Abstract
Purpose: To report the 36-month outcomes from the prospective, multicenter, single-arm IN.PACT Global Study (ClinicalTrials.gov identifier NCT01609296) evaluating the performance of the IN.PACT Admiral drug-coated balloon (DCB) in real-world patients with femoropopliteal occlusive disease. Materials and Methods: The IN.PACT Global Study was conducted at 64 international sites and enrolled 1535 patients with complex lesions, which included bilateral disease, multiple lesions, de novo in-stent restenosis, long lesions, and chronic total occlusions. The predefined full clinical cohort included 1406 patients (mean age 68.6 years; 67.8% men) with claudication or rest pain treated with the study DCB. Mean lesion length was 12.09±9.54 cm; 18.0% had in-stent restenosis, 35.5% were totally occluded, and 68.7% were calcified. Freedom from clinically-driven target lesion revascularization (CD-TLR) was evaluated through 36 months. The safety composite endpoint was freedom from device- and procedure-related death through 30 days and freedom from major target limb amputation and clinically-driven target vessel revascularization within 36 months. All safety and revascularization events were reviewed by an independent clinical events committee. Results: The Kaplan-Meier estimate of freedom from CD-TLR through 36 months was 76.9%. The composite safety endpoint was achieved in 75.6% of patients. The 36-month all-cause mortality rate was 11.6%, and the major target limb amputation rate was 1.0%. The Kaplan-Meier estimate of freedom from CD-TLR through 36 months was significantly lower in patients with chronic limb-threatening ischemia (CLTI) compared with claudicants (67.6% vs 78.0%; p=0.003). Lesions affecting both the superficial femoral artery (SFA) and popliteal artery had lower Kaplan-Meier freedom from CD-TLR through 36 months (69.2%) than either isolated SFA (79.7%) or popliteal artery lesions (76.5%; log- rank p<0.001). Predictors of CD-TLR through 36 months included increased lesion length, reference vessel diameter ≤4.5 mm, in-stent restenosis, bilateral disease, CLTI, and hyperlipidemia. Conclusion: DCB angioplasty with the IN.PACT Admiral DCB for femoropopliteal disease in a diverse and complex real-world population is associated with sustained clinical efficacy and low rates of reinterventions at 3 years after the initial procedure.
Keywords: angioplasty, drug-coated balloon, femoropopliteal segment, mortality, peripheral artery disease, popliteal artery, superficial femoral artery, target lesion revascularization
Introduction
In recent years, endovascular treatments for patients with peripheral artery disease (PAD) of the lower extremities have gained growing acceptance among all vascular specialties and have become the primary mode of revascularization in both claudicants and patients with chronic limb-threatening ischemia (CLTI).1,2 The continuous development of endovascular technologies has offered a variety of different modalities and devices for the treatment of peripheral atherosclerosis.3–11 In the femoropopliteal segment, the introduction of paclitaxel drug-coated balloons (DCBs) has led to a paradigm shift from primary scaffolding with permanent metallic implants to a “leave nothing behind” or “leave less behind” approach.
The antiproliferative agent paclitaxel reduces the risk of restenosis by interrupting the cell cycle, activating apoptosis, and consequently inhibiting the proliferation and migration of smooth muscle cells.12 Numerous randomized controlled trials (RCTs) have demonstrated a clear benefit of DCBs over uncoated balloon angioplasty in terms of improved patency and reduced reintervention and bailout stent rates.3,4,11,13–18 Additionally, paclitaxel-based modalities seem to provide important economic benefits compared to their non-antiproliferative counterparts.19,20 Thus, DCBs have become the treatment of choice for both de novo and restenotic TransAtlantic Inter-Society Consensus II (TASC) A and B femoropopliteal lesions and represent a viable alternative to scaffolds for complex disease.21,22
Despite the promising outcomes of DCBs in the framework of many RCTs, the lesions included in these studies were predominantly single, short, and not severely calcified.13 Single-arm prospective studies and registries of real-world patients have shown a clinical benefit of DCBs in complex lesions.1,23–25 However, there are only limited long-term data beyond 2 years on the use of DCBs for the treatment of complex lesions. Moreover, risk factors in this population that affect the durability of DCB treatment have not been adequately evaluated.
The IN.PACT Global Study is a large prospective study evaluating the performance of the IN.PACT Admiral drug (paclitaxel)-coated balloon (Medtronic, Dublin, Ireland) in the treatment of real-world patients with atherosclerotic disease of the superficial femoral artery (SFA) and/or the entire popliteal artery. The 12- and 24-month results have already shown a sustained clinical benefit of this particular DCB.1,23 This article reports the 36-month outcomes of the full clinical cohort of the IN.PACT Global Study.
Materials and Methods
Study Design
The IN.PACT Global Study is a prospective, multicenter, international, single-arm clinical study assessing the safety and effectiveness of the IN.PACT Admiral DCB for the treatment of real-world patients with femoropopliteal atherosclerotic disease. Details of the study design have been described previously.1,23 Briefly, patients eligible for enrollment presented with intermittent claudication and/or ischemic rest pain [Rutherford category (RC) 2 to 4] and angiographic evidence of severe stenosis or occlusion [length ≥2 cm, de novo or restenosis (in-stent or native artery)] of the femoropopliteal vessels.
All major adverse events (MAEs), clinically-driven target lesion revascularizations (CD-TLRs), and clinically-driven target vessel revascularizations (CD-TVRs) through 36 months after the index procedures were independently adjudicated by an independent Clinical Events Committee (CEC; Syntactx, New York, NY, USA). Statistical methods were designed by the study sponsor; the raw data were transferred to the Baim Institute for Clinical Research (formerly HCRI; Boston, MA, USA) and analyzed independently.
The institutional review board or ethics committee at each study site approved the study protocol. Informed consent was obtained from all patients prior to enrollment. The study was conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines, and applicable laws as specified by all relevant governmental bodies. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov identifier: NCT01609296).
Endpoints and Definitions
The primary safety and effectiveness study endpoints have been previously reported.26 The effectiveness endpoint of this study, freedom from CD-TLR within 36 months, was defined as any reintervention within the target lesion(s) because of symptoms or drop of ankle-brachial index (ABI) of ≥20% or >0.15 when compared with post-index procedure baseline ABI. The safety endpoint was a composite of freedom from device- and procedure-related mortality through 30 days and freedom from major target limb amputation and CD-TVR within 36 months after the index procedure. CD-TVR was defined as any reintervention within the target vessel due to symptoms or drop of ABI ≥20% or >0.15 when compared with the post procedure baseline ABI. All repeat interventions on the target limbs including TLR, TVR, and the clinically-driven status were reviewed and adjudicated by the CEC.
Secondary endpoints included primary sustained clinical improvement, secondary sustained clinical improvement, CD-TLR, CD-TVR, any TLR, any TVR, and the incidence of MAEs (all-cause mortality, CD-TVR, major target limb amputation, and thrombosis at the target lesion site) evaluated at 36 months. Primary sustained clinical improvement was defined as a sustained upward shift of at least 1 RC compared to baseline without the need for repeated TLR or surgical revascularization in amputation-free surviving patients. Secondary sustained clinical improvement was defined as a sustained upward shift of at least 1 RC compared with baseline, including the need for repeated TLR or surgical revascularization in amputation-free surviving patients. The CEC adjudicated all MAEs. Functional assessments included evaluation of walking capacity using the Walking Impairment Questionnaire and quality of life using the EuroQol health status measurement tool in 5 dimensions (EuroQol-5D) index.
Patient Population
A total of 1535 patients were enrolled across 64 sites in 25 countries from Europe, the Middle East, Asia, Australia, Canada, and Latin America from 2012 to 2014. The outcomes reported here are from the 1416 consecutively enrolled full clinical cohort that included patients from the prespecified imaging cohorts as well as those patients without imaging (Figure 1). Patients from the 150-mm DCB cohort were enrolled nonconsecutively and were used for regulatory purposes. Within the clinical cohort, 1406 patients (mean age 68.6 years; 67.8% men) with 1773 lesions treated with the study DCB were analyzed on an intent-to-treat (ITT) basis. Due to protocol violations, this group included an RC 1 and 36 RC 5 patients.
Figure 1.
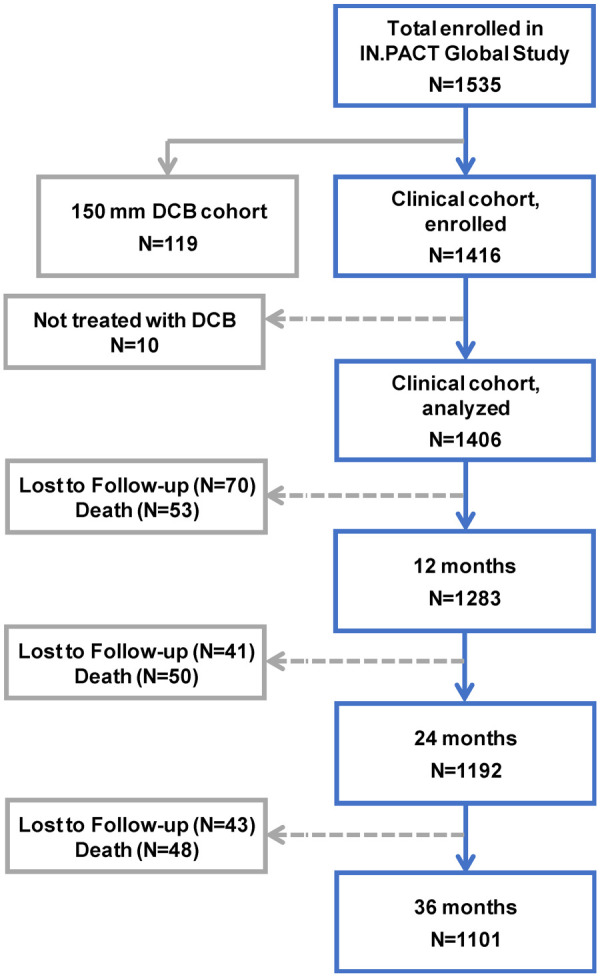
Patient flowchart in the IN.PACT Global Study though 36 months (±60 days). In the full clinical cohort, 1406 patients were treated with the IN.PACT Admiral DCB and will be followed for 5 years.
Overall, 88.8% of the ITT patients (1246/1403) presented with lifestyle-limiting claudication (RC 2/3) and 11.1% (156/1403) with CLTI (RC 4/5). (Note that CLI was used during the data collection; however, in the present report, this is referred to as CLTI to reflect the updated vascular guidelines.) Baseline demographics and lesion characteristics of the full clinical cohort are reported in Tables 1 and 2. Within the full cohort, 39.9% of patients had diabetes mellitus, 40.5% had coronary artery disease, 11.2% had chronic kidney disease, and 8.4% had bilateral disease in the femoropopliteal arteries. Mean lesion length was 12.09±9.54 cm. Among all lesions, 74.3% were de novo, 7.7% were restenotic (native artery), and 18.0% were in-stent restenoses (ISR). The majority (68.7%) of lesions were calcified, and 10.2% were severely calcified (circumference ≥180° on both sides of the vessel at the same location and lengths greater than or equal to half of the total lesion length).
Table 1.
Baseline Demographics and Clinical Characteristics of 1406 Intent-to-Treat Patients in the Clinical Cohort.a,b
| Age, y (n=1396) | 68.6±10.1 |
| Men | 67.8 (953/1406) |
| BMI, kg/m2 (n=1391) | 26.7±4.5 |
| Obesity (BMI ≥30) | 20.5 (285/1391) |
| Diabetes | 39.9 (560/1402) |
| Insulin dependent | 17.8 (249/1402) |
| Hypertension | 83.4 (1169/1401) |
| Dyslipidemia | 70.5 (960/1362) |
| Current smoker | 31.8 (447/1406) |
| Coronary heart disease | 40.5 (540/1332) |
| Carotid artery disease | 20.2 (241/1196) |
| Chronic kidney diseasec | 11.2 (136/1217) |
| Previous peripheral revascularization | 52.4 (737/1406) |
| Below-the-knee disease of target leg | 45.3 (594/1310) |
| Rutherford category | |
| 0 | 0 |
| 1 | 0.1 (1/1403)d |
| 2 | 31.1 (436/1403) |
| 3 | 57.7 (810/1403) |
| 4 | 8.6 (120/1403) |
| 5 | 2.6 (36/1403)d |
| 6 | 0 |
| ABI per target limbe (n=1395) | 0.68±0.22 |
| Bilateral disease | 8.4 (118/1406) |
Abbreviations: ABI, ankle-brachial index; BMI, body mass index.
Continuous data are presented as the mean ± standard deviation with the sample size; categorical data are given as the percentage (number/sample).
Summaries are based on non-missing assessments.
Baseline creatinine ≥1.5 mg/dL.
Due to protocol violations, 1 patient classified as Rutherford category 1 and 36 patients classified as category 5 were enrolled and included in the analysis.
For patients with bilateral disease, ABI is included for each target limb.
Table 2.
| Preprocedure | |
| Lesion typec | |
| De novo | 74.3 (1317/1773) |
| Restenotic (native artery) | 7.7 (136/1773) |
| In-stent restenosis | 18.0 (320/1773) |
| Vesseld | |
| Superficial femoral artery | 87.6 (1553/1773) |
| Popliteal artery | 27.3 (484/1773) |
| Lesion length,d cm (n=1773) | 12.09±9.54 (range 0.5–54.0; median 9.7) |
| Occludedd | 35.5 (629/1773) |
| Calcificationd | 68.7 (1217/1771) |
| Severed,e | 10.2 (181/1771) |
| RVD,d mm (n=1773) | 5.19±0.68 |
| Diameter stenosis,d % (n=1773) | 88.8±12.3 |
| Procedure | |
| DCBs per lesionc (n=1766) | 1.7±1.0 |
| Predilationc | 78.0 (1097/1406) |
| Postdilationc | 35.1 (491/1397) |
| Provisional stentingc | 21.2 (373/1761) |
| Postprocedure | |
| Device successf | 99.4 (2984/3002) |
| Procedural successg | 99.4 (1749/1760) |
| Clinical successh | 98.8 (1379/1396) |
| Dissectionsd | |
| 0 | 56.8 (1006/1772) |
| A-C | 35.4 (627/1772) |
| D-F | 7.8 (139/1772) |
Abbreviations: DCBs, drug-coated balloons; RVD, reference vessel diameter.
Continuous data are presented as the mean ± standard deviation with the sample size; categorical data are given as the percentage (number/sample).
Summaries are based on nonmissing assessments.
Data reported by the investigational sites.
Data reported by independent core imaging laboratories.
Severe calcification was defined as calcification with circumference ≥180° (both sides of the vessel at the same location) and a length greater than or equal to half of the total lesion length.
Device success was defined as successful delivery, inflation, deflation, and retrieval of the intact study balloon device without burst below the rated burst pressure. This analysis is device (balloon) based.
Procedural success was defined as residual stenosis ≤50% for non-stented patients or ≤30% for stented patients by core laboratory assessment (site-reported estimate was used if not available). This analysis is lesion based.
Clinical success was defined as procedural success without complications (death, major target limb amputation, thrombosis of the target lesion, or target vessel revascularization) prior to discharge. This analysis is patient based.
The flow of clinical follow-up through 36 months is shown in Figure 1. A total of 154 patients were lost to follow-up or withdrew consent and 151 died within the 36-month follow-up window. Of the remaining 1101 patients eligible for the 36-month evaluations, 856 (77.7%) completed the 36-month follow-up visit within the window. Endpoints were assessed based on patients with evaluable data within 36 months.
Statistical Analysis
All analyses were based on the intent-to-treat principle. All summaries were based on nonmissing assessments. Unless otherwise specified, all baseline demographics and clinical characteristics were summarized on a patient basis; lesion characteristics were summarized on a lesion basis. For baseline characteristics, continuous variables are described as mean ± standard deviation; dichotomous and categorical variables are described as counts and proportions. The outcome analysis was performed at a patient level.
The Kaplan-Meier method was used to evaluate time-to-event data for freedom from CD-TLR and freedom from mortality over the 36-month follow-up period. The difference in the survival curves across subgroups was assessed using the log-rank test. Estimates are presented with the 95% confidence interval (CI) where applicable. For other outcomes, proportion rates were reported. The Fisher exact test was used to compare binary outcomes between subgroups; the Wilcoxon rank-sum test or Student t test was used for continuous outcomes. For event rates that were expressed as a proportion, the number of patients with events within 36 months was the numerator, and the total number of patients with events or at least 34 months of clinical follow-up was the denominator.
For assessment of clinical characteristics at 36 months, patients were required to have data at baseline and 36 months. For the multivariable analysis, a Cox proportional hazards model with potential baseline predictors was performed for CD-TLR through 36 months; a stepwise selection process with an entry criterion of 0.20 and a stay criterion of 0.10 was used. Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
Results
Efficacy Outcomes
The Kaplan-Meier estimate of freedom from CD-TLR through 36 months was 76.9% (95% CI 74.5% to 79.2%; Figure 2). The rate of CD-TLR at 36 months was 22.9% (289/1262; Table 3). The mean time to first CD-TLR was 15.8±9.6 months. Primary and secondary sustained clinical improvement rates were 59.3% (601/1014) and 81.1% (762/940), respectively, at 36 months (Table 3).
Figure 2.
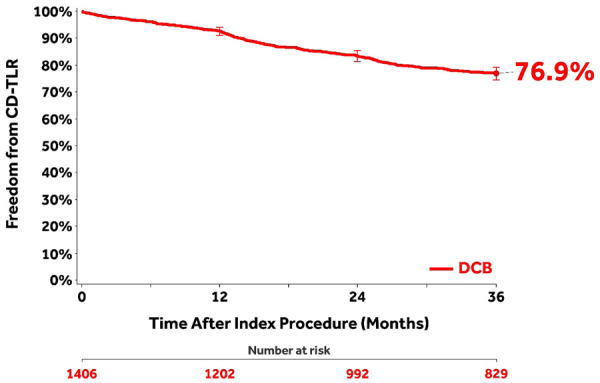
Kaplan-Meier curve of freedom from clinically-driven target lesion revascularization (CD-TLR) in the clinical cohort through 36 months. Bars represent the 95% confidence intervals. All target lesion revascularization events were adjudicated by the independent Clinical Events Committee.
Table 3.
CEC-Adjudicated 36-Month Outcomes in 1406 Patients.a
| Outcomes | Summaries |
|---|---|
| Primary sustained clinical improvementb | 59.3 (601/1014) |
| Secondary sustained clinical improvementc | 81.1 (762/940) |
| Safety composite endpointd freedom from | 75.6 (954/1262) |
| 30-d device- and procedure-related death | 0.2 (3/1402) |
| 36-mo major target limb amputation | 1.0 (12/1262) |
| 36-mo CD-TVRe | 23.7 (299/1262) |
| Cumulative 36-mo complications | |
| MAE compositef | 34.8 (439/1262) |
| Death (all-cause) | 11.6 (147/1262) |
| CD-TVRe | 23.7 (299/1262) |
| Major target limb amputation | 1.0 (12/1262) |
| Thrombosis | 5.6 (71/1262) |
| CD-TLRg | 22.9 (289/1262) |
| Any TVR | 24.2 (306/1262) |
| Any TLR | 23.4 (295/1262) |
| Other 36-mo secondary outcomes | |
| Time to first CD-TLR, mo | 15.8±9.6 (n=289) |
| Change in QoL by EQ-5Dh | 0.13±0.33 (n=823) |
| WIQ,h % | 74.4±30.6 (n=831) |
Abbreviations: CD, clinically driven; CEC, Clinical Events Committee; EQ-5D, EuroQol in 5 dimensions; MAE, major adverse events; QoL, quality of life; RC, Rutherford category; TLR, target limb revascularization; TVR, target vessel revascularization; WIQ, Walking Impairment Questionnaire.
Continuous data are presented as the mean ± standard deviation with the sample size; categorical data are given as the percentage (number/sample). All target lesion revascularizations and adverse events were adjudicated by the independent Clinical Events Committee.
Primary sustained clinical improvement was defined as a sustained upward shift of at least 1 RC compared to baseline without the need for repeated TLR or surgical revascularization in amputation-free surviving patients.
Secondary sustained clinical improvement is defined as a sustained upward shift of at least 1 RC compared to baseline including the need for repeated TLR or surgical revascularization in amputation-free surviving patients.
Safety composite endpoint consists of freedom from device- and procedure-related death to 30 days, freedom from major target limb amputation within 36 months, and freedom from CD-TVR within 36 months.
CD-TVR is defined as any reintervention at the target vessel due to symptoms or drop of ABI ≥20% or >0.15 when compared with post-index procedure baseline ABI.
MAEs are defined as all-cause death, CD-TVR, major target limb amputation, and thrombosis at the target lesion site at 36 months.
CD-TLR is defined as any reintervention within the target lesion due to symptoms or drop in ABI ≥20% or >0.15 when compared to post-index procedure baseline ABI.
Not adjudicated.
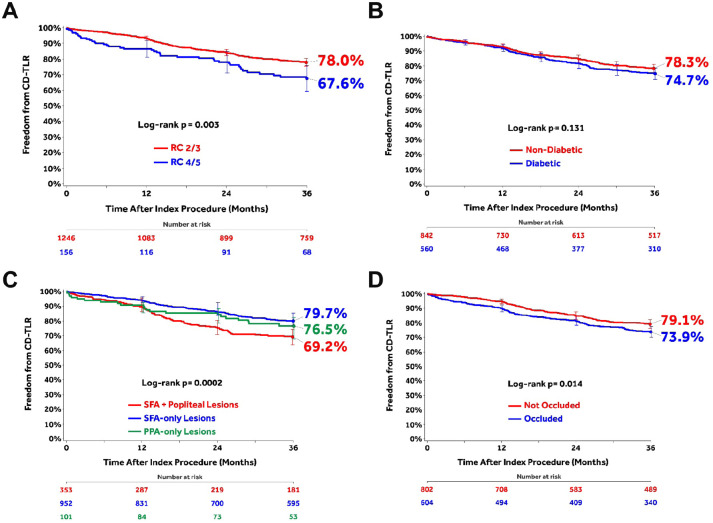
The post hoc analyses of subgroups stratified by baseline clinical or lesion characteristics (Figure 3) found that CLTI patients had significantly lower 36-month freedom from CD-TLR compared to claudicant patients (Figure 3A). Freedom from CD-TLR was not different between diabetic and nondiabetic patients (Figure 3B). Lesions affecting both the SFA and popliteal arteries showed lower freedom from CD-TLR than isolated SFA or popliteal lesions (Figure 3C). Freedom from CD-TLR was significantly higher in not-occluded lesions compared with occluded lesions (Figure 3D).
Figure 3.
Subgroup analyses of freedom from clinically-driven target lesion revascularization (CD-TLR) using the Kaplan-Meier method through 36 months: (A) chronic limb-threatening ischemia [Rutherford category (RC) 4/5] compared with claudicants (RC 2/3); (B) diabetics vs nondiabetics; (C) lesions affecting both the superficial femoral artery (SFA) and popliteal artery vs SFA-only vs popliteal artery–only lesions; and (D) not-occluded vs occluded lesions. Bars represent 95% confidence intervals. All target lesion revascularization events were adjudicated by the independent Clinical Events Committee.
Safety Outcomes
The safety composite endpoint at 36 months was achieved in 75.6% of patients (954/1262; Table 3). The major adverse event rate was 34.8% (439/1262). Thrombosis occurred in 5.6% (71/1262) of patients and the rate of CD-TVR was 23.7% (299/1262). At 36 months, 12 patients (1.0%, 12/1262) underwent a major target limb amputation at an average time to amputation of 15.4±10.6 months. Three patients with a major target limb amputation between 0 and 12 months were classified as RC 3, RC 4, and RC 5 at baseline. Of the 6 patients who required a major amputation between 12 and 24 months, 2 were classified as RC 2, 3 as RC 3, and 1 as RC 4 at baseline. Three patients with amputation between 24 and 36 months were RC 2, RC 3, and RC 4. Of the 12 patients, 6 underwent a CD-TLR prior to the amputation, and 3 patients had thrombosis at the target lesion site prior to CD-TLR. The 3-year major amputation rate in patients with baseline CLTI was 2.9% (4/139) compared with 0.7% (8/1120) for claudicants (p=0.035).
The CEC-adjudicated overall all-cause mortality was 11.6% (147/1262) through 36 months [10.4% (116/1120) for claudicants and 22.3% (31/139) for CLTI; p<0.001). The CEC-adjudicated causes27 of the 147 deaths were cardiovascular (n=67), malignancy (n=20), respiratory (n=5), neurological (noncardiovascular; n=1), pancreatic (n=1), hepatobiliary (n=1), gastrointestinal (n=2), infection/inflammatory (n=11), hemorrhage (excluding cardiovascular bleeds or stroke; n=1), along with other (n=2) or undetermined (n=36) causes. Independent adjudication by the CEC determined that none of the deaths was related to the study device; 6 were possibly or potentially related to the study procedure. Of these, 2 deaths occurred between 24 and 36 months. A post hoc analysis of freedom from all-cause mortality through 36 months (Figure 4) captured patients who were adjudicated by the CEC per study protocol as well as the survival status of an additional 90 patients originally lost to follow-up but collected post hoc at the request of the Food and Drug Administration (FDA) for the Circulatory System Devices Panel.28
Figure 4.
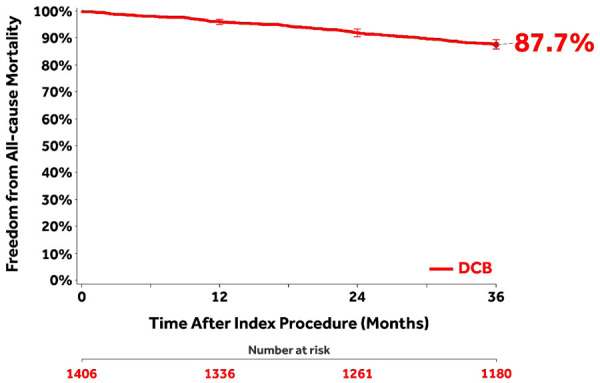
A post hoc Kaplan-Meier analysis of freedom from all-cause mortality through 36 months capturing patients who were adjudicated by the Clinical Events Committee per study protocol as well as the survival status of an additional 90 patients originally lost to follow-up (22 of these patients had died within 36 months). Bars represent 95% confidence intervals.
Functional Outcomes
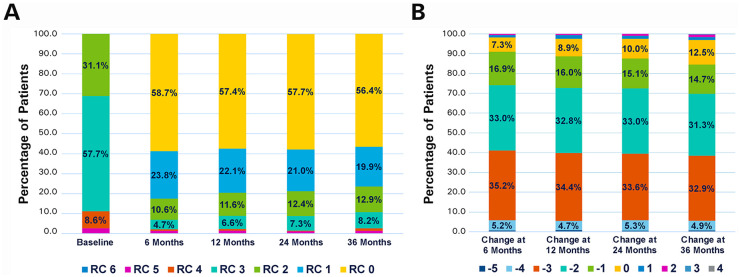
Changes in the RC between baseline and 36 months (Figure 5A) were statistically significant (p<0.001), with the majority of patients (84.5%, 775/917) having ≥1 level improvement (Figure 5B). Mean ABI was 0.68±0.22 at baseline (1395 target limbs) and 0.87±0.22 (872 target limbs) at 36 months (mean change 0.19±0.27 in 821 target limbs, p<0.001). Mean change from baseline in the EuroQol-5D index score at 36 months was 0.13±0.33 (n=823). The overall walking impairment score was 74.4%±30.6% (n=831) at 36 months.
Figure 5.
(A) Distribution of Rutherford category (RC) at baseline and at the follow-up intervals. (B) Changes in RC at the follow-up intervals compared with baseline to illustrate percentage of patients showing improvement.
Predictors of CD-TLR
The multivariable Cox proportional hazards regression analysis (Table 4) found that increasing lesion length, ISR, bilateral disease, hyperlipidemia, CLTI, and reference vessel diameter ≤4.5 mm were associated with an increased risk for CD-TLR within 36 months. SFA-only lesions and the presence of hypertension were associated with reduced risk for CD-TLR. There was a higher trend of risk for CD-TLR in target lesions with moderate to severe calcification, but the correlation was statistically nonsignificant.
Table 4.
Predictors of Clinically-Driven Target Lesion Revascularization Through 36 Months.
| Variable | Coefficient | Hazard Ratio [95% CI] | p |
|---|---|---|---|
| Lesion length >20 vs ≤20 cm | 0.715 | 2.045 [1.543 to 2.710] | <0.001 |
| Lesion location: SFA only | −0.510 | 0.601 [0.454 to 0.795] | <0.001 |
| Lesion type: ISR vs native artery restenosis | 0.535 | 1.707 [1.257 to 2.317] | <0.001 |
| Bilateral vs unilateral lesions | 0.646 | 1.908 [1.259 to 2.891] | 0.002 |
| Age | −0.019 | 0.981 [0.969 to 0.995] | 0.006 |
| RVD ≤4.5 vs >4.5 mm | 0.458 | 1.580 [1.133 to 2.204] | 0.007 |
| Hyperlipidemia | 0.379 | 1.461 [1.052 to 2.029] | 0.024 |
| Hypertension | −0.383 | 0.682 [0.480 to 0.969] | 0.032 |
| CLTI vs claudication (≥RC 4 vs <RC 4) | 0.396 | 1.486 [1.024 to 2.157] | 0.037 |
| Calcification: moderate/severe vs severe | 0.286 | 1.331 [0.975 to 1.815] | 0.071 |
| Posterior tibial artery pulse absent | −0.285 | 0.752 [0.539 to 1.048] | 0.093 |
Abbreviations: CI, confidence interval; CLTI, chronic limb-threatening ischemia; ISR, in-stent restenosis; RC, Rutherford category; RVD, reference vessel diameter; SFA, superficial femoral artery.
Discussion
The current analysis evaluated the clinical efficacy and safety of the IN.PACT Admiral DCB in the treatment of femoropopliteal atherosclerosis in a real-world cohort including patients with complex, calcified, long, and sequential lesions. The results revealed sustained clinical outcomes and low rates of reinterventions through 3 years after the initial procedure; the majority of the patients remained asymptomatic, with a significant improvement in their walking capacity and Rutherford category. Given that the primary goal of revascularization in claudicants and patients with rest pain is to improve the functional status and the quality of life of the patient, these results confirm a sustained, durable clinical efficacy of DCB angioplasty. Most important, these promising outcomes were observed in the framework of a challenging “real-world” cohort. The results of this study additionally suggest that the anatomical distribution of the disease, as well as certain lesion characteristics, such as the length and the calcification grade, might influence the performance of an antiproliferative agent.
There are numerous endovascular options for the treatment of femoropopliteal disease. However, data to 3 years are limited to RCTs or RCT-like settings. Regulatory-restricted inclusion criteria for these studies are often limited to short, less-complex lesions with minimum calcification. Nonetheless, clinical benefit of DCBs in this real-world study is comparable to other studies with morphologically simple lesions. Through 36 months, the Kaplan-Meier estimate of freedom from CD-TLR was 76.9% in the IN.PACT Global Study compared to 84.5% for DCBs in the IN.PACT SFA RCT,16 83.6% to 85.3% for drug-eluting stents (DES),8,29 and 69.7% to 75.5% for bare metal stents (BMS).30,31 However, mean lesion lengths of the aforementioned studies were in the range of only 6.64 to 8.94 cm8,16,29–31 vs 12.1 cm in the IN.PACT Global Study. Moreover, ISR lesions and patients with higher rates of comorbidities were included in the IN.PACT Global study. The 22.9% rate of 36-month CD-TLR in the IN.PACT Global Study is lower than that reported for BMS (31.1% CD-TLR31) and stent-grafts (34.7% TLR32) at 3 years.
Despite the inclusion of CLTI and complex lesions in this study, more than 75% of patients achieved the safety composite endpoint, with low rates of major amputations and reocclusion through 36 months. No device-related death as adjudicated by the CEC was reported. The CEC-adjudicated all-cause mortality of 11.6% in this study is consistent with the range of 3-year rates (3.9% to 12.5%) in other contemporary studies of both drug-coated and uncoated devices.30–34 Causes of death were varied and congruent with the age and the high level of comorbidities in this patient population. A study-level meta-analysis35 reported an association between paclitaxel-coated devices (DCB and DES) and mortality. The concern regarding the mortality safety signal led to the convening of various society forums36,37 and a public advisory committee meeting of the Circulatory System Devices Panel.28 Analyses by the FDA and Vascular Interventional Advances (VIVA) of available data from FDA-approved devices for use in the femoropopliteal segment showed a late mortality signal.38,39 However, a mechanism linking paclitaxel with late mortality could not be established in any of these analyses. It should be noted that these RCTs35,39 and FDA analyses38 were originally not designed or powered to show a superior/inferior mortality risk between the two groups of patients. In contrast, patient-level meta-analyses did not find a statistically significant mortality difference and showed no correlation between paclitaxel exposure and increased risk of mortality.40,41 Analyses using large all-comers registries also did not find any mortality safety signal following the use of paclitaxel-coated devices.42–47 Furthermore, the mortality signal was attenuated in the recently updated VIVA analysis with additional vital status follow-up.48 While the presence or causality of the late mortality signal is unclear, the benefit-risk analysis clearly demonstrated a consistent clinical benefit of paclitaxel-coated devices compared to uncoated devices across all studies.38,41,49–51 Along this line, a recent update from the FDA recommends that for individual patients judged to be at particularly high risk for restenosis and repeat femoropopliteal interventions, clinicians may determine that the benefits of using a paclitaxel-coated device outweigh the risk of late mortality.52 There is no doubt, however, that further investigations with large datasets are required and more research should be encouraged.
The current post hoc analyses and previous publications have shown that DCBs provide a consistent clinical benefit in various lesion types or clinical characteristics, including ISR, CLTI, severely calcified, and diabetic subgroups.1,23,53 However, multivariable risk analysis revealed that the presence of CLTI and circumferential calcification was associated with an increased risk for CD-TLR through 36 months. Lesions in CLTI patients are often more complex and associated with a higher calcium burden. Severe calcification alters the morphology and the compliance of the vessel wall and increases the risk for flow-limiting dissections after angioplasty.54,55 Additionally, the increased calcium burden may limit the drug uptake in the arterial wall and decrease the antiproliferative effect of paclitaxel. Both clinical and experimental data suggested an added benefit from “vessel preparation” prior to DCB angioplasty in severely calcified lesions. Tzafriri et al56 observed increased paclitaxel uptake following orbital atherectomy in calcified femoropopliteal vessels, while an Italian group reported promising patency rates following directional atherectomy with antirestenotic therapy in both severely calcified SFA and common femoral artery disease.57,58 In this context, the use of vessel preparation techniques might further expand the application of DCBs in severely calcified lesions and lead to better clinical and radiological outcomes.
Moreover, increased lesion length and a vessel diameter <4.5 cm can decrease the effectiveness of DCB angioplasty. Despite the satisfying 1- and 2-year results in the SFA Long Study59 and the long lesion imaging subcohort of the IN.PACT Global Study,60 at 3 years, a longer lesion length is associated with increased risk for CD-TLR. Of note, in the DEFINITIVE AR pilot study,61 there was a trend toward improved results with the use of directional atherectomy prior to DCB angioplasty in longer lesions. Thus, debulking might improve the performance of the “leave nothing behind” strategies in these lesions. This concept is being investigated currently in the ongoing REALITY trial (ClinicalTrials.gov identifier: NCT02850107).
Interestingly, the diameter of the treated vessel also affected the outcomes of DCB angioplasty. It should be noted that in the framework of this study the reported vessel diameters were based on visual estimation of the angiograms. It is, on the other hand, well known that angiography underestimates the degree of stenosis and consequently of the vessel diameter.62 This may also lead to a potential undersizing of the selected uncoated balloon angioplasty and DCB catheters. Adjunctive imaging modalities, such as intravascular ultrasound, have the potential to optimize device sizing and improve the outcomes of peripheral interventions.
Furthermore, the lesion location might be an independent risk factor for repeated reinterventions. In the majority of the published studies, the outcomes of endovascular therapy for lesions in the SFA and popliteal artery are evaluated together as femoropopliteal lesions. Nonetheless, both the current literature and the findings of this real-world IN.PACT Global Study suggest that the unique anatomical environment in which the popliteal artery is embedded possesses different challenges compared to the proximal and middle SFA.63 It remains, however, difficult to explain why lesions affecting both the SFA and the popliteal artery performed worse than isolated SFA or popliteal disease. We assume that a geometrical mismatch might play a vital role. In long femoropopliteal lesions, physicians tend to treat the affected vessels with a single long balloon (both uncoated and coated). This might lead to an undersizing of the devices proximally and/or oversizing distally. Undersizing of the uncoated balloon and DCB catheters might lead to inadequate acute lumen gain and decreased paclitaxel tissue uptake, respectively, and consequently to treatment failure. Again, effective vessel preparation might improve the patency rates of “leave nothing behind” strategies in this challenging anatomical region, while decreasing the use of permanent scaffolds.
Limitations
The limitations of this study are consistent with other prospective registries as it did not include a control group. In the absence of an active comparator, the results of this study cannot support direct comparison to other endovascular treatment modalities. Evaluation of DCB effectiveness was limited to clinical outcomes in this full clinical cohort. Not all patients in the full clinical cohort had data available for the analysis of anatomical outcomes, such as restenosis rate, as only predefined cohorts (long lesion, de novo ISR, and chronic total occlusion) within the full clinical cohort were planned for prospective duplex ultrasound and imaging analyses.
Conclusion
Despite the short exposure of the vessel wall to an antiproliferative agent, angioplasty with the IN.PACT Admiral DCB catheter for complex femoropopliteal lesions was associated with sustained clinical efficacy and low rates of reinterventions at 3 years after the initial procedure. Disease distribution as well as lesion characteristics influence the performance of DCBs.
Acknowledgments
The authors would like to thank Megan Fox, MBA, and Eric Fernandez, MD, for clinical support; Pei Li, PhD, for statistical review; Bridget Wall, PhD, and Kathleen Cahill, MS, for technical review of the manuscript; and Sangeeta Yendrembam, PhD, for assistance in its preparation.
Footnotes
IN.PACT Global Study Collaborators: Thomas Zeller, Universitäts-Herzzentrum Freiburg - Bad Krozingen, Bad Krozingen, Germany; Giovanni Torsello, St. Franziskus-Hospital Münster, Münster, Germany; Gunnar Tepe, RoMed Klinikum Rosenheim, Rosenheim, Germany; Patrick Peeters, Imeldaziekenhuis, Bonheiden, Belgium; Dierk Scheinert, Universitätsklinikum Leipzig, Leipzig, Germany; Marc Bosiers, AZ Sint-Blasius, Dendermonde, Belgium; Lieven Maene, Onze-Lieve-Vrouwziekenhuis, Aalst, Belgium; Antonio Micari, Maria Eleonora Hospital, Palermo, Italy; Dai-Do Do, Inselspital Universitätsspital Bern, Bern, Switzerland; Jeroen Hendriks, Universitair Ziekenhuis Antwerpen, Edegem, Belgium; Koen Keirse, RZ Tienen, Tienen, Belgium; Marianne Brodmann, Landeskrankenhaus-Universitätsklinikum Graz, Graz, Austria; Bela Merkely, Semmelweis Egyetem AOK, Budapest, Hungary; Jan-Willem Lardenoije, Rijnstate - Locatie Arnhem, Arnhem, the Netherlands; Zoltan Ruzsa, Bács-Kiskun Megyei Kórháza, Kecskemét, Hungary; Britta Vogel, Universitätsklinikum Heidelberg, Heidelberg, Germany; Pierfrancesco Veroux, AO Universitaria Policlinico Vittorio Emanuele Presidio G R, Catania, Italy; Joao Albuquerque e Castro, Hospital de Santa Marta, Lisbon, Portugal; Daniel Periard, Hopital Cantonal HFR, Fribourg, Switzerland; Tomasz Ludyga, Euromedic, Katowice, Poland; Dominique Midy, Groupe Hospitalier Pellegrin, Bordeaux Cedex, France; Donghoon Choi, Severance Hospital, Seoul, South Korea; Wouter Lansink, Ziekenhuis Oost Limburg-Campus Sint-Jan, Genk, Belgium; Dominik Ketelsen, Universitätsklinikum Tübingen, Tübingen, Germany; Steven Dubenec, Royal Prince Alfred Hospital, Sydney, Australia; Martin Banyai, Luzerner Kantosspital, Luzern, Switzerland; Nabil Chakfe, Hôpitaux Universitaires de Strasbourg - Hôpital Civil, Strasbourg, France; Franz Xaver Roithinger, Landesklinikum Thermenregion Mödling, Mödling, Austria; Carlo Trani, Università Cattolica del Sacro Cuore Policlinico Gemelli, Rome, Italy; Hossam Mansour, As-Salam International Hospital, Cairo, Egypt; Seung-Woon Rha, Korea University Guro Hospital, Seoul, South Korea; Frank Vermassen, Universitair Ziekenhuis Gent, Gent, Belgium; Alexander Belenky, Rabin Medical Center-Beilinson Hospital, Petach Tikva, Israel; Lubomir Spak, VUSCH, Kosice, Slovakia; Nicholas Chalmers, Manchester Royal Infirmary, Manchester, UK; Andrew Benko, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Canada; Steven Kum, Changi General Hospital, Singapore; Je Hwan Won, Ajou University Hospital, Seoul, South Korea; Matej Vozar, SUSCCH, Banská Bystrica, Slovakia; Kong Teng Tan, Toronto General Hospital, Toronto, Canada; Mamdouh Labib, Egypt Air Hospital, Cairo, Egypt; Gert-Jan de Borst, Universitair Medisch Centrum Utrecht, Utrecht, the Netherlands; Young-Soo Do, Samsung Medical Center, Seoul, South Korea; Joep Teijink, Catharina Ziekenhuis, Eindhoven, the Netherlands; Juan Fernando Gomez, Clinica Santa Maria, Medellin, Columbia; Aleksander Falkowski, Samodzielny Publiczny Szpital Kliniczny Nr 2 PAM w Szczecini, Szczecin, Poland; Luis Ferreira, Clinica La Sagrada Familia, Buenos Aires, Argentina; Jozef Matela, University Medical Centre Maribor, Maribor, Slovenia; Seung-Whan Lee, Asan Medical Center, Seoul, South Korea; Bart Verhoeven, Jeroen Bosch Ziekenhuis, Hertogenbosch, the Netherlands; Dalit Mannheim, Carmel Medical Centre, Haifa, Israel; Franco Nessi, Azienda Ospedaliera Ordine Mauriziano, Torino, Italy; Ivan Vulev, Narodny ustav srdcovych a cievnych chorob, a.s. (NUSCH), Bratislava, Slovakia; Jean-Paul de Vries, St. Antonius Ziekenhuis, Nieuwegein, the Netherlands; Radovan Maly, Faculty Hospital Hradec Kralove, Hradec Kralove, Czech Republic; Zaza Kavteladze, City Clinical Hospital #71, Moscow, Russia; Douglas Turner, Sheffield Vascular Institute, Sheffield Teaching Hospitals, Sheffield, UK; Oscar Mendiz, Fundacion Favaloro, Buenos Aires, Argentina; Ralf Kolvenbach, Augusta Krankenhaus, Duesseldorf, Germany; Dimitrios Karnabatidis, General University Hospital of Patras, Patras, Greece; Cesar Cuellar, Clinica Medilaser, Huila, Columbia; Maarit Venermo, Helsingin Seudun Yliopistollinen Keskussairaala, Helsinki, Finland; Linas Velicka, Kaunas Medical University Clinic, Kaunas, Lithuania; Goran Lundberg, Karolinska Universitetssjukhuset, Solna, Sweden.
Authors’ Note: This study was presented at CIRSE (Cardiovascular and Interventional Radiological Society of Europe); September 22–25, 2018; Lisbon, Portugal.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Giovanni Torsello received speaking honoraria from W.L. Gore & Associates and Medtronic. Konstantinos Stavroulakis received honoraria from Medtronic, Boston Scientific Corp, Philips, and Biotronik. Marianne Brodmann received speaking honoraria from Bard Peripheral Vascular, Biotronik, Medtronic, Spectranetics, and VIVA Physicians and is a consultant for Bard Peripheral Vascular, Biotronik, Medtronic, and Spectranetics. Antonio Micari is a compensated consultant for Medtronic and Boston Scientific Corp. Gunnar Tepe received research grants from Medtronic and is a compensated advisory board member for Medtronic. Pierfrancesco Veroux received research and clinical trial funds and honoraria from Medtronic, Abbott Vascular, Terumo Medical Corp, Cook Medical, W.L. Gore & Associates, and Bolton Medical. Andrew Benko received speaking honoraria from Medtronic, Boston Scientific Corp, and Cordis and research funding from Medtronic, Boston Scientific Corp, and Soundbite Medical. Frank E.G. Vermassen received speaking honoraria from Abbott Vascular, Boston Scientific Corp, Medtronic, and Philips and is a consultant for Medtronic and Philips. Michael R. Jaff is a noncompensated advisor for Abbott Vascular; a part-time employee of Boston Scientific; an advisor for Biotronik, Medtronic, Philips, Sanofi, Vactronix, and Vascular Therapies; and an equity investor in Embolitech, Gemini, and PQ Bypass. Thomas Zeller received speaking honoraria from Abbott Vascular, Bard Peripheral Vascular, Biotronik, Boston Scientific Corp, Cook Medical, Cordis, GLG, W.L. Gore & Associates, Medtronic, Philips, Spectranetics, Straub Medical, TriReme, Veryan, and VIVA Physicians; he is a consultant for Abbott Vascular, Bard Peripheral Vascular, Boston Scientific Corp, Cook Medical, W.L. Gore & Associates, Medtronic, and Spectranetics; and his clinic has received study funds or funds for research or clinical trials from 480 Biomedical, Abbott Vascular, B. Braun, Bard Peripheral Vascular, Bayer Pharma, Biotronik, Caveo Med, Contego Medical, Cook Medical, CSI, W.L. Gore & Associates, Innora, Intact Vascular, Medtronic, Mercator, Philips, Pluristem, Shockwave, Spectranetics, Terumo, TriReme, and Veryan. Jia Guo and Reka Dobranszki are full-time employees of Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The IN.PACT Global Study was sponsored by Medtronic.
ORCID iDs: Giovanni Torsello  https://orcid.org/0000-0001-7513-5063
https://orcid.org/0000-0001-7513-5063
Konstantinos Stavroulakis  https://orcid.org/0000-0002-9775-9210
https://orcid.org/0000-0002-9775-9210
Gunnar Tepe  https://orcid.org/0000-0002-1239-994X
https://orcid.org/0000-0002-1239-994X
Pierfrancesco Veroux  https://orcid.org/0000-0002-2780-6421
https://orcid.org/0000-0002-2780-6421
Thomas Zeller  https://orcid.org/0000-0003-2704-3871
https://orcid.org/0000-0003-2704-3871
References
- 1. Brodmann M, Keirse K, Scheinert D, et al. Drug-coated balloon treatment for femoropopliteal artery disease: the IN.PACT Global Study de novo in-stent restenosis imaging cohort. JACC Cardiovasc Interv. 2017;10:2113–2123. [DOI] [PubMed] [Google Scholar]
- 2. Stavroulakis K, Borowski M, Torsello G, et al. One-year results of first-line treatment strategies in patients with critical limb ischemia (CRITISCH Registry). J Endovasc Ther. 2018;25:320–329. [DOI] [PubMed] [Google Scholar]
- 3. Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 5. Rocha-Singh KJ, Jaff MR, Crabtree TR, et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. [DOI] [PubMed] [Google Scholar]
- 6. Katsanos K, Spiliopoulos S, Karunanithy N, et al. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J Vasc Surg. 2014;59:1123–1133e8. [DOI] [PubMed] [Google Scholar]
- 7. Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol. 2013;24:165–173. [DOI] [PubMed] [Google Scholar]
- 8. Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133:1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKinsey JF, Zeller T, Rocha-Singh KJ, et al. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv. 2014;7:923–933. [DOI] [PubMed] [Google Scholar]
- 10. Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840. [DOI] [PubMed] [Google Scholar]
- 11. Jia X, Zhang J, Zhuang B, et al. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the AcoArt I trial. JACC Cardiovasc Interv. 2016;9:1941–1949. [DOI] [PubMed] [Google Scholar]
- 12. Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. [DOI] [PubMed] [Google Scholar]
- 13. Katsanos K, Spiliopoulos S, Paraskevopoulos I, et al. Systematic review and meta-analysis of randomized controlled trials of paclitaxel-coated balloon angioplasty in the femoropopliteal arteries: role of paclitaxel dose and bioavailability. J Endovasc Ther. 2016;23:356–370. [DOI] [PubMed] [Google Scholar]
- 14. Laird JR, Schneider PA, Tepe G, et al. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. [DOI] [PubMed] [Google Scholar]
- 15. Iida O, Soga Y, Urasawa K, et al. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider PA, Laird JR, Tepe G, et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11:e005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishnan P, Faries P, Niazi K, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheinert D, Schulte KL, Zeller T, et al. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther. 2015;22:14–21. [DOI] [PubMed] [Google Scholar]
- 19. Salisbury AC, Li H, Vilain KR, et al. Cost-effectiveness of endovascular femoropopliteal intervention using drug-coated balloons versus standard percutaneous transluminal angioplasty: results from the IN.PACT SFA II trial. JACC Cardiovasc Interv. 2016;9:2343–2352. [DOI] [PubMed] [Google Scholar]
- 20. Sridharan ND, Boitet A, Smith K, et al. Cost-effectiveness analysis of drug-coated therapies in the superficial femoral artery. J Vasc Surg. 2018;67:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortese B, Granada JF, Scheller B, et al. Drug-coated balloon treatment for lower extremity vascular disease intervention: an international positioning document. Eur Heart J. 2016;37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 22. Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 23. Micari A, Brodmann M, Keirse K, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC Cardiovasc Interv. 2018;11:945–953. [DOI] [PubMed] [Google Scholar]
- 24. Schroe H, Holden AH, Goueffic Y, et al. Stellarex drug-coated balloon for treatment of femoropopliteal arterial disease-the ILLUMENATE Global study: 12-month results from a prospective, multicenter, single-arm study. Catheter Cardiovasc Interv. 2018;91:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thieme M, Von Bilderling P, Paetzel C, et al. The 24-month results of the Lutonix Global SFA registry: worldwide experience with Lutonix drug-coated balloon. JACC Cardiovasc Interv. 2017;10:1682–1690. [DOI] [PubMed] [Google Scholar]
- 26. Zeller T, Brodmann M, Micari A, et al. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain. Circ Cardiovasc Interv. 2019;12:e007730. [DOI] [PubMed] [Google Scholar]
- 27. Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71:1021–1034. [DOI] [PubMed] [Google Scholar]
- 28. The Food and Drug Administration (FDA) announcement. Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting. June 19–20, 2019. https://www.fda.gov/advisory-committees/advisory-committee-calendar/june-19-20-2019-circulatory-system-devices-panel-medical-devices-advisory-committee-meeting. Accessed September 4, 2019.
- 29. Müller-Hülsbeck S, Keirse K, Zeller T, et al. Long-term results from the majestic trial of the Eluvia paclitaxel-eluting stent for femoropopliteal treatment: 3-year follow-up. Cardiovasc Intervent Radiol. 2017;40:1832–1838. [DOI] [PubMed] [Google Scholar]
- 30. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 31. Rocha-Singh KJ, Bosiers M, Schultz G, et al. A single stent strategy in patients with lifestyle limiting claudication: 3-year results from the Durability II trial. Catheter Cardiovasc Interv. 2015;86:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geraghty PJ, Mewissen MW, Jaff MR, et al. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg. 2013;58:386–395e4. [DOI] [PubMed] [Google Scholar]
- 33. Bunte MC, Cohen DJ, Jaff MR, et al. Long-term clinical and quality of life outcomes after stenting of femoropopliteal artery stenosis: 3-year results from the STROLL study. Catheter Cardiovasc Interv. 2018;92:106–114. doi: 10.1002/ccd.27569. [DOI] [PubMed] [Google Scholar]
- 34. Brescia AA, Wickers BM, Correa JC, et al. Stenting of femoropopliteal lesions using interwoven nitinol stents. J Vasc Surg. 2015;61:1472–1478. [DOI] [PubMed] [Google Scholar]
- 35. Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beckman JA, White CJ. Paclitaxel-coated balloons and eluting stents: is there a mortality risk in patients with peripheral artery disease? Circulation. 2019;140:1342–1351. [DOI] [PubMed] [Google Scholar]
- 37. Dan K, Garcia-Garcia HM, Hideo-Kajita A, et al. Paclitaxel-coated balloons and stents for the treatment of peripheral artery disease: proceedings from the Cardiovascular Research Technologies (CRT) 2019 Town Hall. EuroIntervention. 2019;15:e317–e319. [DOI] [PubMed] [Google Scholar]
- 38. The Food and Drug Administration Executive Summary: Circulatory System Devices Panel Meeting. https://www.fda.gov/media/127698/download Accessed September 4, 2019.
- 39. Rocha-Singh K, Mullin C. Individual patient data meta-analysis of late mortality after paclitaxel containing device exposure. Presented at the Food and Drug Administration: Circulatory System Devices Panel Meeting; June 19, 2019. [Google Scholar]
- 40. Albrecht T, Schnorr B, Kutschera M, et al. Two-year mortality after angioplasty of the femoro-popliteal artery with uncoated balloons and paclitaxel-coated balloons-a pooled analysis of four randomized controlled multicenter trials. Cardiovasc Intervent Radiol. 2019;42:949–955. [DOI] [PubMed] [Google Scholar]
- 41. Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019;73:2550–2563. [DOI] [PubMed] [Google Scholar]
- 42. Secemsky EA, Kundi H, Weinberg I, et al. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol. 2019;4:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Secemsky EA, Kundi H, Weinberg I, et al. Drug-eluting stent implantation and long-term survival following peripheral artery revascularization. J Am Coll Cardiol. 2019;73:2636–2638. [DOI] [PubMed] [Google Scholar]
- 44. Bertges DJ, Sedrakyan A, Sun T, et al. Mortality after paclitaxel coated balloon angioplasty and stenting of superficial femoral and popliteal artery in the Vascular Quality Initiative. Circ Cardiovasc Interv. 2020. Feb;13(2):e008528. doi: 10.1161/CIRCINTERVENTIONS.119.008528. [DOI] [PubMed] [Google Scholar]
- 45. King AH, Ambani RN, Bose S, et al. Paclitaxel-coated peripheral arterial devices are not associated with increased mortality. J Vasc Surg. 2019;70:e57. [DOI] [PubMed] [Google Scholar]
- 46. Donas KP, Sohr A, Pitoulias GA, et al. Long-term mortality of matched patients with intermittent claudication treated by high-dose paclitaxel-coated balloon versus plain balloon angioplasty: a real-world study. Cardiovasc Intervent Radiol. 2020;43:2–7. [DOI] [PubMed] [Google Scholar]
- 47. Freisinger E, Koeppe J, Gerss J, et al. Mortality after use of paclitaxel-based devices in peripheral arteries: a real-world safety analysis [published online October 8, 2019]. Eur Heart J. doi: 10.1093/eurheartj/ehz698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rocha-Singh KJ, Duval S, Jaff MR, et al. Mortality and paclitaxel-coated devices: an individual patient data meta-analysis. Circulation. 2020. May 6. doi: 10.1161/CIRCULATIONAHA.119.044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anantha-Narayanan M, Shah SM, Jelani QU, et al. Drug-coated balloon versus plain old balloon angioplasty in femoropopliteal disease: an updated meta-analysis of randomized controlled trials. Catheter Cardiovasc Interv. 2019;94:139–148. [DOI] [PubMed] [Google Scholar]
- 50. Caradu C, Lakhlifi E, Colacchio EC, et al. Systematic review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease. J Vasc Surg. 2019;70:981–995e10. [DOI] [PubMed] [Google Scholar]
- 51. Dinh K, Gomes ML, Thomas SD, et al. Mortality after paclitaxel-coated device use in patients with chronic limb-threatening ischemia: a systematic review and meta-analysis of randomized controlled trials. J Endovasc Ther. 2020;27:175–185. [DOI] [PubMed] [Google Scholar]
- 52. The Food and Drug Administration Update: Treatment of peripheral arterial disease with paclitaxel-coated balloons and paclitaxel-eluting stents potentially associated with increased mortality. August 7, 2019. https://www.fda.gov/medical-devices/letters-health-care-providers/august-7-2019-update-treatment-peripheral-arterial-disease-paclitaxel-coated-balloons-and-paclitaxel. Accessed September 4, 2019.
- 53. Reijnen M, van Wijck I, Zeller T, et al. Outcomes after drug-coated balloon treatment of femoropopliteal lesions in patients with critical limb ischemia: a post hoc analysis from the IN.PACT Global study. J Endovasc Ther. 2019;26:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tepe G, Beschorner U, Ruether C, et al. Drug-eluting balloon therapy for femoropopliteal occlusive disease: predictors of outcome with a special emphasis on calcium. J Endovasc Ther. 2015;22:727–733. [DOI] [PubMed] [Google Scholar]
- 55. Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37:898–907. [DOI] [PubMed] [Google Scholar]
- 56. Tzafriri AR, Garcia-Polite F, Zani B, et al. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017;264:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cioppa A, Stabile E, Popusoi G, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: One-year single centre clinical results. Cardiovasc Revasc Med. 2012;13:219–223. [DOI] [PubMed] [Google Scholar]
- 58. Cioppa A, Stabile E, Salemme L, et al. Combined use of directional atherectomy and drug-coated balloon for the endovascular treatment of common femoral artery disease: immediate and one-year outcomes. EuroIntervention. 2017;12:1789–1794. [DOI] [PubMed] [Google Scholar]
- 59. Micari A, Nerla R, Vadala G, et al. 2-Year results of paclitaxel-coated balloons for long femoropopliteal artery disease: evidence from the SFA-Long Study. JACC Cardiovasc Interv. 2017;10:728–734. [DOI] [PubMed] [Google Scholar]
- 60. Scheinert D. Drug-coated balloon treatment for femoropopliteal artery disease: the IN.PACT Global Study long lesion imaging cohort. Circ Cardiovasc Interv. 2018;11:e005654. [DOI] [PubMed] [Google Scholar]
- 61. Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017;10:e004848. doi: 10.1161/CIRCINTERVENTIONS.116.004848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kashyap VS, Pavkov ML, Bishop PD, et al. Angiography underestimates peripheral atherosclerosis: lumenography revisited. J Endovasc Ther. 2008;15:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stavroulakis K, Schwindt A, Torsello G, et al. Directional atherectomy with antirestenotic therapy vs drug-coated balloon angioplasty alone for isolated popliteal artery lesions. J Endovasc Ther. 2017;24:181–188. [DOI] [PubMed] [Google Scholar]