cm2, duration 5000 msec, frequency 125 Hz. The patient is more alert, interactive, no longer wears a helmet, and her parents/caregivers report a dramatically better quality of life both for patient and themselves.
Figure 2.
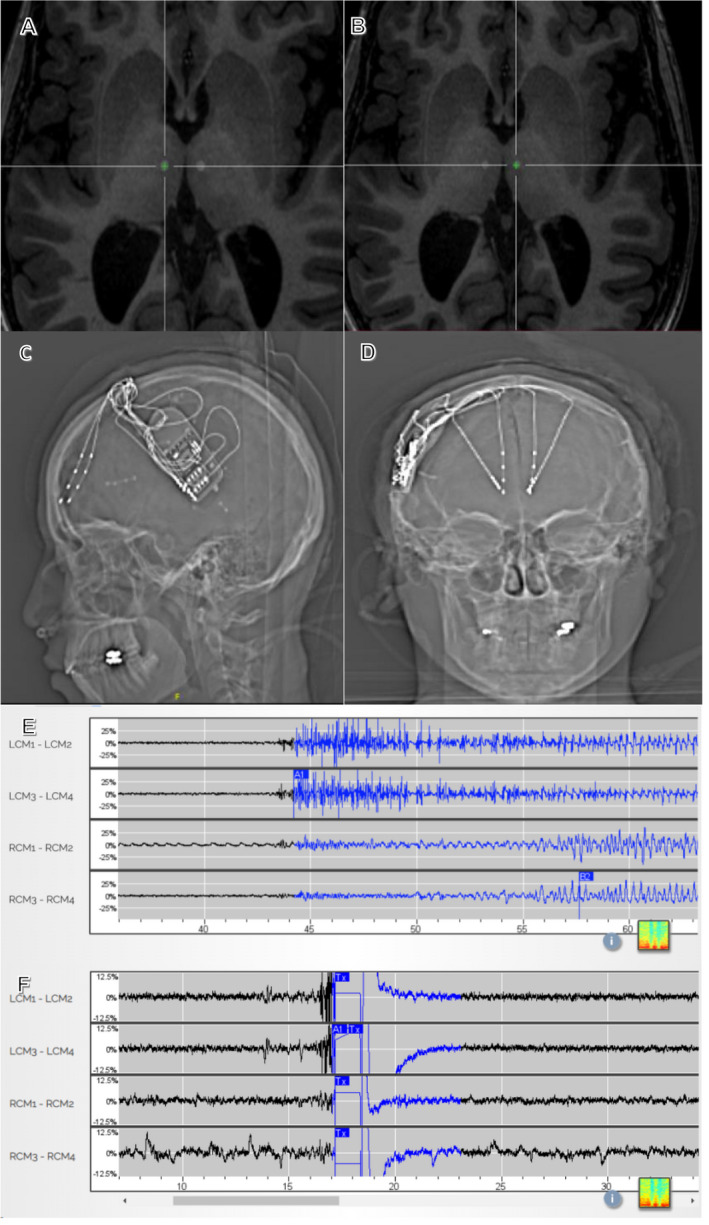
(A) Merged MPRAGE‐CT neuronavigation targeting right CMT; (B) Merged MPRAGE‐CT neuronavigation targeting left CMT; (C) AP X‐ray of RNS in situ with frontal and centromedian thalamic depth electrodes; (D) Lateral X‐ray of RNS in situ, same patient; (E) Seizure detection on thalamic leads before stimulation is activated; (F) Ictal pattern detected, stimulated and stopped from development in thalamic leads. LCM, left centromedian, RCM, right centromedian. L,R CM1‐L,R CM2 = deeper electrodes; L,R CM3‐L,R CM4 = more superficial electrodes.
Patient‐2: A 12‐year‐old boy presented with DRE, LGS, ASD, and severe obstructive sleep apnea. The patient’s epilepsy, which began at age eight, was characterized by mixed seizures including tonic, myoclonic, atonic, and GTC seizures, having up to 50 events daily for three years prior to presentation. The patient was the product of an uneventful pregnancy and delivery but had developmental delay in sensorimotor, language, and cognitive function. Genetic testing revealed dup15q syndrome, a rare genetic disorder commonly associated with ASD and epilepsy. 10 Multiple antiseizure drug trials were performed. His MRI demonstrated callosal dysgenesis and colpocephaly without hydrocephalus, but no other focal findings. His mother sought a second opinion after options of corpus callosotomy and VNS were offered at other centers.
The patient’s vEEG revealed moderate diffuse slowing, slow spike and wave at 1.5–2.5 Hz, bilateral independent temporal lobe spikes more frequent on the left. The patient underwent sEEG placement capturing: (1) frequent diffuse and multifocal spikes, (2) multiple tonic seizures primarily in sleep with diffuse onsets, (3) multiple myoclonic and tonic seizures, more in the morning, with diffuse onset. Given our experience with thalamic stimulation in multifocal and similar diffuse epileptogenic network cases in adults and our experience in Patient 1 above, Patient 2 underwent RNS System placement with bilateral CMT depth leads and bilateral frontal cortical strip leads. The patient was discharged home on postoperative day two. As he had his typical seizures captured by postoperative day 1, the CMT leads were activated immediately to both detect and stimulate (Fig. 2C and D). The patient saw an immediate response from RNS, with his seizure frequency decreasing from 50 daily to 3–4 total seizures in the first 2 weeks following his surgery. The patient’s mother also reported a decrease in sleep latency from 3–4 h to 1–2 h, with no seizures while the patient was awake. At the patient’s 1‐year follow‐up, there was an overall 75–95% seizure reduction (>50/day to 2/day) reported by his mother. Evening seizures that occurred mainly with sleep initiation had dropped dramatically. The daytime seizures had also noticeably decreased from daily seizures to one seizure a week. Overall, there has been >90% improvement in tonic seizures, drop attacks, myoclonus, and 70% improvement in GTCs. The patient had tried six different antiseizure medications before being on valproate and lamotrigine prior to surgery. The patient remains on these two medications at lower doses. The RNS system parameters are as follows: current 0.8 mA, pulse width 80 μsec, charge density 0.4 μC/Cm2, duration 1000 msec, and frequency 62.5 Hz (left CM); current 1 mA, pulse width 80 μsec, charge density 0.5 μc/Cm2, duration 1000 msec, frequency 62.5 Hz (right CM). The patient has had lasting therapeutic benefit from RNS at 13 months, is more alert and interactive, walks independently, and no longer wears a helmet. 11 No surgical complications were experienced for either patient.
Discussion
The burden of DRE coupled with ASD is high. 12 Epilepsy surgery yields significantly greater life expectancy compared to medical treatment in children with DRE, yet it is underutilized. 6 Our group has previously shown the feasibility and safety of epilepsy surgery in children with ASD, emphasizing the potential for better seizure control and associated gains in cognition with reductions in violent behavior. 12 Not surprisingly, children with ASD and MRI‐negative epilepsy, similar to the two presented here, appeared to have worse outcomes than their lesional counterparts after an irreversible ablative treatment. 12
We chose a modulatory approach in the current patients for several reasons. Novel treatments such as RNS have added to our armamentarium in treating epilepsy in adults. 1 The RNS System received FDA approval following the randomized controlled trial of 191 adults with DRE (partial). 13 Long‐term follow‐up found a 75% median seizure reduction at 9 years, and over one‐quarter of these patients experienced at least 6‐month periods of seizure freedom. 14 44% reported improvements in their quality of life at 2 years. 15 Both patients in this report had dramatic reductions in seizure frequency and severity accompanied by behavioral, cognitive, and quality of life improvements from RNS. In Patient 1, we were able to enhance seizure control by changing leads in an outpatient setting, a more appealing option compared to further irreversible resection, disconnection, or ablation. Sudden unexpected death in epilepsy (SUDEP) is a major concern for caregivers. In 2018, a study of 707 patients treated with the RNS System reported a lower than anticipated rate of SUDEP due to a reduction in seizures. 16 The potential to reduce the risk of SUDEP was the driving factor leading the mother of the second patient to seek treatment with RNS.
Stimulation of deep brain structures has been part of common neurosurgical practice for several decades, and Class I evidence exists for the use of deep brain stimulation in the anterior nucleus of the thalamus in patients with DRE (partial). 17 For children, DBS surgery is limited to the treatment of movement disorders. Few case reports of thalamic RNS in the anterior nucleus of the thalamus also exist with favorable results. 18 , 19 The CMT, as part of the cortico‐striato‐thalamic pathway, has widespread connections to the frontal cortex, 20 is involved in cortical excitation, seizure propagation, and plays a role in the loss of consciousness during seizures. 21 Several studies of CMT stimulation have also demonstrated seizure reduction and diminishing frequency in EEG spiking for generalized epilepsy. 22 RNS System use in the CMT has also been recently reported in an adult case of generalized epilepsy. 23 EEG‐fMRI analysis has shown robust and reproducible cortico‐thalamic network activity between medial frontal cortex and the CMT in LGS. 7
We have used a neuromodulatory strategy after sEEG to effectively control epilepsy in focal and regional onset clinical scenarios, 24 but do not feel that RNS System treatment should be limited to only such cases. sEEG based upon seizure semiologies and vEEG histories has given us the opportunity to understand the networks active in patients with multifocal and diffuse onset epilepsy. Rapid, diverse, and diffuse frontotemporal onsets seen in these two patients with sEEG led us to choose the CMT as our therapeutic target. In the first patient, we were not confident in using CMT leads alone; therefore, we added frontal cortical depth leads to ensure early detection of seizure activity. Subsequent experience has shown us that the thalamic leads are capable of detecting epileptiform activity and therefore programmable for responsive stimulation. Applying this principal directly to the second patient and subsequently to the first patient has provided a diagnostic and therapeutic benefit with more widespread neuromodulatory effect, and faster and better seizure control, albeit with a very limited sample size. Brain‐responsive neuromodulation may be an effective treatment with minimal risk for children with multifocal, MRI‐negative epilepsy. In the setting of comorbid ASD, significant gains can be made through better seizure control in a population for whom ablative surgery is not a worthwhile alternative.
Conflict of Interest
S. Ghatan receives honoraria from Neuropace and Monteris for education. F. Panov receives honoraria for education from Zimmer Biomet and Neuropace. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The remaining authors have no conflicts of interest.
Acknowledgment
Kwon is the holder of a Leon Levy Foundation Fellowship.
Funding Information
No funding information provided.
References
- 1. Kwon CS, Jette N, Ghatan S. Perspectives on the current developments with neuromodulation for the treatment of epilepsy. Expert Rev Neurother 2020;20:189–194. [DOI] [PubMed] [Google Scholar]
- 2. Nair DR, Laxer KD, Weber PB, et al. Nine‐year prospective efficacy and safety of brain‐responsive neurostimulation for focal epilepsy. Neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panov F, Ganaha S, Haskell J, et al. Safety of responsive neurostimulation in pediatric patients with medically refractory epilepsy. J Neurosurg Pediatr 2020;1–8. [DOI] [PubMed] [Google Scholar]
- 4. Kokoszka MA, Panov F, La Vega‐Talbott M, et al. Treatment of medically refractory seizures with responsive neurostimulation: 2 pediatric cases. J Neurosurg: Pediatr 2018;21:421–427. [DOI] [PubMed] [Google Scholar]
- 5. Singhal NS, Numis AL, Lee MB, et al. Responsive neurostimulation for treatment of pediatric drug‐resistant epilepsy. Epilepsy Behav Case Rep 2018;10:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duchowny M. Pediatric epilepsy surgery: past, present, and future. Epilepsia 2020;61:228–229. [DOI] [PubMed] [Google Scholar]
- 7. Warren AEL, Harvey AS, Vogrin SJ, et al. The epileptic network of Lennox‐Gastaut syndrome: cortically driven and reproducible across age. Neurology 2019;93:e215–e226. [DOI] [PubMed] [Google Scholar]
- 8. Velasco F, Velasco M, Jimenez F, et al. Predictors in the treatment of difficult‐to‐control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery 2000;47:295–304; discussion 5. [DOI] [PubMed] [Google Scholar]
- 9. Sudhyadhom A, Haq IU, Foote KD, et al. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). NeuroImage 2009;47(Suppl 2):T44–T52. [DOI] [PubMed] [Google Scholar]
- 10. Conant KD, Finucane B, Cleary N, et al. A survey of seizures and current treatments in 15q duplication syndrome. Epilepsia 2014;55:396–402. [DOI] [PubMed] [Google Scholar]
- 11. Wright J. Can preventing seizures alther the course of autism? www.spectrumnews.org/features/deep‐dive/can‐preventing‐seizures‐alter‐the‐course‐of‐autism/ (last accessed May 6, 2020).
- 12. Kokoszka MA, McGoldrick PE, La Vega‐Talbott M, et al. Epilepsy surgery in patients with autism. J Neurosurg Pediatr 2017;19:196–207. [DOI] [PubMed] [Google Scholar]
- 13. Morrell MJ, Group RNSSiES . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. [DOI] [PubMed] [Google Scholar]
- 14. Bergey GK, Morrell MJ, Mizrahi EM, et al. Long‐term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015;84:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meador KJ, Kapur R, Loring DW, et al. Quality of life and mood in patients with medically intractable epilepsy treated with targeted responsive neurostimulation. Epilepsy Behav 2015;45:242–247. [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain‐responsive neurostimulation. Epilepsia 2018;59:555–561. [DOI] [PubMed] [Google Scholar]
- 17. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 18. Herlopian A, Cash SS, Eskandar EM, et al. Responsive neurostimulation targeting anterior thalamic nucleus in generalized epilepsy. Ann Clin Transl Neurol 2019;6:2104–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elder C, Friedman D, Devinsky O, et al. Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment‐resistant multifocal epilepsy. Epilepsia Open 2019;4:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J, Kim Y, Nakajima R, et al. Inhibitory basal ganglia inputs induce excitatory motor signals in the thalamus. Neuron 2017;95:1181–96 e8. [DOI] [PubMed] [Google Scholar]
- 21. Miller R. Cortico‐thalamic interplay and the security of operation of neural assemblies and temporal chains in the cerebral cortex. Biol Cybern 1996;75:263–275. [DOI] [PubMed] [Google Scholar]
- 22. Valentin A, Garcia Navarrete E, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013;54:1823–1833. [DOI] [PubMed] [Google Scholar]
- 23. Kokkinos V, Urban A, Sisterson ND, et al. Responsive neurostimulation of the thalamus improves seizure control in idiopathic generalized epilepsy: a case report. Neurosurgery 2020. [DOI] [PubMed] [Google Scholar]
- 24. Ma BB, Fields MC, Knowlton RC, et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia 2020;61:96–106. [DOI] [PubMed] [Google Scholar]


