Abstract
Maintenance of mitochondrial function and integrity is critical for normal cell survival, particularly in non-dividing cells with a high-energy demand such as cardiomyocytes. Well-coordinated quality control mechanisms in cardiomyocytes, involving mitochondrial biogenesis, mitochondrial dynamics-fission and fusion, and mitophagy, act to protect against mitochondrial dysfunction. Mitochondrial fission, which requires dynamin-related protein 1 (Drp1), is essential for segregation of damaged mitochondria for degradation. Alterations in this process have been linked to cardiomyocyte apoptosis and cardiomyopathy. In this review, we discuss the role of Drp1 in mitophagy and apoptosis in the context of cardiac pathology, including myocardial ischemia and heart failure.
Keywords: Drp1, apoptosis, mitophagy, mitochondrial fission, heart
1. Introduction
Mitochondria are highly abundant in the heart. Adult cardiac mitochondria comprise approximately 30% of the total cell volume and generate vast amounts of ATP through oxidative phosphorylation to maintain contractile function. Cardiomyocytes produce high levels of oxidative stress and are long-lived with infrequent, if not a complete absence of, proliferation [1]. To maintain mitochondrial homeostasis, cardiomyocytes have developed well-coordinated quality control mechanisms involving mitochondrial biogenesis, mitochondrial dynamics, and a mitochondria-specific form of autophagy, termed mitophagy [2–5]. It has been suggested that mitochondrial fission and fusion serve as an early response to mitochondrial dysfunction, acting to overcome regional deterioration in mitochondria [6, 7]. Mitochondria in adult cardiomyocytes are distributed in a more ordered fashion than in other cell types. They are located between myofibrils, around nuclei, and in the subsarcolemmal space, and are referred to as interfibrillar, perinuclear and subsarcolemmal mitochondria, respectively. In particular, intermyofibrillar mitochondria are located in parallel to and in direct contact with myofibers, thereby efficiently supplying ATP to myofibers. Due to this unique spatial organization, it has been speculated that their movement, including fusion, may be limited. Interestingly, however, recent evidence suggests that mitochondrial fusion is rather robust, supporting the exchange of matrix content between adjacent mitochondria in adult cardiomyocytes, which is controlled by cardiac contractility [8]. Furthermore, in mice in which Drp1, a dynamin-related GTPase that critically mediates fission, is downregulated in cardiomyocytes, mitochondria are elongated compared to in wild type mice. Thus, fission and fusion do take place in adult cardiomyocytes in vivo [9]. Our group has been investigating the molecular mechanisms responsible for mitochondrial quality control. In particular, we have focused on the role of Drp1 in the heart because the stressed heart contains fragmented mitochondria with mitochondrial translocation of Drp1 and because Drp1 plays an important role in mediating many aspects of mitochondrial quality control in the heart [9]. In this review, we will discuss the role of Drp1 in the heart.
2. General functions of Drp1
2.1. General features of Drp1
Drp1 belongs to the dynamin family of GTP-binding proteins. The dynamin family includes Dnm1 in yeast and dynamin I, II, III in mammals. Classic Dynamin proteins contain a GTPase domain, a pleckstrin homology motif, and the proline-rich tail and mediate receptor-mediated endocytosis in organisms ranging from insects to vertebrates [10]. Dnm1 in yeast and Drp1 in mammals, which lack the pleckstrin homology motif and the proline-rich region, function in mitochondrial fission [11]. In humans, Drp1 is expressed ubiquitously [10, 12] as multiple alternatively spliced variants [13], with higher expression levels in the heart, skeletal muscle, brain, kidney and testis [10, 13]. Although the majority of Drp1 is localized in the cytosol at baseline, when mitochondria undergo fission, Drp1 is recruited to sites of mitochondrial fission through interactions with mitochondrial outer membrane proteins, including fission 1 (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 KDa (MiD49 and MiD51) [12, 14–16]. Fis1 was initially proposed as a Drp1 receptor based on genetic and biochemical data in yeast [17]. However, two molecular adaptors, Mdv1 and Caf4, are required for Fis1 to localize at mitochondria in yeast and neither of these molecules exists in mammalian cells. This raises the possibility that the role of Fis1 in mammalian cells may be different from that in yeast [14]. Indeed, experiments using Fis1-null mouse embryonic fibroblasts (MEFs) indicated only a minor role for Fis1 in Drp1 recruitment and mitochondrial fission. On the other hand, knockdown of Mff results in mitochondrial elongation and reduces the amount of Drp1 recruited to mitochondria in MEFs [14, 15]. MiD49 and MiD51 induce recruitment of Drp1 and mitochondrial fission even in the absence of Fis1 and Mff [14]. These studies suggest that multiple receptors can recruit Drp1 to mitochondria for mitochondrial fission, depending on cell type and cellular circumstances.
ER-mitochondria communication through contact sites, known as mitochondria-associated ER membranes (MAMs), is important for mitochondrial fission [18–20]. Actin polymerization occurs through ER-localized inverted formin 2 (INF2), promoting initial mitochondrial constriction and determining the site of Drp1 recruitment [19]. Filamin A (FLNa), a protein scaffold for cell adhesion and migration, activates Drp1 through its guanine nucleotide exchange activity and functionally couples Drp1 with actin [21]. During hypoxia, the mitochondrial outer membrane protein FUN14 domain-containing 1 (FUNDC1) accumulates at MAMs and recruits Drp1 to MAMs and mitochondrial fission sites [22]. SNARE protein syntaxin 17 (Syn17) is also present at MAMs, recruits Drp1, and drives mitochondrial fission [23].
2.2. Posttranslational modifications of Drp1
Drp1 contains four domains: an N-terminal GTPase domain, middle domain, variable domain, and C-terminal GTPase effector domain (GED). The recruitment of Drp1 to mitochondria is regulated by reversible phosphorylation at the C-terminal GED domain through interaction with multiple kinases and phosphatases [24, 25], comprising a complex regulatory network for rapid mitochondrial remodeling in response to intrinsic and extrinsic signals. For instance, during mitosis, mitochondrial fission is enhanced through phosphorylation of Drp1 at Ser 616 by Cdk1 [26]. In post-mitotic neurons, CDK5 phosphorylates Drp1 at Ser 616, resulting in its recruitment and subsequent mitochondrial fission associated with neuronal injury [27]. Drp1 is also phosphorylated at Ser 616 by other kinases, including Erk2, Ca2+/calmodulin-dependent kinase II (CaMKII) and Rip1 [27–30]. Phosphorylation of Drp1 at Ser 637 within the C-terminal GED domain by cAMP-dependent protein kinase (PKA) decreases GTPase activity by affecting its intramolecular interaction and inhibits mitochondrial fission in cells [31]. Phospho-mimetic substitution at Ser 616 influences neither the intramolecular interaction between the GTP-binding domain and the GED domain nor the GTPase activity. Thus, Ser 616 phosphorylation likely regulates Drp1 through other mechanisms [26]. The calcium-dependent phosphatase, calcineurin, dephosphorylates Ser 637 of Drp1 and promotes its recruitment to mitochondria [32]. In addition, sumoylation modifies and stabilizes mitochondrial Drp1 and enhances mitochondrial fission [33, 34]. Acetylation of Drp1 at Lys 642 in response to high fat diet consumption induces mitochondrial translocation of Drp1 and cell death in cardiomyocytes [35]. Furthermore, Drp1 interacts with phosphatidic acid (PA) and saturated phospholipids in the mitochondrial membrane, and this interaction prevents Drp1 from initiating the constriction of mitochondria [36, 37].
2.3. Drp1 in peroxisomes
Drp1 is also involved in the fission of peroxisomes, membrane-bound intracellular organelles that play a key role in metabolism and conversion of reactive oxygen species. Increasing lines of evidence suggest that peroxisome dynamics, number, and morphology critically affect cellular function. Association of Drp1 with peroxisomes is increased during peroxisome proliferation [38, 39], whereas downregulation of Drp1 inhibits peroxisomal fission and causes peroxisome elongation [38, 39]. Drp1 interacts with the peroxisomal membrane protein Peroxin 11, thereby playing an important function in mediating peroxisomal fission during the initial phase of fission [40]. However, how the function of Drp1 in peroxisomes contributes to the overall cardiac phenotype in Drp1 KO mice remains to be elucidated.
3. The role of Drp1 in mitophagy
3.1. The general autophagy pathway
Autophagy is an evolutionarily conserved “self-eating” process by which intracellular materials are degraded. The autophagy process begins with initiation and maturation of double-membraned vesicles called autophagosomes that enclose the materials to be degraded. Initiation is promoted by the unc-51-like autophagy-activating kinase (Ulk) complex (comprising Atg13, Ulk1, FIP200 and Atg101), which then activates another complex consisting of Beclin1, Atg14L, Vps34, and Vps15 for expansion of the nascent autophagosome. The final maturation and closure of the autophagosome is mediated by the Atg conjugation system (Atg5, Atg7 and Atg12), which conjugates LC3 to phosphatidylethanolamine to form LC3-II, a component of autophagosomal membranes. After maturation, autophagosomes fuse with lysosomes to degrade the sequestered cargo [41]. When autophagy degrades mitochondria in a selective manner, it is referred to as mitophagy.
3.2. The role of Drp1 in mitophagy
Although the mitochondrial electron transport chain is a major source of ATP, ATP production is accompanied by production of reducing sources. The leakage of electrons under pathophysiological conditions leads to increased oxidative stress, which amplifies rapidly and attenuates mitochondrial function [42]. It has been shown that, at least under some conditions, mitochondria with depolarized mitochondrial membrane potential can be selectively targeted by mitochondrial fission and mitophagy [6, 7]. An important question here is how mitochondrial fission and mitophagy coordinate with one another to maintain the quality of mitochondria. Readers are referred to our recent review describing the connection between mitochondrial fission and mitophagy [43]. It is generally believed that healthy mitochondria can restore mitochondrial membrane potential levels and participate in the fusion-fission cycle, whereas mitochondria that fail to do so are marked and eliminated by mitophagy [6] (Figure 1). According to this theory, mitochondrial division occurs prior to mitophagy so that only damaged parts are degraded by autophagy. What is the role of Drp1 in this process?
Figure 1. The role of Drp1 in mitochondrial quality control and cell death.
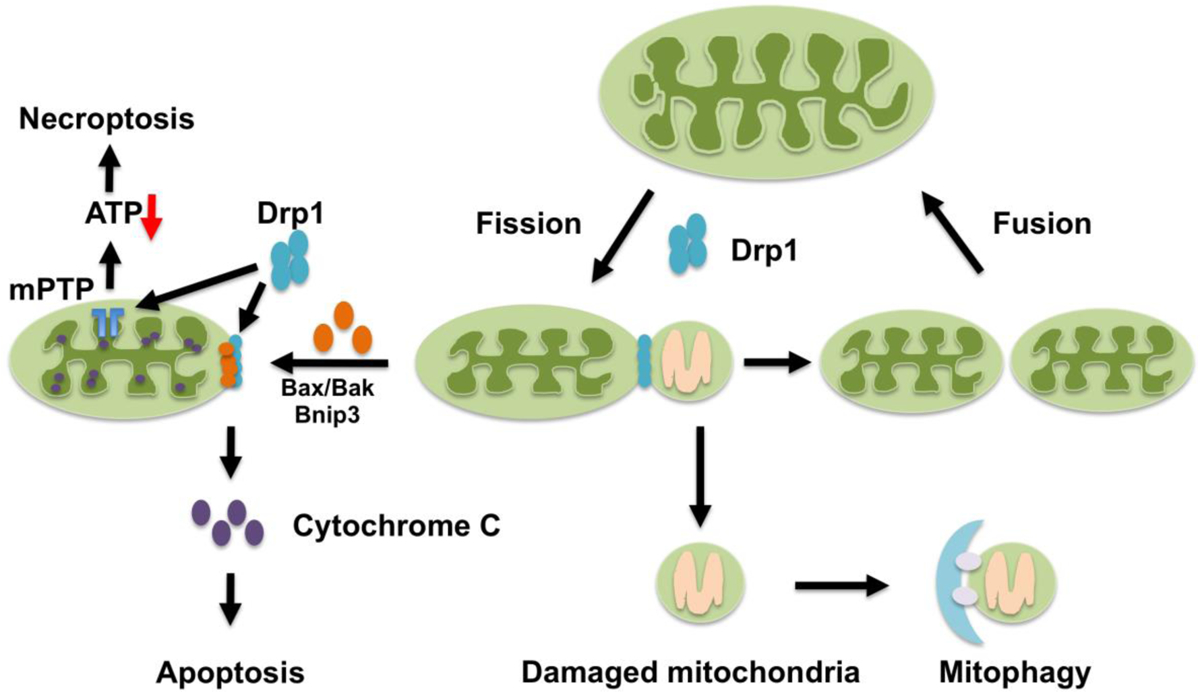
Mitochondrial quality control mechanisms include mitochondrial biogenesis, mitochondrial dynamics - fission and fusion, and mitophagy. Mitochondrial fission, which requires Drp1, is essential for segregation of damaged mitochondria for degradation. Drp1 is involved in Bax/Bak- or Bnip3-dependent apoptosis by controlling outer mitochondrial membrane permeabilization. Drp1 associates with Bax at mitochondrial fission sites during cell death by apoptosis and acts downstream of Bax translocation but upstream of cytochrome c release. In addition, Drp1 promotes mPTP opening and induces necroptosis.
In yeast, Dnm1, a mitochondrial fission protein and a homologue of Drp1, plays a critical role in mediating mitophagy [44]. In response to nutrient starvation, the fission complex is recruited to mitochondria through physical interaction between Dnm1 and Atg11, a scaffold protein that recruits ATG proteins to the site of autophagosome formation, suggesting that the mitochondrial fission and autophagy machineries may act in a coordinated manner to selectively target the mitochondria to be degraded [45]. Mitophagy in B-cells is significantly inhibited in the presence of dominant negative Drp1, downregulation of Fis1 or overexpression of OPA1, suggesting that either Drp1 or mitochondrial fission also plays an essential role in mediating mitophagy in mammalian cells [6].
Consistent with the results in these seminal works, increasing lines of evidence suggest that Drp1 promotes, if not plays an essential role in, mitophagy in many cell types in response to different types of stress. Inhibition of Drp1-mediated mitochondrial fission prevents Parkin-induced mitophagy in HeLa cells and MEFs [7]. The Bcl2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3), an atypical BH3-only protein, induces mitochondrial translocation of Drp1, which in turn promotes translocation of Parkin to mitochondria in a Drp1-dependent manner in adult cardiomyocytes [46]. Thus, Drp1 is required for Bnip3-induced mitophagy by inducing mitochondrial fission and recruitment of Parkin in a coordinated manner.
We have shown recently that downregulation of endogenous Drp1 induces elongated mitochondria, inhibits mitophagy, and induces mitochondrial dysfunction and consequent cell death in the heart both at baseline and under stress conditions such as glucose-deprivation, fasting and ischemia/reperfusion [9]. Drp1 physically interacts with Bcl-xL in cardiomyocytes. When Drp1 is translocated to mitochondria in response to stress, it disrupts the interaction between Beclin1 and Bcl-xL, a negative regulator of Beclin 1, thereby activating autophagy in cardiomyocytes [9]. These results suggest that Drp1 not only induces mitochondrial fission but also directly stimulates autophagy in cardiomyocytes.
Drp1 is also involved in activation of mitophagy during hypoxia in HeLa cells. During hypoxia, FUNDC1, an LC3 receptor, but not the Drp1 receptors Mff, MID49/51, or Fis1, is required for Drp1 to translocate to the MAM site and mediate mitophagy [22]. Interestingly, this form of mitophagy occurs in a Parkin-independent manner.
Mitochondrial dysfunction is commonly observed during heart failure. In the transverse aortic constriction mouse model of heart failure, mitophagy is upregulated in the heart in response to pressure overload (PO). Activation of mitophagy is observed only transiently 3–5 days after the initiation of PO. Although mitochondrial function was maintained during the first 1–2 weeks in this model, mitochondrial dysfunction and left ventricular dysfunction develop thereafter. It is likely that mitophagy is an adaptive mechanism to maintain mitochondrial function during PO. It is currently unknown why mitophagy is activated only transiently. Interestingly, induction of mitophagy in the heart in response to PO was observed only after autophagy that targets cellular components in a non-specific manner, namely general autophagy, was inactivated. Although the exact molecular mechanism by which the time course of mitophagy dissociates from that of general autophagy in response to PO is currently unknown, importantly, mitochondrial autophagy in response to PO coincides with mitochondrial translocation of Drp1, which was also accompanied by phosphorylation of Ser 616 and dephosphorylation of Ser 637 of Drp1. Furthermore, mitophagy is abrogated in mice with cardiac-specific Drp1 knockout (KO) [9, 47, 48] (Figure 2a). These results suggest that endogenous Drp1 plays an important role in mediating mitophagy in response to PO and that this mitophagy is most likely mediated by a mechanism distinct from that mediating general autophagy.
Figure 2. Mitophagy is mediated by either Drp1-dependent or -independent mechanisms.
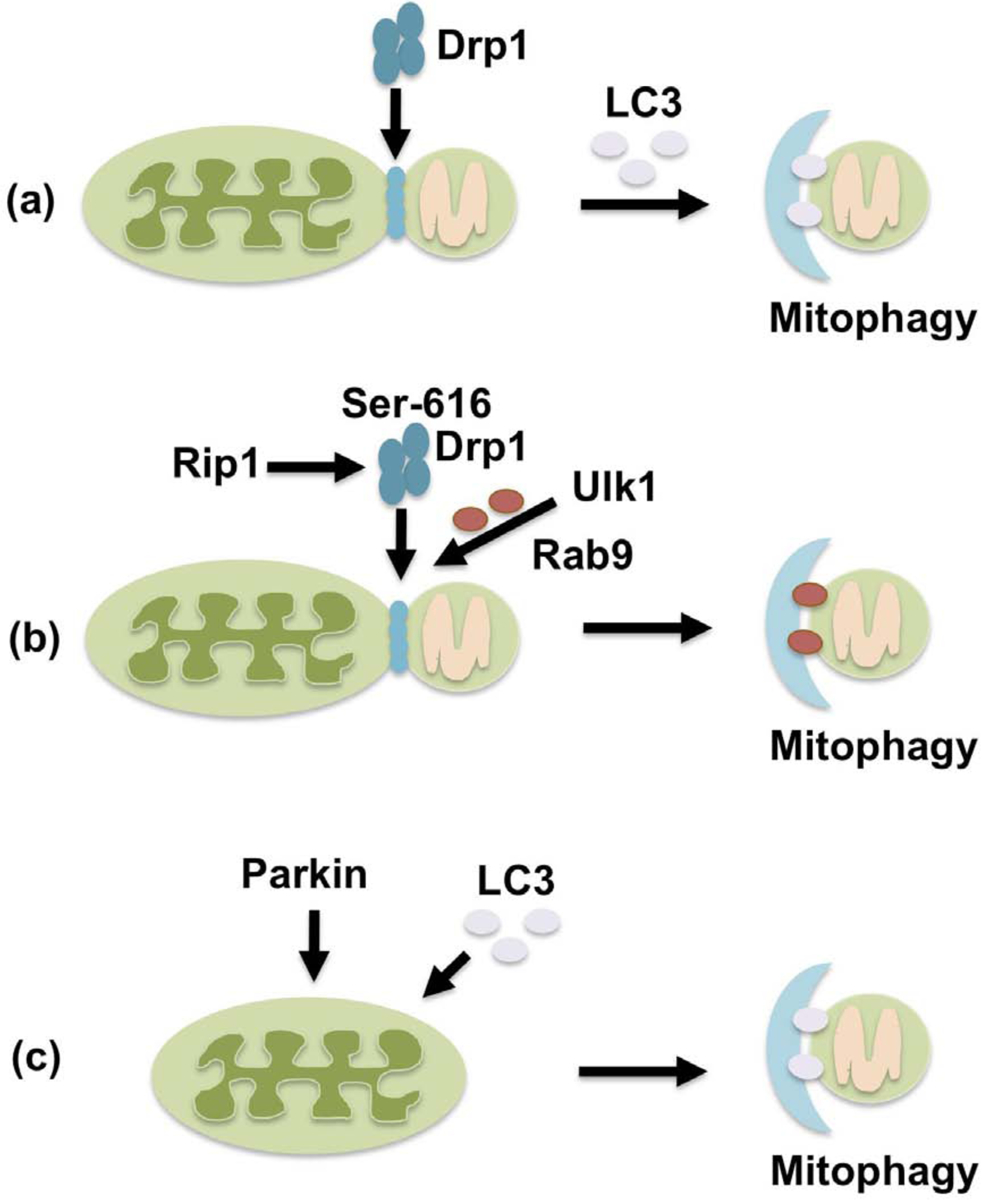
In Drp1-dependent mitophagy, mitochondrial fission mediated by Drp1 segregates damaged mitochondria, which are then degraded by LC3-dependent autophagy (a). Drp1 is also involved in alternative mitophagy, which occurs through a Ulk1/Rab9/Rip1-dependent mechanism. In this case, Drp1 is translocated to mitochondria through Rip1-mediated phosphorylation at Ser616 and forms a complex with Rab9 in an Ulk1-dependent manner (b). In Drp1-independent mitophagy, Parkin marks depolarized mitochondria, and separation and sequestration of damaged mitochondria into autophagosomes occur through a common mechanism in an LC3-dependent manner (c).
Dissociation between general autophagy and mitophagy was also observed in the heart during ischemia. We recently showed that mitophagy during myocardial ischemia is mediated predominantly through autophagy characterized by Rab9-associated autophagosomes, rather than LC3-dependent autophagy [30]. This form of mitophagy plays a more important role in protecting the heart against ischemia than LC3-dependent autophagy and is mediated through the Ulk1-Rab9-Rip1-Drp1 pathway rather than Atg7- and LC3-dependent mechanisms (Figure 2b). This form of mitophagy is similar to the alternative autophagy originally reported by Honda et al. [49]. Drp1 is phosphorylated at Ser 616 by Rip1, a serine/threonine kinase, in response to hypoxia and Rip1 associates with Drp1 in an Ulk1- and Rab9-dependent manner. Furthermore, Ulk1-dependent phosphorylation of Rab9 at Ser 179 plays an important role in mediating the Rab9-Rip1 interaction and Ser 616 phosphorylation of Drp1 by Rip1. Thus, Ser 616-phosphorylated Drp1 forms a complex with autophagosomes marked with Rab9 in an Ulk1-dependent manner through a series of phosphorylation events [30]. This interaction between the fission machinery and the autophagy machinery is reminiscent of the Dnm1-Atg11 interaction during mitophagy in yeast.
Following the recruitment of Drp1 to mitochondria, Drp1 interacts with the Zip1-MCU complex, which promotes the entry of Zn2+ into the mitochondrial matrix, thereby leading to decreases in the local mitochondrial membrane potential [50]. Upon fission by Drp1, the fate of fragmented mitochondria is determined by their ability to restore mitochondrial membrane potential; healthy mitochondria can restore mitochondrial membrane potential and participate in the fusion-fission cycle again, whereas mitochondria that fail to do so are marked and eliminated by mitophagy [50].
Different observations have been reported regarding the timing of mitochondrial fission and mitophagy relative to the timing of mitochondrial depolarization. For example, mitochondrial depolarization induces Drp1 recruitment, which in turn promotes mitochondrial fission in response to carbonyl cyanide m-chlorophenylhydrazone, a mitochondrial uncoupler [7]. In this case, fission occurs after mitochondrial depolarization. On the other hand, mitochondrial depolarization occurs well after mitochondria are completely sequestered into mitophagosomes during nutrient deprivation, where mitophagy is mediated by phosphatidylinositol-3-kinase in coordination with fission in hepatocytes [51]. It is likely that cell type-specific distinct molecular mechanisms are involved in the induction of mitochondrial fission and mitophagy. Further investigation is needed to elucidate the stimulus-specific mechanisms mediating mitochondrial fission and mitophagy and how they relate to Drp1 and mitochondrial depolarization.
In addition, Vps13D, a ubiquitin binding protein, has been shown to regulate mitochondrial size and mitophagy by acting downstream of Drp1 and MFF [52]. Thus, Drp1 appears to have multiple targets that allow it to fully accomplish its mitochondrial quality control function through fission and mitophagy.
3.3. Drp1-independent mitophagy
It should be noted that Drp1 is dispensable in some forms of mitophagy [53–55]. For example, hypoxia-induced mitophagy can take place in cell lines in which Drp1 is downregulated and in Drp1 KO MEFs, although deletion of Drp1 slightly decreased mitophagy and prolonged the time required for autophagosomes to sequestrate mitochondria [54]. Importantly, factors needed for isolation membranes to be formed and extended, namely FIP200, ATG14 and WIPI, are required for mitochondrial division during mitophagy [54]. These observations suggest that mitochondrial division can be accomplished as a part of autophagosome formation during mitophagy, even in the absence of a separate mechanism dedicated to mitochondrial fission (Figure 2c).
Kageyama et al. proposed the existence of two types of mitophagy in the heart and neurons, namely Drp1-dependent mitophagy and Parkin-dependent mitophagy, which synergistically maintain mitochondrial homeostasis [47]. In their model, Parkin-dependent mitophagy becomes more critical for the maintenance of mitochondrial respiratory function in the absence of Drp1-dependent mitophagy. In theory, Parkin-dependent mitophagy should use Drp1-independent mitochondrial fission when depolarized mitochondria are separated [56]. However, Song et al. [53] reported that Parkin-dependent mitophagy is hyper-activated in Drp1-deficient mouse hearts. In this study, the authors concluded that hyper-activation of mitophagy is detrimental for the heart, which is the opposite of what was reported by Kageyama et al. [47]. Molecular mechanisms through which Parkin-dependent mitophagy becomes hyperactive in the absence of Drp1 remain to be clarified. It has been shown that downregulation of Drp1 induces constitutive recruitment of Parkin to elongated mitochondria and increases degradation of healthy mitochondria [57]. This suggests that the most important physiological role of endogenous Drp1 is to protect healthy mitochondria from mitophagy. It should be noted that the level of mitophagy activation in Drp1-deficient mice differs significantly among investigators [9, 47, 53]. The reason for this discrepancy remains to be clarified.
Increasing lines of evidence suggest that damaged mitochondria are degraded through multiple mechanisms. One other such mechanism is the transfer of a small portion of mitochondria to lysosomes via mitochondrial-derived vesicles (MDV). In this mechanism, although depolarized mitochondria are sequestrated into small vesicles in a Parkin-dependent manner, degradation takes place even in the absence of Drp1 [58].
4. The role of Drp1 in apoptosis and necroptosis
4.1. Apoptosis
Mitochondrial fragmentation is a conserved aspect of apoptosis from yeast, C.elegans, Drosophila to mammals [59–64]. Since mitochondrial fragmentation is often observed when cells are under stress or when they are dying, Drp1 has been implicated in the pathogenesis of cell death [65]. For example, during apoptosis, activation of proapoptotic signaling BH3-only BIK induced mitochondrial cristae remodeling, loss of mitochondrial membrane potential and fragmentation, which is Drp1-dependent [66]. PKA-mediated phosphorylation of Drp1 at Ser 637 induces mitochondrial elongation and resistance to apoptotic stimuli, whereas dephosphorylation of Ser 637 by calcineurin promotes mitochondrial fragmentation and increases cell vulnerability to apoptosis in rat pheochromocytoma PC12 cells [32]. Although death may take place secondary to mitochondrial dysfunction caused by dysregulated fission, increasing lines of evidence suggest that Drp1 is more directly involved in apoptosis by controlling outer mitochondrial membrane permeabilization when it stably associates with mitochondria. For example, Drp1 associates with Bax at mitochondrial fission sites during apoptosis in HeLa cells. However, neither Drp1 nor mitochondrial fission itself may be sufficient for cell death [64]. Loss of Drp1 prevents mitochondrial fragmentation and apoptosis by inhibiting cytochrome c release but without affecting Bax translocation into mitochondria in HeLa cells, suggesting that Drp1 controls cell death by acting downstream of Bax translocation but upstream of cytochrome c release [67]. During apoptosis, Drp1 stably associates with the mitochondrial membrane in a Bax/Bak-dependent manner but independent of mitochondrial fragmentation in HeLa cells. This allows remodeling of both the inner and outer membranes and cristae that precedes the permeabilization of the outer membrane, the complete release of cytochrome c and eventual loss of ΔΨ [68] (Figure 1).
4.2. Necroptosis
Necroptosis is a form of regulated necrotic cell death mediated by Rip1 (receptor-interacting protein kinase 1), Rip3, and MLKL (mixed-lineage kinase domain-like pseudokinase) [69]. PGAM5, a mitochondrial phosphatase localized in a Rip1- and Rip3-containing protein complex, mediates necroptosis in response to multiple stimuli by activating Drp1 and mitochondrial fragmentation [70] (Figure 1). However, other studies found that, although PGAM5 promotes inflammasome activation, it is dispensable for necroptosis in macrophages [71]. The Rip1-Rip3-Drp1 signaling pathway is required for RNA virus-induced activation of the NLRP3 inflammasome. However, MLKL and PGAM5 are not involved in this process [72]. Thus, further investigation is required to elucidate how Drp1 is activated and mediates necroptosis.
Recent evidence suggests that inhibition of Drp1 translocation to mitochondria is protective against hypoxia/reoxygenation-induced death in cardiomyocytes if the intervention is given as pretreatment. However, if inhibition of Drp1 translocation is initiated at the time of reoxygenation, it has been found to promote necroptosis, suggesting that association of Drp1 with mitochondria has time-dependent consequences in the regulation of cell death [73]. Further investigation is necessary to elucidate how Drp1 regulates multiple forms of cell death.
5. The function of Drp1 in the heart
Homozygous deletion of Drp1 in mice leads to embryonic lethality between embryonic days (E) 9.5 and 11.5, with elongated mitochondria, reduced cell proliferation, and decreased developmentally regulated apoptosis [74]. Postnatal cardiac specific knock out of Drp1 induces dilated cardiomyopathy and rapid lethality in mice [9, 47, 75]. In Drp1KO cardiomyocytes, mitochondria exhibited increased connectivity, accumulation of ubiquitinated proteins, and decreased respiration [9, 47, 75]. These results suggest that endogenous Drp1 plays an important role in mediating mitochondrial quality control and the survival of cardiomyocytes at baseline.
Drp1 is upregulated during pathological conditions in the heart and excessive fission of mitochondria appears to be detrimental for the heart. For example, excessive mitochondrial fission occurs within 60 minutes of myocardial reperfusion after transient ischemia, leading to mitochondrial dysfunction and decreasing cardiac contractility [76]. Ser 637 of Drp1 is dephosphorylated during reperfusion, which in turn induces mitochondrial translocation of Drp1 and increased mitochondrial fission [77]. Treatment of the heart with chemical inhibitors of Drp1, including Mdivi-1 and P110, protects the heart against ischemia/reperfusion (I/R) injury [76, 78]. The Drp1 inhibitors preserve mitochondrial morphology, decrease cytosolic calcium, inhibit mitochondrial permeability transition pore (mPTP) opening and prevent apoptosis in cardiomyocytes, thereby decreasing myocardial injury [78]. It should be noted that the effect of Drp1 inhibition appears to be dose- and time-dependent. For example, complete or prolonged inhibition of Drp1 through genetic deletion induces harmful effects through suppression of essential mitochondrial quality control mechanisms [9]. In addition, the effects of chemical inhibitors of Drp1 upon other molecules, including Bax/Bak [79] and delayed rectifier K+ channel [80], may contribute to their protective effects against I/R injury. Thus, caution should be exercised when interpreting results obtained using chemical inhibitors of Drp1. FK506, a calcineurin inhibitor, prevents dephosphorylation of Drp1 Ser 637 and preserves cardiac function during I/R [77]. During ischemia, downregulation of endogenous miR-499 activates calcineurin, which dephosphorylates Drp1, and increases mitochondrial fragmentation, thereby inducing apoptosis. Overexpression of exogenous miR-499, however, inhibits cardiomyocyte apoptosis through its suppression of calcineurin-mediated dephosphorylation of Drp1 [81]. Hyperfission of mitochondria is also observed in the heart after myocardial infarction. Hypoxia induces association between FLNa and Drp1, leading to hyperfission of mitochondria and myocardial senescence [21].
Activation of Drp1 is also implicated in cardiac hypertrophy and heart failure. Phosphorylation of Drp1 by CaMKII at Ser 616 stimulates mPTP opening during chronic β-adrenergic receptor (β-AR) stimulation [29]. Protein kinase D (PKD), a serine/threonine kinase implicated in the development of cardiac hypertrophy and heart failure, is translocated to mitochondria in response to hypertrophic stimuli and induces phosphorylation of Drp1 at Ser 637, which leads to mitochondrial dysfunction and cardiomyocyte apoptosis. The detailed mechanism by which Ser 637 phosphorylation of Drp1 by PKD mediates fission, but not fusion, remains to be clarified [82].
Doxorubicin-induced cardiotoxicity is accompanied by mitochondrial translocation of Bnip3 and Drp1, mitochondrial fragmentation and mPTP opening[83] (Figure 1). Cardiac-specific Drp1 KO mice are protected from doxorubicin-induced cardiac damage, suggesting that Drp1 promotes cardiac damage in response to doxorubicin. Strategies that limit mitochondrial fission in the physiologic range may help reduce doxorubicin-induced cardiotoxicity [84]. Cellular accumulation with proteins carrying polyglutamine repeats in Huntington’s disease activates Drp1-mediated excessive mitochondrial fission, which in turn induces cell death, including apoptosis and necrosis, which is accompanied by accumulation of damaged mitochondria in the lysosome that was coupled with lysosomal dysfunction [85]. Drp1 also contributes to the pathogenesis of hypertensive cardiac hypertrophy through production of reactive oxygen species (ROS) and suppression of Drp1 may be effective in preventing cardiac hypertrophy [86]. Drp1 has also been shown to be involved in the development of pathologic fission and mitochondrial dysfunction in sepsis-induced cardiomyopathy [87]. These results are all consistent with the notion that excessive activation of mitochondrial fission causes progression of cardiomyopathy and that normalizing this process by inhibiting Drp1 is effective for treating such conditions. Excessive activation of mitochondrial fission also occurs through epigenetic dysregulation of MiD49 and MiD51, cellular receptors of Drp1, which drives pathological proliferation and apoptosis resistance in pulmonary artery smooth muscle cells. This mechanism has been implicated in the pathogenesis of pulmonary arterial hypertension [88].
Although many studies highlight the detrimental aspects of mitochondrial fission mediated by Drp1, which often occurs to an excessive degree during pathological conditions in the heart, other studies have shown that Drp1 is important in maintaining mitochondrial homeostasis under some conditions [89–91]. For example, Drp1 maintains or positively stimulates mitochondrial respiration, bioenergetics and ROS signaling in adult cardiomyocytes at baseline [91]. During exercise, mitochondrial fission enhances, rather than impairs, mitochondrial function in the heart. This physiological fission is mediated by Drp1 and, unlike with pathological fragmentation, mitochondrial membrane potential is maintained and mitophagy is not activated [90]. Increased oxidative stress leads to activation of RhoA, increases phosphorylation of Drp1 at Ser 616, and leads to localization of Drp1 at mitochondria. Thus, activation of Drp1 mediates the protective effect of RhoA against oxidative stress in cardiomyocytes [92]. We have shown previously that persistent inhibition of Drp1 by genetic deletion or repetitive application of Mdivi-1 in the heart exacerbates myocardial I/R injury [9]. Thus, it is likely that although dysregulated activation of Drp1 in response to stress is detrimental for the heart, persistent inhibition of Drp1 is also detrimental since it would abolish homeostatic functions of Drp1, including induction of mitophagy and its positive effects upon mitochondrial respiration in the heart.
It is generally believed that fused mitochondria are more efficient at producing ATP than fragmented ones due to mixing of the matrix and inner membrane in response to acute demands. Mitochondrial fusion also allows complementation of gene products to compensate for the accumulation of mitochondrial mutations over the long-term [93]. Thus, excessive mitochondrial fission, which is supposed to exhibit opposite properties, has been proposed as an underlying mechanism of cardiac dysfunction in many animal studies based upon the presence of mitochondrial fission or sensitivity to chemical inhibitors of Drp1, including mdivi-1. However, since Drp1 may have fission-independent functions [91], and given that chemical inhibitors of Drp1 appear to also have some Drp1-independent effects [94], further investigation is needed to clarify whether cardiac dysfunction is induced primarily by excessive fission and whether other molecules besides Drp1 are also involved in its pathogenesis.
6. Concluding remarks
In this review we have discussed cellular functions of Drp1 with an emphasis on its role in mitochondrial quality control mechanisms and the pathophysiological roles of Drp1 in the heart. It is becoming clear that Drp1 influences the survival and death of cardiomyocytes, possibly through its fission-independent effects on mitophagy, apoptosis and respiration. However, further investigations are required to clarify whether the proposed cellular actions of Drp1, including mitophagy, apoptosis and regulation of respiration, are truly mediated through fission-independent mechanisms, and, if so, what the underlying molecular mechanisms are. Precise localization of Drp1 in mitochondria and the identity of proteins interacting with Drp1 need to be clarified. Posttranslational modifications and other regulatory mechanisms by which Drp1 switches between physiological and pathological roles need to be identified. Further investigation regarding these issues would allow for control of Drp1 function with enhanced precision and the development of improved interventions to achieve better mitochondrial quality control in patients with heart disease.
Highlights.
Drp1 critically regulates mitochondrial function through fission, autophagy/mitophagy, and regulation of respiration.
Drp1 controls cell death, including apoptosis and necroptosis, through fission-dependent and -independent mechanisms.
Dysregulation of Drp1 in cardiomyocytes contributes to cardiac pathology, including heart failure and myocardial injury.
Funding:
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL112330, HL138720, HL144626, and AG23039 (J.S.), the American Heart Association 2020 Merit Award 20 Merit35120374 (J.S.), and by the Fondation Leducq Transatlantic Network of Excellence 15CBD04 (J.S).
List of abbreviations
- I/R
ischemia/reperfusion
- mPTP
mitochondrial permeability transition pore
- KO
knockout
- MAM
mitochondria-associated ER membrane
- MDV
mitochondrial-derived vesicles
- MEF
mouse embryonic fibroblast
- PO
pressure overload
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest:
The authors have declared that no conflict of interest exists.
References
- [1].Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S, Mitochondrial dynamics, mitophagy and cardiovascular disease, J Physiol 594(3) (2016) 509–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Friedman JR, Nunnari J, Mitochondrial form and function, Nature 505(7483) (2014) 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pickles S, Vigie P, Youle RJ, Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance, Current biology : CB 28(4) (2018) R170–r185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashrafi G, Schwarz TL, The pathways of mitophagy for quality control and clearance of mitochondria, Cell death and differentiation 20(1) (2013) 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Picca A, Mankowski RT, Burman JL, Donisi L, Kim JS, Marzetti E, Leeuwenburgh C, Mitochondrial quality control mechanisms as molecular targets in cardiac ageing, Nature reviews. Cardiology 15(9) (2018) 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS, Fission and selective fusion govern mitochondrial segregation and elimination by autophagy, Embo J 27(2) (2008) 433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ, Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin, J Cell Biol 191(7) (2010) 1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eisner V, Cupo RR, Gao E, Csordas G, Slovinsky WS, Paillard M, Cheng L, Ibetti J, Chen SR, Chuprun JK, Hoek JB, Koch WJ, Hajnoczky G, Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity, Proceedings of the National Academy of Sciences of the United States of America 114(5) (2017) E859–e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J, Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress, Circ Res 116(2) (2015) 264–78. [DOI] [PubMed] [Google Scholar]
- [10].Imoto M, Tachibana I, Urrutia R, Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p, Journal of cell science 111 (Pt 10) (1998) 1341–9. [DOI] [PubMed] [Google Scholar]
- [11].Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM, A human dynamin-related protein controls the distribution of mitochondria, The Journal of cell biology 143(2) (1998) 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shin HW, Shinotsuka C, Torii S, Murakami K, Nakayama K, Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p, Journal of biochemistry 122(3) (1997) 525–30. [DOI] [PubMed] [Google Scholar]
- [13].Yoon Y, Pitts KR, Dahan S, McNiven MA, A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells, The Journal of cell biology 140(4) (1998) 779–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loson OC, Song Z, Chen H, Chan DC, Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission, Molecular biology of the cell 24(5) (2013) 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoon Y, Krueger EW, Oswald BJ, McNiven MA, The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1, Mol Cell Biol 23(15) (2003) 5409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K, Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells, J Cell Biol 191(6) (2010) 1141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mozdy AD, McCaffery JM, Shaw JM, Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p, The Journal of cell biology 151(2) (2000) 367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK, ER tubules mark sites of mitochondrial division, Science 334(6054) (2011) 358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Korobova F, Ramabhadran V, Higgs HN, An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2, Science (New York, N.Y.) 339(6118) (2013) 464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ji WK, Chakrabarti R, Fan X, Schoenfeld L, Strack S, Higgs HN, Receptor-mediated Drp1 oligomerization on endoplasmic reticulum, The Journal of cell biology 216(12) (2017) 4123–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nishimura A, Shimauchi T, Tanaka T, Shimoda K, Toyama T, Kitajima N, Ishikawa T, Shindo N, Numaga-Tomita T, Yasuda S, Sato Y, Kuwahara K, Kumagai Y, Akaike T, Ide T, Ojida A, Mori Y, Nishida M, Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence, Science signaling 11(556) (2018). [DOI] [PubMed] [Google Scholar]
- [22].Wu W, Lin C, Wu K, Jiang L, Wang X, Li W, Zhuang H, Zhang X, Chen H, Li S, Yang Y, Lu Y, Wang J, Zhu R, Zhang L, Sui S, Tan N, Zhao B, Zhang J, Li L, Feng D, FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions, The EMBO journal 35(13) (2016) 1368–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arasaki K, Shimizu H, Mogari H, Nishida N, Hirota N, Furuno A, Kudo Y, Baba M, Baba N, Cheng J, Fujimoto T, Ishihara N, Ortiz-Sandoval C, Barlow LD, Raturi A, Dohmae N, Wakana Y, Inoue H, Tani K, Dacks JB, Simmen T, Tagaya M, A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division, Developmental cell 32(3) (2015) 304–17. [DOI] [PubMed] [Google Scholar]
- [24].Pitts KR, McNiven MA, Yoon Y, Mitochondria-specific function of the dynamin family protein DLP1 is mediated by its C-terminal domains, The Journal of biological chemistry 279(48) (2004) 50286–94. [DOI] [PubMed] [Google Scholar]
- [25].Chang CR, Blackstone C, Drp1 phosphorylation and mitochondrial regulation, EMBO reports 8(12) (2007) 1088–9; author reply 1089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K, Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission, J Biol Chem 282(15) (2007) 11521–9. [DOI] [PubMed] [Google Scholar]
- [27].Jahani-Asl A, Huang E, Irrcher I, Rashidian J, Ishihara N, Lagace DC, Slack RS, Park DS, CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death, Human molecular genetics 24(16) (2015) 4573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, Counter CM, Kashatus DF, Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth, Molecular cell 57(3) (2015) 537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu S, Wang P, Zhang H, Gong G, Gutierrez Cortes N, Zhu W, Yoon Y, Tian R, Wang W, CaMKII induces permeability transition through Drp1 phosphorylation during chronic beta-AR stimulation, Nature communications 7 (2016) 13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saito T, Nah J, Oka SI, Mukai R, Monden Y, Maejima Y, Ikeda Y, Sciarretta S, Liu T, Li H, Baljinnyam E, Fraidenraich D, Fritzky L, Zhai P, Ichinose S, Isobe M, Hsu CP, Kundu M, Sadoshima J, An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia, The Journal of clinical investigation 129(2) (2019) 802–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang CR, Blackstone C, Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology, The Journal of biological chemistry 282(30) (2007) 21583–7. [DOI] [PubMed] [Google Scholar]
- [32].Cribbs JT, Strack S, Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death, EMBO reports 8(10) (2007) 939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L, Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria, Proc Natl Acad Sci U S A 105(41) (2008) 15803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Harder Z, Zunino R, McBride H, Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission, Current biology : CB 14(4) (2004) 340–5. [DOI] [PubMed] [Google Scholar]
- [35].Hu Q, Zhang H, Gutierrez Cortes N, Wu D, Wang P, Zhang J, Mattison JA, Smith E, Bettcher LF, Wang M, Lakatta EG, Sheu SS, Wang W, Increased Drp1 Acetylation by Lipid Overload Induces Cardiomyocyte Death and Heart Dysfunction, Circulation research (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Adachi Y, Iijima M, Sesaki H, An unstructured loop that is critical for interactions of the stalk domain of Drp1 with saturated phosphatidic acid, Small GTPases 9(6) (2018) 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Adachi Y, Itoh K, Yamada T, Cerveny KL, Suzuki TL, Macdonald P, Frohman MA, Ramachandran R, Iijima M, Sesaki H, Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division, Molecular cell 63(6) (2016) 1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Koch A, Thiemann M, Grabenbauer M, Yoon Y, McNiven MA, Schrader M, Dynamin-like protein 1 is involved in peroxisomal fission, The Journal of biological chemistry 278(10) (2003) 8597–605. [DOI] [PubMed] [Google Scholar]
- [39].Li X, Gould SJ, The dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11, The Journal of biological chemistry 278(19) (2003) 17012–20. [DOI] [PubMed] [Google Scholar]
- [40].Williams C, Opalinski L, Landgraf C, Costello J, Schrader M, Krikken AM, Knoops K, Kram AM, Volkmer R, van der Klei IJ, The membrane remodeling protein Pex11p activates the GTPase Dnm1p during peroxisomal fission, Proceedings of the National Academy of Sciences of the United States of America 112(20) (2015) 6377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sciarretta S, Maejima Y, Zablocki D, Sadoshima J, The Role of Autophagy in the Heart, Annu Rev Physiol 80 (2018) 1–26. [DOI] [PubMed] [Google Scholar]
- [42].Zorov DB, Juhaszova M, Sollott SJ, Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol Rev 94(3) (2014) 909–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ikeda Y, Shirakabe A, Brady C, Zablocki D, Ohishi M, Sadoshima J, Molecular mechanisms mediating mitochondrial dynamics and mitophagy and their functional roles in the cardiovascular system, J Mol Cell Cardiol 78 (2015) 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kanki T, Wang K, Baba M, Bartholomew CR, Lynch-Day MA, Du Z, Geng J, Mao K, Yang Z, Yen WL, Klionsky DJ, A genomic screen for yeast mutants defective in selective mitochondria autophagy, Mol Biol Cell 20(22) (2009) 4730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mao K, Wang K, Liu X, Klionsky DJ, The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy, Dev Cell 26(1) (2013) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee Y, Lee HY, Hanna RA, Gustafsson AB, Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes, Am J Physiol Heart Circ Physiol 301(5) (2011) H1924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H, Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain, EMBO J 33(23) (2014) 2798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J, Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure, Circulation 133(13) (2016) 1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Honda S, Arakawa S, Nishida Y, Yamaguchi H, Ishii E, Shimizu S, Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes, Nature communications 5 (2014) 4004. [DOI] [PubMed] [Google Scholar]
- [50].Cho HM, Ryu JR, Jo Y, Seo TW, Choi YN, Kim JH, Chung JM, Cho B, Kang HC, Yu SW, Yoo SJ, Kim H, Sun W, Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System, Molecular cell 73(2) (2019) 364–376.e8. [DOI] [PubMed] [Google Scholar]
- [51].Lemasters JJ, Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3), Redox Biol 2 (2014) 749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Anding AL, Wang C, Chang TK, Sliter DA, Powers CM, Hofmann K, Youle RJ, Baehrecke EH, Vps13D Encodes a Ubiquitin-Binding Protein that Is Required for the Regulation of Mitochondrial Size and Clearance, Current biology : CB 28(2) (2018) 287–295.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ, Dorn GW 2nd, Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts, Circ Res 117(4) (2015) 346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yamashita SI, Jin X, Furukawa K, Hamasaki M, Nezu A, Otera H, Saigusa T, Yoshimori T, Sakai Y, Mihara K, Kanki T, Mitochondrial division occurs concurrently with autophagosome formation but independently of Drp1 during mitophagy, The Journal of cell biology 215(5) (2016) 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, Yasui H, Ueda H, Akazawa Y, Nakayama H, Taneike M, Misaka T, Omiya S, Shah AM, Yamamoto A, Nishida K, Ohsumi Y, Okamoto K, Sakata Y, Otsu K, Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation, Nature communications 6 (2015) 7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Roy M, Kageyama Y, Iijima M, Sesaki H, PARK2/Parkin becomes critical when DNM1L/Drp1 is absent, Autophagy 11(3) (2015) 573–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Burman JL, Pickles S, Wang C, Sekine S, Vargas JNS, Zhang Z, Youle AM, Nezich CL, Wu X, Hammer JA, Youle RJ, Mitochondrial fission facilitates the selective mitophagy of protein aggregates, The Journal of cell biology 216(10) (2017) 3231–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA, Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control, EMBO J 33(4) (2014) 282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basanez G, Hardwick JM, Mitochondrial fission proteins regulate programmed cell death in yeast, Genes & development 18(22) (2004) 2785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ivanovska I, Hardwick JM, Viruses activate a genetically conserved cell death pathway in a unicellular organism, The Journal of cell biology 170(3) (2005) 391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jagasia R, Grote P, Westermann B, Conradt B, DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans, Nature 433(7027) (2005) 754–60. [DOI] [PubMed] [Google Scholar]
- [62].Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K, Mitochondrial disruption in Drosophila apoptosis, Developmental cell 12(5) (2007) 793–806. [DOI] [PubMed] [Google Scholar]
- [63].Goyal G, Fell B, Sarin A, Youle RJ, Sriram V, Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster, Developmental cell 12(5) (2007) 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R, Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis, Mol Cell 16(1) (2004) 59–68. [DOI] [PubMed] [Google Scholar]
- [65].Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ, The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis, Dev Cell 1(4) (2001) 515–25. [DOI] [PubMed] [Google Scholar]
- [66].Germain M, Mathai JP, McBride HM, Shore GC, Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis, The EMBO journal 24(8) (2005) 1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ, Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis, Molecular biology of the cell 15(11) (2004) 5001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wasiak S, Zunino R, McBride HM, Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death, The Journal of cell biology 177(3) (2007) 439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Galluzzi L, Kepp O, Chan FK, Kroemer G, Necroptosis: Mechanisms and Relevance to Disease, Annual review of pathology 12 (2017) 103–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang Z, Jiang H, Chen S, Du F, Wang X, The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways, Cell 148(1–2) (2012) 228–43. [DOI] [PubMed] [Google Scholar]
- [71].Moriwaki K, Farias Luz N, Balaji S, De Rosa MJ, O’Donnell CL, Gough PJ, Bertin J, Welsh RM, Chan FK, The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages, Journal of immunology (Baltimore, Md. : 1950) 196(1) (2016) 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R, RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway, Nature immunology 15(12) (2014) 1126–33. [DOI] [PubMed] [Google Scholar]
- [73].Dong Y, Undyala VVR, Przyklenk K, Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment, Basic research in cardiology 111(5) (2016) 59. [DOI] [PubMed] [Google Scholar]
- [74].Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H, The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice, J Cell Biol 186(6) (2009) 805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Song M, Dorn GW 2nd, Mitoconfusion: Noncanonical Functioning of Dynamism Factors in Static Mitochondria of the Heart, Cell Metab 21(2) (2015) 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D, Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction, Journal of the American Heart Association 2(5) (2013) e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL, Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission, FASEB J 28(1) (2014) 316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ, Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury, Circulation 121(18) (2010) 2012–22. [DOI] [PubMed] [Google Scholar]
- [79].Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J, Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization, Developmental cell 14(2) (2008) 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW 2nd, Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin, Circ Res 114(2) (2014) 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF, miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1, Nat Med 17(1) (2011) 71–8. [DOI] [PubMed] [Google Scholar]
- [82].Jhun BS, J OU, Adaniya SM, Mancini TJ, Cao JL, King ME, Landi AK, Ma H, Shin M, Yang D, Xu X, Yoon Y, Choudhary G, Clements RT, Mende U, Sheu SS, Protein kinase D activation induces mitochondrial fragmentation and dysfunction in cardiomyocytes, The Journal of physiology 596(5) (2018) 827–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dhingra A, Jayas R, Afshar P, Guberman M, Maddaford G, Gerstein J, Lieberman B, Nepon H, Margulets V, Dhingra R, Kirshenbaum LA, Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes, Free radical biology & medicine 112 (2017) 411–422. [DOI] [PubMed] [Google Scholar]
- [84].Catanzaro MP, Weiner A, Kaminaris A, Li C, Cai F, Zhao F, Kobayashi S, Kobayashi T, Huang Y, Sesaki H, Liang Q, Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy, FASEB journal : official publication of the Federation of American Societies for Experimental Biology (2019) fj201802663R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Joshi AU, Ebert AE, Haileselassie B, Mochly-Rosen D, Drp1/Fis1-mediated mitochondrial fragmentation leads to lysosomal dysfunction in cardiac models of Huntington’s disease, Journal of molecular and cellular cardiology 127 (2019) 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hasan P, Saotome M, Ikoma T, Iguchi K, Kawasaki H, Iwashita T, Hayashi H, Maekawa Y, Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats, Journal of molecular and cellular cardiology 121 (2018) 103–106. [DOI] [PubMed] [Google Scholar]
- [87].Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D, Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy, Journal of molecular and cellular cardiology 130 (2019) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen KH, Dasgupta A, Lin J, Potus F, Bonnet S, Iremonger J, Fu J, Mewburn J, Wu D, Dunham-Snary K, Theilmann AL, Jing ZC, Hindmarch C, Ormiston ML, Lawrie A, Archer SL, Epigenetic Dysregulation of the Dynamin-Related Protein 1 Binding Partners MiD49 and MiD51 Increases Mitotic Mitochondrial Fission and Promotes Pulmonary Arterial Hypertension: Mechanistic and Therapeutic Implications, Circulation 138(3) (2018) 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang W, Fernandez-Sanz C, Sheu SS, Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart, Biochimica et biophysica acta. Molecular basis of disease 1864(5 Pt B) (2018) 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Coronado M, Fajardo G, Nguyen K, Zhao M, Kooiker K, Jung G, Hu DQ, Reddy S, Sandoval E, Stotland A, Gottlieb RA, Bernstein D, Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand, Circulation research 122(2) (2018) 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhang H, Wang P, Bisetto S, Yoon Y, Chen Q, Sheu SS, Wang W, A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration, Cardiovasc Res 113(2) (2017) 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Brand CS, Tan VP, Brown JH, Miyamoto S, RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes, Cellular signalling 50 (2018) 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Westermann B, Bioenergetic role of mitochondrial fusion and fission, Biochimica et biophysica acta 1817(10) (2012) 1833–8. [DOI] [PubMed] [Google Scholar]
- [94].Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A, Yadava N, Ge SX, Francis TC, Kennedy NW, Picton LK, Kumar T, Uppuluri S, Miller AM, Itoh K, Karbowski M, Sesaki H, Hill RB, Polster BM, The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species, Developmental cell 40(6) (2017) 583–594.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


