Abstract
In 301 treatment-naïve patients with pulmonary arterial hypertension stratified by the European Society of Cardiology/European Respiratory Society risk score, further stratification of intermediate-risk patients based on six-minute walk distance and the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio identified a subset with mortality rates comparable to low-risk patients.
Keywords: pulmonary hypertension, hemodynamics, echocardiography, risk factors, mortality/survival
Introduction
Risk stratification is increasingly important in pulmonary hypertension (PH).1–5 The current European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines therefore recommend a risk stratification scheme to facilitate initial treatment decisions and monitoring of treatment response in patients with pulmonary arterial hypertension (PAH).1 The scheme classifies patients into three risk groups reflecting mortality, and has been validated in several PAH cohorts.3–5 However, it remains unclear how patients with an intermediate risk should be treated—initial monotherapy and initial combination therapy are both options in the current ESC/ERS guidelines.1 Furthermore, the aim of medical treatment is to shift high- and intermediate-risk patients into the low-risk group by improving their hemodynamic function and, concordantly, their life expectancy.1,3,4,6 Usually, this goal is not achieved since many patients remain in the intermediate-risk group3–5 or have a mixed pattern of low and intermediate risk. The current guidelines do not clearly specify how to manage PH in those patients: should they be treated as high-risk patients considering therapy escalation or as low-risk patients with frequent follow-up until deterioration?1,7
We therefore analyzed whether further stratification of intermediate-risk patients would allow a more decisive prediction of their mortality.
Methods
The raw data that underpin this study are available from the corresponding author on reasonable request.
We retrospectively analyzed patients with PAH who entered the Giessen PH Registry8 between 2008 and 2018. All patients were treatment-naïve and underwent diagnostic right heart catheterization in our clinic including measurements of right atrial pressure, cardiac index, and mixed venous oxygen saturation. Right heart catheterization parameters obtained after a short waiting period were used for risk stratification to avoid methodological issues.9 Transthoracic echocardiography data as well as six-minute walk distance (6MWD) and brain natriuretic peptide values were also considered for risk stratification, if available. Missing data (6MWD, brain natriuretic peptide, right atrial pressure, cardiac index, mixed venous oxygen saturation, and the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP) ratio) were addressed by multiple imputation. All patients were diagnosed according to the current guidelines,1 and all diagnoses of PH were assessed by a multidisciplinary board including pulmonary physicians and radiologists. Patients were followed until December 2018. Survival status was determined by contacting the patient or their primary care physician. The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the Faculty of Medicine at our University. All participating patients gave written informed consent to be enrolled in the Giessen PH Registry. All authors had full access to all the data in the study and take responsibility for its integrity and the data analysis. Patients were classified as low, intermediate, or high risk based on the parameters listed above, using ESC/ERS cut-off values1 and the strategy described by Kylhammar and co-workers.4
For focused risk stratification of intermediate-risk patients, we developed two models. In the first model, 6MWD was considered alone for advanced risk stratification using the threshold mentioned in the ESC/ERS risk stratification scheme (high risk: <165 m).1 In the second model, 6MWD (optimal cut-off (Youden’s index): <270 m) was combined with the TAPSE/PASP ratio (optimal cut-off (Youden’s index): <0.24 mm/mmHg). If at least one of the two included parameters was below the optimal cut-off, the patient was assigned an overall classification of high-intermediate risk; otherwise, the patient was classified as low-intermediate risk. Kaplan–Meier analysis and log-rank tests were performed to evaluate survival differences between risk groups. Cox regression analysis was performed to assess hazard ratios with low-risk patients as the reference group. The optimal cut-offs for 6MWD and TAPSE/PASP were derived from patients at intermediate risk.
The prognostic abilities of the original and advanced risk stratification systems were evaluated by calculating the c statistic10 and compared by calculating the integrated discrimination improvement. The integrated discrimination improvement measures the extent to which a new model changes the difference in estimated risk of an event between individuals with and without an event.11
Statistical analyses were done using R (The R Foundation, Vienna, Austria) and SPSS (Version 26, IBM, Armonk, USA). Normally distributed parameters are shown as mean ± standard deviation.
Results
Overall, 301 patients with PAH were included in the analysis. The patients were 58 ± 15 years old and 65% were female. Right heart catheterization measurements, brain natriuretic peptide values, 6MWD, and echocardiographic parameters were available in 268 (89%), 179 (59%), 234 (78%), and 274 (91%) patients, respectively. TAPSE/PASP was measurable in 261 patients (87%). Baseline characteristics showed hemodynamic impairment (mean pulmonary arterial pressure: 45 ± 13 mmHg; right atrial pressure: 6.3 ± 4.7 mmHg; pulmonary vascular resistance: 8.9 ± 5.0 Wood Units). Seventy-eight patients (26%) were classified as low risk, 191 patients (63%) as intermediate risk, and 32 patients (11%) as high risk. The intermediate- and high-risk groups had older patients, a greater proportion of men, and more severe hemodynamic impairment than the low risk group.
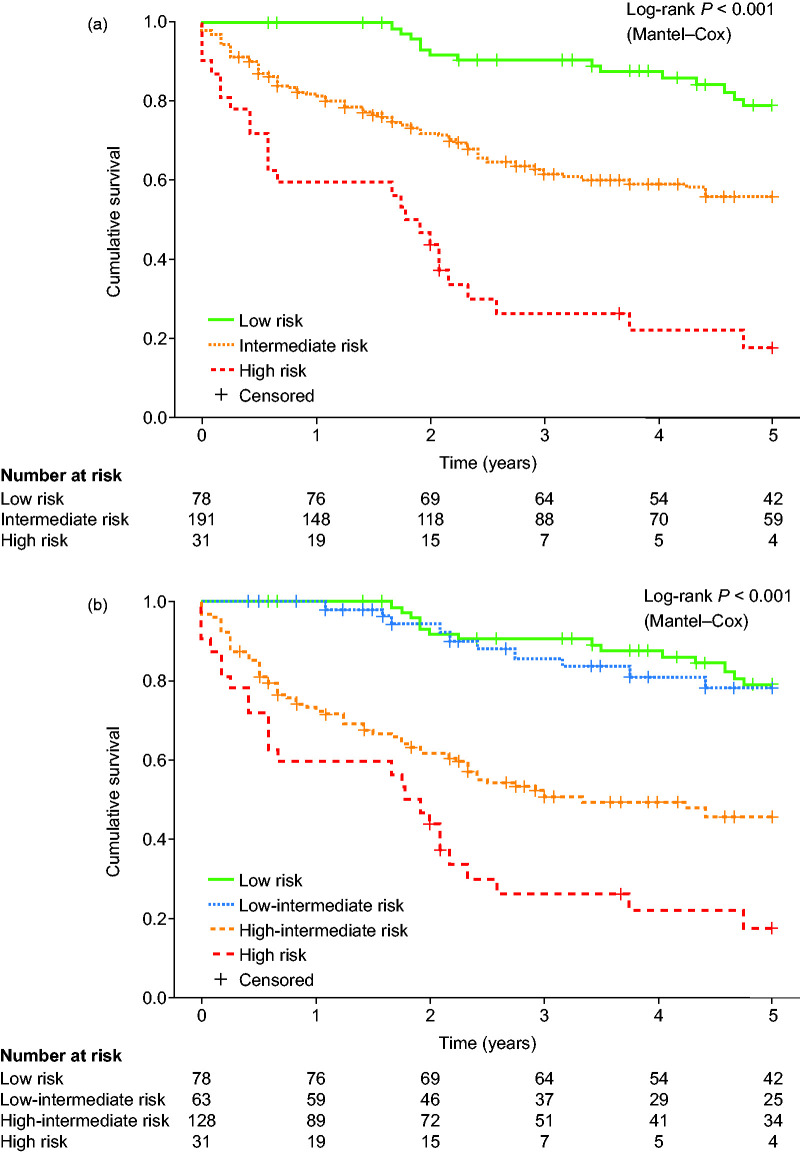
The survival of the ESC/ERS risk groups differed significantly (log-rank P < 0.001). One-, three-, and five-year survival were 100%, 91%, and 79%, respectively, in the low-risk group, 81%, 62%, and 56%, respectively, in the intermediate-risk group, and 59%, 26%, and 17%, respectively, in the high-risk group (Fig. 1a).
Fig. 1.
Advanced risk stratification of patients with PAH in the Giessen pulmonary hypertension registry. (a) Five-year survival of patients with PAH classified according to the ESC/ERS risk stratification scheme. (b) Five-year survival of patients with PAH classified according to the ESC/ERS risk stratification scheme combined with a further sub-stratification of intermediate-risk patients into low-intermediate- and high-intermediate-risk groups using 6MWD and TAPSE/PASP as surrogates of right ventricular–arterial coupling. Stratification was performed after multiple imputation of missing data.
Classification of low-intermediate- and high-intermediate-risk groups based on 6MWD alone did not reflect survival when using non-imputed data (log-rank P > 0.05 between low-intermediate- and high-intermediate-risk groups, plot not shown). However, after multiple imputation of missing data, 6MWD was able to differentiate between low-intermediate- and high-intermediate-risk groups (log-rank P = 0.013, plot not shown). Interestingly, the second risk stratification model combining 6MWD with TAPSE/PASP was sufficient for advanced risk stratification in the intermediate-risk group with and without imputation of missing data. Of the intermediate-risk patients, 63 were classified as low-intermediate risk and 128 as high-intermediate risk based on 6MWD and TAPSE/PASP after multiple imputation. One-, three-, and five-year survival was 100%, 86%, and 78%, respectively, in the low-intermediate-risk group and 72%, 50%, and 45%, respectively, in the high-intermediate-risk group (log-rank P < 0.001).
Right atrial pressure and cardiac index did not differ between the low-intermediate- and high-intermediate-risk groups, whereas mean pulmonary arterial pressure and pulmonary vascular resistance were increased and mixed venous oxygen saturation was decreased in the high-intermediate-risk group (mean pulmonary arterial pressure: 42 ± 12 mmHg vs 48 ± 13 mmHg, P = 0.006; right atrial pressure: 5.9 ± 3.9 mmHg vs 6.4 ± 4.3 mmHg, P = 0.441; pulmonary vascular resistance: 7.6 ± 4.0 Wood Units vs 9.9 ± 4.9 Wood Units, P = 0.003; cardiac index: 1.7 ± 5.5 L/min/m2 vs 2.4 ± 3.6 L/min/m2, P = 0.673; and mixed venous oxygen saturation: 66 ± 7% vs 63 ± 6%, P = 0.002).
Overall, the stratification into four risk groups using 6MWD and TAPSE/PASP was prognostic (Fig. 1b, log-rank P < 0.001). The survival distribution showed a significant difference between the low-intermediate- and high-intermediate-risk groups (log-rank P < 0.001) but not between the low-intermediate and low-risk groups (log-rank P = 0.719). Patients at high risk showed impaired survival compared with patients at high-intermediate risk (log-rank P = 0.006). Accordingly, Cox regression (with the low risk group as the reference) revealed that low-intermediate risk was not associated with increased mortality (hazard ratio (95% confidence interval (CI)): 1.049 (0.466–2.362), P = 0.908), whereas patients at high-intermediate and high risk showed impaired survival (hazard ratio (95% CI): 4.173 (2.336–7.454), P < 0.001 (high-intermediate risk) and 8.065 (4.151–15.670), P < 0.001 (high risk)).
The c statistic for the ESC/ERS risk prediction model was c = 0.66 (95% CI: 0.64–0.68), while the combination of 6MWD and TAPSE/PASP in the advanced prediction model showed superior prognostic information (c = 0.72 (95% CI: 0.70–0.74)) with an integrated discrimination improvement of 0.07 (95% CI: 0.04–0.10, P < 0.001).
Discussion
Overall, this registry study gives further evidence for the usefulness of the ESC/ERS risk stratification scheme for PAH. For the first time, a large cohort including echocardiographic parameters was analyzed.3–5 As described previously, patients at low risk had significantly higher survival than patients at intermediate or high risk.3,4 Analogous results were observed by Boucly et al. who defined low-risk patients as those meeting 3–4 out of 4 low-risk criteria.5 Furthermore, we introduced a new possibility to sub-stratify patients at intermediate risk into low-intermediate- and high-intermediate-risk groups. We chose 6MWD as the main parameter for our advanced stratification because it was previously shown to be one of the main predictors of mortality in the ESC/ERS risk score.3 Of note, 6MWD was sufficient for stratification only after imputation of missing data, whereas 6MWD combined with TAPSE/PASP was able to stratify intermediate-risk patients adequately with and without imputation of missing data. TAPSE/PASP is a reproducible marker for right ventricular-arterial coupling which is a well-known predictor of mortality in PH.12–15 We suggest that including right ventricular-arterial coupling mirrored by TAPSE/PASP increases the reliability of the presented method. Both 6MWD and TAPSE/PASP are non-invasive parameters that can be determined easily. Since we found no significant survival difference between low and low-intermediate-risk groups, we conclude that using these well-established predictors of mortality for advanced risk stratification is a feasible method for initial decision-making,1,16–19 depending on local availability in expert centers. Our study provides evidence that almost one-third of intermediate-risk patients have similar mortality to low-risk patients. Therefore, our simple advanced risk stratification method allows the identification of a subset of intermediate-risk patients who may benefit from early treatment escalation, and improves the prognostic ability of the commonly used ESC/ERS risk stratification scheme. The presented method for risk stratification into four risk groups is comparable to the risk stratification scheme presented in the current guidelines for pulmonary embolism.20
Study limitations
Limitations of the current study include its retrospective, single-center design. Owing to missing data, the World Health Organization functional class was not assessed for risk stratification; for other parameters, missing data were imputed. Further prospective studies are therefore needed to confirm our findings.
Conclusions
The presented scheme is a straightforward and simple tool for advanced risk stratification in PAH. Prospective evaluation is warranted.
Acknowledgments
Editorial assistance was provided by Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK), funded by the University of Giessen.
Contributorship
Dr. Henning Gall conceived the idea for the analyses detailed in this manuscript. All authors contributed to the design and data collection in the study. Mr. Athiththan Yogeswaran and Dr. Henning Gall undertook statistical analyses of the data in the manuscript. All authors contributed to drafting and critical review of the manuscript. All authors approved the manuscript for submission.
Ethical approval
The investigation was approved by the ethics committee of the Faculty of Medicine at the University of Giessen.
Conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mr. Athiththan Yogeswaran reports non-financial support from the University of Giessen during the conduct of the study. Dr. Manuel J. Richter reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and grants from United Therapeutics, grants and personal fees from Bayer, and personal fees from Actelion, Mundipharma, Roche, and OMT outside the submitted work. Dr. Natascha Sommer reports personal fees from Actelion outside the submitted work. Dr. Hossein A. Ghofrani reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Bayer, Actelion, Pfizer, Merck, GSK, and Takeda, grants and personal fees from Novartis, Bayer HealthCare, and Encysive/Pfizer, and grants from Aires, the German Research Foundation, the Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research outside the submitted work. Dr. Werner Seeger reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Pfizer and Bayer Pharma AG outside the submitted work. Dr. Khodr Tello reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion and Bayer outside the submitted work. Dr. Henning Gall reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics outside the submitted work.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Projektnummer 268555672—SFB 1213, Project B08.
ORCID iDs
Athiththan Yogeswaran https://orcid.org/0000-0001-9505-8608
Henning Gall https://orcid.org/0000-0001-7016-7373
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Delcroix M, Staehler G, Gall H, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J 2018; 52: 1800248. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 4.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 5.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 6.Olsson KM, Richter MJ, Kamp JC, et al. Intravenous treprostinil as an add-on therapy in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2019; 38: 748–756. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957–967. [DOI] [PubMed] [Google Scholar]
- 9.Yogeswaran A, Richter MJ, Sommer N, et al. Evaluation of pulmonary hypertension by right heart catheterisation: does timing matter? Eur Respir J 2020; 56: 1901892. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 12.Richter MJ, Peters D, Ghofrani HA, et al. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2020; 201: 116–119. [DOI] [PubMed] [Google Scholar]
- 13.Tello K, Axmann J, Ghofrani HA, et al. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol 2018; 266: 229–235. [DOI] [PubMed] [Google Scholar]
- 14.Tello K, Ghofrani HA, Heinze C, et al. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur Respir J 2019; 54: 1802435. [DOI] [PubMed] [Google Scholar]
- 15.Tello K, Wan J, Dalmer A, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 2019; 12: e009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 17.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161: 487–492. [DOI] [PubMed] [Google Scholar]
- 19.Oudiz RJ, Barst RJ, Hansen JE, et al. Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol 2006; 97: 123–126. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019; 54: 1901647. [DOI] [PubMed] [Google Scholar]