Abstract
A novel member of human RNA coronavirus, which is an enveloped betacoronavirus, has been termed severe acute respiratory syndrome coronavirus-2 (SARS COV-2). The illness caused by SARS COV-2 is referred to as the coronavirus disease 2019 (COVID-19). It is a highly contagious disease that has resulted in a global pandemic. The clinical spectrum of COVID-19 ranges from asymptomatic illness to acute respiratory distress syndrome, septic shock, multi-organ dysfunction, and death. The most common symptoms include fever, fatigue, dry cough, dyspnea, and diarrhea. Neurological manifestations have also been reported. However, the data on the association of Guillain-Barré syndrome (GBS) with COVID-19 are scarce. We report a rare case of a COVID-19-positive 36-year-old immunocompromised male who presented with clinical features of GBS. His clinical examination showed generalized weakness and hyporeflexia. The cerebrospinal fluid (CSF) analysis showed albuminocytological dissociation. Intravenous immunoglobulin (IVIG) was administered based on the high clinical suspicion of GBS. The patient’s neurological condition worsened with progression to bulbar weakness and ultimately neuromuscular respiratory failure requiring mechanical ventilation. His nerve conduction studies were consistent with demyelinating polyneuropathy. He received five plasma exchange treatments and was successfully weaned from mechanical ventilation. A brain and cervical spine magnetic resonance imaging was obtained to rule out other causes, which was normal. COVID-19 is believed to cause a dysregulated immune system, which likely plays an important role in the neuropathogenesis of GBS.
Keywords: COVID-19, Guillain-Barré syndrome, respiratory failure, GBS
Introduction
Severe acute respiratory syndrome coronavirus-2 emerged as a global pandemic in 2020 spreading from Wuhan city, Hubei province of China in December of 2019.1 At the time of writing this article, robust data are being generated from all corners of the globe on a daily basis to understand this new human pathogen. Patients affected by COVID-19 viral illness have displayed a wide range of symptoms ranging from mild flu-like symptoms to severe life-threatening respiratory failure. Less is known at this juncture regarding the pathogenesis behind neurological complications and long-term sequelae related to COVID-19. To date, only a few case reports have been published citing the incidence of Guillain-Barré syndrome (GBS) in COVID-19 patients.2-8 With this report, we highlight a rare case of GBS in a young patient with COVID-19.
Case Report
A 36-year-old male with a past medical history of hypertension and two renal transplants (2009 and 2012) secondary to renal dysplasia presented to the emergency department (ED) with worsening respiratory distress. The patient was diagnosed with COVID-19 by nasopharyngeal polymerase chain reaction (PCR) around 10 days prior when he developed fever, chills, myalgias, and cough. He self-quarantined at home; however, his respiratory distress gradually progressed, which prompted him to come to the ED. He was on tacrolimus 2 mg twice a day, mycophenolate 540 mg twice a day, and prednisone 5 mg/day for immunosuppression. When he was first diagnosed with COVID-19, his mycophenolate dose was reduced to 360 mg twice a day. His tacrolimus dosing was also adjusted based on daily serum tacrolimus levels. His chest X-ray showed diffuse bilateral pulmonary opacities. He was admitted for acute hypoxemic respiratory failure requiring supplemental oxygen via high-flow nasal cannula. He also received remdesivir with an initial dose of 200 mg, followed by 100 mg daily for the next 4 days. The patient showed a remarkable improvement in his condition and was discharged home after a hospital stay of 6 days without any home oxygen. At the time of discharge, the patient did not have any neurological deficits.
Two days after discharge, the patient began to develop numbness, tingling over his fingers, toes, and perioral region, which then progressed to weakness in legs. Two days later, he presented to the ED and was admitted for progressive and ascending weakness in his legs along with difficulty walking. Neurological examination revealed intact cranial nerves, decreased muscle strength with Medical Research Council (MRC) scale of 3/5 in his lower extremities (LE) and 4/5 in upper extremities (UE) without a significant difference between the proximal and distal muscle groups, areflexia in LE, hyporeflexia in LE, intact sensations, and no evidence of ataxia. Cerebrospinal fluid (CSF) analysis showed a CSF protein of 117 mg/dL, white blood cell count of 1/mm3, glucose of 66 mg/dL, a CSF immunoglobulin G (IgG) index of 14.67 (Ref: <0.86) and high IgG synthesis rate of 293.5 (Ref: 9.2 mg/day) with normal albumin levels. Oligoclonal bands were absent in the CSF. The constellation of neurological examination findings and albuminocytologic dissociation in CSF raised suspicion for acute inflammatory polyneuropathy. The CSF analysis was negative for viral and bacterial meningitis including syphilis and West Nile virus antibodies. His serum antinuclear antibody, thyroid-stimulating hormone, hemoglobin A1c, protein electrophoresis, and tacrolimus level were normal. Serum vitamin B12 level was normal at 660 ng/mL (Ref: 180-914 pg/mL) and folic acid level was 9 ng/mL (Ref: >5.8 ng/mL). His serum HIV, treponemal antibody, urine, and blood cultures were negative. Magnetic resonance imaging (MRI) of the brain and cervical spine were normal. He was found to be moderately hyponatremic (122 mEq/L) on admission, urine studies were consistent with the syndrome of inappropriate antidiuretic hormone secretion (SIADH), and it was gradually corrected over a few days. His other serum electrolytes, kidney, and liver function tests were normal. A nasopharyngeal swab during the current admission continued to test positive for COVID-19 by PCR.
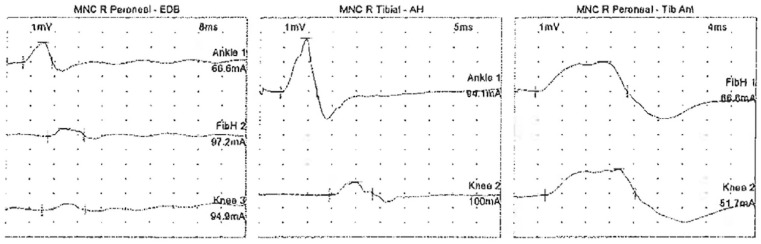
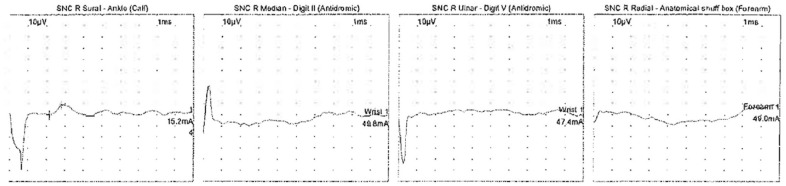
The patient was evaluated by a neurologist on admission. He was initiated on intravenous immunoglobulin (IVIG) therapy for five days at a dose of 0.4 mg/kg/day given the high suspicion for GBS. The patient, however, continued to have progressive weakness including bulbar involvement with dysarthria and soft speech. He continued to have worsening dyspnea, and his chest X-ray showed improving multifocal bilateral patchy pulmonary opacities. On day 6 of his current admission, he developed increased work of breathing with a declining negative inspiratory force (−15 cm of water) and vital capacity requiring intubation for neuromuscular respiratory failure. Nerve conduction studies performed on the right upper and lower extremity revealed decreased motor amplitude and conduction block between the ankle and knee for right tibial and peroneal motor conduction studies (Figure 1). The right median and ulnar motor conduction studies showed decreased amplitude, normal distal latencies, and prolonged F-wave latencies (Tables 1 and 2). The sensory responses of the right median, ulnar, and radial nerves were absent with sural sparing (Figure 2). Needle electromyography was deferred as the patient was on sedation on the ventilator. Patchy, multifocal demyelination with unequivocal conduction block, prolonged F-wave latencies, and sural sparing pointed toward acute inflammatory demyelinating polyneuropathy. Although the patient had completed a course of IVIG, given his rapid decline, plasma exchange of 4000 mL for five cycles was initiated with replacement with 3 L of 5% albumin and 1 L of fresh frozen plasma. He showed an improvement from a respiratory and neuromuscular standpoint after receiving 5 plasma exchange treatments. His creatine kinase (CK) enzyme level was normal on admission, transiently elevated up to 990 U/L while on propofol infusion, and quickly returned to normal within two days. The patient’s course was complicated by superimposed bacterial pneumonia from Haemophilus influenzae and Staphylococcus aureus, for which he received broad-spectrum antibiotics (vancomycin and cefepime). His tracheal aspirate COVID-PCR was negative on day 15 of his hospital stay. He required mechanical ventilation for a total of 13 days. He was successfully extubated on day 19 of his hospital stay when he had a NIF of −41cm of water and was able to pass the spontaneous breathing trial. Following extubation, his motor strength continued to improve, and he was noted to have muscle strength with MRC scale of 4/5 in proximal and 5/5 in distal muscle groups bilaterally before discharge. He was discharged to a rehabilitation facility for physical therapy in a stable condition without any supplemental oxygen after a hospital stay of 23 days.
Figure 1.
Motor nerve conduction (MNC) study of the right peroneal and tibial nerve between the ankle and knee showing decreased amplitude and conduction block. AH, abductor hallucis muscle; EDB, extensor digitorum brevis muscle; FibH, fibular head; Tib Ant, tibialis anterior muscle.
Table 1.
Motor Nerve Conduction Study Findings of the Right (R) Peroneal, Tibial, Median, and Ulnar Nerve.
| Nerve/sites | Muscle | Latency (ms) | Amplitude (mV) | Distance (mm) | Velocity (m/s) |
|---|---|---|---|---|---|
| R peroneal nerve—EDB | |||||
| Ankle | EDB | 5.5 | 1.3 | 70 | |
| FibH | EDB | 13.9 | 0.5 | 300 | 35.8 |
| Knee | EDB | 12.1 | 0.5 | 80 | 45.2 |
| R tibial nerve—AH | |||||
| Ankle | AH | 4.4 | 3.7 | 70 | |
| Knee | AH | 14.5 | 0.9 | 390 | 38.4 |
| R peroneal nerve—Tib Ant | |||||
| FibH | Tib Ant | 3.5 | 2.0 | 100 | |
| Knee | Tib Ant | 5.3 | 1.8 | 90 | 52.4 |
| R median nerve—APB | |||||
| Wrist | APB | 5.8 | 2.1 | 70 | |
| Elbow | APB | 10.6 | 1.7 | 230 | 48.0 |
| R ulnar nerve—ADM | |||||
| Wrist | ADM | 3.3 | 3.3 | 70 | |
| BEL | ADM | 7.9 | 3.1 | 260 | 56.7 |
Abbreviations: ADM, abductor digiti minimi muscle; AH, abductor hallucis muscle; APB, abductor pollicis brevis muscle; BEL, below elbow; EDB, extensor digitorum brevis muscle; FibH, fibular head; Tib Ant, tibialis anterior muscle.
Table 2.
F-Wave Latency of the Right (R) Tibial, Median, and Ulnar Nerve.
| Nerve | F-wave latency (ms) |
|---|---|
| R tibial—AH | 44.6 |
| R median—APB | 35.8 |
| R ulnar—ADM | 37.2 |
Abbreviations: ADM, abductor digiti minimi muscle; AH, abductor hallucis muscle; APB, abductor pollicis brevis muscle.
Figure 2.
Sensory nerve conduction (SNC) study showing a normal response for the right sural nerve, and an absent response for the right median, ulnar, and radial nerve.
Discussion
COVID-19 is a novel coronavirus infection that has been reported to affect multi-organ systems. The major known clinical manifestations of this disease are fever, myalgias, respiratory, and gastrointestinal involvement.1,9 Neurological manifestations in a study by Mao et al were seen in 36.4% of the 214 patients and included headache, dizziness, stroke, and hyposmia.10 GBS following COVID-19 illness has been reported in a few patients around the world so far,2-8 and there have been efforts to create an International Neuromuscular COVID-19 database.
The interval between onset of COVID-19 viral illness and the first symptoms of GBS reported in the literature so far ranges from five days to three weeks.3,5 Our patient developed flaccid hyporeflexic symmetrical tetraparesis within 18 days of the initial COVID-19 diagnosis, and it quickly progressed to neuromuscular respiratory failure requiring mechanical ventilation. While his COVID-19 continued to remain positive from the nasopharyngeal swab, his respiratory compromise was most likely related to neuromuscular weakness as evidenced by his pulmonary mechanics. The likelihood of an infectious etiology remained low as evidenced by a negative infectious workup. Our patient had electrophysiologic evidence of acquired demyelinating polyneuropathy. There is no clear predilection for axonal or demyelinating variants of GBS in COVID-19 in the reported literature so far.3
Interestingly, our patient’s CSF findings suggested possible intrathecal IgG synthesis in addition to albuminocytologic dissociation. Abnormal concentrations of IgG in the CSF and oligoclonal bands are usually attributable to a dysfunctional blood-brain barrier or damaged nerve roots.11,12 Given no clear evidence of a breakdown of the blood-brain barrier, the etiology of elevated IgG in CSF is unclear. Critical illness myopathy is a consideration, but it was unlikely in our patient as our patient developed weakness prior to his critical illness. His CK enzyme was only mildly elevated during the hospital stay and returned to normal range within two days. A normal brain and cervical spine MRI excluded a central cause of his neurological symptoms. Multifocal demyelinating polyneuropathy following tacrolimus therapy has been rarely reported.13 However, our patient’s tacrolimus level was within the therapeutic range and, therefore, could not explain his presentation.
We believe that our patient developed postinfectious acute inflammatory demyelinating polyneuropathy following COVID-19 infection. Emerging literature suggests that dysregulated immune response related to COVID-19 may result in the development of GBS. GBS is a well-described autoimmune disorder causing acute paralytic peripheral neuropathy. It is usually triggered by an infection or other immune stimulus that incites an aberrant immune response against peripheral nerves and/or spinal nerve roots due to molecular mimicry. It is unclear whether COVID-19 drives a humoral response against the gangliosides; a T-cell-mediated disorder; or a direct neuroinvasive phenomenon leading to GBS.4,14 The genetic and environmental factors (such as immunosuppression in our patient) that predispose a patient to develop GBS are unclear.15,16 GBS has been well documented in several autoimmune disorders and has been rarely reported in patients with organ transplants following Cytomegalovirus infection or reactivation.17
GBS is a neurological emergency and clinical diagnosis. Quick recognition of symptoms and diagnosis is important in the management of these patients. We propose that close attention to neurologic complications including GBS should be given in COVID-19 patients. It is becoming increasingly evident that COVID-19 is a multisystem disease with dysregulated immune response, and further studies are needed to understand the neuropathophysiology of this novel infection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. doi: 10.1212/NXI.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204-207. doi: 10.1111/jns.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sedaghat Z, Karimi N. Guillain-Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233-235. doi: 10.1016/j.jocn.2020.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574-2576. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain-Barré syndrome. Rev Neurol (Paris). 2020;176:516-518. doi: 10.1016/j.neurol.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267:1877-1879. doi: 10.1007/s00415-020-09849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Virani A, Rabold E, Hanson T, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20:e00771. doi: 10.1016/j.idcr.2020.e00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. Published online April 13, 2020. doi: 10.1177/0145561320920762 [DOI] [PubMed] [Google Scholar]
- 10. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:71-79. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matà S, Galli E, Amantini A, Pinto F, Sorbi S, Lolli F. Anti-ganglioside antibodies and elevated CSF IgG levels in Guillain-Barré syndrome. Eur J Neurol. 2006;13:153-160. doi: 10.1111/j.1468-1331.2006.01161.x [DOI] [PubMed] [Google Scholar]
- 12. Segurado OG, Krüger H, Mertens HG. Clinical significance of serum and CSF findings in the Guillain-Barré syndrome and related disorders. J Neurol. 1986;233:202-208. doi: 10.1007/BF00314019 [DOI] [PubMed] [Google Scholar]
- 13. Wilson JR, Conwit RA, Eidelman BH, Starzl T, Abu-Elmagd K. Sensorimotor neuropathy resembling CIDP in patients receiving FK506. Muscle Nerve. 1994;17:528-532. doi: 10.1002/mus.880170510 [DOI] [PubMed] [Google Scholar]
- 14. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. doi: 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15:671-683. doi: 10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717-727. doi: 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 17. El-Sabrout RA, Radovancevic B, Ankoma-Sey V, Van Buren CT. Guillain-Barré syndrome after solid organ transplantation. Transplantation. 2001;71:1311-1316. doi: 10.1097/00007890-200105150-00023 [DOI] [PubMed] [Google Scholar]