Abstract
We assessed safety and potential efficacy of a chamomile gel compared with urea cream to prevent acute radiation dermatitis in head and neck cancer patients. We assessed safety and potential efficacy of the chamomile gel in escalating concentrations of 2.50%, 5.00% and 8.35% of chamomile. Concentration of 8.35% was chosen for a randomized trial comparing chamomile gel (8.35%) with urea cream (n = 24 per group), for potential efficacy to delay or prevent radiation dermatitis in these patients. Preliminary results demonstrate a delayed onset of dermatitis, with onset of Grade 2 dermatitis at 5.1 (1.3) weeks in the chamomile group and 4.5 (1.3) weeks in the urea group (effect size of 0.46). Itching, burning and hyperpigmentation were more frequently reported in the urea group. Results indicates a potential efficacy of the chamomile gel. Further studies are needed to confirm the effect of the chamomile gel in reducing or delaying the occurrence of radiation dermatitis.
Keywords: radiodermatitis, head and neck neoplasms, matricaria, urea, skin care
Introduction
Radiation dermatitis (RD) is one of the main toxicities induced by radiation therapy that occurs in approximately 95% of patients exposed to ionizing radiation.1,2 RD affects especially head and neck cancer patients where the skin is part of the target volume.3,4
RD can cause pain, pruritus, burning, and discomfort, thus affecting the quality of life of a patient.5 Acute injury occurs within hours after radiation exposure. A large number of basal keratinocytes are destroyed, resulting in an imbalance in the process of cellular repopulation of the epidermis. Continued exposure to ionizing radiation does not allow time for cells to repair tissue or DNA damage. Erythema and dry desquamation are the first signs of acute RD due to the injury of the epithelium and underlying structures of the skin. With dose accumulation over several radiation therapy sessions, several skin changes occur, such as severe erythema, hyperpigmentation, and dry or moist desquamation.2 Acute RD may lead to premature interruption of radiation therapy and to a reduction of the planned ionizing radiation dose, which in turn could be detrimental to the treatment outcome.6
A systematic review found several randomized clinical trials have investigated the use of topical agents for RD prevention and treatment in head and neck cancer patients undergoing radiation therapy.7 The topical agents included trolamine, aloe vera, allantoin, lianbai liquid, sucralfate, Na-sucrose octasulfate, olive oil, hyaluronic acid, and dexpanthenol. However, the review showed that there is limited or no evidence for the efficacy of any of these topical agents in preventing RD.
RD is an inflammatory reaction and research on prevention and treatment should focus on anti-inflammatory agents. Chamomilla recutita is a medicinal plant from the Asteraceae family, whose flowers have anti-inflammatory properties.8-10 The anti-inflammatory action of this plant has been evaluated for different dermatological conditions, such as contact dermatitis,11 eczema,12 peristomal skin lesions in colostomy,13 phlebitis,8 and dermatitis induced by ultra-violet irradiation,14 with positive effects in the reduction of inflammatory signs. Therefore, topical formulations containing chamomile might be an alternative for management of RD.
One formulation of chamomile cream that already exists in the market contains apigenin-7-glicosideo and levomenol as active ingredients. Chamomile has other anti-inflammatory ingredients that could be incorporated in a formulation. In addition, gel formulations are easier to apply and remove than cream formulations,15 and might be easier to use by the patient.
The last author (PEDR) has developed a gel formulation based on several ingredients present in chamomile.16 In the present work, we conducted 2 studies. In the first, we have prospectively compared 3 concentrations of the developed chamomile gel to assess its safety and to stablish the dose for the subsequent randomized trial. In the next study, we conducted a randomized trial to compare the chamomile gel (Chamomile Recutita) with urea cream (which is used in the institutional usual care) to assess the potential efficacy of the gel in delaying the occurrence of acute RD in patients with head and neck cancer, and to estimate effect sizes for a future larger confirmatory study.
Materials and Methods
In the first study, we prospectively compared 3 concentrations of chamomile gel, based on the formulation provided by the last author (PEDR), to assess its safety and potential efficacy, and to determine an appropriate starting dose for the second study. A gel preparation was chosen because it gives a refreshing sensation and it is oil-free, which is a more appropriate formulation to apply in skin exposed to radiation therapy, avoiding a bolus-like effect and making it easier to remove before the next radiation therapy session. In the second study, we compared the chamomile gel at the dose determined in the first study (concentration 8.35%) with a urea cream to prevent acute RD in head and neck skin exposed to radiation therapy. Both studies were conducted at the Center of High Complexity Oncology, University Hospital, Federal University of Brasília, Brasília, Brazil. The study was registered with Clinical Trials Registry (ClinicalTrials.gov Identifier: NCT02249884 and NCT02247830). A detailed protocol has been published elsewhere.17
Study 1: Safety and Potential Efficacy Study
Participants
Thirty participants with head and neck cancer receiving radiation therapy with or without concomitant chemotherapy, who were treated at the radiation therapy center were recruited for this study. Inclusion criteria were adults 18 years old or older undergoing radiation therapy for the first time for head and neck cancer. Exclusion criteria included: presenting without intact skin in the radiation area, having history of hypersensitivity or adverse reaction to chamomile or any plant of the Asteraceae or Compositae family, or receiving any type of medical prescription for prevention of RD. In addition, the person would be removed from the trial if he/she received medical prescription for prevention of RD during any point of the study. The study was approved by the Committee on Research Ethics of the School of Health Sciences of the University of Brasília, approval number 24692813.6.0000.0030.
Dose escalation of gel
In dose escalation studies, a modified Fibonacci scheme18 is usually followed, where dose levels increase by sequentially decreasing increments as the doses increase. This plan sets the second dose level at double the initial dose, the third level at 1.67 times higher than the second, the fourth at 1.5 times higher than the third, and each subsequent level is increased by 1.33 times. In this type of study, there was no control group, as the main objective was the safety of the gel.
Reis et al8 found that a 2.50% dose was active in the reduction of the duration of erythema in patients with phlebitis due to infusion chemotherapy. Therefore, we started the dose escalation at 2.50%, and used the modified Fibonacci scheme to increase the doses of the chamomile gel. The World Health Organization (WHO) establishes that concentration of doses should not be above 10% in semisolid formulations, such as gels.19 Therefore, we tested the following concentrations: 2.50%, 5.00% and, 8.35%, and we stopped the escalation, as the next value (12.53%) would be above the limit established by WHO.
The gel was produced by a compounding pharmacy according to a formulation developed by the last author.16 According to the Brazilian Health Surveillance Agency (Agência de Vigilância Sanitária - ANVISA), products made of chamomile do not require proof of safety because chamomile is already registered at the Brazilian Simplified Registry of Traditional Phytotherapic Products.20,21 However, since we were testing increasing doses for efficacy, any adverse events possibly related to the gel were also evaluated.
Recruitment
Potential participants were approached for enrollment between April 2014 and April 2015. After receiving a detailed explanation of the study, eligible individuals who agreed to participate completed an informed consent and were then provided with instructions for usual post-radiation self-care and for the gel application in the irradiated skin.
In dose escalation studies, a group of participants receives the lowest dose, and if there is no toxicity, a second group of participants receives the next higher dose, and so on. We started with 10 participants at 2.50% dose, followed by 10 participants for 5.00% dose and 10 participants for 8.35% dose. The assessment of each participant lasted 3 weeks.
Procedures
The study protocol was the same for all enrolled participants, except for the dose concentration of chamomile gel as explained above. Participants had an initial consultation with a nurse and learned how to perform self-care after radiation therapy, including oral hydration (approximately 2 L of water per day) and skin care with a moisturizing soap (DoveTM), followed by drying the area with a soft towel. They were instructed to avoid sun exposure, and for men, it was recommended that they only use electrical shavers if necessary. Skin care also included restrictions on applying any products to the irradiated area to avoid undesirable bolus effects. Participants were instructed to apply the chamomile gel topically 3 times a day (morning, afternoon and night) on the skin of the irradiated area for the entire period of the radiation therapy.
The gel was delivered in tubes, labeled with the product name (without specifying the concentration), expiration date, pharmacist’s name, and instructions for use. Participants and nurse evaluators were kept blinded regarding the gel dose received by each specific person. Nurse evaluators were not aware of escalation nature of the study design.
Radiation therapy was delivered using a 6MV photon beam from a Siemens Primus linear accelerator, in three-dimensional conformal radiation therapy (3D-CRT) technique, with thermoplastic mask.
Outcomes
Primary safety outcomes were presence of toxicity (presence [yes/no] of skin rash, papules, vesicle, bubbles and, pustule), dry desquamation (yes/no), and severity of erythema as assessed by the Radiation Therapy Oncology Group (RTOG) criteria (no reaction = 0, mild erythema = 1, moderate erythema = 2, and severe erythema = 3).22
The secondary outcome was time to development of erythema, measured as number of sessions of radiation therapy before erythema development.
Data collection/assessment
The participants were followed from the first radiation therapy session to the third week (15 sessions). Radiation therapy sessions occurred from Monday to Friday. At each session of radiation therapy, participants were evaluated by nurses and the occurrence of any of the outcomes was noted. The assessment included a physical exam of skin according to the RTOG criteria. Participants were also asked to report any symptoms related to the gel.
To document the evolution of the outcomes, at each radiation therapy session, photos were taken of the following regions: frontal region of head and neck, right and left lateral profiles, and neck posterior region. Photos were standardized, using the same distance from the participant, same type and intensity of light, and same camera (P510 Nikon). Only persons who agreed (on the signed informed consent) had their photos taken, and their identity was concealed in the photo.
Statistical analysis
Descriptive analyses (numeric and visual displays) were performed for all data. To compare demographic and clinical characteristics of the 3 dose groups and establish that they were comparable, we used Kruskal–Wallis non-parametric test for medians for age, and the Fisher exact test for all the categorical variables. To compare the average number of sessions from radiation therapy initiation to development of RD, we used the Kruskal–Wallis test since we had 3 groups and we could not assume normality of the data given the small sample size. Significance level for all tests was 0.05 and no multiple comparisons adjustment were made because this was a study to assess preliminary safety and potential efficacy of each dose level. Data were analyzed using IBM SPSS software (version 26.0 for Mac).
Study 2: Randomized Trial for Efficacy of Chamomile Gel versus Cream of Urea
Design and sample
Participants were recruited among patients in the unit where the study was performed. Inclusion and exclusion criteria for study 2 were the same as study 1.
Participants were randomly assigned to one of two groups: chamomile gel or urea cream. The outcome evaluators were blind to the group allocation.
Since this was a preliminary study, and no measures of efficacy were available for the chamomile gel or the urea cream groups, a formal sample size calculation was not possible. We aimed to enroll the maximum number that our time and resources allowed. Our study had 48 participants (two groups of 24). The study was approved by the Committee on Research Ethics of the School of Health Sciences of the University of Brasília.
Recruitment
Between July 2015 and May 2017, patients who were scheduled to receive radiation therapy for the first time, were approached by one of the investigators and invited to participate in the study. Inclusion criteria were checked, and the study was explained to the participant if he/she was eligible. Patients who agreed to participate signed an informed consent after receiving a detailed explanation of the study and having any of their questions answered.
Participants were randomized into the chamomile or the urea group. The randomization was conducted using a blocked approach with random assignments inserted in numbered opaque envelopes and then sealed. At the time of the randomization of each participant, the research nurse opened the next numbered envelope and assigned the participant to a group.
Procedure
Participants received the same written and oral instructions regarding self-care of the irradiated area as in Study 1. Individuals in both groups were instructed to apply the assigned product topically, 3 times a day, on the skin at the irradiated area, during the entire period of the radiation treatment. Both products were delivered in tubes labeled “cream for radiation dermatitis,” with the expiration date of the product, instructions for its use, and the name of the pharmacist. Although the participant was blind to the product he/she was receiving, the urea cream and the chamomile gel had different textures and colors, and there was a possibility that the participants might have guessed or known what they were receiving.
Participants received radiation therapy 5 days a week for 6 to 8 weeks, according to their prescription plan. Radiation therapy was delivered using a 6MV photon beam linac, using conformal planning (3D-CRT), with a thermoplastic mask.
A nurse, blind to the product received, evaluated the person’s skin weekly. To document the evolution of the outcomes during the treatment sessions, photographs were taken of the regions of the participant’s head and neck on a weekly basis (if the participant gave permission). The first evaluation was on the first radiation day, and each subsequent evaluation happened every 7 days thereafter until the end of treatment.
The cream of urea (the institution’s usual care) was produced in the compounding pharmacy of the hospital. The chamomile gel was produced in a private compounding pharmacy, and followed the formulation provided by the last author (PEDR). To reduce the possibility of degradation of the products over time (both had validity of 3 months), we ordered the products in several batches according to the enrollment of patients. Both pharmacies followed standardized procedures and had strict control of quality of their products; thus, we did not expect variations in the product that would affect the outcomes.
Outcomes
The primary outcome was time from beginning of radiation to the first occurrence (in weeks) of grade 1 or grade 2 RD, during the first 6 weeks of treatment. Grading was assessed by the RTOG criteria.22 Grade 1 was defined as follicular, mild erythema/ epilation/dry desquamation/decreased sweating, and Grade 2 was defined as tender or bright erythema, patchy moist desquamation/ moderate edema. Each grade was analyzed separately.
Secondary outcomes were: participant self-report of presence of tenderness, discomfort or pain (yes/no), presence of itching (yes/no), presence of burning (yes/no), and interference in daily activities (yes/no). In addition, the nurse evaluator assessed presence of hyperpigmentation (yes/no).
While the primary outcomes addressed only Grades 1 and 2, we followed all participants to the end of their treatments (up to 8 weeks) and collected data on occurrence of Grade 3 (confluent, moist desquamation other than skin folds, pitting edema) and Grade 4 (ulceration, hemorrhage, necrosis), if they occurred.
Other variables collected
Sociodemographic and clinical variables collected were: age (in years), sex (female or male), tumor site, diabetes (yes/no), smoking (smoker, ex-smoker, never smoked), alcohol use (alcoholic, ex-alcoholic, never drank), current treatment (radical radiation therapy or chemoradiation therapy), treatment technique (2DRT, 3D-CRT), total dose (cumulative measured in Gray - Gy), fractioned dose (received per day in each session, measured in Gy), and total number of fractions.
Statistical analysis
Descriptive analysis was performed for all variables. To compare the demographic and clinical characteristics of the 2 groups we used t-test for independent samples for mean age, the Mann–Whitney non-parametric test to compare the distributions of total dose and number of fractions, and the Fisher exact test for all other variables. To compare the 2 groups on the primary outcome, we calculated Kaplan–Meier curves for each intervention group and compared them using log-rank test. We set the significance level to 0.05. The effect size was calculated for the primary outcome as the difference in groups mean time divided by the standard deviation of the urea group (control). For adverse events (our secondary outcomes) we calculated the percentage of individuals presenting with the adverse event (at any level) in each group at each week and plotted those percentages over time. We tested the differences in distribution (proportion) using a Fisher’s exact test (using Monte Carlo simulation) at each time point of each adverse event. However, we want to emphasize that these tests should not be considered as confirmatory of any hypothesis test, as the secondary outcomes are of exploratory nature, the sample size is relatively small, and at later weeks, there were individuals with missing values, further reducing the sample size at those points. The figures present important information on the adverse events, that can be used to improve the study design of future studies. Data were analyzed using IBM SPSS software (version 26.0 for Mac) and figures were produced using RStudio (version 1.1.383).
Ethical considerations
Each participant provided written consent prior to starting the procedures.
Results
Study 1: Safety and Potential Efficacy Study
For this study, we planned to have 10 participants in the lowest dose, and if no serious adverse events were observed, we would go to the next higher dose with 10 more participants and repeat the process, until the third and highest dose. Using this process, 66 participants were assessed for eligibility, of whom 24 did not meet inclusion criteria and 4 refused to participate. In addition, 8 participants had to be excluded because of technical problems with the linear accelerator, which interrupted their treatment. The remaining 30 participants were included in the study as follows: the first 10 received 2.50% chamomile gel, the next 10 received 5.00% chamomile gel, and the last 10 received 8.35% chamomile gel. Two participants were lost to follow-up and were not included in the final analysis of safety and potential efficacy: one in the 2.50% group had radiation therapy suspended due to a surgery and one in the 5.00% group did not use the chamomile gel.
Most participants developed erythema during the 3-week assessment (8 out of 9 for 2.50%, 8 out of 9 for 5.00%, and 8 in 10 for 8.35%), and there was no statistically significant difference in proportions between the dose groups (P = 1.0). There was a longer time to develop erythema in the 8.35% gel group (mean = 10.7 sessions, standard deviation [SD] = 4.4, median = 11.5 sessions), followed by the 5.00% gel group (7.8, SD = 4.9, median = 7.0) and the 2.5% gel group (6.2, SD = 4.4, median = 5.0). The test for difference in median times to develop erythema was not statistically significant (P = 0.12), though the median times increased with increasing doses. Among participants who developed erythema, only one evolved from mild to moderate erythema during the follow-up time of 16 radiotherapy sessions. None of the participants had dry desquamation or skin toxicity related to the gel.
Figure 1 shows the number of radiation therapy sessions before development of erythema for each participant in each chamomile gel dose group. Currently, at our clinic, the usual treatment to prevent RD is urea cream. Our clinic records show that, on average, a person who uses that cream develops RD after 5.5 radiation therapy sessions. The vertical solid line in Figure 1 depicts that average. Although the median number of sessions for development of RD was not statistically different for the 3 groups, Figure 1 shows that there seems to be a possible delay in development as the concentrations of dose increases. Given those results and the fact that no adverse effects were observed, we chose the 8.35% dose of chamomile gel for Study 2.
Figure 1.
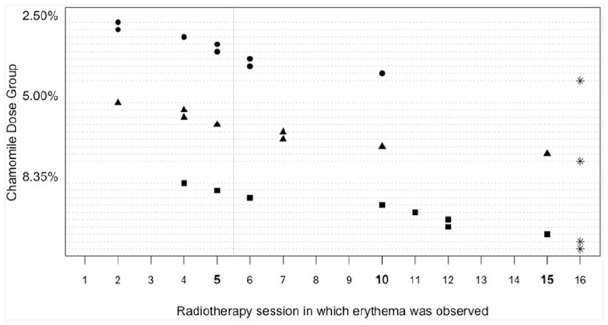
Time to development of erythema by concentrations of chamomile.
Each dashed line depicts the timeline for a person, with a marker at the radiotherapy session in which the erythema was first observed. Stars depict individuals who did not develop erythema by session 16. The vertical solid line depicts the average time for development for erythema when the usual cream of urea is used for prevention.
Study 2: Randomized Pilot Trial for Efficacy of Chamomile Gel versus Urea Cream
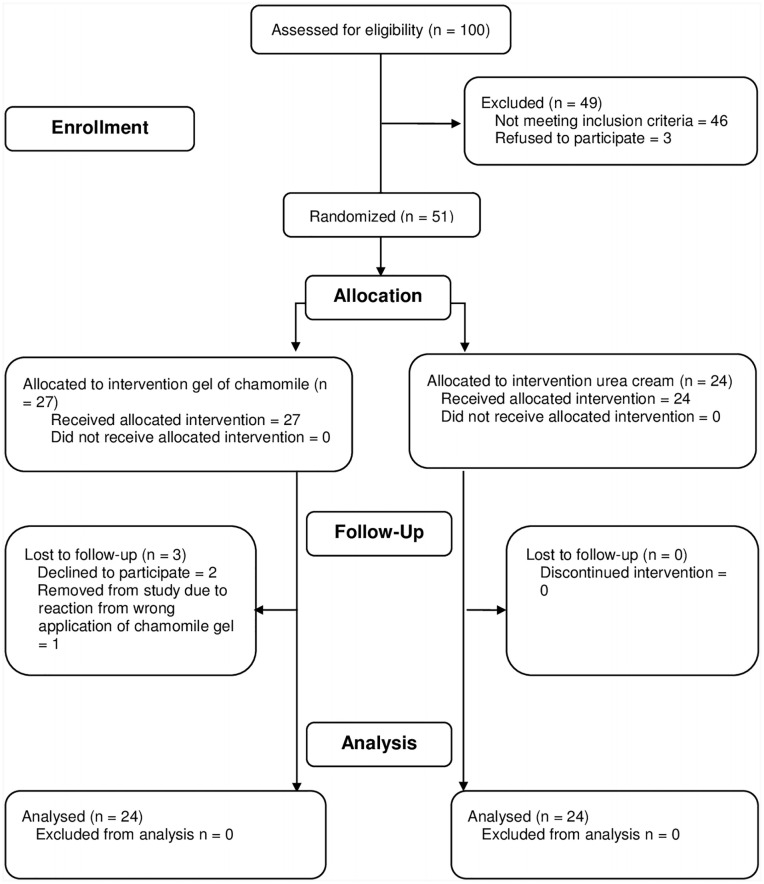
Figure 2 shows the CONSORT flow chart for the randomized study. One hundred participants were assessed for eligibility. Forty-six participants did not meet the eligibility criteria, and 3 participants refused to participate. From the 51 participants enrolled and randomized, 2 withdrew participation in the first week and 1 mistakenly applied chamomile gel near the eye region and was removed from the study to treat the adverse reactions (edema and pruritus in the periorbital region). The final analysis had 48 participants, with 24 participants in each group.
Figure 2.
CONSORT flow diagram for study 2.
Table 1 shows the distribution of demographic and clinical characteristics by group. No statistical differences were found between the 2 groups for age, smoking, drinking status, diabetes, tumor site, current treatment, radiation therapy technique, total dose, fractioned dose, and number of fractions.
Table 1.
Demographic and Clinical Characteristics by Intervention group (n = 48).
| Characteristics | Chamomile gel n = 24 | Urea cream n = 24 | P-value* |
|---|---|---|---|
| Age, in years, Mean (Standard deviation) Median (min, max) |
56 (16) 59 (19, 83) |
59 (13) 60 (26, 79) |
.57 |
| Sex, n (% male) | 16 (67) | 17 (71) | 1.00 |
| Tumor site, n (%) | |||
| Nasal cavity | 1 (4) | 0 (0) | .92 |
| Oral cavity | 9 (38) | 10 (42) | |
| Larynx | 6 (25) | 7 (29) | |
| Pharynx (nasopharynx, oropharynx and, hypopharynx) | 7 (29) | 5 (21) | |
| Other sites | 1 (4) | 2 (8) | |
| Diabetes, n (% yes) | 5 (21) | 3 (12) | .70 |
| Smoking, n (%) | |||
| Current or former smoker | 19 (79) | 19 (79) | 1.00 |
| Never smoked | 5 (21) | 5 (21) | |
| Alcohol consumption, n (%) | |||
| Current or former drinker | 16 (67) | 19 (79) | .52 |
| Never drank | 8 (33) | 5 (21) | |
| Current treatment, n (%) | |||
| Radical radiotherapy | 8 (33) | 9 (38) | 1.00 |
| Chemoradiotherapy | 16 (67) | 15 (62) | |
| Radiotherapy type, n (%) | |||
| 2DRT | 2 (8) | 1 (4) | 1.00 |
| 3D-CRT | 22 (92) | 23 (96) | |
| Total dose (Gy), Median (min, max) | 70 (36, 70) | 70 (30, 70) | .37 |
| Fractioned sose (Gy), n (%) | |||
| 1.80 | 7 (29) | 6 (25) | 1.00 |
| 2.00 | 16 (67) | 17 (71) | |
| 2.25 | 0 (0) | 1 (4) | |
| 2.50 | 1 (4) | 0 (0) | |
| Fractions, Median (min, max) | 35 (20, 39) | 35 (15, 39) | .45 |
P-value from Student t-test for equality of means of age, from Mann–Whitney test for equality of distribution of total dose and fractions, and from Fisher Exact test for all others.
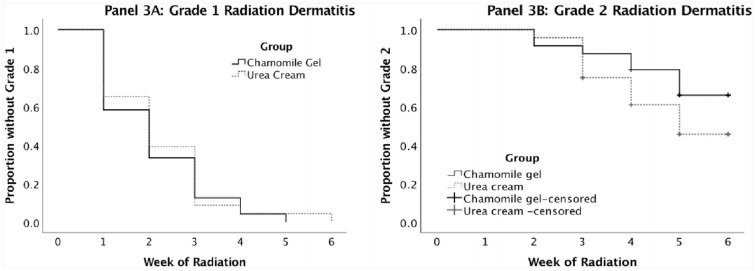
Figure 3 shows the Kaplan–Meyer curves for time to occurrence of Grade 1 (Panel 3A) and Grade 2 (Panel 3B) RD, respectively. The log-rank tests were not statistically significant for Grade 1 (P = 0.67) or Grade 2 (P = 0.17). Grade 1 started developing at the end of the first week of radiation therapy in both groups, and all participants had at least Grade 1 by the end of the study. For the individuals who did not develop Grade 1 or 2, the time was considered as 6 weeks (the maximum possible). Mean time to develop Grade 1 was 2.08 weeks (SD = 1.18) in the chamomile group and 2.22 (1.24) in the urea group. The effect size was −0.11, a small effect size. Grade 2 started developing in week 2 in both groups, but the urea group had a slightly faster rate of occurrence over time, though not statistically significant. Mean time to develop Grade 2 was 5.08 (1.28) in the chamomile group and 4.48 (1.31) in the urea group. In both groups, there were participants who did not develop Grade 2 before the end of follow-up (those observations are denominated “censored”). The effect size for Grade 2 was 0.46, a moderate effect size in the direction of longer time to develop Grade 2 in the gel group. There were no occurrences of Grades 3 and 4 throughout the entire study.
Figure 3.
Kaplan–Meier curves comparing time to occurrence of grade 1 (Panel A) and grade 2 (Panel B) radiation dermatitis in the chamomile and urea groups.
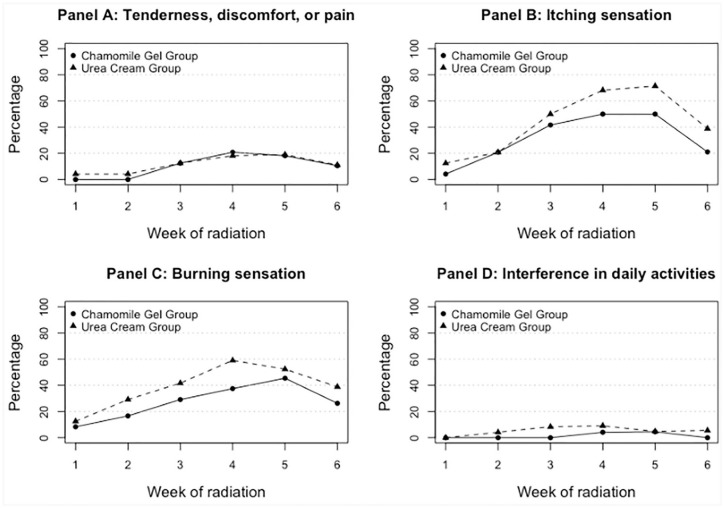
Figure 4 shows percentages of participants self-reporting presence of adverse events (yes/no) by intervention group over the 6-week study period. None of the differences between groups at each point in time were statistically significant. However, the figures present important information that might be useful in designing future studies. Tenderness, discomfort and pain (Panel 4A) had similar patterns in the 2 groups from week 3 and on, with maximum percentage observed of about 19% at week 4. Differences (chamomile gel minus urea cream) varied between −4% and 2% (negative percentages represent lower percentages for the gel group). Itching sensation (Panel 4B) was reported more frequently in the urea group starting on week 3, with about 17% to 21% more in the urea than the gel group for weeks 4 to 6. Maximum reported was 71% for urea group and 50% in the gel group, both at week 5. Burning sensation was reported more often in the urea group starting in the first week, with a peak in the urea group on week 4 (59%) and a peak in the gel group in week 5 (45%). Differences (chamomile gel minus urea cream) varied from −4% to −22% (on week 4), showing a consistently smaller percentage in the gel group. Interference with daily activities was not frequently reported (maximum of 9% in the urea and 4% in gel groups), though the first reported interference occurred earlier (week 2) for the urea group than for the gel group (week 4).
Figure 4.
Percentage of participants reporting presence of tenderness, discomfort or pain (Panel A), itching sensation (Panel B), burning sensation (Panel C), and interference in daily activities (D), by in chamomile and urea groups, over the 6-week period of intervention.
Figure 5 shows the percentage of individuals with hyperpigmentation as evaluated by the research nurse. Both groups had similar percentages for the first 2 weeks (from 8% to 25%). Starting in week 3, the urea group presented a higher percentage of hyperpigmentation than the gel group, with 20% to 31% higher percentages in the urea group. The peak occurred on week 6 (94%) for the urea group, and on week 5 (68%) for the gel group. The groups were not statistically different at all point in times, except for week 6 (Monte Carlo simulated P = 0.04, 95% confidence interval: 0.03-0.04), despite the loss to follow-up of 5 individuals in the gel group and 6 in the urea group.
Figure 5.
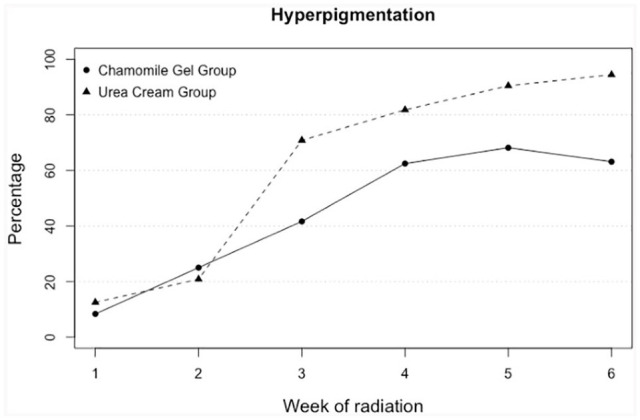
Percentage of participants presenting with hyperpigmentation in the chamomile and urea groups, by week of treatment.
Discussion
In our first study, we found that the gel containing 8.35% was still safe when compared to concentrations of 2.5% and 5.0% used by participants receiving radiation therapy for head and neck cancer. We also showed that increasing concentrations tended to delay the development of erythema in those participants.
In the second study, a formulation of 8.35% chamomile gel was not statistically different from urea cream in the delay the development of grades 1 and 2 RD, though the effect size of delay of Grade 2 was of moderate size. No statistical differences over time were seen between the 2 groups on adverse events. However, visually, the figures showed that the chamomile gel might delay or reduce itching and burning sensations, and the development of hyperpigmentation as evaluated by the research nurse.
The development and severity of acute RD depends on multiple factors intrinsic and extrinsic to the person, including age, sex, diabetes, smoking, tumor site, current treatment, treatment technique, total dose, and fractioned dose.1,5,23 In this pilot study, the 2 groups evaluated (chamomile gel and urea cream) were balanced on all extrinsic and intrinsic factors that we measured and that could contribute to differences in susceptibility to RD. Therefore, we expect that those factors were not the source of the differences we observed in the figures. Our results can be used to design a larger confirmatory study of superiority of the chamomile gel over urea cream for the purpose of delaying the development of RD and reducing severity of symptoms in head and neck cancer patients.
Our results are important for several reasons. First, the systematic reviews of interventions for prevention and treatment of RD that have been published24-29 for topical interventions had mixed results, and most studies were not specific for head and neck cancer. We found few studies that identified topical interventions that potentially can prevent RD in head and neck cancer. Abbas et al30 assessed the efficacy of trolamine against usual supportive care (keep the area clean with the option to use a moisturizer) in 30 participants and found that trolamine was efficacious for preventing RD from increasing in severity from RTOG grade 2 to grade 3 (only 3 of 15 participants developed grade 3 in the trolamine group versus 8 of 15 in the control group). Pallati et al31 evaluated the efficacy of topical application of a cream containing turmeric and sandal wood oil that was shown to be effective in preventing RTOG grade 1 RD in 3 of 22 participants with head and neck cancer (12%) when compared with 10 of 24 that used Johnson’s baby oil (42%). Recently, one study that evaluated StrataXRT® versus 10% Glycerin showed positive results for preventing and managing RD in patients with head and neck cancer. The StrataXRT® group had a 12% lower risk of experiencing grade 2 skin toxicity (P = 0.031); and a 36% lower risk of experiencing grade 3 skin toxicity (P = 0.025).32 Although those interventions were considered effective, some studies had small sample sizes30,31 and the new products are not available worldwide (none of them are available in Brazil).
Second, in Brazil, there is no standardized topical intervention that is recommended or used for prevention of RD. In addition, the majority of the population has low economic status, and there is a need for low-cost and efficacious interventions to prevent RD in patients undergoing radiation therapy. The chamomile gel is an affordable product and if shown effective, it would be beneficial to patients undergoing radiation therapy for head and neck cancer.
Third, we compared the chamomile gel against the oil-free urea cream, thus avoiding a comparison that could be biased to a possible bolus effect of the comparator. One study in Brazil tested calendula (Calendula officinalis) against essential fatty acids in the prevention and treatment of RD in this patient population.33 The study showed that calendula was more effective than essential fatty acids (oil lotion) in the prevention of RD and showed that the patients in the group that used the oil lotion developed RD grade 2 and 3.33 In our pilot study, no participant developed grade 3 in either group. We think that oils should not be used on irradiated skin in order to avoid the bolus effect, and therefore, calendula should have been compared with an oil-free topical intervention.
The lack of low-cost efficacious interventions for RD in patients with head and neck cancer was the main motivator for the development of the chamomile gel and for the 2 studies reported here. There is some evidence that newer technologies for intensity-modulated radiation therapy (IMRT) might decreases the severity of RD.34 However, such new technologies are either of limited access or not available to populations from mid/low income countries, and low-cost solutions for prevention and treatment of RD are still needed.
Chamomile has been used in traditional medicine for a long time to treat a wide variety of diseases and has a low cost because it is a widely cultivated medicinal plant. Pre-clinical studies in vitro showed evidence of the anti-inflammatory action of chamomile by inhibiting the production of interleukin-6,35 prostaglandins, and cyclooxygenase, by a mechanism of action similar to non-steroidal anti-inflammatory drugs.36 In various studies in rats, chamomile showed anti-inflammatory effects similar to benzydamine, reduced edema, and inhibited dermatitis induced by noxious agents, suggesting that chamomile may be a potential topical anti-inflammatory agent.37
In human studies, the anti-inflammatory action of chamomile has been evaluated for different inflammatory conditions of the skin, such as eczema,12,38,39 phlebitis,8 and dermatitis,11,40,41 with positive effects in reduction of inflammatory signs. The erythema is usually the most common primary outcome evaluated among the studies.
Kamillosan®, a cream developed in Germany that contains 2% ethanol extract from chamomile flowers, was evaluated in comparison to almond ointment in 48 women with breast cancer undergoing radiation therapy who developed RD. There was a delay in the onset of erythema, and dry and moist desquamation in the group treated with Kamillosan®. However, most of the reactions appeared in the second or third week in both groups and the interventions, and moist desquamation, a reaction that led to the interruption of the treatment, occurred in 4 (8%) patients in the Kamillosan group, and in 3 (6%) of the almond group. The use of the products was stopped in all of those cases.40 In our study, none of the patients developed moist desquamation, and we conjecture that the reason was that our formulations were oil free. Formulations that countains oil are difficult to remove before the next radiation therapy session, which needs clean skin for the radiation therapy. In the Kamillosan study, both creams had oil (almond oil in the almond cream and maize oil in the Kamillosan®).
Our second study presented a few limitations that should be taken into account when designing a larger confirmatory study. We measured development of RD on a weekly basis, but it might be more informative to measure it daily to have a more precise estimate of the difference in time effects of the 2 interventions. For example, it is possible that the difference in development of RD is of a magnitude of days and not weeks. Another limitation was the difficulty in monitoring the amount of chamomile gel and urea cream applied by each participant, as well as the frequency and quality of the applications that were carried out at home. Measuring compliance is notoriously difficult, as many patients manipulate the information due to social desirability (patient wants to give the information that they think the research wants to hear). We dealt with this problem by using intent-to-treat data analysis, which assumes the individual used the treatment as prescribed, whether this is true or not. If the gel is efficacious when correctly used and some individuals did not completely comply with the prescriptions, then we might have underestimated its effect. It is possible that due to differences in appearance between the 2 products, such as color, texture and smell, some participants might have guessed their intervention and be more or less inclined to use it as prescribed according to what they believed they received as intervention. We tried to minimize this effect (if it existed) by keeping evaluators blind to the intervention and by using intent-to-treat analysis. In future studies of the same type, we might ask the individual to report what treatment they thought they received, and include some means (dairies, for example) to stablish whether the person used the product or not. A final limitation is the instrument to measure the development of RD. The RTOG criteria was the only instrument available at the time of the study to measure RD. In our experience, however, the instrument is not fine enough to evaluate gradual small but clinically important changes in RD. An instrument more responsive to small but clinically important changes would be useful for research in this area.
The differences between groups that we found were in the desired direction, although not statistically significant. The moderate effect size of the chamomile gel against urea cream of 0.46 in delaying Grade 2 is encouraging and should be further tested. If it is confirmed, it would mean that Grade 2 could be delayed for a few days. Before this study, we did not have data to allow us to design a confirmatory study, which should be the next logical step in this research area. We showed that this type of study is feasible in a population seen in a public healthcare system like the one in Brazil. The rate of refusal was very small (only 3 participants before randomization and 2 after), and only one participant was lost due to incorrect application of the cream. The results or various outcomes are promising, showing a good potential for the chamomile gel to have a better preventive action than urea cream, in situations where a two-dimensional RT or three-dimensional conformal RT is used, as in many mid- and low-income countries. In addition, in our study, the cost of the gel was about a third of the cost of the urea cream, making it a desirable product (if proven efficacious) for our low-income population. This study provides the motivation and the estimates necessary to design a larger confirmatory study.
Conclusion
In our first study, we observed that a higher concentration of 8.35% dose of chamomile gel seemed to be potentially more efficacious than 2.50% and 5.00% concentrations, while not showing adverse effects. In the second study, there was no statistically significant difference in time to occurrence of RD grade 1 and 2, although the time for the development of RD grade 2 was longer in the chamomile group, and that the outcomes reported by the participants as well as the hyperpigmentation observed by the nurses occurred less often in the chamomile group than in the urea cream group. These findings justify a call for a larger study of efficacy of chamomile gel to prevent RD when compared to the institutional usual care (cream of urea).
Supplemental Material
Supplemental material, CONSORT_2010_checklist_of_information_to_include_when_reporting_a_randomised_trial for Chamomile Gel versus Urea Cream to Prevent Acute Radiation Dermatitis in Head and Neck Cancer Patients: Results from a Preliminary Clinical Trial by Elaine B. Ferreira, Marcia A. Ciol, Amanda G. de Meneses, Priscila de S. M. Bontempo, Jeanne M. Hoffman and Paula E. D. dos Reis in Integrative Cancer Therapies
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the CNPq (National Council for Scientific and Technological Development) [grant number 8268792905342559].
ORCID iD: Elaine B. Ferreira  https://orcid.org/0000-0003-0428-834X
https://orcid.org/0000-0003-0428-834X
References
- 1. Iacovelli NA, Naimo S, Bonfantini F, et al. Preemptive treatment with Xonrid®, a medical device to reduce radiation induced dermatitis in head and neck cancer patients receiving curative treatment: a pilot study. Support Care Cancer. 2017;25:1787-1795. [DOI] [PubMed] [Google Scholar]
- 2. Seité S, Bensadoun R-J, Mazer J-M. Prevention and treatment of acute and chronic radiodermatitis. Breast Cancer. 2017;9:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tao Y, Auperin A, Sire C, et al. Multicenter randomized double-blind, placebo-controlled trial GORTEC (Groupe Oncologie Radiotherapie Tete et Cou) 2009-01 evaluating the effect of the regenerating agent on radiodermatitis of head and neck cancer patients. Int J Radiat Oncol. 2017;99:590-595. [DOI] [PubMed] [Google Scholar]
- 4. O’Donovan A, Coleman M, Harris R, Herst P. Prophylaxis and management of acute radiation-induced skin toxicity: a survey of practice across Europe and the USA. Eur J Cancer Care. 2015;24:425-435. [DOI] [PubMed] [Google Scholar]
- 5. Villavicencio M, Granados-García M, Vilajosana E, Domínguez-Cherit J. Management of radiodermatitis associated with cetuximab in squamous cell carcinomas of the head and neck. Int J Dermatol. 2017;56:602-609. [DOI] [PubMed] [Google Scholar]
- 6. Narvaez C, Doemer C, Idel C, et al. Radiotherapy related skin toxicity (RAREST-01): Mepitel® film versus standard care in patients with locally advanced head-and-neck cancer. BMC Cancer. 2018;18:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira EB, Vasques CI, Gadia R, et al. Topical interventions to prevent acute radiation dermatitis in head and neck cancer patients: a systematic review. Support Care Cancer. 2017;25:1001-1011. [DOI] [PubMed] [Google Scholar]
- 8. Reis PED, Carvalho EC, Bueno PCP, Bastos JK. Clinical application of Chamomilla recutita in phlebitis: dose response curve study. Rev Lat Am Enfermagem. 2011;19:3-10. [DOI] [PubMed] [Google Scholar]
- 9. Singh M, Alavi A, Wong R, Akita S. Radiodermatitis: a Review of our current understanding. Am J Clin Dermatol. 2016;17:277-292. [DOI] [PubMed] [Google Scholar]
- 10. Santos DS, Barreto RSS, Serafini MR, et al. Phytomedicines containing Matricaria species for the treatment of skin diseases: a biotechnological approach. Fitote. 2019;138:104267. [DOI] [PubMed] [Google Scholar]
- 11. Arsić I, Tadić V, Vlaović D, et al. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother Res. 2011;25:228-233. [DOI] [PubMed] [Google Scholar]
- 12. Shimelis ND, Asticcioli S, Baraldo M, Tirillini B, Lulekal E, Murgia V. Researching accessible and affordable treatment for common dermatological problems in developing countries. An Ethiopian experience. Int J Dermatol. 2012;51:790-795. [DOI] [PubMed] [Google Scholar]
- 13. Charousaei F, Dabirian A, Mojab F. Using chamomile solution or a 1% topical hydrocortisone ointment in the management of peristomal skin lesions in colostomy patients: results of a controlled clinical study. Ostomy Wound Manage. 2011;57:28-36. [PubMed] [Google Scholar]
- 14. Korting HC, Schäfer-Korting M, Hart H, Laux P, Schmid M. Anti-inflammatory activity of hamamelis distillate applied topically to the skin. Influence of vehicle and dose. Eur J Clin Pharmacol. 1993;44:315-318. [DOI] [PubMed] [Google Scholar]
- 15. Mayba J, Gooderham J. A guide to topical vehicle formulations. J Cutan Med Surg. 2018;22:207-212. [DOI] [PubMed] [Google Scholar]
- 16. REIS, P.E.D. Therapeutic Topic Use of Chamomilla Recutita in Phlebitis Due to Peripheral Intravenous Therapy [Doctoral thesis]. University of São Paulo at Ribeirão Preto College of Nursing, Ribeirão Preto, SP, Brasil, 2008: 229. [Google Scholar]
- 17. Ferreira EB, Ciol MA, Vasques CI, et al. Gel of chamomile vs. urea cream to prevent acute radiation dermatitis in patients with head and neck cancer: a randomized controlled trial. J Adv Nurs. 2016;72:1926-1934 [DOI] [PubMed] [Google Scholar]
- 18. Penel N, Kramar A. What does a modified-Fibonacci dose-escalation actually correspond to? BMC Med Res Methodol. 2012;12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. WHO monographs on selected medicinal plants, vol 1. World Heal Organization; 1999:1 https://apps.who.int/medicinedocs/pdf/s2200e/s2200e.pdf. Accessed March 24, 2019. [Google Scholar]
- 20. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. [Resolution - RDC No. 14, of March 31, 2010. Provides for the registration of herbal medicines 2010]. http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2014/rdc0026_13_05_2014.pdf. Accessed March 24, 2019.
- 21. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. [Normative Instruction No. 2 of May 13, 2014. Publishes the “List of herbal medicines for simplified registration” and “List of traditional herbal products for simplified registration”] 2014. http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2014/int0002_13_05_2014.pdf Accessed March 24, 2019.
- 22. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol. 1995;31:1341-1346. [DOI] [PubMed] [Google Scholar]
- 23. Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Zhang S, Shao X. Topical agent therapy for prevention and treatment of radiodermatitis: a meta-analysis. Support Care Cancer. 2013;21:1025-1031. [DOI] [PubMed] [Google Scholar]
- 26. Salvo N, Barnes E, van Draanen J, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17:94-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson J, Smith JE, McIntyre M, Thomas R, Pilkington K. Aloe vera for preventing radiation-induced skin reactions: a systematic literature review. Clin Oncol. 2005;17:478-484. [DOI] [PubMed] [Google Scholar]
- 28. Bolderston A, Lloyd NS, Wong RKS, Holden L, Robb-Blenderman L. Supportive care guidelines group of cancer care ontario program in evidence-based care. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006;14:802-817. [DOI] [PubMed] [Google Scholar]
- 29. Meghrajani CF, Co HCS, Ang-Tiu CMU, Roa FC. Topical corticosteroid therapy for the prevention of acute radiation dermatitis: a systematic review of randomized controlled trials. Expert Rev Clin Pharmacol. 2013;6:641-649. [DOI] [PubMed] [Google Scholar]
- 30. Abbas H, Bensadoun R-J. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer. 2012;20:185-190. [DOI] [PubMed] [Google Scholar]
- 31. Palatty PL, Azmidah A, Rao S, et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol. 2014;87:20130490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan RJ, Blades R, Jones L, et al. A single-blind, randomised controlled trial of StrataXRT – A silicone-based film-forming gel dressing for prophylaxis and management of radiation dermatitis in patients with head and neck cancer. Radiother Oncol. 2019;139:72-78. [DOI] [PubMed] [Google Scholar]
- 33. Schneider F, Tannia M, Danski R, Vayego SA. Usage of Calendula officinalis in the prevention and treatment of radiodermatitis: a randomized double-blind controlled clinical trial. J Sch Nurs. 2015;49:221-228. [DOI] [PubMed] [Google Scholar]
- 34. Kole AJ, Kole L, Moran MS. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer. 2017;9:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew). Food Chem Toxicol. 2003;41:1381-1390. [DOI] [PubMed] [Google Scholar]
- 36. Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85:663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petronilho S, Maraschin M, Coimbra MA, Rocha SM. In vitro and in vivo studies of natural products: a challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind Crops Prod. 2012;40:1-12. [Google Scholar]
- 38. Patzelt-Wenczler R, Ponce-Pöschl E. Proof of efficacy of Kamillosan(R) cream in atopic eczema. Eur J Med Res. 2000;5:171-175. [PubMed] [Google Scholar]
- 39. Aertgeerts P, Albring M, Klaschka F, et al. Comparative testing of Kamillosan cream and steroidal (0.25% hydrocortisone, 0.75% fluocortin butyl ester) and non-steroidal (5% bufexamac) dermatologic agents in maintenance therapy of eczematous diseases. Z Hautkr. 1985;60:270-277. [PubMed] [Google Scholar]
- 40. Maiche AG, Gröhn P, Mäki-Hokkonen H. Effect of chamomile cream and almond ointment on acute radiation skin reaction. Acta Oncol. 1991;30:395-396. [DOI] [PubMed] [Google Scholar]
- 41. Nissen HP, Biltz H, Kreysel HW. Profilometry, a method for the assessment of the therapeutic effectiveness of Kamillosan ointment. Z Hautkr. 1988;63:184-190. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CONSORT_2010_checklist_of_information_to_include_when_reporting_a_randomised_trial for Chamomile Gel versus Urea Cream to Prevent Acute Radiation Dermatitis in Head and Neck Cancer Patients: Results from a Preliminary Clinical Trial by Elaine B. Ferreira, Marcia A. Ciol, Amanda G. de Meneses, Priscila de S. M. Bontempo, Jeanne M. Hoffman and Paula E. D. dos Reis in Integrative Cancer Therapies