Abstract
The ketamine metabolite (2R,6R)-hydroxynorketamine has been proposed to have rapid and persistent antidepressant actions in rodents, but its mechanism of action is controversial. We have compared the ability of (R,S)-ketamine with the (2S,6S)- and (2R,6R)-isomers of hydroxynorketamine to affect the induction of N-methyl-d-aspartate receptor–dependent long-term potentiation in the mouse hippocampus. Following pre-incubation of these compounds, we observed a concentration-dependent (1–10 μM) inhibition of long-term potentiation by ketamine and a similar effect of (2S,6S)-hydroxynorketamine. At a concentration of 10 μM, (2R,6R)-hydroxynorketamine also inhibited the induction of long-term potentiation. These findings raise the possibility that inhibition of N-methyl-d-aspartate receptor–mediated synaptic plasticity is a site of action of the hydroxynorketamine metabolites with respect to their rapid and long-lasting antidepressant-like effects.
Keywords: Ketamine, hydroxynorketamine, N-methyl-d-aspartate receptors, long-term potentiation, antidepressant
The remarkably rapid and long-lasting antidepressant actions of ketamine (Berman et al., 2000) have prompted an intensive effort into understanding its mode of action, to aid with the design of improved therapeutic agents for the treatment of depression and prevention of suicide. It has been known for almost 40 years that ketamine is an NMDAR antagonist (Anis et al., 1983) and that NMDARs are major triggers for synaptic plasticity (Collingridge et al., 1983). Therefore, a logical explanation for the rapid and persistent antidepressant effects of ketamine is that antagonism of NMDARs modulates synaptic plasticity in pathways that regulate mood (Collingridge et al., 2017).
More recently, it was reported that certain ketamine metabolites, in particular (2R,6R)-HNK, have rapid antidepressant actions in rodents at concentrations below those that affect NMDARs (Lumsden et al., 2019; Zanos et al., 2016; but see Kavalali and Monteggia, 2018). A potential explanation is that the metabolites enhance AMPA receptor (AMPAR) function downstream of NMDARs, potentially by engaging LTP-like mechanisms.
In this study, we investigated whether HNK regulates LTP of synaptic transmission. We studied the hippocampal Schaffer collateral-commissural pathway to the CA1, as LTP in this pathway is well characterised and known to be sensitive to the actions of ketamine (e.g. Ingram et al., 2018; Stringer and Guyenet, 1983). We found that, like (R,S)-ketamine, both the (2S,6S)- and (2R,6R)-isomers of HNK inhibited LTP at these synapses. These data support the idea that interference with NMDAR-mediated synaptic plasticity may contribute to the rapid antidepressant-like actions in rodents of both ketamine and these two metabolites.
Experiments were performed on mouse hippocampal slices in accordance with the Institutional Animal Care and Use Committee of Seoul National University, as described previously (Kang et al., 2017). Briefly, hippocampal slices were kept in an interface recording chamber and continuously perfused at 2 mL/min with artificial cerebrospinal fluid that comprised (in mM) 124 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 MgSO4, 10 d-glucose and 2 CaCl2 (oxygenated with 95% O2 and 5% CO2 at 32°C). Gamma-aminobutyric acid (GABA) antagonists were not added to the perfusate. Field excitatory postsynaptic potential (fEPSPs) slope measurements were obtained from stratum radiatum in response to test stimulation of the Schaffer collateral-commissural pathway delivered at 0.033 Hz. LTP was induced by a brief period of theta burst stimulation (TBS, three episodes with an inter episode interval of 10 s; each episode comprising five pulses at 100 Hz delivered five times at 5 Hz). We studied LTP in slices from young (P14) mice, so that a comparison between LTP and long-term depression (LTD) could be made in future experiments.
Although it is well known that ketamine is an NMDAR antagonist (Anis et al., 1983) and can block the induction of NMDAR-dependent LTP (e.g. Ingram et al., 2018; Stringer and Guyenet, 1983), there are inconsistencies in the literature that might relate to the experimental conditions. We therefore first tested the ability of ketamine to prevent the induction of LTP in slices obtained from our colony of P14 male C57BL/6 mice. In order to ensure equilibrium of ketamine in our hippocampal slice preparation (see Kang et al., 2017), we pre-incubated slices for at least 2 h and continuously perfused slices with ketamine throughout the remainder of each experiment. In interleaved control experiments, TBS invariably resulted in a robust LTP of 156% ± 8% of baseline (n = 7). Ketamine (1–10 µM) resulted in a concentration-dependent inhibition of LTP, with complete inhibition of LTP observed at between 3 and 10 µM (Figures 1 and 2(d) and (e)).
Figure 1.
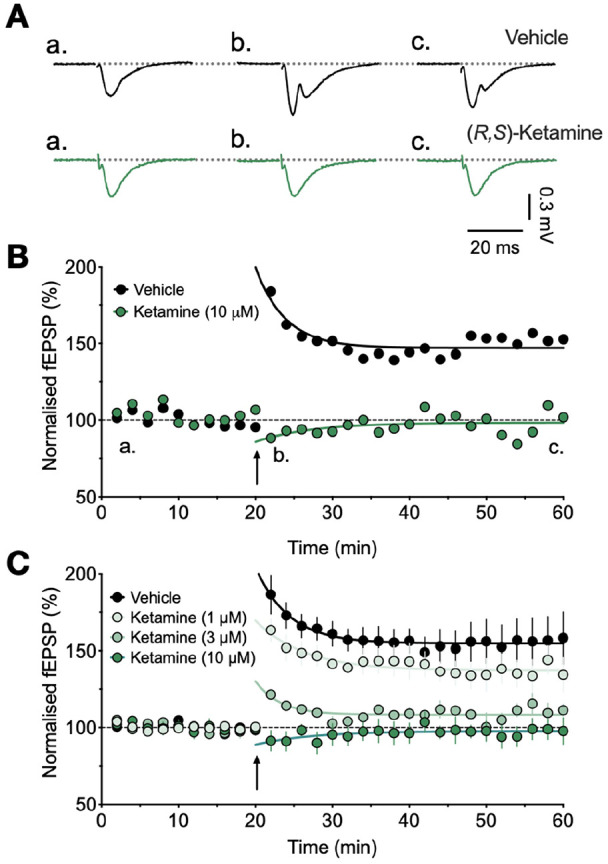
Concentration-dependent inhibition of LTP at hippocampal CA3-CA1 synapses by ketamine. (A) Representative fEPSP traces (averages of four successive sweeps at the time indicated by a–c in panel B) for vehicle control (black) and 10 µM ketamine (green). (B) Time course normalised plots for these experiments. Each point plots the average of four successive sweeps. (C) Pooled data for control (n = 7), and ketamine applied at 1 µM (n = 4), 3 µM (n = 4) and 10 µM (n = 5). TBS was delivered at the time indicated by an arrow.
Figure 2.
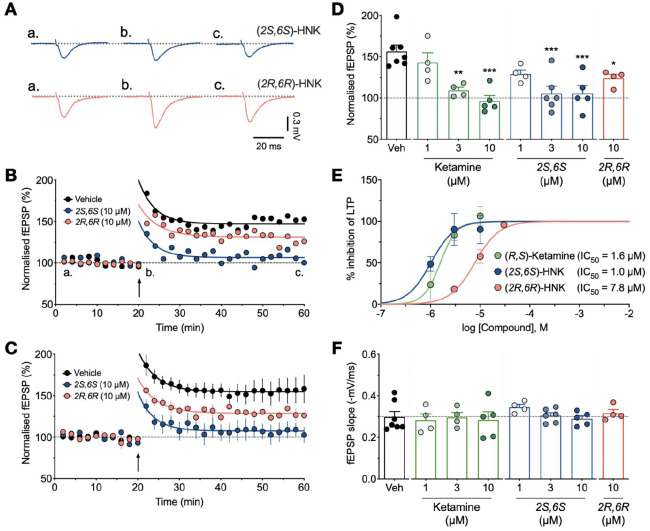
(2S,6S)-HNK and (2R,6R)-HNK inhibit the induction of LTP. (A) Representative traces of fEPSPs for (2S,6S)-HNK (upper) and (2R,6R)-HNK (lower), both at 10 µM. (B) Time-course plots of single experiments for a control (black), (2S,6S)-HNK (blue) and (2R,6R)-HNK (red) experiment. (C) Pooled data for control (n = 7), 10 µM (2S,6S)-HNK (n = 5) and 10 µM (2R,6R)-HNK (n = 4). (D) Summary histograms of the level of LTP, measured 20 min after delivery of TBS. *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA with Bonferroni’s correction compared to vehicle control). (E) Concentration–response curves for inhibition of LTP by ketamine (13 slices), (2S,6S)-HNK (15 slices) and (2R,6R)-HNK (6 slices). A comparison of IC50 values showed that (2S,6S)-HNK inhibited LTP with a greater potency than (2R,6R)-HNK (p < 0.05, extra sum-of-squares F test). (F) Baseline fEPSP slope measurements prior to delivery of TBS for the different experimental conditions.
In this series of experiments, using the same pre-incubation protocol, we also tested the (2S,6S)- and (2R,6R)-isomers of HNK (Figure 2). We found that (2S,6S)-HNK (1–10 µM) had a similar concentration-dependent effect on LTP (Figure 2(a)–(e)). For example, the level of LTP was 129% ± 5% at 1 µM (n = 4) and 106% ± 10% at 10 µM (n = 5). We also observed inhibition of LTP when we used (2R,6R)-HNK, though this isomer appeared less potent (Figure 2(a)–(e)). Using a concentration of 10 µM, the level of LTP was reduced to 124% ± 5% (n = 4). Quantification of the level of LTP obtained for all three compounds, measured 20 min following TBS, is presented in Figure 2(d). A concentration–response curve was plotted to show the relative potency for each compound with respect to its ability to inhibit LTP (Figure 2(e)). The effects on LTP were not due to alterations in baseline AMPAR-mediated synaptic transmission, since these were similar for all conditions (Figure 2(f)).
There is considerable interest in the use of ketamine metabolites as rapid antidepressants, with the hope that they might retain the beneficial effects but with reduced side effects. In particular, (2R,6R)-HNK has been proposed to retain the antidepressant effects with a lesser side-effect profile (Zanos et al., 2016). It was proposed that its action is not due to inhibition of NMDARs, but rather it potentiates AMPAR function. Since its action requires ERK signalling, it is possible that (2R,6R)-HNK is tapping into an LTP process downstream of NMDAR activation that is also expressed as an increase in AMPAR function. However, it has also been reported that (2R,6R)-HNK is an NMDAR antagonist and that its actions can be explained via a ketamine-like effect on NMDARs (Kavalali and Monteggia, 2018). In this study, we addressed the question as to whether HNK isomers affect NMDAR-dependent LTP and, if so, in which direction.
The results we present here demonstrate inhibition of NMDAR-dependent LTP by both ketamine and its HNK metabolites. Our findings are consistent with an action of the HNK isomers on NMDARs. However, it has been reported that NMDAR-mediated fEPSPs at CA3-CA1 synapses are only weakly affected by HNK isomers, with IC50 values of approximately 50 and 200 µM for the (2S,6S) and (2R,6R) isomers, respectively (Lumsden et al., 2019). There are a number of possible explanations for these differences. First, we pre-incubated slices with the compounds for 2 h, due to the long time required for effects of ketamine to stabilise (Kang et al., 2017), whereas the other study (Lumsden et al., 2019) made measurements after 30 min. Second, we made measurements in slices from 14-day-old mice as opposed to 8- to 10-week-old mice, where the NMDAR subtype composition may be different and where it may be easier to obtain a steady-state concentration at the site of action. Third, we measured LTP, which is non-linearly related to NMDAR-mediated synaptic transmission such that a small inhibition of the synaptic response may lead to a larger inhibition of LTP. Thus, although we cannot discount an action on LTP that is unrelated to antagonism of NMDARs, we consider that the most likely explanation for the effects of the HNK isomers on LTP is inhibition of NMDAR-mediated synaptic function.
Understanding how, first, ketamine and, second, its HNK metabolites exert their antidepressant actions is not a straightforward matter. Ketamine has multiple actions in man and rodents that include dissociative anaesthesia and analgesia in addition to its rapid antidepressant effects. In small mammals, the rapid antidepressant effects of both ketamine and (2R,6R)-HNK are obtained with doses in the range of 10 mg/kg (i.p.) and this yields a concentration in the brain of approximately 4–8 µM (Lumsden et al., 2019; Zanos et al., 2018), which is within the concentration range used in this study. It should be noted that the doses required to obtain the anaesthetic, analgesic and rapid antidepressant effects in humans are much lower than in rodents, reflecting differences in pharmacokinetics and pharmacodynamics (Zanos et al., 2018).
Multiple mechanisms have been proposed to explain the rapid antidepressant effects of ketamine. Although these have included NMDAR-independent mechanisms, most mechanisms are based on an action on NMDARs or on NMDAR-triggered processes downstream of the NMDAR per se. For example, it has been proposed that ketamine enhances AMPAR-mediated synaptic transmission by engaging signalling molecules downstream of NMDARs (Autry et al., 2011) and that it can facilitate LTP in ex vivo slices following in vivo administration in a rodent model of depression (Aleksandrova et al., 2020). One potential mechanism by which ketamine may facilitate LTP is via a preferential reduction in feedforward inhibition, which can augment the induction of LTP (Bliss and Collingridge, 1993). Notably, NMDARs on certain classes of interneurons contain GluN2D subunits and these are more sensitive than GluN2A and GluN2B subunits to the action of ketamine in the presence of physiological concentrations of Mg2+ (Kotermanski and Johnson, 2009). A preferential reduction in the excitatory drive onto GABAergic interneurons could therefore promote both synaptic excitation and synaptic plasticity. Another potential site of action is short-term potentiation (STP), which paradoxically can be long-lasting in nature, since it is more sensitive than LTP to the effects of ketamine by virtue of the involvement of GluN2D-containing NMDARs (Ingram et al., 2018).
In conclusion, our present work builds on previous studies that have shown that ketamine blocks the induction of NMDAR-mediated LTP. In particular, we demonstrate that the HNK isomers have similar effects to ketamine in this regard. If inhibition of NMDAR-mediated synaptic plasticity is indeed a site of action of these compounds with respect to their antidepressant effects, it will be important to establish which forms of NMDAR-dependent plasticity are primarily affected in relevant brain regions, such as the lateral habenula (Yang et al., 2018). NMDARs mediate the induction of STP, as well as several forms of LTP, depotentiation and long-term depression (Bliss and Collingridge, 1993). These synaptic plasticity processes underlie multiple forms and components of learning and memory, including re-consolidation. Therefore, as suggested previously (Collingridge et al., 2017), inhibition of the synaptic mechanisms underlying reconsolidation of memories could underlie the effectiveness of ketamine. In other words, in the presence of ketamine an individual ‘forgets’ about their depression, because reconsolidation of the negative mood state is prevented. We propose a similar mechanism of action of the HNK isomers.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the following grants: (1) CIHR (Canadian Institutes of Health Research) Foundation Grant #154276 (G.L.C.), (2) ‘The Neuroscience Catalyst’ research programme of the Centre for Collaborative Drug Research (CCDR) at the University of Toronto (G.L.C. and J.G.) and (3) The National Honor Scientist Program (NRF-2012R1A3A1050385) of Korea (B.-K.K.).
ORCID iDs: Patrick Tidball  https://orcid.org/0000-0002-8983-1905
https://orcid.org/0000-0002-8983-1905
John Georgiou  https://orcid.org/0000-0003-4503-6384
https://orcid.org/0000-0003-4503-6384
Graham L. Collingridge  https://orcid.org/0000-0002-9572-5359
https://orcid.org/0000-0002-9572-5359
References
- Aleksandrova LR, Wang YT, Phillips AG. (2020) Ketamine and its metabolite, (2R,6R)-HNK, restore hippocampal LTP and long-term spatial memory in the Wistar-Kyoto rat model of depression. Molecular Brain 13(1): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, et al. (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. British Journal of Pharmacology 79(2): 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354): 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47(4): 351–354. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (1993) A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 361(6407): 31–39. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. (1983) Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. The Journal of Physiology 334(1): 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Lee Y, Bortolotto ZA, et al. (2017) Antidepressant actions of ketamine versus hydroxynorketamine. Biological Psychiatry 81(8): e65–e67. [DOI] [PubMed] [Google Scholar]
- Ingram R, Kang H, Lightman S, et al. (2018) Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity. Neuropharmacology 142: 30–40. [DOI] [PubMed] [Google Scholar]
- Kang H, Park P, Bortolotto ZA, et al. (2017) Ephenidine: A new psychoactive agent with ketamine-like NMDA receptor antagonist properties. Neuropharmacology 112(Pt A): 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. (2018) The ketamine metabolite 2R,6R-hydroxynorketamine blocks NMDA receptors and impacts downstream signaling linked to antidepressant effects. Neuropsychopharmacology 43(1): 221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. (2009) Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. Journal of Neuroscience 29(9): 2774–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, et al. (2019) Antidepressant-relevant concentrations of the ketamine metabolite (2 R,6 R)-hydroxynorketamine do not block NMDA receptor function. Proceedings of the National Academy of Sciences of the United States of America 116(11): 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JL, Guyenet PG. (1983) Elimination of long-term potentiation in the hippocampus by phencyclidine and ketamine. Brain Research 258(1): 159–164. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, et al. (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554(7692): 317–322. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, et al. (2018) Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacological Reviews 70(3): 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]