Abstract
Heart failure (HF) is recognized as one of the leading causes of morbidity and mortality worldwide. Every year about 500,000 new cases of HF are diagnosed in the United States. The predominant etiology of death in HF patients include sudden cardiac death (SCD) and pump failure. Prediction of mode of death may help in devising management decisions. In patients with HF, the presence of myocardial fibrosis has been a known risk factor for SCD and thus it could be used as a criterion in risk stratification for SCD. However, the underlying pathophysiology of SCD is uncertain and controversial, which makes it necessary to develop newer tools to enhance SCD risk stratification. The newer tools should be innovative enough either to complement or to replace the currently available tools. In this scoping review, we highlighted the utilization of novel biomarkers galectin-3 (gal-3) and soluble ST2 (sST2) and discussed that how they might complement currently available tools such as, cardiac MRI (CMR) for SCD risk stratification in HF patients.
Keywords: Sudden cardiac death, cardiac magnetic resonance, heart failure
Introduction
Sudden cardiac death (SCD) can be defined as a death within an hour of symptom onset or during sleep in a patient who was previously stable.1 The risk stratification and prevention of SCD remains quite challenging in the management of heart failure (HF) patients. About 50% of all HF deaths are due to SCD, which mainly affects patients with mild-to-moderate symptomatology.2 One of the important underlying pathological processes that may enhance the risk of SCD is myocardial fibrosis and stress.3 The presence of myocardial scar detected by cardiac MRI (CMR) provides adequate prognostic information in patients with nonischemic cardiomyopathy (NICM).4 However, the underlying mechanism of SCD is uncertain and additional diagnostic tests to complement or to replace current tools are required for better prediction of SCD risk in HF patients.5
Plasma biomarkers, such as galectin-3 (Gal-3), soluble ST2 (sST2) and amino-terminal pro-brain natriuretic peptide (NT-proBNP), reflect the degree of myocardial fibrosis, stress and may help in enhancing SCD risk stratification.5 Although advances in diagnostic modalities have made progress in risk stratification for SCD in HF patients, tools to detect the disease early on and to predict prognosis are lacking.6 Therefore, in this review, we mainly focused on novel biomarkers (Gal-3 and sST2), occasionally discussed in the literature, as compared to traditional biomarkers such as NT-proBNP. This scoping review consists of the descriptional individual roles of CMR, Gal-3 and ST2 in detecting myocardial fibrosis and their significance as SCD risk stratification tools. Finally, the possible roles of Gal-3 and ST2 as complementary tools to CMR for SCD risk stratification are discussed.
Cardiac magnetic resonance imaging
Cardiac Magnetic Resonance (CMR) imaging is a rapidly evolving diagnostic tool that has emerged as a novel noninvasive imaging for HF patients. In addition to its diagnostic role, CMR plays an important role in monitoring HF therapy and assessment of prognosis. CMR is now the gold standard tool for the assessment and detection of myocardial anatomy, global and regional and wall function as well as viability in HF patients. Additionally, it provides information about edema, fibrosis, infiltration, necrosis and wall perfusion. Moreover, it helps in detecting the underlying etiology of HF and monitoring disease progression.7
Besides the routine use of CMR in anatomic assessment of cardiac structures, its pertinent finding, late gadolinium enhancement (LGE), may assist in determination of regions of myocardial fibrosis that generate life-threatening ventricular tachyarrhythmias. LGE-CMR has shown diagnostic accuracy in assessment of myocardial fibrosis in different types of cardiomyopathies. LGE-CMR has shown its prognostic value to predict recovery of myocardium in ischemic cardiomyopathy (ICM) and in other types of cardiomyopathy.8 Assomull et al.9 demonstrated that myocardial fibrosis shown by LGE-CMR correlated with an increase in SCD or ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy (NIDCM) [hazard ratio 5.2, p = 0.03). Furthermore, Rubinshtein et al.10 stated that LGE was significantly associated with arrhythmias and subsequent SCD after adjusting other risk factors in hypertrophic cardiomyopathy (HCM). Their study showed that patients with LGE were more likely to have episodes of nonsustained ventricular tachycardia (34 of 126 [27%] versus 8 of 94 [8.5%], p < 0.001), had more episodes of nonsustained ventricular tachycardia per patient (4.5 ± 12 versus 1.1 ± 0.3, p = 0.04) and had higher frequency of ventricular extrasystoles/24 hours (700 ± 2080 versus 103 ± 460, p = 0.003). On follow-up, SCD occurred in 4 patients and an additional 4 patients received appropriate ICD discharges. All 8 patients were LGE positive (event rate of 0.94%/year, p = 0.01 versus LGE negative). Two additional HF-related deaths were recorded among LGE-positive patients. LGE is also associated with adverse cardiac events in patients undergoing aortic valve replacement (AVR) and patients with cardiac amyloidosis.11,12 Additionally, prognostic value of LGE was demonstrated recently in asymptomatic patients with diabetes mellitus and hypertension with a preserved left ventricular (LV) ejection fraction.8
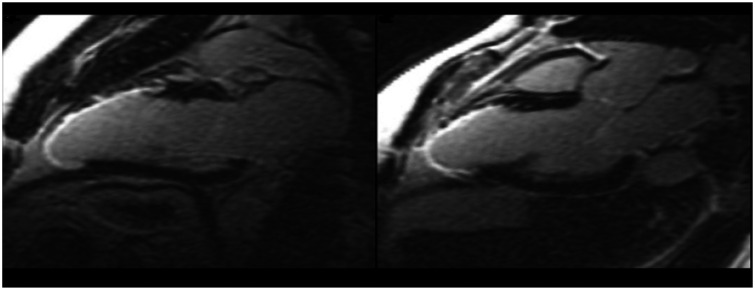
LGE-CMR is the most accurate method to detect myocardial replacement fibrosis, but it is less sensitive for diffuse interstitial fibrosis (Figure 1). In LGE-CMR, image contrast relies on the difference in signal intensity between the fibrotic and normal myocardium, and such differences may not be present if the process is diffuse.8 Gulati et al.13 conducted a study with the objective to determine whether myocardial fibrosis (detected by LGE-CMR) is an incremental and independent predictor of mortality and SCD in NIDCM. They postulated that the assessment of myocardial fibrosis with LGE-CMR provided independent prognostic information in patients with NIDCM and LGE-CMR improved risk stratification for SCD and all-cause mortality when compared to LV ejection fraction. However, it is advisable to further investigate the potential clinical utility of myocardial fibrosis detected by LGE-CMR in the risk stratification.
One of the emerging CMR techniques include estimation of myocardial longitudinal magnetic relaxation time (T1) known as myocardial T1 mapping. The emergence of T1 mapping further improves clinical assessment and information of myocardial fibrosis provided by LGE-CMR. It may serve to detect subclinical myocardial changes before the onset of diastolic and systolic dysfunction. This advancement may improve to stratify patients with relatively lower cardiovascular risk.8 LGE works by the principle that scarred tissue passively accumulates more gadolinium-based contrast agent which shortens its T1 mapping compared to normal healthy myocardium and this could be visible with a particular imaging sequence. Inclusion of T1 mapping further supports this principle of LGE.14 Therefore, combining T1 mapping with LGE may precisely enhance myocardial tissue characterization which may eventually help the physician to better understand the underlying cardiomyopathic process. This information leads to an improvement of clinical outcomes by providing early diagnosis, enhancing therapeutic strategies and enabling a direct monitoring system.15 However, more prospective studies are required to elucidate the clinical value of this combined imaging technique, especially in large population data.8
Inflammatory biomarkers
In addition to LGE-CMR, plasma biomarkers play an important role in the SCD risk stratification of HF patients. From the results of several large studies, the United States Food and Drug Administration (FDA) has cleared 3 biomarkers for assessing prognosis of HF patients: natriuretic peptides such as NT-proBNP and B-type natriuretic peptide (BNP), Gal-3 and sST216 (Table 1) In general, the natriuretic peptides (NT-proBNP and BNP) are released in response to myocardial wall stress and have shown to be powerful markers of prognosis in HF.17 On the other hand, it was necessary to identify and to evaluate novel biomarkers (Gal-3 and sST2) that may reflect different pathophysiological mechanisms to improve risk stratification (Table 2). In one cohort study, Ahmad et al.18 evaluated the relationships between elevated levels of NT-proBNP, Gal-3, ST2 and mode of death in 813 HF patients who were on optimal medical therapy. The novel biomarkers (Gal-3 and sST2) were found to be significantly associated with increased risk of death from SCD but not pump failure. As per their study, clinical variables along with NT-proBNP levels were stronger predictors of pump failure (C statistic: 0.87) than SCD (C statistic: 0.73) and the addition of Gal-3 and sST2 improved the net risk classification by 11% for SCD, but not pump failure in chronic HF patients. Thus, the information provided by novel biomarkers improved the predictability of SCD risk, which suggests that they can be used clinically to focus on enhancing SCD risk stratification.
Galectin-3 (gal-3)
Gal-3 is a beta-galactosidase-binding lectin and an emerging prognostic biomarker of HF. Recent experimental studies suggested its role as a mediator of cardiac fibrosis.19 The administration of Gal-3 in animal models led to progressive fibrosis and LV systolic dysfunction and hence it has a role in promoting ventricular remodeling. With these observations, Gal-3 has evolved as a potential HF biomarker that could reflect ongoing ventricular remodeling. At the cellular level, it is secreted by activated macrophages in the stunned cardiac myocytes that initiates the cascade for necrosis and ultimately fibrosis.19 It was further studied as a novel biomarker to predict disease progression and prognosis in acute HF syndrome along with its role in estimating adverse outcomes.20
This hypothesis was further elaborated by Ho et al.21 who suggested that higher concentrations of Gal-3 are associated with an increased risk for HF incidence and mortality (HR 1.28 per 1 standard deviation increase in log-Gal-3, 95% CI 1.14–1.43, p < 0.0001). In contrast, a recent review of prognostic studies concluded that although Gal-3 carries a prognostic role in acute as well as chronic HF but its association with outcomes is attenuated when adjusted for renal function or cardiac-specific biomarkers.22 In several cohorts, it has been suggested that increased Gal-3 level is associated with higher evidence of decompensated HF which is indicative of worse prognosis.23 In the era of patient tailored management, Gal-3 has an essential role in guiding physicians to identify HF patients at risk of decompensation, readmission and death, thus enabling clinicians to adjust management goals. This is based on the fact that Gal-3 expression is maximal at peak fibrosis and virtually absent after recovery. De Boer et al.24 in a large HF cohort study, postulated that Gal-3 has a promising prognostic role in estimating disease advancement and decompensation in both HF preserved ejection fraction [HFpEF] as well as HF reduced ejection fraction [HFrEF] groups.
As discussed earlier, Gal-3 has a key role in diagnostic and prognostic algorithm of HF and it may be considered as a target for intervention.23 Yu et al.24 suggested that unlike BNP, Gal- 3 is not only the aftermath of cardiac remodeling, but it influences the development of fibrosis and ultimately impacts HF progression. They also suggested that the use of drugs binding to Gal-3 may prove as a potential therapeutic option for the prevention or reversal of HF with extensive fibrosis.24 In murine and rat models of HF, genetic disruption and pharmacological inhibition of Gal-3 attenuated the progression of cardiac remodeling.24 Additionally, inhibition of Gal-3 largely preserved diastolic and systolic function by inhibiting myocardial fibrosis and decreasing collagen production, processing and cross-linking. However, the data is lacking in terms of Gal-3 as a therapeutic target in a HF population.
Table 1.
Comparison of characteristics of cardiac biomarkers.
| GALECTIN-3 | Soluble ST2 |
|---|---|
| Member of lectin family19 | IL-1 family receptor26 |
| Marker of fibrosis | Marker of fibrosis |
| Can be elevated liver cirrhosis and pulmonary fibrosis21 | Can be elevated in pulmonary diseases and systemic infection or inflammation27 |
| Has diagnostic role in HF when combined with NT-proBNP16 | Less role in HF diagnosis16 |
| Role as a prognostic marker in HF is debatable16 | Useful marker of prognosis in patients with HF and MI16,27 |
| Predictor of all-cause mortality21 | Better predictor of cardiovascular mortality than overall mortality31 |
Table 2.
Studies showing role of cardiac biomarkers and CMR in determining myocardial fibrosis and risk of HF/SCD.
| Studies | Title | Year | Ref No. | Results/Outcome | |
|---|---|---|---|---|---|
| CMR | Karamitsos TD et al.Type of the study: Review Article | The role of cardiovascular magnetic resonance imaging in heart failure | 2009 | 7 | CMR is now the gold standard tool for detection and assessment of myocardial anatomy, regional and global wall function and viability in HF patients. Moreover, it helps in detecting the underlying etiology of HF as well as monitoring disease progression. |
| Mewton N, Liu CY et al.Type of the study: Review Article | Assessment of myocardial fibrosis with cardiovascular magnetic resonance | 2011 | 8 | LGE-CMR has shown its prognostic power to predict myocardial recovery in ischemic cardiomyopathy (ICM) and in other types of cardiomyopathy. The emergence of T1 mapping further improves clinical assessment and information of myocardial fibrosis provided by LGE-CMR. | |
| Assomull RG, Prasad SK, Lyne J, et al. Type of the study: prospective longitudinal studyStudy population: 101 | Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy | 2006 | 9 | Midwall fibrosis was present in 35% of patients and was associated with a higher rate of all-cause death and hospitalization for a cardiovascular event (hazard ratio 3.4, p = 0.01) which were the predefined primary combined end points. Midwall fibrosis also predicted secondary outcome measures of SCD or ventricular tachycardia (VT) (hazard ratio 5.2, p = 0.03) | |
| Rubinshtein R, Glockner JF, Ommen SR, et al. Type of the study: Retrospective cohort studyStudy population: 424 | Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. | 2010 | 10 | LGE-positive patients were more likely to have episodes of nonsustained ventricular tachycardia (34 of 126 [27%] versus 8 of 94 [8.5%], P<0.001), more episodes of nonsustained ventricular tachycardia per patient (4.5±12 versus 1.1±0.3, P=0.04) and had higher frequency of ventricular extrasystoles in 24 hour monitoring (700±2080 versus 103±460, P=0.003). | |
| Gal-3 | Sharma UC, Pokharel S, van Brakel TJ, et al. Type of the study: Microarray study performed in Rat model | Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. | 2004 | 19 | Exogenous recombinant galectin-3 significantly increased cardiac fibroblast proliferation and lead to progressive fibrosis and left ventricular systolic dysfunction. |
| Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ et al. Type of the study: Prospective cohort studyStudy population: 3353 | Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. | 2012 | 21 | Levels of Gal-3 was associated with increased risk of incident HF and remained significant after adjustment for clinical variables and BNP (HR: 1.23; 95% CI: 1.04 to 1.47; p = 0.02). | |
| Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B et al.Type of the study: Experimental study in Mouse | Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis | 2013 | 24 | Genetic disruption and pharmacological inhibition of galectin-3 attenuates cardiac fibrosis, LV dysfunction, and subsequent heart failure development. | |
| ST-2 | Pascual-Figal DA, Ordonez-Llanos J, Tornel PL et al. Type of the study: Nested case control studyStudy population: 36 cases of SCD and 63 controls | Soluble st2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction | 2009 | 30 | Elevated sST2 concentrations are predictive of SCD (+0.1 ng/ml, odds ratio: 1.39, 95% confidence interval: 1.09 to 1.78, p = 0.006) in patients with chronic HF and provide complementary information to NT-proBNP levels. |
| Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C et al. Type of the study: Pooled data analysisStudy population: 1239 | Complementary roles for biomarkers of biomechanical strain st2 and n-terminal prohormone b-type natriuretic peptide in patients with st-elevation myocardial infarction. | 2008 | 31 | After adjustment for baseline characteristics and NT-proBNP levels, an ST2 level above the median was associated with a significantly greater risk of SCD, cardiovascular death or heart failure in patients with ST-elevation myocardial infarction. (third quartile: adjusted odds ratio, 1.42; 95% confidence interval, 0.68 to 3.57; fourth quartile: adjusted odds ratio, 3.57; 95% confidence interval, 1.87 to 6.81; P<0.0001 for trend). | |
| Ahmed T, Fiuzat et al.Type of the study: Observational studyStudy population: 813 | Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. | 2014 | 18 | Clinical variables along with NT-proBNP levels were stronger predictors of pump failure (C statistic: 0.87) than sudden cardiac death (C statistic: 0.73). Addition of ST2 and galectin-3 led to improved net risk classification of 11% for sudden cardiac death, but not pump failure in chronic heart failure patients. |
Figure 1.
Cardiac MRI showing late gadolinium enhancement.
ST2
ST2 is a novel biomarker for cardiac strain. Its transmembrane form is thought to play a role in the development of immunological tolerance and modulatory responses of T helper type 2 cells. Its soluble form is upregulated in growth-stimulated fibroblasts.25 It is an interleukin-1 (IL-1) receptor family member and is released when cardiac fibroblasts and cardiomyocytes are undergoing biomechanical strain.26 Thus, sST2 has been identified as a biomarker of cardiac fibrosis, remodeling and wall stress.27 The circulating form of ST2, a transmembrane ligand (ST2L), signals through a complex involving interleukin-33 (IL-33).27 This IL-33/ST2L signaling is expected to have antifibrotic actions and play a cardioprotective role in mechanical overload.28
Sanada et al.29 suggested that the sST2 protein acts as a decoy receptor, thus reversing the effects of IL-33/ST2L in a dose-dependent manner, presumably by binding IL-33 and preventing signaling through ST2L. Therefore, sST2 could be considered as a potential pathophysiological mediator of myocardial fibrosis. Additionally, the concentrations of sST2 have been found to have prognostic value in predicting the mortality rate in acute coronary syndromes (ACS). It serves to predict morbidity in long-term follow-up of patients after an acute HF event.29 However, it was unknown if sST2 could play a role in the identifying patients at risk of SCD. Pascual-Figal et al.30 conducted a case control study to investigate the prognostic value of sST2 in patients with mild-to-moderate HF with LV systolic dysfunction. The study showed that concentrations of sST2 were greater among decedents (0.23 ng/mL [interquartile range 0.16–0.43 ng/ml] versus 0.12 ng/mL [interquartile range 0.06–0.23 ng/mL], p = 0.001) and were predictive of experiencing SCD (+0.1 ng/mL, odds ratio: 1.39, 95% confidence interval: 1.09–1.78, p = 0.006). This suggests that elevated sST2 levels tend to predict SCD in a HF population and has the ability to improvise diagnostic and prognostic strategies in management of HF. The study emphasized the use of newer biomarkers in conjunction with traditional biomarkers to formulate therapeutic goals in HF. However, the study did not provide information regarding the role of sST2 in decompensated HF. In another study, Sabatine et al.31 showed that in patients with an ACS (ST-segment elevation myocardial infarction-STEMI), elevated baseline sST2 levels had a higher risk of SCD because it could predict contractile function and myocardial reserve. sST2 is known to be a significant predictor of cardiovascular and HF mortality, independent of NT-proBNP levels and baseline clinical characteristics. They further concluded that the combination of sST2 and NT-proBNP is a potent marker of cardiovascular risk stratification and prediction of adverse outcomes.31 Based on these data, the authors again stressed the increased prognostic value of risk stratification models utilizing various complementary biomarkers in the management of STEMI.31
How inflammatory biomarkers complement CMR
Despite the advancements in medical therapy for HF, prognosis remain poor with a 5-year mortality rate of nearly 50% in symptomatic patients, which may further compel physicians to make a decision about more expensive and complex interventions, such as automatic implantable cardioverter defibrillator (AICD) and cardiac assist device placement. Thus, risk stratification of HF patients becomes critical in order to select the patients most likely to benefit from such interventions. Currently available risk stratification tools only focus on predicting total mortality among HF patients but lack sensitivity and specificity when predicting cause-specific death.32 Severe LV systolic dysfunction has been recognized as a risk factor for SCD; however, LV ejection fraction (LVEF) has a poor correlation in SCD risk stratification requiring more potential indicators.33 Meanwhile, there is some evidence that prolonged QRS duration and the presence of nonsustained ventricular tachycardia may assist in identifying patients at a higher risk of SCD.34
The SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) evaluated if AICD therapy is beneficial for the primary prevention of SCD in subjects with ICM or NICM, LVEF ≤35% and NYHA functional class II or III. Analysis of this trial showed that although there was a mortality risk reduction, only 20–25% of patients received an appropriate AICD shock in 5 years.35 Therefore, the number needed to treat (NNT) in this trial was very high if subjects were selected only based on LVEF criteria. Many eligible subjects did not receive benefit from the AICD therapy.35 Additionally, community-based studies from the United States and Europe demonstrated that up to 70% of SCD victims had preserved LVEF prior to the sudden cardiac arrest and did not meet current LVEF criteria for AICD implantation for primary prevention of SCD.36 Thus, it is worth mentioning that current risk stratification tools are not appropriate in identifying a large group of patients who are at risk of SCD. The lapse in the prediction models require another revised model that can efficiently predict and stratify a population at risk of SCD irrespective of LVEF.
One of the common histological features of the failing heart is myocardial fibrosis. Myocardial fibrosis in animal and human studies is associated with worsening ventricular systolic function, increased ventricular stiffness and abnormal cardiac remodeling.37 In recent clinical studies, fibrosis has been shown to be a major independent predictor of adverse cardiac outcomes.9 A review study by Kim et al.,4 summarized data from 15 studies published in the last 7–8 years with more than 2700 patients and demonstrated that myocardial scar was associated with a 2.5-fold increased risk of all-cause mortality and a more than 3-fold increase in the risk of major cardiovascular events.4 More importantly, they found the strongest association with a near 5-fold increased rate of ventricular tachyarrhythmias or SCD in NICM patients who had myocardial scar. In patients with NICM, the average prevalence of myocardial scar was 41%. Given the poor specificity of LVEF, utilizing myocardial scar for risk stratification appears to be appealing particularly while identifying appropriate AICD recipients for prevention of SCD.
Although there has been a decrease in the proportion of SCDs due to coronary artery disease, the incidence of SCD has not decreased as the proportion of SCDs resulting from nonischemic causes has increased.38 A diverse group of heart diseases, such as coronary artery disease, hypertensive heart disease, dilated cardiomyopathy, healed myocarditis and inherited structural disorders can lead to secondary myocardial fibrosis and thus are associated with increased risk of SCD.39 However, in some cases, autopsies have shown myocardial fibrosis without any known underlying heart disease (primary myocardial fibrosis). One study has shown that patients were found to have myocardial fibrosis and genetic variants of various forms of cardiomyopathy during autopsy but were not showing any autopsy findings of those cardiomyopathies. These findings could be explained due to early phenotypic expression of genes in the form of myocardial fibrosis leading to SCD even before the primary disease develops.39 A recent study has demonstrated left ventricular fibrosis as a major cause of SCD in young athletes.40 Major genetic advances in rare cardiovascular diseases caused by structural or ion channel abnormalities have been made which help to identify such rare diseases earlier in the family at potential risk for SCD.41–43
There are few open questions before myocardial fibrosis or scar can be used widely for SCD risk stratification, such as if the presence of scar alone is sufficient as the criterion for optimized risk stratification or a scar cutoff needs to be considered as well. Gulati et al.13 have shown that presence of scar itself is a strong risk parameter and thus may obviate the need to quantify scar. On the other hand, several studies have shown a quantitative relationship between extent of scar and adverse outcome. Therefore, these questions need to be addressed in the future.
Binas et al.44 conducted a study in which they measured serum concentrations of Gal-3 and sST2 in 262 subjects with dilated cardiomyopathy (DCM) and survival rates were determined for all-cause mortality and cardiac mortality. The study showed that sST2 predicts all-cause mortality and cardiac mortality in patients with nonischemic HF, especially in patients with an inflammatory background. Also, the study showed that intermediate levels of Gal-3 allow for better prognosis. Furthermore, Hu et al.45 conducted a study in 192 patients with NICM, including 85 subjects with DCM and 107 with HCM with a goal to determine whether LGE in conjunction with Gal-3 level offered more precise prognosis of NICM in comparison to LGE alone.45 Their study showed that the combination of LGE and Gal-3 levels offered a more significant prognostic value than using LGE alone for both DCM and HCM (DCM p = 0.001< 0.012; HCM p = 0.037< 0.040).
LGE-CMR and plasma cardiac biomarkers (NT-proBNP, Gal-3 and sST2) play important roles in detecting myocardial fibrosis and thus in risk stratification of SCD. Perhaps, both tools might have some interesting association between them, which may ultimately be utilized to improve risk stratification. To investigate such possibility, a study was conducted with the aim to determine the association of biomarkers for fibrosis and myocardial fibrosis detected by LGE-CMR and their relationship to LV diastolic filling measured by tissue Doppler echocardiography in patients with stable coronary artery disease.46 The investigators found a significant association between the elevated levels of cardiac fibrosis biomarkers and myocardial fibrosis imaged with LGE-CMR in patients with uncomplicated coronary artery disease. Additionally, it was found that LV interstitial fibrosis plays an essential role in impaired diastolic function among patients with coronary artery disease. Thus, the estimate of cardiac diastolic dysfunction can be assessed by utilizing serum biomarkers of fibrosis.46 Although the study was applied to patients with coronary artery disease, we can extrapolate these results to HF patients as far as the association between fibrosis biomarkers and LGE-CMR is concerned. Nevertheless, it is worth to consider here the possibility that the novel biomarkers (Gal-3 and ST2) might complement LGE-CMR for the detection of myocardial fibrosis and thus for SCD risk stratification in HF patients.(Table 2) However, it is necessary to further investigate such a possibility by designing an appropriate prospective randomized clinic study in the near future.
Summary
From this review it is evident that about one-half of the deaths in HF patients are due to SCD, and thus risk stratification for SCD in this population is of utmost importance. We have several tools available for the detection of myocardial fibrosis that can be used for SCD risk stratification. Among these tools, LGE-CMR has proved its diagnostic accuracy for assessing replacement myocardial fibrosis but carries limited sensitivity for detecting diffuse interstitial fibrosis. Similarly, novel cardiac biomarkers, such as Gal-3 and sST2 have been identified as the fibrosis biomarkers and thus can serve as a powerful risk stratification tool. These biomarkers can enhance the risk stratification process by detecting fibrosis at an early stage. Additionally, such novel biomarkers might even overcome the poor sensitivity of LGE-CMR for assessment of diffuse interstitial fibrosis. Thus, we conclude that novel biomarkers (Gal-3 and sST2) can be excellent complementary tools to CMR for SCD risk stratification in HF patients and such possibility needs to be investigated thoroughly in a well-designed prospective study.
Acknowledgements
None.
Contributorship
Timothy J Vittorio conceived with an idea about this work and approved the final manuscript; Niel N Shah was responsible for drafting the outline and main manuscript as well as editing of the main manuscript; Puvanalingam Ayyadurai, Muhammad Saad, Constantine E. Kosmas, Muhammad U. Dogar, Upen Patel were responsible for critically reviewing the manuscript for intellectual content and editing of the main manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
None.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
TJV.
ORCID iDs
Puvanalingam Ayyadurai https://orcid.org/0000-0001-7927-655X
Muhammad Saad https://orcid.org/0000-0002-3827-2218
References
- 1.Marc W, Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res 2015; 116: 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uretsky BF, Sheahan RG. Primary prevention of sudden cardiac death in heart failure: will the solution be shocking? J Am Coll Cardiol 1997; 30: 1589– 1597. [DOI] [PubMed] [Google Scholar]
- 3.Havmoller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circ Arrhythm Electrophysiol 2012; 5: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EK, Chattranukulchai P, Klem I. Cardiac magnetic resonance scar imaging for sudden cardiac death risk stratification in patients with Non-Ischemic cardiomyopathy. Korean J Radiol 2015; 16: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane RE, Cowie MR, Chow AWC. Prediction and prevention of sudden cardiac death in heart failure. Heart 2005; 91: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickstein K, Cohen-Solal A, Filippatos G, et al. For ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European society of intensive care medicine (ESICM). Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 7.Karamitsos TD, Francis JM, Myerson S, et al. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol 2009; 54: 1407–1424. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011; 57: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006; 48: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 10.Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010; 3: 51–58. [DOI] [PubMed] [Google Scholar]
- 11.Austin BA, Tang WH, Rodriguez ER, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging 2009; 2: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 12.Azevedo CF, Nigri M, Higuchi ML, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010; 56: 278–287. [DOI] [PubMed] [Google Scholar]
- 13.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309: 896896. [DOI] [PubMed] [Google Scholar]
- 14.Haaf P, Garg P, Messroghli DR, et al. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016; 18: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díez J, Querejeta R, López B, et al. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002; 105: 2512–2517. [DOI] [PubMed] [Google Scholar]
- 16.van Kimmenade RR, Januzzi JL., Jr. Emerging biomarkers in heart failure. Clin Chem 2012; 58: 127–138. [DOI] [PubMed] [Google Scholar]
- 17.Doust JA, Pietrzak E, Dobson A, et al. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005; 330: 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad T, Fiuzat M, Neely B, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail 2014; 2: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004; 110: 3121–3128. [DOI] [PubMed] [Google Scholar]
- 20.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 2006; 48: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 21.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 2012; 60: 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filipe MD, Meijers WC, Rogier van der Velde A, et al. Galectin-3 and heart failure: prognosis, prediction & clinical utility. Clin Chim Acta 2015; 443: 48–56. [DOI] [PubMed] [Google Scholar]
- 23.de Boer RA, Voors AA, Muntendam P, et al. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009; 11: 811–817. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013; 6: 107–117. [DOI] [PubMed] [Google Scholar]
- 25.Januzzi JL, Jr, Peacock WF, Maisel AS, et al. Measurement of the interleukin family member st2 in patients with acute dyspnea: results from the pride (probrain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am CollCardiol 2007; 50: 607–613. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg EO, Shimpo M, De Keulenaer GW, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002; 106: 2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac biomarker: ST2: a review. Molecules 2013; 18: 15314–15328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masson S, Latini R, Anand IS, on behalf of the Val-HeFT Investigators et al. Direct comparison of b-type natriuretic peptide (bnp) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (VAL-HEFT) data. Clin Chem 2006; 52: 1528–1538. [DOI] [PubMed] [Google Scholar]
- 29.Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117: 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, et al. Soluble st2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol 2009; 54: 2174–2179. [DOI] [PubMed] [Google Scholar]
- 31.Sabatine MS, Morrow DA, Higgins LJ, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone b-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation 2008; 117: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapanainen JM, Lindgren KS, Mäkikallio TH, et al. Natriuretic peptides as predictors of nonsudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol 2004; 43: 757–763. [DOI] [PubMed] [Google Scholar]
- 33.Zipes DP, Camm AJ, Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol 2006; 48: e247–e346. [DOI] [PubMed] [Google Scholar]
- 34.Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation 2004; 110: 766–769. [DOI] [PubMed] [Google Scholar]
- 35.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009; 120: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon sudden unexpected death study. J Am Coll Cardiol 2006; 47: 1161–1166. [DOI] [PubMed] [Google Scholar]
- 37.Conrad CH, Brooks WW, Hayes JA, et al. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 1995; 91: 161–170. [DOI] [PubMed] [Google Scholar]
- 38.Junttila MJ, Hookana E, Kaikkonen KS, et al. Temporal trends in the clinical and pathological characteristics of victims of sudden cardiac death in the absence of previously identified heart disease. Circ Arrhythm Electrophysiol 2016; 9: 10–1161. [DOI] [PubMed] [Google Scholar]
- 39.Junttila MJ, Holmström L, Pylkäs K, et al. Primary myocardial fibrosis as an alternative phenotype pathway of inherited cardiac structural disorders. Circulation 2018; 137: 2716–2726. [DOI] [PubMed] [Google Scholar]
- 40.Finocchiaro G, Papadakis M, Robertus JL, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol 2016; 67: 2108–2115. [DOI] [PubMed] [Google Scholar]
- 41.Bagnall RD, Weintraub RG, Ingles J, et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med 2016; 374: 2441–2452. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez O, Campuzano O, Fernández-Falgueras A, et al. Natural and undetermined sudden death: value of post-mortem genetic investigation. PLoS One 2016; 11: e0167358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahrouchi N, Raju H, Lodder EM, et al. Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol 2017; 69: 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binas D, Daniel H, Richter A, et al. The prognostic value of sST2 and galectin-3 considering different aetiologies in non-ischaemic heart failure. Open Heart 2018; 5: e000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu DJ, Xu J, Du W, Zhang JX, et al. Cardiac magnetic resonance and galectin-3 level as predictors of prognostic outcomes for non-ischemic cardiomyopathy patients. Int J Cardiovasc Imaging 2016; 32: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 46.Samuli Lepojärvi E, Piira O-P, Pääkkö E, et al. Serum PINP, PIIINP, galectin-3, and ST2 as surrogates of myocardial fibrosis and echocardiographic left ventricular diastolic filling properties. Front physiol 2015; 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]