Abstract
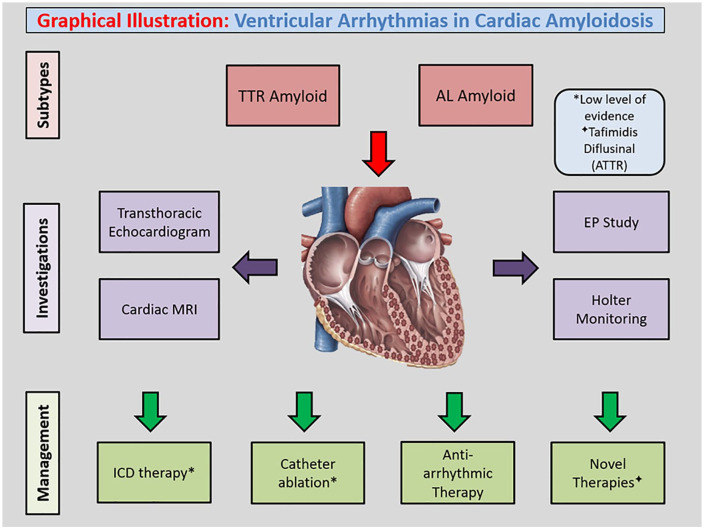
Cardiac Amyloidosis is an infiltrative cardiomyopathy which occurs secondary to deposition of mis-folded protein in the myocardium, with the two most common subtypes being AL amyloidosis and TTR amyloidosis. The pathogenesis of the disease is multifaceted and involves a variety of mechanisms including an inflammatory response cascade, oxidative stress and subsequent separation of myocyte fibrils. Cardiac Amyloidosis frequently results in congestive cardiac failure and arrhythmias, from a disruption in cardiac substrate with subsequent electro-mechanical remodelling. Disease progression is usually demonstrated by development of progressive pump failure, which may be seen with a high arrhythmic burden, usually portending a poor prognosis. There is a paucity of literature on the clinical implications of ventricular arrhythmias in the context of cardiac amyloidosis. The important diagnostic investigations for these patients include transthoracic echocardiography, cardiac magnetic resonance imaging and an electrophysiology study. Whilst there are no robust management guidelines, studies have indicated benefits from contemporary pharmacological therapy and case-by-case catheter ablation. There are novel directed therapies available for TTR amyloidosis that have shown to improve overall survival. The role of ICD therapy in cardiac amyloidosis is controversial, with benefits seen predominantly in early phases of the disease process. The only definitive surgical therapy includes heart transplantation, but is largely indicated for progressive decompensated heart failure (Figure 1). Further large-scale studies are required to better outline management paradigms for treating ventricular arrhythmias in cardiac amyloidosis.
Keywords: Cardiac amyloidosis, Ventricular Tachycardia
Introduction
Cardiac amyloidosis (CA) is characterised by the aggregation of misfolded protein deposits in the extracellular space with a resultant distortion of the myocardium.1 The prevalence of CA is approximately 6 to 10 per million population, and is largely a disease of middle-age and elderly population groups.2 The leading cause of morbidity and mortality in these patients is from development of restrictive cardiomyopathy with progressive congestive cardiac failure (CCF).2 The two main forms of amyloidosis are transthyretin (TTR) and light chain (AL).3 Amyloidosis resulting from TTR is termed ATTR and has two distinct phenotypes: ATTR wild-type (also termed senile ATTR), in which the TTR deposition occurs over decades, and a familial ATTR caused by a mutation in the TTR gene.1-3 On the contrary, AL amyloidosis is a haematological disorder resulting in an excessive proliferation of an abnormal plasma cell clone, overproducing lambda or kappa light chains.
Whilst both forms of amyloid subtypes have a predilection to the development of amyloid cardiomyopathy, the underlying electrical burden and arrhythmogenic substrate varies between the two, carrying distinct clinical implications.4 The resultant disruption of the cardiac conduction system in patients with CA predisposes to arrhythmias, with the most frequent identified as atrial fibrillation (45-65%) and less frequently, atrioventricular conduction delays (3.5%) and ventricular tachyarrhythmias (VT 9.9% and VF 0.7% respectively).5,6 Literature however currently states the patients with AL CA have a higher propensity to develop arrhythmias.3 There is currently a paucity of literature on the prognostic benefit of treatment of ventricular arrhythmias in CA.
Epidemiology of Ventricular Arrhythmias
Conduction abnormalities may represent terminal events for patients with CA, especially those with AL subtype.7 The prevalence of non-sustained ventricular tachycardia (NSVT) in AL amyloidosis ranges from 5 to 27% with routine monitoring,7-9 and 100% during the stem cell transplant period.10 See Table 1. The prevalence of VT in the ATTR population is less often reported, but small studies suggest a prevalence of approximately 17%.11 See Table 1. Additionally, as AL amyloidosis is more likely to cause end-stage CCF when compared to TTR, there is a higher preponderance to development of ventricular arrhythmias. The clinical significance of ventricular arrhythmias are varied with some studies suggesting a prognostic role,7,10 with some studies reporting no such correlation,12 and other studies suggesting an inverse relationship between the presence of premature ventricular conduction (PVC) and survival.13
Table 1.
Summary of studies investigating the prevalence of ventricular tachy-arrhythmias in cardiac amyloidosis.
| Study summary | Number of patients | Type of cardiac amyloid | Method of monitoring | Non-sustained VT in % of patients | Other related findings |
|---|---|---|---|---|---|
| Palladini et al7 | 51 | AL | 24-hour Holter monitoring | Non-sustained VT in 18% of patients. | Ventricular tachycardia was a significant prognostic determinant for survival (P = 0.04). |
| Sayed et al8 | 20 | AL with symptoms of pre-syncope or syncope | Implantable loop recorder | Non-sustained VT in 5% of patients | Terminal syncopal event was marked by bradycardia, not tachycardia, in every available recording. |
| Murtagh et al9 | 127 | AL | Electrocardiogram | Non-sustained VT in 1% of patients | Premature ventricular contractions were noted in 13% of patients. |
| Goldsmith et al10 | 24 | AL monitored peri autologous stem cell transplantation | Telemetry, average of 24 days | Non-sustained VT in 100% of patients | In the deceased patients (n = 3), VT/VF events were the highest of all 24 patients. There was a correlation between VT and serum BNP levels before SCT (r = 0.47, P = 0.019) and during admission for SCT (r = 0.62, P = 0.0012), serum creatinine before SCT (r = 0.62, P = 0.001), and inverse relationship with cardiac output (r = –0.72, P < 0.001) |
| Dubrey et al12 | 232 | AL | 24-hour Holter monitoring | Non-sustained VT in 26.7% of patients | Ventricular tachycardias were not associated with increased risk of sudden cardiac death. |
| Hörnsten et al11 | 30 | ATTR (ATTR Val30Met trait), before liver transplant | 24-hour Holter monitoring | Non-sustained VT in 16.7% of patients | – |
| Varr et al45 | 31 | AL 77%, ATTR 23% (V122I mutation 10%, wild type 13%) | ICD, permanent pacemaker, telemetry | VT in 74% of patients | ICD therapy was successful in most patients, with therapy in 4 of 5 (80%) patients resulting in the termination of the arrhythmia. |
| Kristen et al13 | 19 | AL with ICD insitu | ICD | VT/VF in 11% of patients | Ventricular extra beats (grade IVa or higher) were present more often in non-survivors than in survivors (P < 0.05). |
| Hamon et al49 | 45 | Familial ATTR 60%, AL 27%, senile ATTR 13%. All with ICD in situ | ICD | VT/VF in 27% of patients | Inappropriate shocks was uncommon and occurred in 2 patient (4.4%). |
Pathophysiology of Ventricular Arrhythmias
There are a variety of mechanisms that form the fundamental pathogenesis for ventricular arrhythmias in CA. Firstly, amyloid fibril deposition with a resultant inflammatory response and oxidative stress leads to a separation of myocytes resulting in remodelling and left ventricular (LV) fibrosis, which progressively develops arrhythmogenic potential.1-2,4 Secondly, amyloid fibril deposition in the conduction system can potentiate arrhythmias.14 Thirdly, micro- and macro- vascular myocardial ischaemia from amyloid deposition, can be demonstrated by regional myocardial dysfunction as assessed by cardiac MRI.15
Ventricular Arrhythmias: Diagnostics
There are several key investigations that may assist in predicting VT in patients with CA and allow early stratification of these high-risk population groups. On ventricular EP analysis, Orini et al. demonstrated that patients with CA have a marked reduced epicardial signal amplitude (P < 0.001), slower intraventricular conduction time (P < 0.001) and higher markers of conduction abnormality (P < 0.001) when compared to healthy volunteers.3
Figure 1.
Graphical Illustration: Ventricular Arrhythmias in Cardiac Amyloidosis.
There have also been studies assessing the relationship between cardiac monitoring findings and VT in CA. In a recent study of 239 patients with AL CA, whilst the presence of NSVT conferred worse survival prior to stem cell transplant, cardiac monitoring only detected its presence in approximately 25% of patients.16 Sayed et al. found that amongst a prospective series of 20 patients with syncope in the context of severe CA, only one episode of VT was documented, with an overall 13 deaths. The remaining arrhythmias detected were either atrial arrhythmias or atrio-ventricular conduction block.8
It is well known that patients with structural heart disease have a lower threshold in developing arrhythmias, and this may be the case for patients with CA, specifically the AL subtype.17 Several echocardiographic correlates of structural heart disease are known to result in VT, for example evidence of elevated LVIDs (P = 0.02), impaired LVEF (<30%, P = 0.03), widened QRS (>125 m-sec, P = 0.049), age > 65 (P = 0.047) a prior history of CCF (P = 0.03), and active diuretic therapy (P = 0.02), when compared to patients with absence of VT.17-18 These specific clinical and echocardiographic findings are frequently seen in patients with CA, and therefore such high risk populations should undergo frequent monitoring.18 Similarly, a study by Falk et al. has shown that in patients with CA who present with high grade ventricular arrhythmias, there is more likely to be a history of congestive cardiac failure (P < 0.001) and an abnormal echocardiogram (P < 0.001), when compared to patients without arrhythmias.19 Whilst a reduction in the mean longitudinal strain by echocardiography has been shown to be an independent predictor of cardiovascular death, there is a lack of data on the underlying mechanism.20 These findings highlight the relationship between structural heart disease, heart failure and ventricular tachycardia. Therefore, structural assessment in these patients may provide prognostic value and allow for more appropriate medical therapy.
There is a growing role for cardiac MRI (CMR) in the assessment of CA, especially in displaying late gadolinium enhancement (LGE), which typically occurs in a global subendocardial pattern.21 This is important as LGE is increasingly being recognised as a marker of amyloidogenesis and fibrosis and a surrogate of arrhythmogenic potential. This substrate has been associated with increased risk LGE-predicted death (HR 5.4; 95% CI, 2.1-13.7; P < 0.0001), which may represent both heart failure and potential lethal arrhythmias.22 In addition to this, T2 imaging for assessment of myocardial oedema is a surrogate of active inflammation, and has been intrinsically linked to arrhythmogenic potential.23 The finding of LGE-associated fibrosis on CMR has been shown be significantly increase the risk of sudden cardiac death and recurrent ventricular arrhythmias.24 Therefore, CMR is useful as both a diagnostic and prognostic tool for patients with CA.
Electrophysiology (EPS) study is a useful assessment tool in CA, as it can determine the extent of conduction disease, risk of sudden death, determine underlying rhythm and stratify populations to guide catheter ablation therapies. A study of 25 biopsy-proven AL CA by Reisinger et al. has shown that 92% of patients had a prolonged His-ventricular (HV) interval (>55 ms), which is indicative of distal conduction disease.25 Importantly, a prolonged HV interval during EPS and / or the presence of a late potential (on signal averaged ECG) was shown to be an independent predictor of sudden death (P = 0.05) on multivariate analysis amongst patients with AL CA amyloid.23 Another major predictor of sudden death is presence of pre-syncope or syncope (P = 0.026), which may be caused by underlying conduction disease or ventricular arrhythmia. The main identified cause of death in this study was ventricular arrhythmias and/or electromechanical dissociation.25
Interestingly, inducibility of VT on EP study was not associated with a significant increase in sudden death (P = 0.77). The other important aspect of performing an EP study is assessment of distal conduction disease and VT inducibility, as CA predisposes to variety of conduction diseases including VT.26 EP studies are also useful in stratifying high risk populations in order to guide catheter ablation therapies.
The different subtypes of CA manifest with specific conduction abnormalities identified by EPS. Using electrocardiographic imaging, Orini et al showed that ventricular conduction and repolarisation abnormalities were more pronounced in AL amyloidosis compared to ATTR.3 Specifically, those with AL amyloidosis had more epicardial abnormalities in the form of lower epicardial signal amplitudes (1.07/0.46 vs 1.83/1.26 mV, P = 0.02), higher degree of epicardial signal fractionation (P = 0.02) and longer dispersion of repolarisation (187.6/65 vs 158.3/40 ms, P = 0.06). These findings indicate that patients with AL amyloidosis have an increased risk of developing ventricular arrhythmias.
Prognosis
Amyloid infiltration is more severe in ATTR when compared to AL amyloidosis, which ultimately results in a greater LV mass, and higher rates of congestive cardiac failure.2 Despite these phenotypic differences, AL amyloidosis confers a worse prognosis with high rates of SCD (~33% of patients) in the first 3 months of diagnosis.8 This may be secondary to higher spatial heterogenous conduction and repolarisation, a marker of arrhythmogenesis and overall worse prognosis, as shown by Orini et al.3
Management
The management of ventricular arrhythmias in CA requires a different approach to standard ventricular arrhythmia guidelines in the context of heart failure or conventional cardiomyopathies. There are several arrhythmias that may occur in the context of CA, with atrial arrhythmias being the most common. The most frequent ventricular arrhythmia in CA however is VT.17,27 The treatment of VT can be subdivided into cardiac anti-arrhythmic medications, procedural-based therapy and surgical modalities. Patients presenting with VT frequently undergo extensive investigation, including cardiac MRI to evaluate myocardial LGE or scar, as it provides prognostic information and may identify a site of origin of VT that can then be effectively targeted by catheter ablation.28 This work-up may be of benefit in patients with CA presenting with ventricular arrhythmias. Conventional pharmacotherapies that are used in treatment of VT include oral anti-arrhythmic agents and beta-blockade.
Beta-blockers, whilst used widely in the suppression of VT with beta-adrenergic antagonist activity, may be detrimental with a resultant loss in cardiac output, in patients with CA.5 Amiodarone, another commonly used, and effective therapy for VT, may result in complications when treating patients with CA, namely prolongation of QTc and torsades de pointes, worsening of systolic function through inherent beta antagonistic activity and complete heart block when compared to patients without CA, as shown in a study (43.8% vs 30.0%, P < 0.0001).29 These findings indicate poorer overall outcomes with conventional pharmacological therapy for VT, when used in patients with CA.
A study by Le Bras et al. demonstrated that dexamethasone as an induction agent for treatment of AL CA patients may potentiate fluid retention and promote arrhythmias, hence resulting in an arrhythmogenic ventricular substrate.30 Similarly, high dose chemotherapy, specifically cyclophosphamide and bortezomib, in AL amyloidosis can be cardio-toxic in nature and result in VT due to myocardial dysfunction.31,32 Accordingly, it is important to consider the primary aetiology of CA, as directed treatment may precipitate ventricular arrhythmias.
The advent of disease modifying therapy for TTR amyloidosis, have revolutionised management protocols. Tafimidis, a transthyretin stabiliser, prevents overall tetramer dissociation and amyloidogenesis and therefore has been shown to reduce all-cause mortality and cardiovascular hospitalisations.33 Similarly, diflusinal is a non-steroidal drug that also stabilises the TTR tetramer and has shown to stabilise LVmass (g), (baseline vs followup; 384 vs 331, P = 0.36) and LVEF (%) (baseline vs followup; 48 vs 50, P = 0.61) indicating a halt in progression of cardiac amyloidogenesis.34 By effectively preventing amyloidogenesis, these 2 therapies have been shown to result in reduction in incidence of sudden cardiac death with the potential to mitigate ventricular arrhythmias in the TTR population.
Additionally, close monitoring for Torsades de Pointes is important as there are reports of patients who present with polymorphic ventricular tachycardia from an amyloidosis - induced prolongation of QTc. The proposed mechanism is thought to be due to amyloid fibril deposition in the intrinsic myocardial conduction system.32,33 Treatment in these patients should be targeted at avoiding QT prolonging medications, correcting electrolyte abnormalities and appropriate cardiac monitoring.35,36 Furthermore, digoxin and calcium channel blockers should be avoided in these patients as they may result in toxicity and impaired myocardial contractility.
Whilst the role of catheter ablation for treatment for atrial arrhythmias is evolving, with current literature showing potential benefits in terms of recurrence rate and overall survival, there is a lack of data on its use in ventricular arrhythmias.37,38 Other than case reports of successful radiofrequency VT ablation, there have been no large-scale studies assessing the role of VT/VF ablation in CA.39,40 Catheter ablation of VT has been shown to have some utility patients with other infiltrative cardiomyopathies, such as cardiac sarcoidosis, and therefore it may be a viable option for patients with CA.41 Further large-scale studies are required to assess the risk and benefits of catheter ablation for ventricular tachycardia in CA, with no current data showing a mortality benefit.
The development of refractory heart failure is usually seen with incessant VT and portends a poor prognosis. Epicardial mapping and catheter ablation are also options for management of incessant VT.42 The definitive surgical therapy for treatment of end-stage heart failure, which may be seen with recurrent ventricular arrhythmias, is an orthotopic cardiac transplantation, an option in the setting of failed medical management.43 Heart transplantation is an effective therapy for end-stage CA with good follow-up survival rates, with absence of recurrent amyloid deposition in cardiac allograft.44
Device Therapy
The Stanford Amyloid Center’s ICD implantation criteria is a proposed standard for appropriate implantation in patients with CA. It assesses patients who have high risk of SCD, but a good quality of life and minimal heart failure symptoms as assessed by the New York Heart Association classification (NYHA).45 It is currently however unknown which subtype of CA benefits the most from ICD therapy. Available evidence however current supports the use of Stanford Amyloid Center’s ICD implantation criteria for all subtypes, with a stronger focus for secondary prevention, as opposed to primary prevention.20
Sudden cardiac death due to conduction-system disease is a common cause of mortality amongst patients with CA, especially those with AL amyloidosis and elevated biomarkers, including cardiac troponins and NT-pro BNP. Increased burden of ventricular arrhythmias are associated with increased mortality, and so detection of NSVTs and reports of syncope should prompt immediate clinical concerns. As such, ICDs are proposed to mitigate some risk of sustained VT as both primary and secondary prevention strategies.45,46
Currently, there is no consensus on the absolute benefit that ICDs can confer patients with CA. Whilst its potential to eliminate fatal ventricular arrhythmias is supported by select and anecdotal cases, the overall effectiveness of ICDs and their safety remains unclear.47 Most decisions made regarding ICD insertions are largely patient-centred and based upon local expertise. Proponents of ICDs suggest that patients with CA with previously identified NSVTs are most likely to receive appropriate ICD discharges, thus advocating for its use as secondary prevention.45,48 In a study of 45 people, of whom the majority (73%) had TTR CA, ICD discharges were most appropriate in those with less advanced cardiomyopathy. However, overall survival was not impacted by the burden of ventricular arrhythmias.49 In another study, ICD therapy was not associated with prolonged survival in patients with CA when compared to patients without ICD therapy (44 vs 40 months, P = 0.76). Compared to patients without CA, patients with the disease had a significantly higher mortality (40.5 vs 26.2%, P < 0.045), despite ICD therapy.50
There is an increasing awareness that the primary driver of mortality in these patients is electromechanical dissociation, as opposed to ventricular tachycardia.51,52 It should however be noted that despite a lack of data on ventricular arrhythmias in this patient group, several studies have found that the majority of detected ventricular arrhythmias are non-sustained.10 In a study of 19 patients with AL amyloidosis, only 2 patients received appropriate ICD discharges for ventricular tachyarrhythmias whilst 6 patients died from sudden cardiac deaths due to electromechanical dissociation despite ICD insertion. Electromechanical dissociated is likely a manifestation of progressive deterioration in heart failure not amenable to ICD therapy.13
When non-sustained VT and syncope are captured and documented, ICD implantation for prevention of SCD in CA is most likely a reasonable approach.13,46,52 Specifically, this would be most appropriate for those with AL amyloidosis whereby ICD implantation was is associated with a high rate of appropriate discharges for ventricular arrhythmias (32% in the first year, in one series). However, a significant mortality benefit has not been observed for this group.13
For primary prevention in CA, the decision to implant an ICD does require a stringent patient selection criterion. That is, an ICD is likely suitable in early stages of AL amyloidosis whereby predicted survival is favourable with less impairment in LV systolic function and minimally raised cardiac - specific biomarkers. This can be assessed using biomarkers such as troponin and NT-pro BNP, to indicate an earlier stage of CA with absence of cardiac failure.28 This is likely due to the impression that ICD placement would offer no further benefit in those with lower LVEF, a strong indicator of progressive heart failure and an increased risk of electromechanical dissociation.13 Finally, specifically for AL amyloidosis, ICD implantation for primary prevention may also be reasonable for patients awaiting heart transplantation or a mechanical left ventricular assist device either as destination therapy or bridge-to-transplantation. This is only if perceived survival is meaningful (greater than 1 year), NT-pro BNP is less than 8500 ng/L and NYHA Functional Class is less than IV.53
Future therapies for the management of arrhythmias in CA are being particularly investigated with disease modifying treatments in the TTR population. These management paradigms are aimed at reducing overall arrhythmogenic burden in this population group.54
Conclusion
CA, specifically the AL subtype is associated with higher rates of ventricular arrhythmias, especially VT, when compared to ATTR. Despite current pharmacological therapies and small studies indicating benefits from catheter ablation, there are no robust guidelines on management of ventricular arrhythmias in CA. The benefit of ICD implantation varies from a case-to-case basis, with benefit seen only in early stages of CA. Further studies are required to delineate an optimal diagnostic pathway and appropriate management paradigm for this select population.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Shaun Khanna  https://orcid.org/0000-0002-6741-1096
https://orcid.org/0000-0002-6741-1096
References
- 1. Dubrey S, Hawkins P, Falk R. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75-84. [DOI] [PubMed] [Google Scholar]
- 2. Martinez-Naharro A, Hawkins PN, Fontana M. Cardiac amyloidosis. Clin Med (Northfield Il). 2018;18:s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orini M, Graham AJ, Martinez-Naharro A, et al. Noninvasive mapping of the electrophysiological substrate in cardiac amyloidosis and its relationship to structural abnormalities. J Am Heart Assoc. 2019;8:e012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cappelli F, Vignini E, Martone R, et al. Baseline ECG features and arrhythmic profile in transthyretin versus light chain cardiac amyloidosis. Circ Heart Fail. 2020;13:e006619. [DOI] [PubMed] [Google Scholar]
- 5. Rochlani YM, Nishi SN, Hakeem A, Bhatti S. Burden of arrhythmias in patients hospitalized with cardiac amyloidosis. Circulation. 2015;132:A20097. [Google Scholar]
- 6. Sanchis K, Cariou E, Colombat M, et al. Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid. 2019;26:128-138. [DOI] [PubMed] [Google Scholar]
- 7. Palladini G, Malamani G, Co F, et al. Holter monitoring in AL amyloidosis: prognostic implications. Pacing Clin Electrophysiol. 2001;24:1228-1233. [DOI] [PubMed] [Google Scholar]
- 8. Sayed RH, Rogers D, Khan F, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36:1098-1105. [DOI] [PubMed] [Google Scholar]
- 9. Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535-537. [DOI] [PubMed] [Google Scholar]
- 10. Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo RL, Steingart RM. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;104:990-994. [DOI] [PubMed] [Google Scholar]
- 11. Hörnsten R, Wiklund U, Olofsson B-O, Jensen SM, Suhr OB. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation. 2004;78:112-116. [DOI] [PubMed] [Google Scholar]
- 12. Dubrey S, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141-157. [DOI] [PubMed] [Google Scholar]
- 13. Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235-240. [DOI] [PubMed] [Google Scholar]
- 14. Ridolfi RL, Bulkley BH, Hutchins GM. The conduction system in cardiac amyloidosis: clinical and pathologic features of 23 patients. Am J Med. 1977;62:677-686. [DOI] [PubMed] [Google Scholar]
- 15. Li R, Yang Z-g, Wen L-y, et al. Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: assessment with 3T cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2016;18:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sidana S, Tandon N, Brady PA, et al. Prognostic significance of Holter monitor findings in patients with light chain amyloidosis. Mayo Clin Proc. 2019. doi: 10.1016/j.mayocp.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 17. John R. Arrhythmias in cardiac amyloidosis. J Innov Card Rhythm Manag. 2018;9:3051-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagourelias ED, Mirea O, Duchenne J, et al. Echo parameters for differential diagnosis in cardiac amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging. 2017;10:e005588. [DOI] [PubMed] [Google Scholar]
- 19. Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3:107-113. [DOI] [PubMed] [Google Scholar]
- 20. Bellavia D, Pellikka PA, Al-Zahrani GB, et al. Independent predictors of survival in primary systemic (Al) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banypersad SM. The evolving role of cardiovascular magnetic resonance imaging in the evaluation of systemic amyloidosis. Magn Reson Insights. 2019;12:1178623X19843519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fluechter S, Kuschyk J, Wolpert C, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2010;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amano Y, Tachi M, Tani H, Mizuno K, Kobayashi Y, Kumita S. T2-weighted cardiac magnetic resonance imaging of edema in myocardial diseases. Sci World J. 2012;2012:194069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dawson DK, Hawlisch K, Prescott G, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6:335-344. [DOI] [PubMed] [Google Scholar]
- 25. Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol. 1997;30:1046-1051. [DOI] [PubMed] [Google Scholar]
- 26. Catanzaro JN, Makaryus JN, Makaryus AN, et al. Echocardiographic predictors of ventricular tachycardia. Clin Med Insights Cardiol. 2014;8(suppl 4):S18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chamarthi B, Dubrey SW, Cha K, Skinner M, Falk RH. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80:1242-1245. [DOI] [PubMed] [Google Scholar]
- 28. Tilz RR, Lenarczyk R, Scherr D, et al. Management of ventricular tachycardia in the ablation era: results of the European Heart Rhythm Association survey. EP Europace. 2018;20:209-213. [DOI] [PubMed] [Google Scholar]
- 29. Alkindi S, Almasoud A, Younes A, et al. Increased risk of heart block in patients with cardiac amyloidosis on amiodarone. J Card Fail. 2015;21:S125. [Google Scholar]
- 30. Le Bras F, Molinier-Frenkel V, Guellich A, et al. Sequential cyclophosphamide-bortezomib-dexamethasone unmasks the harmful cardiac effect of dexamethasone in primary light-chain cardiac amyloidosis. Eur J Cancer. 2017;76:183-187. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood. 1986;68:1114-1118. [PubMed] [Google Scholar]
- 32. Xiao Y, Yin J, Wei J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007-1116. [DOI] [PubMed] [Google Scholar]
- 34. Castaño A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18:315-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilotra NA, Chow GV, Cingolani OH. Cardiac amyloidosis presenting with prolonged QT interval and recurrent polymorphic ventricular tachycardia. Tex Heart Inst J. 2013;40:193. [PMC free article] [PubMed] [Google Scholar]
- 36. Perlini S, Salinaro F, Cappelli F, et al. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol. 2013;167:2156-2161. [DOI] [PubMed] [Google Scholar]
- 37. Tan NY, Mohsin Y, Hodge DO, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2016;27:1167-1173. [DOI] [PubMed] [Google Scholar]
- 38. Donnellan E, Wazni O, Kanj M, et al. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. EP Europace. 2020;22:259-264. [DOI] [PubMed] [Google Scholar]
- 39. Chung F-P, Lin Y-J, Kuo L, Chen S-A. Catheter ablation of ventricular tachycardia/fibrillation in a patient with right ventricular amyloidosis with initial manifestations mimicking arrhythmogenic right ventricular dysplasia/cardiomyopathy. Korean Circ J. 2017;47:282-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mlcochova H, Saliba WI, Burkhardt DJ, et al. Catheter ablation of ventricular fibrillation storm in patients with infiltrative amyloidosis of the heart. J Cardiovasc Electrophysiol. 2006;17:426-430. [DOI] [PubMed] [Google Scholar]
- 41. Muser D, Santangeli P, Pathak RK, et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9:e004333. [DOI] [PubMed] [Google Scholar]
- 42. Shumway SJ, Johnson EM, Svendsen CA, Kriett JM, Ring WS. Surgical management of ventricular tachycardia. Ann Thorac Surg. 1997;63:1589-1591. [DOI] [PubMed] [Google Scholar]
- 43. Estep JD, Bhimaraj A, Cordero-Reyes A, Bruckner B, Loebe M, Torre-Amione G. Heart transplantation and end-stage cardiac amyloidosis: a review and approach to evaluation and management. Methodist Debakey Cardiovasc J. 2012;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Donnelly J, Soltesz E, Tong M, et al. Heart transplantation in cardiac amyloidosis: a ten year experience. J Heart Lung Transplant. 2019;38:S389. [Google Scholar]
- 45. Varr BC, Zarafshar S, Coakley T, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11:158-162. [DOI] [PubMed] [Google Scholar]
- 46. Falk RH, Dubrey SW. Amyloid Heart Disease. Amyloidosis: Springer; 2010:107-128. [Google Scholar]
- 47. Lin G, Dispenzieri A, Brady PA. Successful termination of a ventricular arrhythmia by implantable cardioverter defibrillator therapy in a patient with cardiac amyloidosis: insight into mechanisms of sudden death. Eur Heart J. 2010;31:1538. [DOI] [PubMed] [Google Scholar]
- 48. Chen J, Johnson G, Hellkamp AS, et al. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden cardiac death in heart failure trial). J Am Coll Cardiol. 2013;61:2161-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamon D, Algalarrondo V, Gandjbakhch E, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562-568. [DOI] [PubMed] [Google Scholar]
- 50. Kim EJ, Holmes B, Huang S, et al. Does an implantable defibrillator alter outcomes in patients with cardiac amyloidosis? J Am Coll Cardiol. 2019;73:837. [Google Scholar]
- 51. Cheung CC, Roston TM, Andrade JG, Bennett MT, Davis MK. Arrhythmias in cardiac amyloidosis: challenges in risk stratification and treatment. Can J Cardiol. 2019;36:416-423. [DOI] [PubMed] [Google Scholar]
- 52. Bart NK, Thomas L, Korczyk D, Atherton JJ, Stewart GJ, Fatkin D. Amyloid cardiomyopathy. Heart Lung Circ. 2019;29:575-583. [DOI] [PubMed] [Google Scholar]
- 53. John RM, Stern DL. Use of implantable electronic devices in patients with cardiac amyloidosis. Can J Cardiol. 2019;36:408-415. [DOI] [PubMed] [Google Scholar]
- 54. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020; 142:e7–e22. [DOI] [PubMed] [Google Scholar]