Abstract
Purpose: To investigate the outcomes of orbital atherectomy (OA) for the treatment of patients with peripheral artery disease (PAD) manifesting as claudication or chronic limb-threatening ischemia (CLTI). Materials and Methods: The database from the LIBERTY study (ClinicalTrials.gov identifier NCT01855412) was interrogated to identify 503 PAD patients treated with any commercially available endovascular devices and adjunctive OA for 617 femoropopliteal and/or infrapopliteal lesions. Cox regression analyses were employed to examine the association between baseline Rutherford category (RC) stratified as RC 2-3 (n=214), RC 4-5 (n=233), or RC 6 (n=56) and all-cause mortality, target vessel revascularization (TVR), major amputation, major adverse event (MAE), and major amputation/death at up to 3 years of follow-up. The mean lesion lengths were 78.7±73.7, 131.4±119.0, and 95.2±83.9 mm, respectively, for the 3 groups. Results: After OA, balloon angioplasty was used in >98% of cases, with bailout stenting necessary in 2.0%, 2.8%, and 0% of the RC groups, respectively. A small proportion (10.8%) of patients developed angiographic complications, without differences based on presentation. During the 3-year follow-up, claudicants were at lower risk for MAE, death, and major amputation/death than patients with CLTI. The 3-year Kaplan-Meier survival estimates were 84.6% for the RC 2-3 group, 76.2% for the RC 4-5 group, and 63.7% for the RC 6 group. The 3-year freedom from major amputation was estimated as 100%, 95.3%, and 88.6%, respectively. Among CLTI patients only, the RC at baseline was correlated with the combined outcome of major amputation/death, whereas RC classification did not affect TVR, MAE, major amputation, or death rates. Conclusion: Peripheral artery angioplasty with adjunctive OA in patients with CLTI or claudication is safe and associated with low major amputation rates after 3 years of follow-up. These results demonstrate the utility of OA for patients across the spectrum of PAD.
Keywords: amputation, balloon angioplasty, critical limb ischemia, endovascular treatment/therapy, femoropopliteal segment, infrapopliteal arteries, mortality, orbital atherectomy, peripheral artery disease, stent
Introduction
Peripheral artery disease (PAD) affects more than 8 million people in the United States1,2 and has increased in prevalence by almost 24% from 2000 to 2010.2,3 PAD is associated with morbidity and mortality rates similar to or higher than coronary artery disease.4–6 Approximately 20% of patients older than 50 years are afflicted, and it is even more common in diabetics, smokers, and patients with chronic kidney disease (CKD).6–9 The degree of PAD severity can be categorized according to the Rutherford category (RC)10 as mild to severe intermittent claudication (RC 1-3) and chronic limb-threatening ischemia (CLTI) with/without tissue loss (RC 4-6). About 10% of patients with PAD suffer from CLTI.11,12
CLTI is a multilevel disease that has been associated with up to 45% 1-year mortality13–15 and high amputation rates,16,17 significantly increasing overall health care costs.18–20 The American College of Cardiology/American Heart Association guidelines21 have recommended that revascularization is a reasonable treatment option for patients with RC >2 symptoms when optimal medical therapy and/or exercise fail to improve symptoms. However, data regarding best revascularization strategies for CLTI are sparse.22–24
Extensive arterial calcification remains a major challenge for peripheral interventions owing to the increased risk of periprocedural complications (eg, flow-limiting dissections25,26), higher restenosis rates,26–32 and slow flow or spasm, especially when treating infrapopliteal lesions.33 Endovascular treatment of calcified plaques has also been associated with distal embolization, particularly when treating CLTI patients with mildly calcified, fibrotic, soft-plaque lesions.34 Thus, vessel calcification is directly correlated to the overall implications of PAD.35,36 As such, numerous treatment methods have been developed for plaque modification before angioplasty31,37–41 in order to minimize periprocedural complications, enhance vessel patency, and minimize the need for provisional stenting.42
Orbital atherectomy (OA) is an effective device for vessel preparation before balloon angioplasty (BA) and was designed for the treatment of infrainguinal arteries with severe calcification.31,41,43–48 The current study investigated the short- and long-term outcomes of OA for the treatment of severe claudication and CLTI using a subgroup of patients from the LIBERTY study.49 In addition to describing overall procedural safety and 3-year outcomes, the results were also stratified by Rutherford category.
Materials and Methods
Study Design and Patient Enrollment
LIBERTY 360 was a prospective, observational, multicenter study investigating the clinical and economic outcomes of patients with symptomatic PAD who underwent lower extremity endovascular interventions with any approved or cleared device between 2013 and 2016. A steering committee, including principal investigators, representatives from the study core laboratories, and the sponsor (Cardiovascular Systems, Inc, St Paul, MN, USA), developed the study’s protocol; the sponsor was also responsible for effective oversight of the research process. The institutional review board of each site approved the protocol. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov; identifier NCT01855412).
The detailed inclusion and exclusion criteria of the LIBERTY 360 study were previously reported.50 Angiographic data were adjudicated by SynvaCor/Prairie Educational and Research Cooperative (PERC; Springfield, IL, USA). When data from the core laboratory evaluation were not available, site-reported data were used for the analysis. The current study included only patients treated with adjunctive OA (Diamondback/Stealth; Cardiovascular Systems, Inc) and comparisons were stratified by RC. Patients treated with more than one atherectomy device were excluded from the analysis, and as such only cases treated solely with OA were included. Lesions above and below the knee were revascularized, while the target area at the infrapopliteal segment was any lesion in a native vessel located within or extending to 10 cm above the medial epicondyle to the digital arteries.
A total of 503 patients from the LIBERTY trial met the criteria and were included in this analysis. Patient demographics and lesion characteristics stratified by RC are illustrated in Tables 1 and 2, respectively. There were 214 patients with claudication (RC 2-3) and 289 patients with CLTI (233 with RC 4-5 and 56 with RC 6) who underwent endovascular treatment for 617 lesions (251 RC 2-3, 289 RC 4-5, and 77 RC 6). Over half of the lesions (54.1%) were located solely at the infrapopliteal segment, a third of the lesions (32.6%) were in the femoropopliteal arteries, while 13.3% of the treated lesions involved diffuse segments above and below the knee.
Table 1.
Baseline Characteristics of the Participants.a
| Characteristics | RC 2-3: Claudicantsb (n=214) | RC 4-5: CLTI (n=233) | RC 6: CLTI (n=56) | p |
|---|---|---|---|---|
| Age, y | 70.4±9.6 | 71.3±10.9 | 67.9±14.2 | 0.106 |
| Men | 149/214 (69.6) | 138/233 (59.2) | 40/56 (71.4) | 0.154 |
| Race | ||||
| American Indian or Alaska Native | 1/214 (0.5) | 1/233 (0.4) | 0/56 | >0.99 |
| Asian | 1/214 (0.5) | 1/233 (0.4) | 1/56 (1.8) | 0.467 |
| Black or African American | 26/214 (12.1) | 26/233 (11.2) | 13/56 (23.2) | 0.068 |
| Native Hawaiian or Other Pacific Islander | 0/214 | 1/233 (0.4) | 0/56 | >0.99 |
| White | 181/214 (84.6) | 198/233 (85.0) | 42/56 (75.0) | 0.178 |
| Other | 5/214 (2.3) | 6/233 (2.6) | 0/56 | 0.753 |
| BMI, kg/m2 | 28.8±5.5 | 29.1±6.7 | 29.3±7.1 | 0.785 |
| eGFR, mg/dL/1.73 m2 | 69.0±28.6 | 58.3±26.4 | 57.2±34.4 | <0.001 |
| Smoking history | 0.128 | |||
| Current | 44/214 (20.6) | 41/233 (17.6) | 10/56 (17.9) | 0.731 |
| Former | 111/214 (51.9) | 100/233 (42.9) | 26/56 (46.4) | 0.166 |
| Diabetes | 109/214 (50.9) | 157/233 (67.4) | 44/56 (78.6) | <0.001 |
| Hyperlipidemia | 195/214 (91.1) | 207/233 (88.8) | 38/56 (67.9) | <0.001 |
| Hypertension | 201/214 (93.9) | 218/233 (93.6) | 52/56 (92.9) | 0.931 |
| Renal disease | 55/214 (25.7) | 99/233 (42.5) | 23/56 (41.1) | <0.001 |
| Hemodialysis | 9/55 (16.4) | 20/99 (20.2) | 10/23 (43.5) | 0.033 |
| Coronary artery disease | 132/214 (61.7) | 157/233 (67.4) | 28/56 (50.0) | 0.047 |
| Myocardial infarction | 46/214 (21.5) | 66/233 (28.3) | 9/56 (16.1) | 0.085 |
| Stroke/TIA | 39/214 (18.2) | 36/233 (15.5) | 4/56 (7.1) | 0.112 |
| Runoff vesselsc | 179 | 215 | 47 | 0.141 |
| Pretreatment | ||||
| 3 | 33/179 (18.4) | 32/215 (14.9) | 8/47 (17.0) | 0.654 |
| 2 | 69/179 (38.5) | 82/215 (38.1) | 18/47 (38.3) | >0.99 |
| 1 | 67/179 (37.4) | 83/215 (38.6) | 12/47 (25.5) | 0.232 |
| 0 | 10/179 (5.6) | 18/215 (8.4) | 9/47 (19.1) | 0.018 |
| Posttreatment | 0.96 | |||
| 3 | 32/147 (21.8) | 38/181 (21.0) | 7/36 (19.4) | 0.982 |
| 2 | 61/147 (41.5) | 80/181 (44.2) | 14/36 (38.9) | 0.785 |
| 1 | 51/147 (34.7) | 61/181 (33.7) | 15/36 (41.7) | 0.653 |
| 0 | 3/147 (2.0) | 2/181 (1.1) | 0/36 | 0.795 |
| Previous EVT of target limb | 58/214 (27.1) | 71/233 (30.5) | 14/56 (25.0) | 0.172 |
| Previous bypass surgery of target limb | 9/214 (4.2) | 9/233 (3.9) | 4/56 (7.1) | 0.397 |
| Prior stent placed in target limb | 31/214 (14.5) | 28/233 (12.0) | 7/56 (12.5) | 0.889 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; EVT, endovascular therapy; ICU, intensive care unit; RC, Rutherford category; TIA, transient ischemic attack.
Continuous data are presented as the mean ± standard deviation (sample size if different from the group number); categorical data are given as the number/sample (percentage). Sample sizes may differ from the group number owing to missing data.
Two patients (with a total of 3 wounds) were classified as RC 2-3 by the treating physician and therefore were analyzed with that group.
As determined by the core laboratory.
Table 2.
Baseline Lesion Characteristics.a
| Characteristics | RC 2-3: Claudicants (214 patients, 251 lesions) | RC 4-5: CLTI (233 patients, 289 lesions) | RC 6: CLTI (56 patients, 77 lesions) | p |
|---|---|---|---|---|
| Lesion location ATK or BTK | <0.001 | |||
| ATK only | 111/251 (44.2) | 71/289 (24.6) | 19/77 (24.7) | <0.001 |
| ATK and BTK | 32/251 (12.7) | 44/289 (15.2) | 6/77 (7.8) | 0.225 |
| BTK only | 108/251 (43.0) | 174/289 (60.2) | 52/77 (67.5) | <0.001 |
| Lesion location | <0.001 | |||
| SFA only | 30/251 (12.0) | 5/289 (1.7) | 2/77 (2.6) | <0.001 |
| SFA to popliteal | 35/251 (13.9) | 31/289 (10.7) | 8/77 (10.4) | 0.489 |
| Popliteal only | 46/251 (18.3) | 35/289 (12.1) | 9/77 (11.7) | 0.106 |
| SFA to BTK | 0/0 | 12/289 (4.2) | 1/77 (1.3) | 0.001 |
| Popliteal to BTK | 32/251 (12.7) | 32/289 (11.1) | 5/77 (6.5) | 0.315 |
| Lesion length, mm | 78.7±73.7 (n=240) | 131.4±119.0 (n=273) | 95.2±83.9 (n=73) | <0.001 |
| <40 | 94/240 (39.2) | 67/273 (24.5) | 25/73 (34.2) | 0.002 |
| 40–99 | 85/240 (35.4) | 80/273 (29.3) | 15/73 (20.5) | 0.047 |
| ≥100 | 61/240 (25.4) | 126/273 (46.2) | 33/73 (45.2) | <0.001 |
| Distal RVD, mm | 3.7±1.2 (n=243) | 3.2±1.2 (n=277) | 2.9±0.9 (n=74) | <0.001 |
| MLD, mm | 0.8±0.8 (n=245) | 0.7±0.9 (n=279) | 0.6±0.6 (n=76) | 0.019 |
| Stenosis, % | 77.9±19.4 (n=245) | 80.7±20.3 (n=280) | 81.6±17.9 (n=76) | 0.187 |
| Chronic total occlusion | 55/245 (22.4) | 100/280 (35.7) | 26/76 (34.2) | 0.004 |
| TASC type | <0.001 | |||
| A | 169/246 (68.7) | 141/278 (50.7) | 39/73 (53.4) | <0.001 |
| B | 38/246 (15.4) | 41/278 (14.7) | 21/73 (28.8) | 0.02 |
| C | 26/246 (10.6) | 61/278 (21.9) | 8/73 (11.0) | 0.001 |
| D | 13/246 (5.3) | 35/278 (12.6) | 5/73 (6.8) | 0.012 |
| Predominantly calcified plaque | 168/232 (72.4) | 176/261 (67.4) | 46/72 (63.9) | 0.286 |
| PARC stenosis | 0.043 | |||
| Mild | 23/245 (9.4) | 21/280 (7.5) | 4/76 (5.3) | 0.506 |
| Moderate | 56/245 (22.9) | 56/280 (20.0) | 18/76 (23.7) | 0.634 |
| Severe | 111/245 (45.3) | 103/280 (36.8) | 28/76 (36.8) | 0.117 |
| Occluded | 55/245 (22.4) | 100/280 (35.7) | 26/76 (34.2) | 0.002 |
| PARC concentric calcification | 0.069 | |||
| Focal | 17/220 (7.7) | 19/244 (7.8) | 2/67 (3.0) | 0.4 |
| Mild | 47/220 (21.4) | 28/244 (11.5) | 6/67 (9.0) | 0.005 |
| Moderate | 35/220 (15.9) | 42/244 (17.2) | 15/67 (22.4) | 0.45 |
| Severe | 57/220 (25.9) | 70/244 (28.7) | 18/67 (26.9) | 0.79 |
| Inflow vessel disease (>50% stenosis) | 83/214 (38.8) | 70/233 (30.0) | 37/56 (66.1) | <0.001 |
Abbreviations: ATK, above the knee; BTK, below the knee; MLD, minimum lumen diameter; PARC, Peripheral Academic Research Consortium; RC, Rutherford category; RVD, reference vessel diameter; SFA, superficial femoral artery; TASC, TransAtlantic Inter-Society Consensus.
Continuous data are presented as the mean ± standard deviation (sample size if different from the group number); categorical data are given as the number/sample (percentage). Sample sizes may differ from the group number owing to missing data.
Study Outcomes
Primary outcomes were lesion success and the incidence of major adverse events (MAEs). Lesion success was assessed by the angiographic core laboratory as <50% residual stenosis without significant angiographic complications [ie, flow-limiting dissection (types C-F), perforation, distal embolization, abrupt closure]. MAEs were defined as death within 30 days of the primary procedure, unplanned major amputation of the target limb, or clinically-driven target vessel revascularization (CD-TVR) as assessed by the angiographic core laboratory when angiographic images were available.
Secondary endpoints were TVR, death, major amputation, death or major amputation combined, and wound healing up to 3 years of follow-up. A wound was defined as healed at the follow-up visit if the area was reduced to zero, including cases where amputation occurred and the surgical site completely healed. The type of wound (eg, ischemic, neurogenic) was not captured; involvement of a wound care specialist was reported only when the wound was located on the target limb. No adjustments were made for missing data. The ankle-brachial index (ABI), toe-brachial index (TBI), and RC were also assessed during follow-up; however, as the 3-year evaluation was a phone visit, these measures could be assessed only up to 2 years.
Statistical Analysis
Descriptive statistics were used for baseline and lesion characteristics. All summary data are based on non-missing assessments. Categorical variables are presented as the number per sample (percentage) and were compared with Monte Carlo approximation of the Fisher exact test. Continuous data are presented as mean ± standard deviation and were compared using the analysis of variance or paired t test. Discrete data were compared with the Kruskal-Wallis test; paired data were evaluated using the Wilcoxon signed rank test. Significant angiographic complications for procedural and lesion success outcomes were imputed using site data when the core laboratory was unable to perform angiographic assessment.
Cox regression models (RC 2-3 vs RC 4-5 vs RC 6) were synthesized for MAE, death, major amputation, and major amputation or death combined in up to 36 months of follow-up. Results are presented as the hazard ratio (HR) with a 95% confidence interval (CI). Kaplan-Meier survival curves were plotted for primary and secondary outcomes. A p<0.05 was considered statistically significant for all tests. Statistical analyses were conducted by NAMSA (Northwood, OH, USA).
Results
Comparison of Patient and Lesion Characteristics
Patients with CLTI (RC 4-6) had significantly lower estimated glomerular filtration rates at baseline compared with claudicants. The RC 2-3 group included more patients with smoking history than the RC 4-5 group (p=0.009). Moreover, patients with CLTI (RC 4-5: 67.4% and RC 6: 78.6%) were more likely to be diabetic compared to patients with claudication (RC 2-3: 50.9%).
At baseline, RC 4-5 [22/233 (9.4%)] and RC 6 [8/56 (14.3%)] patients more often had noncompressible vessels than RC 2-3 patients [10/214 (4.7%)], so ABI was measurable in fewer RC 4-5 (p=0.004) and RC 6 cases (p=0.002) compared with RC 2-3. Therefore, at baseline the average ABI of the target limb was similar among all 3 groups (RC 2-3: 0.84±0.26; RC 4-5: 0.86±0.29; RC 6: 0.86±0.32), whereas the average TBI at baseline was significantly worse (RC 2-3 vs RC 6: p=0.005; RC 4-5 vs RC 6: p=0.037) in patients with more severe disease (RC 2-3: 0.54±0.22; RC 4-5: 0.52±0.26; RC 6: 0.41±0.19).
Overall, patients with CLTI had longer average target lesion lengths, smaller mean reference vessel diameters, and more stenosed lesions, with a mean minimum lumen diameter at the target lesion of 0.6±0.6 mm for the RC 6 vs 0.8±0.8 mm for the RC 2-3 vs 0.7±0.9 mm for the RC 4-5 groups. In addition, chronic total occlusions were more often observed among patients with CLTI, without any difference between the RC 4-5 and RC 6 groups. Inflow vessel disease (>50% stenosis) was also more prevalent among RC 6 cases. Predominately calcified lesions were observed in 390 of 565 lesions (69.0%), without any difference between the groups (p=0.286).
Procedure Characteristics
Most endovascular procedures were performed through a contralateral retrograde access and utilized an antegrade crossing approach (Table 3). All patients treated with OA were included; details regarding the crown size of the atherectomy devices are presented in Supplementary Table 1 (available in the online version of the article). After OA, balloon angioplasty was used in >98% of cases, with bailout stenting necessary in 2.0%, 2.8%, and 0% of the RC 2-3, RC 4-5, and RC 6 groups, respectively.
Table 3.
Procedure Characteristics and Device Use.a
| Characteristics | RC 2-3: Claudicants (214 patients, 251 lesions) | RC 4-5: CLTI (233 patients, 289 lesions) | RC 6: CLTI (56 patients, 77 lesions) | p |
|---|---|---|---|---|
| Access site | 0.005 | |||
| Femoral | 254/267 (95.1) | 278/307 (90.6) | 76/81 (93.8) | 0.106 |
| Popliteal | 1/267 (0.4) | 0/307 | 1/81 (1.2) | 0.113 |
| Tibial | 11/267 (4.1) | 29/307 (9.4) | 2/81 (2.5) | 0.012 |
| Pedal | 5/267 (1.9) | 15/307 (4.9) | 3/81 (3.7) | 0.153 |
| Approach | 0.014 | |||
| Ipsilateral | 71/267 (26.6) | 96/307 (31.3) | 17/81 (21.0) | 0.155 |
| Contralateral | 192/267 (71.9) | 194/307 (63.2) | 62/81 (76.5) | 0.02 |
| Dual access | 4/267 (1.5) | 17/307 (5.5) | 2/81 (2.5) | 0.026 |
| Access site position relative to lesion | 0.007 | |||
| Antegrade | 248/267 (92.9) | 257/307 (83.7) | 75/81 (92.6) | 0.001 |
| Retrograde | 15/267 (5.6) | 33/307 (10.7) | 4/81 (4.9) | 0.049 |
| Dual access | 4/267 (1.5) | 17/307 (5.5) | 2/81 (2.5) | 0.025 |
| Inflow treatment in target limb | 44/151 (29.1) | 24/136 (17.6) | 10/38 (26.3) | 0.065 |
| Target lesions per subjectb | 1.2±0.4 (n=214) | 1.2±0.5 (n=233) | 1.4±0.7 (n=56) | 0.04 |
| Devices used per subject | 2.8±1.3 (n=214) | 3.2±1.9 (n=233) | 3.1±1.8 (n=56) | 0.151 |
| Balloons used in target lesions | 242/245 (98.8) | 277/283 (97.9) | 73/73 (100) | 0.509 |
| BA | 203/245 (82.9) | 241/283 (85.2) | 43/73 (58.9) | <0.001 |
| DCB | 22/245 (9.0) | 12/283 (4.2) | 5/73 (6.8) | 0.074 |
| Cutting | 15/245 (6.1) | 18/283 (6.4) | 16/73 (21.9) | <0.001 |
| Focal force | 33/245 (13.5) | 41/283 (14.5) | 18/73 (24.7) | 0.069 |
| Scoring | 2/245 (0.8) | 1/283 (0.4) | 1/73 (1.4) | 0.352 |
| Maximum nominal balloon diameter, mm | 4.2±1.3 (n=242) | 3.8±1.2 (n=277) | 3.7±1.2 (n=73) | <0.001 |
| Maximum balloon length, mm | 105.7±68.7 (n=242) | 140.3±74.2 (n=277) | 115.1±64.4 (n=73) | <0.001 |
| Bailout stenting | 5/245 (2.0) | 8/283 (2.8) | 0/73 | 0.454 |
| Lesions treated with atherectomy | 245/245 (100) | 283/283 (100) | 73/73 (100) | - |
| Lesions treated with stent | 26/245 (10.6) | 26/283 (9.2) | 13/73 (17.8) | 0.124 |
| DES | 10/245 (4.1) | 8/283 (2.8) | 5/73 (6.8) | 0.238 |
| BMS | 17/245 (6.9) | 17/283 (6.0) | 8/73 (11.0) | 0.324 |
| Covered | 0/245 | 2/283 (0.7) | 0/73 | 0.613 |
| Maximum stent diameter, mm | 6.1±1.0 (n=27) | 5.1±1.3 (n=26) | 5.2±0.8 (n=13) | 0.003 |
| Maximum stent length, mm | 97.7±39.3 (n=27) | 85.7±42.2 (n=26) | 87.5±36.1 (n=13) | 0.52 |
| Postprocedure MLD, mm | 2.8±1.2 (n=241) | 2.3±1.2 (n=269) | 2.2±1.1 (n=72) | <0.001 |
| Acute MLD gain, mm | 2.0±1.1 (n=238) | 1.6±1.0 (n=268) | 1.6±0.9 (n=72) | <0.001 |
| Postprocedure stenosis, % | 29.9±16.1 (n=241) | 33.6±20.1 (n=269) | 29.8±18.8 (n=72) | 0.054 |
| Procedure time, min | 66.7±39.3 (n=213) | 76.5±39.8 (n=232) | 70.8±44.6 (n=56) | 0.037 |
| Fluoroscopy time, min | 22.3±17.0 (n=213) | 25.9±17.3 (n=231) | 22.1±19.7 (n=56) | 0.072 |
| Contrast volume, mL | 157.1±75.9 (n=214) | 161.7±95.1 (n=230) | 123.6±79.4 (n=56) | 0.011 |
| Antiplatelet therapy at discharge | 205/214 (95.8) | 220/233 (94.4) | 44/56 (78.6) | <0.001 |
| Aspirin | 173/214 (80.8) | 189/233 (81.1) | 38/56 (67.9) | 0.093 |
| Clopidogrel | 156/214 (72.9) | 183/233 (78.5) | 31/56 (55.4) | 0.003 |
| Prasugrel | 4/214 (1.9) | 12/233 (5.2) | 1/56 (1.8) | 0.137 |
| Other | 16/214 (7.5) | 10/233 (4.3) | 0/56 | 0.051 |
| Dual | 139/214 (65.0) | 166/233 (71.2) | 26/56 (46.4) | 0.002 |
| Anticoagulants at discharge | 18/214 (8.4) | 26/233 (11.2) | 4/56 (7.1) | 0.523 |
| Warfarin | 9/214 (4.2) | 14/233 (6.0) | 3/56 (5.4) | 0.681 |
| Other | 9/214 (4.2) | 12/233 (5.2) | 1/56 (1.8) | 0.634 |
| Antihyperlipidemic drugs at discharge | 176/214 (82.2) | 196/233 (84.1) | 39/56 (69.6) | 0.054 |
| Antihypertensive drugs at discharge | 197/214 (92.1) | 213/233 (91.4) | 50/56 (89.3) | 0.779 |
| Hospitalization | 61/214 (28.5) | 108/233 (46.4) | 31/56 (55.4) | <0.001 |
| ICU admission | 13/61 (21.3) | 9/108 (8.3) | 7/31 (22.6) | 0.025 |
| Length of hospital stay, h | 13.3±14.1 (n=213) | 33.9±72.8 (n=233) | 99.0±151.0 (n=55) | <0.001 |
Abbreviations: BA, plain balloon angioplasty; BMS, bare metal stents; DCB, drug-coated balloon; DES, drug-eluting stents; MLD, minimum lumen diameter; RC, Rutherford category.
Continuous data are presented as the mean ± standard deviation (sample size if different from the group number); categorical data are given as the number/sample (percentage). Sample sizes may differ from the group number owing to missing data.
As determined by the core laboratory.
Core laboratory–adjudicated data regarding stent or balloon utilization were available for 245 RC 2-3, 283 RC 4-5, and 73 RC 6 cases. Conventional balloons were used in the majority of cases in all groups (Table 3). Stents, predominately bare metal models, were used in 27 RC 2-3 cases (10.6%), 27 RC 4-5 (9.2%), and 13 RC 6 cases (17.8%). Drug-eluting technology [drug-coated balloons (DCB) or drug-eluting stents (DES)] was used infrequently but were equally utilized between the 3 groups. The mean procedure durations were longer for patients with CLTI, while the overall fluoroscopy times were not different between the 3 groups.
Periprocedural Complications
Overall, angiographic complications (ie, dissection, perforation, distal embolization, abrupt closure) occurred in 65 of 609 lesions (10.7%), without any differences between the 3 groups (Table 4). Target lesion success was 83.8% (201/240) in the RC 2-3 group vs 80.8% (219/271) in the RC 4-5 or 78.9% (56/71) in the RC 6 group (p=0.551). The hospitalization and admission to the intensive care unit (ICU) rates were higher among patients with CLTI and RC 6 at baseline, with a mean 99.0±151.0 hours of hospitalization (p<0.001).
Table 4.
Complications and In-Hospital Outcomes.a
| Characteristics | RC 2-3: Claudicants (214 patients, 251 lesions) | RC 4-5: CLTI (233 patients, 289 lesions) | RC 6: CLTI (56 patients, 77 lesions) | p |
|---|---|---|---|---|
| Procedure successb per patient | 170/205 (82.9) | 169/213 (79.3) | 38/50 (76.0) | 0.434 |
| Lesion successb | 201/240 (83.8) | 219/271 (80.8) | 56/71 (78.9) | 0.551 |
| Severe angiographic complications (per lesion) | 23/248 (9.3) | 32/287 (11.1) | 10/74 (13.5) | 0.669 |
| Severe dissection (types C-F) | 4/251 (1.6) | 7/289 (2.4) | 2/77 (2.6) | 0.741 |
| Perforation | 3/251 (1.2) | 6/289 (2.1) | 1/77 (1.3) | 0.707 |
| Distal embolization | 16/247 (6.5) | 16/287 (5.6) | 6/74 (8.1) | 0.487 |
| Abrupt closure | 1/251 (0.4) | 7/289 (2.4) | 1/77 (1.3) | 0.115 |
| MAE | 0/211 | 0/227 | 3/55 (5.5) | 0.001 |
| Death | 0/211 | 0/227 | 2/55 (3.6) | 0.015 |
| Major amputation | 0/211 | 0/227 | 1/55 (1.8) | 0.109 |
| TVR | 0/211 | 0/227 | 0/55 |
Abbreviations: MAE, major adverse events; RC, Rutherford category; TVR, target vessel revascularization.
Data are given as the number/sample (percentage). Sample sizes may differ from the group number owing to missing data.
Success defined as <50% residual stenosis, without significant angiographic complications.
No in-hospital MAE, death, major amputation, or TVR occurred for the RC 2-3 and RC 4-5 groups. Among patients with RC 6 at baseline, 3 experienced in-hospital MAEs: 2 died and one underwent major amputation during hospitalization. Claudicants were at lower risk for 30-day MAE compared to patients with CLTI (RC 2-3 vs RC 4-5: HR 0.24, 95% CI 0.05 to 1.11, p=0.068; RC 2-3 vs RC 6: HR 0.10, 95% CI 0.02 to 0.53, p=0.006); however, MAE rates between RC 4-5 and RC 6 groups did not differ significantly (HR 0.42, 95% CI 0.14 to 1.25, p=0.119). No differences were observed between the groups in terms of 30-day death, major amputation, TVR, or combined major amputation/death risk rates. Odds ratios for periprocedural complications and short-term outcomes are illustrated in Supplementary Table 2.
Outcomes in Follow-up
At 30 days, the mean ABI of each group was improved compared to the corresponding preprocedural values (mean change from baseline: RC 2-3: 0.16±0.25; RC 4-5: 0.12±0.32; and RC 6: 0.12±0.29); however, no difference was observed between groups (RC 2-3: 0.99±0.24 vs RC 4-5: 0.97±0.30 vs RC 6: 0.94±0.21; p=0.565). Nonetheless, at 6 months, the ABI values remained similar between the groups (RC 2-3: 0.93±0.25; RC 4-5: 0.94±0.31; RC 6: 0.83±0.28). However, at 12 months, the mean ABI of the RC 6 group (0.77±0.32) was significantly worse than the values of the RC 2-3 (0.97±0.29; p=0.005) and RC 4-5 groups (0.95±0.30; p=0.017). The average ABI remained similar among the RC 2-3 (0.97±0.25) and RC 4-5 (0.91±0.25) groups during 2 years of follow-up (p=0.078).
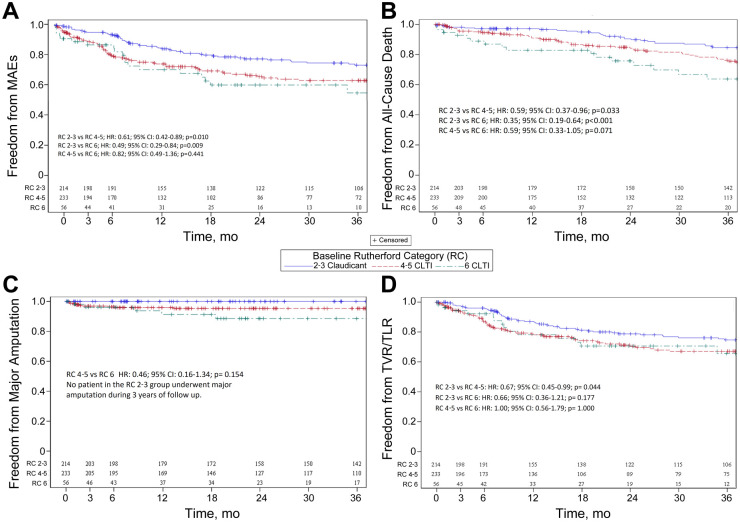
At 3 years’ follow-up, claudicants were at significantly lower risk for any MAE compared to CLTI patients (RC 2-3 vs RC 4-5: HR 0.61, 95% CI 0.42 to 0.89, p=0.010; RC 2-3 vs RC 6: HR 0.49, 95% CI 0.29 to 0.84, p=0.009). Nonetheless, the RC at baseline was not associated with MAE risk among patients with CLTI at 3-year follow-up (RC 4-5 vs RC 6: HR 0.82, 95% CI 0.49 to 1.36, p=0.441). The 3-year Kaplan-Meier estimates of freedom from MAE (Figure 1A) were 74.1% (95% CI 67.6% to 80.6%), 64.3% (95% CI 57.1% to 71.4%), and 56.3% (95% CI 40.0% to 72.5%) for the RC 2-3, RC 4-5, and RC 6 groups, respectively. Moreover, patients among the RC 2-3 group had a lower risk for all-cause death during 3 years of follow-up (RC 2-3 vs RC 4-5: HR 0.59, 95% CI 0.37 to 0.96, p=0.033; RC 2-3 vs RC 6: HR 0.35, 95% CI 0.19 to 0.64, p<0.001).
Figure 1.
Kaplan-Meier estimates of freedom from (A) major adverse events (MAEs), (B) all-cause death, (C) major amputation, and (D) target vessel/limb revascularization (TVR/TLR). CLTI, chronic limb-threatening ischemia.
An analysis of CLTI patients demonstrated that there was no significant difference between RC 4-5 and RC 6 patients in terms of late mortality, but patients with RC 6 had a strong trend toward higher risk for all-cause death (RC 4-5 vs RC 6: HR 0.59, 95% CI 0.33 to 1.05, p=0.071). The 3-year Kaplan-Meier survival estimates (Figure 1B) were 84.6% (95% CI 79.3% to 89.9%) for the RC 2-3 group, 76.2% (95% CI 69.9% to 82.6%) for the RC 4-5, and 63.7% (95% CI 48.9% to 78.6%) for the RC 6 group. Furthermore, no statistically significant difference was detected in terms of major amputation risk at 3 years in RC 4-5 vs RC 6 (HR 0.46, 95% CI 0.16 to 1.34, p=0.154). No patient in the RC 2-3 group underwent major amputation during 3 years of follow-up. The 3-year freedom from amputation estimates (Figure 1C) were 100%, 95.3% (95% CI 92.5% to 98.2%), and 88.6% (95% CI 79.1% to 98.1%) for the RC 2-3, RC 4-5, and RC 6 groups, respectively. However, the risk for the combined outcome of major amputation/death was significantly higher among the RC 6 group compared with RC 2-3 (HR 0.26, 95% CI 0.14 to 0.46, p<0.001) and RC 4-5 groups (HR 0.55, 95% CI 0.33 to 0.92, p=0.024). Patients with RC 4-5 at baseline were also at higher risk for major amputation or death at the end of follow-up compared to claudicants (RC 2-3 vs RC 4-5: HR 0.48, 95% CI 0.31 to 0.77, p=0.002).
The 3-year Kaplan-Meier estimates of freedom from major amputation/death were 84.6% (95% CI 79.3% to 89.9%) for the RC 2-3 group, 72.3% (95% CI 65.8% to 78.9%) for the RC 4-5 group, and 56.5% (95% CI 41.5% to 71.6%) for the RC 6 group. TVR was less frequently required among patients with RC 2-3 compared to patients with RC 4-5 (HR 0.67, 95% CI 0.45 to 0.99, p=0.044). Interestingly, no difference in the risk for TVR was observed between the RC 6 and other groups during 3 years of follow-up (Figure 1D).
Wound Healing
At 6 months, 5 of 174 RC 2-3 (2.9%), 35 of 172 RC 4-5 (20.3%), and 10 of 32 RC 6 (31.3%) patients were seeing a wound care specialist. At 6 months, wounds identified at baseline on the target limb had completely healed in two-thirds of RC 2-3 (66.7%), 51 of 71 RC 4-5 (65.4%), and 18 of 31 RC 6 (58.1%).
At 12 months, 3 of 165 RC 2-3 patients (1.8%), 32 of 152 RC 4-5 patients (21.1%), and 5 of 22 RC 6 patients (22.7%) were seeing a wound care specialist. At this time, wounds identified at baseline on the target limb had completely healed in 1 of 1 (100%), 17 of 27 (63.0%), and 9 of 12 (75.0%), respectively.
At 24 months, 2 of 136 RC 2-3 patients (1.5%), 12 of 112 RC 4-5 patients (10.7%), and 1 of 19 RC 6 patients (5.3%) were seeing a wound care specialist. At 24 months, wounds identified at baseline on the target limb had completely healed in 9 of 10 RC 4-5 (90%) and 2 of 2 RC 6 (100%).
Discussion
The evidence from this study supports peripheral BA with adjunctive OA in patients with CLTI or intermittent claudication as demonstrated by the low rates of periprocedural complications, the high procedural success rates, and the low major amputation rates after 3 years of follow-up. BA compared with bypass surgery has demonstrated similar limb salvage rates with shorter hospital stay and fewer periprocedural complications, even when treating patients with advanced PAD.51,52 Therefore, endovascular intervention has been increasingly utilized for the treatment of severe claudication or CLTI.53 Expeditious and appropriate evaluation has led to an increase in revascularization rates and subsequent reduction in amputation rates.54,55
Although BA with or without bare metal stenting for femoropopliteal disease has had an acceptable safety profile, the rates of restenosis are still considerable in CLTI patients.56–58 Modification of calcified plaque and vessel preparation prior to endovascular intervention may decrease the risk of periprocedural complications, improve success rates, and achieve sustained patency over time.25,41,48,59 The use of OA has been associated with improved angioplasty results, with low bailout stenting rates,31,60 and has provided similar clinical outcomes (ie, dissection, slow-flow, vessel spasm, distal embolization) in patients with higher RC status at baseline and complex lesions vs patients with milder disease.33,34,60,61 This system employs a 360° rotational device with a diamond-coated crown that orbits eccentrically within the vessel.43 This circumferential calcium removal by OA has been hypothesized to improve vessel compliance, facilitating angioplasty and as such leading to favorable periprocedural outcomes (ie, lower rates of complications, better vessel expansion).46,47
The Orbital Atherectomy System for the Treatment of Peripheral Vascular Stenosis (OASIS) study, the first-in-human study investigating the outcomes of OA in infrapopliteal lesions, showed 90% procedural success and 97.4% freedom from TLR at 6 months of follow-up.45 A prospective analysis of 3135 patients from the CONFIRM registry demonstrated that OA followed by BA performed at lower pressures was safe and was associated with a low 3.8% to 5.8% provisional stent rate.62 In our real-world study, with most lesions being isolated calcified infrapopliteal lesions, the periprocedural complication rate was low in all 3 groups despite the unfavorable baseline and lesion characteristics of the 2 CLTI groups; bailout stenting was required in only 2.2% of all lesions. Thus, this study suggested that OA is a safe and effective modality in all RC populations, significantly decreasing the plaque burden of any type of atherosclerotic lesion in patients with any degree of PAD severity. Similarly, the Comparison of Orbital Atherectomy Plus Balloon Angioplasty vs Balloon Angioplasty Alone in Patients with Critical Limb Ischemia (CALCIUM 360°) trial, comparing the outcomes of BA + OA vs BA alone, showed fewer periprocedural complications in the combination group than the BA only arm, indicating that OA not only improves the result of an endovascular intervention but also minimizes the risk for any complications.31
Up to 10% of patients with PAD have CLTI, and 5% to 10% of patients with intermittent claudication or mild asymptomatic PAD will eventually progress to CLTI after 5 years.11,12 Thus, delay of intervention may lead to PAD progression and the development of diffuse, difficult to treat lesions (ie, CLTI) and overall poor prognosis.63 This study showed 100% 3-year freedom from amputation when utilizing BA with adjunctive OA for RC 2-3 patients, indicating that early treatment of claudication with BA + OA might benefit this population by obtaining desirable angioplasty results; however, future prospective studies are needed to evaluate these findings.
CLTI has been associated with 20% 1-year all-cause mortality and 20% 1-year limb loss,24 while several studies have reported up to a 50% incidence of major amputation or death within the first year of diagnosis.16,17 In this study utilizing OA prior to BA, the 1-year major amputation rates (RC 2-3: 0%; RC 4-5: 4.1%; RC 6: 8.7%) were significantly improved relative to historical data, indicating that OA is safe and effective regardless of PAD severity.
Furthermore, the CALCIUM 360° randomized trial demonstrated that the combination of OA and BA was superior to BA alone when treating patients with CLTI.31 The combined arm of the study demonstrated 93.3% 1-year freedom from TLR/TVR, 100% 1-year survival, and 93.3% 1-year freedom from MAE.31 In contrast, in our study all 3 groups demonstrated lower freedom from MAE and from TVR/TLR at 1 year. The small sample size of the CALCIUM 360° study and the differences in baseline characteristics between the populations might have contributed to this discrepancy. However, in our study, combined therapy with BA + OA among CLTI patients still had acceptable survival rates (RC 4-5 76.2% and RC 6 63.7%) and very low amputation rates (4.7% and 11.4%, respectively) after 3 years. The similar risk for death or major amputation at 3 years between the 2 CLTI groups suggested that BA + OA can be effective, with acceptable hemodynamic improvement, even in difficult to treat RC 6 subjects, significantly minimizing the need for amputation in this high-risk subgroup. Additionally, the numbers of healed wounds on the target limb within the first 2 years after the primary procedure were encouraging, indicating that a good angioplasty result with adjuvant OA might contribute to better wound healing. Further research should investigate whether optimal vessel preparation with OA might lead to similar outcomes between RC 4-5 and RC 6 patients, validating our results.
Limitations
The LIBERTY study was a multicenter, core laboratory–adjudicated study that included patients who are typically excluded from large clinical trials. However, our results should be interpreted in the context of several limitations. First, the LIBERTY study was an observational nonrandomized study of endovascular therapies.49 Second, site and patient participation bias may have occurred because not all patients treated for PAD were enrolled. Furthermore, the operator’s choice regarding the most optimal therapeutic approach (eg, stenting vs BA, antithrombotic therapy, statin therapy) and the treatment or not of postangioplasty dissections might have affected the outcomes. Thereby, future studies with adequate follow-up should determine the role of statin use and optimized medical therapy (eg, antiplatelet coverage) in preventing limb ischemic events and cardiovascular mortality.
Third, it should be noted that the combined outcome of major amputation/death was significantly different between RC 4-5 and RC 6 patients, with RC 4-5 patients being at lower risk for this endpoint during follow-up, although separate analyses for death and major amputation did not show any difference between RC 4-5 and RC 6. Thus, our study might be underpowered in order to demonstrate differences in the outcomes of BA treatment between RC 6 vs RC 4-5 groups.
Moreover, at the time of patient enrollment, drug-eluting technology was not widely applied; therefore, the different rates of DCB angioplasty among the groups should be taken into account when interpreting the results of this study. Additionally, significant inflow and outflow disease (>50% stenosis) was treated at the discretion of the operator, which might have affected the outcomes. Last, it should be noted that although the lesion location exhibited high heterogeneity, with below-the-knee lesions being more prevalent among CLTI patients, sensitivity analyses for lesions limited to the infrapopliteal or femoropopliteal segment could not be synthesized.
Conclusion
The use of OA, adjunctive to BA, has been associated with improved angioplasty results, with low bailout stenting rates, and has provided similar periprocedural complications (ie, dissection, perforation, distal embolization) regardless of PAD severity. Claudication was associated with lower risk of adverse events during a 3-year follow-up among patients undergoing endovascular revascularization with adjuvant OA. Among CLTI patients, the RC at presentation was correlated only with the combined outcome of major amputation or death; no differences were identified between RC 4-5 vs RC 6 in terms of late all-cause death, MAE, TVR, or major amputation. Further studies should validate our results and investigate whether early combined BA + OA of patients with claudication is associated with improved overall prognosis. Additional studies should identify if there is any difference among patients with higher RC classifications at baseline and complex lesions vs patients with milder disease.
Supplemental Material
Supplemental material, 19-0584_supplementary_materals for Three-Year Outcomes of Orbital Atherectomy for the Endovascular Treatment of Infrainguinal Claudication or Chronic Limb-Threatening Ischemia by Stefanos Giannopoulos, Eric A. Secemsky, Jihad A. Mustapha, George Adams, Robert E. Beasley, George Pliagas and Ehrin J. Armstrong in Journal of Endovascular Therapy
Acknowledgments
The authors thank Ann Behrens, BS, and Brad J. Martinsen, PhD, of Cardiovascular Systems, Inc, for editing and critical review of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Eric A. Secemsky reports grants and modest consulting fees given by Medtronic. Jihad A. Mustapha is a consultant to Bard Peripheral Vascular, Boston Scientific, Cardiovascular Systems Inc (CSI), Medtronic, Spectranetics, and Terumo. George Pliagas is a consultant to Cook, Philips, CSI, and Medtronic. Ehrin J. Armstrong is a consultant to Abbott Vascular, Boston Scientific, CSI, Medtronic, Philips, and PQ Bypass.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cardiovascular Systems, Inc.
ORCID iDs: Stefanos Giannopoulos  https://orcid.org/0000-0002-1942-911X
https://orcid.org/0000-0002-1942-911X
Ehrin J. Armstrong  https://orcid.org/0000-0002-1381-4754
https://orcid.org/0000-0002-1381-4754
Supplementary Material: The online materials are available at http://journals.sagepub.com/doi/suppl/10.1177/1526602820935611
References
- 1. Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet. 2013;382:1312–1314. [DOI] [PubMed] [Google Scholar]
- 4. Subherwal S, Patel MR, Kober L, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22:317–325. [DOI] [PubMed] [Google Scholar]
- 5. McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002;17: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen DC, Singh GD, Armstrong EJ, et al. Long-term comparative outcomes of patients with peripheral artery disease with and without concomitant coronary artery disease. Am J Cardiol. 2017;119:1146–1152. [DOI] [PubMed] [Google Scholar]
- 7. Shammas AN, Jeon-Slaughter H, Tsai S, et al. Major limb outcomes following lower extremity endovascular revascularization in patients with and without diabetes mellitus. J Endovasc Ther. 2017;24:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong EJ, Chen DC, Westin GG, et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3:e000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 11. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 12. Levin SR, Arinze N, Siracuse JJ. Lower extremity critical limb ischemia: a review of clinical features and management. Trends Cardiovasc Med. 2020;30:125–130. [DOI] [PubMed] [Google Scholar]
- 13. Becker F, Robert-Ebadi H, Ricco JB, et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur J Vasc Endovasc Surg. 2011;42(suppl 2):S4–S12. [DOI] [PubMed] [Google Scholar]
- 14. Conte SM, Vale PR. Peripheral arterial disease. Heart Lung Circ. 2018;27:427–432. [DOI] [PubMed] [Google Scholar]
- 15. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. [DOI] [PubMed] [Google Scholar]
- 16. TASC Steering Committee, Jaff MR, White CJ, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Endovasc Ther. 2015;22:663–677. [DOI] [PubMed] [Google Scholar]
- 17. Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 18. Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005-2008. Prev Chronic Dis. 2011;8:A141. [PMC free article] [PubMed] [Google Scholar]
- 19. Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. [DOI] [PubMed] [Google Scholar]
- 20. Allie DE, Hebert CJ, Lirtzman MD, et al. Critical limb ischemia: a global epidemic. A critical analysis of current treatment unmasks the clinical and economic costs of CLTI. EuroIntervention. 2005;1:75–84. [PubMed] [Google Scholar]
- 21. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 22. Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J. 2014;167:489–498.e7. [DOI] [PubMed] [Google Scholar]
- 23. Utsunomiya M, Takahara M, Iida O, et al. Limb-based patency after surgical vs endovascular revascularization in patients with chronic limb-threatening ischemia [published online May 20, 2020]. J Endovasc Ther. doi: 10.1177/1526602820923388 [DOI] [PubMed] [Google Scholar]
- 24. Swaminathan A, Vemulapalli S, Patel MR, et al. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag. 2014;10: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64–70. [DOI] [PubMed] [Google Scholar]
- 26. Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37:898–907. [DOI] [PubMed] [Google Scholar]
- 27. Bishop PD, Feiten LE, Ouriel K, et al. Arterial calcification increases in distal arteries in patients with peripheral arterial disease. Ann Vasc Surg. 2008;22:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halwani DO, Anderson PG, Brott BC, et al. The role of vascular calcification in inducing fatigue and fracture of coronary stents. J Biomed Mater Res B Appl Biomater. 2012;100:292–304. [DOI] [PubMed] [Google Scholar]
- 29. Schillinger M, Minar E. Percutaneous treatment of peripheral artery disease: novel techniques. Circulation. 2012;126:2433–2440. [DOI] [PubMed] [Google Scholar]
- 30. Adlakha S, Sheikh M, Wu J, et al. Stent fracture in the coronary and peripheral arteries. J Interv Cardiol. 2010;23:411–419. [DOI] [PubMed] [Google Scholar]
- 31. Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19:480–488. [DOI] [PubMed] [Google Scholar]
- 32. Mattesini A, Di Mario C. Calcium: a predictor of interventional treatment failure across all fields of cardiovascular medicine. Int J Cardiol. 2017;231:97–98. [DOI] [PubMed] [Google Scholar]
- 33. Lee MS, Beasley R, Adams GL. Impact of advanced age on procedural and acute angiographic outcomes in patients treated for peripheral artery disease with orbital atherectomy: a CONFIRM Registries subanalysis. J Invasive Cardiol. 2015;27:381–386. [PubMed] [Google Scholar]
- 34. Adams GL, Das T, Lee MS, et al. Subanalysis of the CONFIRM Registries: acute procedural outcomes in claudicant and critical limb ischemia patients with varying levels of calcification treated for peripheral arterial disease with orbital atherectomy. J Invasive Cardiol. 2015;27:516–520. [PubMed] [Google Scholar]
- 35. Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. [DOI] [PubMed] [Google Scholar]
- 36. Erbel R, Schmermund A. Clinical significance of coronary calcification. Arterioscler Thromb Vasc Biol. 2004;24:e172. [DOI] [PubMed] [Google Scholar]
- 37. Kim TI, Schneider PA. New innovations and devices in the management of chronic limb-threatening ischemia. J Endovasc Ther. 2020;27(4):524–539. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong EJ, Alam S, Henao S, et al. Multidisciplinary care for critical limb ischemia: current gaps and opportunities for improvement. J Endovasc Ther. 2019;26:199–212. [DOI] [PubMed] [Google Scholar]
- 39. Armstrong EJ, Shishehbor MH. Commentary: Contemporary outcomes of endovascular interventions for peripheral artery disease: the LIBERTY to determine optimal treatment strategies. J Endovasc Ther. 2019;26:155–157. [DOI] [PubMed] [Google Scholar]
- 40. Topfer LA, Spry C. New technologies for the treatment of peripheral artery disease. 2018 Apr 1. In: CADTH Issues in Emerging Health Technologies. Canadian Agency for Drugs and Technologies in Health; 2016, p172 Accessed June 1, 2020 https://www.ncbi.nlm.nih.gov/books/NBK519606/ [PubMed] [Google Scholar]
- 41. Foley TR, Cotter RP, Kokkinidis DG, et al. Mid-term outcomes of orbital atherectomy combined with drug-coated balloon angioplasty for treatment of femoropopliteal disease. Catheter Cardiovasc Interv. 2017;89:1078–1085. [DOI] [PubMed] [Google Scholar]
- 42. Li F, McDermott MM, Li D, et al. The association of lesion eccentricity with plaque morphology and components in the superficial femoral artery: a high-spatial-resolution, multi-contrast weighted CMR study. J Cardiovasc Magn Reson. 2010;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams GL, Khanna PK, Staniloae CS, et al. Optimal techniques with the Diamondback 360 degrees system achieve effective results for the treatment of peripheral arterial disease. J Cardiovasc Transl Res. 2011;4:220–229. [DOI] [PubMed] [Google Scholar]
- 44. Heuser RR. Treatment of lower extremity vascular disease: the Diamondback 360 degrees orbital atherectomy system. Expert Rev Med Devices. 2008;5:279–286. [DOI] [PubMed] [Google Scholar]
- 45. Safian RD, Niazi K, Runyon JP, et al. Orbital atherectomy for infrapopliteal disease: device concept and outcome data for the OASIS trial. Catheter Cardiovasc Interv. 2009;73:406–412. [DOI] [PubMed] [Google Scholar]
- 46. Korabathina R, Mody KP, Yu J, et al. Orbital atherectomy for symptomatic lower extremity disease. Catheter Cardiovasc Interv. 2010;76:326–332. [DOI] [PubMed] [Google Scholar]
- 47. Makam P. Use of orbital atherectomy treatment in a high-volume clinical practice modifies non-compliant plaque to deliver durable long-term results. J Invasive Cardiol. 2013;25:85–88. [PubMed] [Google Scholar]
- 48. Kokkinidis DG, Jawaid O, Cantu D, et al. Two-year outcomes of orbital atherectomy combined with drug-coated balloon angioplasty for treatment of heavily calcified femoropopliteal lesions. J Endovasc Ther. 2020;27:492–501. [DOI] [PubMed] [Google Scholar]
- 49. Mustapha J, Gray W, Martinsen BJ, et al. One-year results of the LIBERTY 360 study: evaluation of acute and midterm clinical outcomes of peripheral endovascular device interventions. J Endovasc Ther. 2019;26:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams GL, Mustapha J, Gray W, et al. The LIBERTY study: design of a prospective, observational, multicenter trial to evaluate the acute and long-term clinical and economic outcomes of real-world endovascular device interventions in treating peripheral artery disease. Am Heart J. 2016;174:14–21. [DOI] [PubMed] [Google Scholar]
- 51. Antoniou GA, Georgiadis GS, Antoniou SA, et al. Bypass surgery for chronic lower limb ischaemia. Cochrane Database Syst Rev. 2017;4:cd002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goksel OS, Karpuzoglu E, Issever H, et al. Midterm results with drug-coated balloons for SFA lesions in patients with CLTI: comparison with conventional bypass surgery. Int Angiol. 2018;37:365–369. [DOI] [PubMed] [Google Scholar]
- 53. Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 54. Hallett JW, Jr, Byrne J, Gayari MM, et al. Impact of arterial surgery and balloon angioplasty on amputation: a population-based study of 1155 procedures between 1973 and 1992. J Vasc Surg. 1997;25:29–38. [DOI] [PubMed] [Google Scholar]
- 55. Nehler MR, Coll JR, Hiatt WR, et al. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003;38:7–14. [DOI] [PubMed] [Google Scholar]
- 56. Writing Committee M, Gerhard-Herman MD, Gornik HL, et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary. Vasc Med. 2017;22:NP1–NP43. [DOI] [PubMed] [Google Scholar]
- 57. Katsanos K, Kitrou P, Spiliopoulos S, et al. Comparative effectiveness of plain balloon angioplasty, bare metal stents, drug-coated balloons, and drug-eluting stents for the treatment of infrapopliteal artery disease: systematic review and Bayesian network meta-analysis of randomized controlled trials. J Endovasc Ther. 2016;23:851–863. [DOI] [PubMed] [Google Scholar]
- 58. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. [DOI] [PubMed] [Google Scholar]
- 59. Shammas NW, Dippel EJ, Coiner D, et al. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15:270–276. [DOI] [PubMed] [Google Scholar]
- 60. Lee MS, Heikali D, Mustapha J, et al. Acute procedural outcomes of orbital atherectomy for the treatment of common femoral artery disease: sub-analysis of the CONFIRM Registries. Vasc Med. 2017;22:301–306. [DOI] [PubMed] [Google Scholar]
- 61. Lee MS, Canan T, Rha SW, et al. Pooled analysis of the CONFIRM Registries: impact of gender on procedure and angiographic outcomes in patients undergoing orbital atherectomy for peripheral artery disease. J Endovasc Ther. 2015;22:57–62. [DOI] [PubMed] [Google Scholar]
- 62. Das T, Mustapha J, Indes J, et al. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc Interv. 2014;83:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circ Res. 2015;116:1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 19-0584_supplementary_materals for Three-Year Outcomes of Orbital Atherectomy for the Endovascular Treatment of Infrainguinal Claudication or Chronic Limb-Threatening Ischemia by Stefanos Giannopoulos, Eric A. Secemsky, Jihad A. Mustapha, George Adams, Robert E. Beasley, George Pliagas and Ehrin J. Armstrong in Journal of Endovascular Therapy