Abstract
Objective
The increasing number of spinal surgeries being performed in the elderly has increased the incidence of postoperative delirium. The prediction of delirium is complex, and few studies have been performed to examine the preoperative risk factors for delirium after spinal surgery in the elderly. This study was performed to clarify such risk factors in patients aged ≥75 years undergoing spinal surgery.
Method
This retrospective observational study included 299 patients aged ≥75 years. Comorbidities, medication history, preoperative examination findings, surgery-related characteristics, and health scale assessments, including the 36-Item Short-Form Survey (SF-36) score and prognostic nutritional index (PNI), were examined as potential risk factors for delirium.
Results
Delirium occurred in 53 patients (17.7%). The preoperative risk factors for delirium were a history of stroke and mental disorders, hypnotic drug use, malnutrition, hyponatremia, anemia, respiratory dysfunction, and cervical surgery. Logistic regression analysis demonstrated that the independent predictors of delirium were a history of stroke, non-benzodiazepine hypnotic drug use, preoperative hyponatremia, the PNI, and the SF-36 physical component summary (PCS) score.
Conclusions
Independent preoperative predictors of delirium in elderly patients undergoing spinal surgery included a history of stroke, non-benzodiazepine hypnotic drug use, preoperative hyponatremia, the PNI, and the SF-36 PCS score.
Keywords: Delirium, preoperative risk factor, complication, elderly, spinal surgery, predictive factor
Introduction
Postoperative delirium is one of the most common complications in perioperative patients.1 According to previous reports, the incidence of delirium after spinal surgery widely ranges from 0.84% to 24.30%.1–7 Delirium is defined as a disturbance of consciousness and is characterized by an acute onset and fluctuating course of inattention. It is accompanied by either a change in cognition or a perceptual disturbance such that a patient’s ability to receive, process, store, and recall information is impaired.8 In the postoperative setting, delirium has been associated with poor cognitive and functional recovery, increased mortality, and other complications.5,6,9 Delirium has also been associated with longer hospital stays and greater hospital costs and caregiver burdens.10,11 Therefore, prevention of delirium is important to reduce these problems and the associated costs.
The cause of postoperative delirium is multifactorial and has been attributed to numerous risk factors; however, the exact pathophysiology and etiology remain unclear.12 Predisposing factors such as pre-existing dementia, drug or alcohol abuse, and abnormal sodium and potassium levels as well as precipitating factors such as postoperative pain, surgery, anesthesia, and hypoxia have been reported as risk factors for delirium.5,12 Several studies to date have investigated the risk factors for delirium occurring after spinal surgery, and old age has been the most frequently identified factor.1,2,5,7,13 However, because age is a difficult factor to control, it is important to consider risk factors other than old age.
With the increasing frequency of spinal surgeries being performed among patients in a growing elderly population, the rise in the incidence of postoperative delirium after spinal surgery has become a matter of concern. Japan is a super-aged society with the world’s oldest population, and the late-elderly (aged ≥75 years as defined by the Japanese government) accounted for 11.6% of Japan’s population in the 2010 national population census. It has been proposed that 65 to 74 years of age be defined as “pre-old age” and that ≥75 years of age be defined as “old age.”14 It should also be noted that the incidence of complications of spinal surgery increase in the late-elderly.15 Because the late-elderly are often nutritionally weaker than younger people16,17 and consume multiple sleeping pills and other medications, preoperative evaluation and management of each patient’s health condition is extremely important to prevent postoperative delirium. However, few studies have been performed to examine the preoperative risk factors for delirium in elderly patients. The purpose of this study was to examine such preoperative risk factors in elderly patients undergoing spinal surgery because an understanding of these factors may help to prevent postoperative delirium.
Materials and methods
This retrospective observational study included 299 consecutive patients aged ≥75 years who underwent spinal surgery at our hospital from January 2014 to March 2017. The study was approved by the Ethics Review Board of Tokyo Medical and Dental University on 17 July 2018 (approval number #M2018-101). The requirement for informed consent was waived because of the anonymous nature of the data. For patients in whom multiple delirium episodes occurred during the study period, the second and subsequent episodes were excluded. All surgeries were performed under general anesthesia with propofol, fentanyl, and rocuronium bromide. Sevoflurane or desflurane was used as the inhalation anesthetic. All cervical and thoracic surgeries were performed under total intravenous anesthesia for monitoring of combined intraoperative motor evoked potentials, whereas lumbar surgeries were performed under total intravenous anesthesia or inhalation anesthesia at the discretion of the anesthesiologist. All patients were supplied with oxygen (3–5 L/min) through a mask for at least 3 hours postoperatively. They were allowed to raise the bed to 90 degrees the day after the surgery and ambulate from the second postoperative day. All patients who underwent anterior cervical spine surgery stayed overnight in the intensive care unit. None of the patients were still on a ventilator after surgery. The diagnosis of delirium was based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition.8 When a patient exhibited symptoms of suspected delirium, the primary doctor or nurses consulted with a liaising psychiatry team; the psychiatrists then diagnosed the patients with delirium and began treatment. The patients were divided into a delirium group and non-delirium group and statistically compared as described below.
The background factors compared between the two groups were age, sex, and comorbidities (diabetes mellitus, renal failure with dialysis, stroke, cardiovascular disease, and mental disorders). The medication factors were the use of hypnotic drugs (benzodiazepines, non-benzodiazepines, suvorexant, and ramelteon), mood stabilizers, antipsychotic drugs, antidepressants as psychotropic drugs, antithrombogenic drugs, H2 blockers, beta blockers, anticholinergic drugs, dopamine agonists, and other drugs commonly used by the elderly. The preoperative examination factors among respiratory function tests were the percentage vital capacity and forced expiratory volume, and those among blood tests were the hemoglobin concentration, hematocrit, sodium concentration, albumin concentration, total cholesterol concentration, total lymphocyte count, blood urea nitrogen concentration, and creatinine concentration. The operative factors were surgical lesions (cervical, thoracic, or lumbar), operative time, and intraoperative blood loss volume. The health scale factors were the Medical Outcomes Study Short-Form 36-Item Health Survey (SF-36) score, the controlling nutritional status (CONUT) score, and the prognostic nutritional index (PNI). The SF-36 questionnaire was distributed on the first day of hospitalization, and several subscale scores were calculated: the physical component summary (PCS), mental component summary, and role/social component summary scores. The CONUT score and PNI were calculated to comprehensively evaluate the nutritional status.18,19 The CONUT score was evaluated using the albumin concentration, total cholesterol concentration, and total lymphocyte count.18 The PNI was calculated as follows: PNI = 10 × albumin concentration + 0.005 × total lymphocyte count.19
Furthermore, during a 1-year postoperative follow-up, the patients were investigated with respect to the length of hospital stay, postoperative complications, and adverse events.
Statistical analysis
Neurological and radiographic assessments were performed by observers other than the principal surgeon. Statistical analyses were performed using JMP Version 14.2 (SAS Institute, Cary, NC, USA). Intergroup comparisons were performed using the χ2 test or Mann–Whitney U test. A multivariable logistic regression analysis was performed using the forward-backward stepwise method to determine the independent risk factors for postoperative delirium. The model was constructed using variables in a univariate analysis. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. A P-value of <0.05 was considered statistically significant.
Results
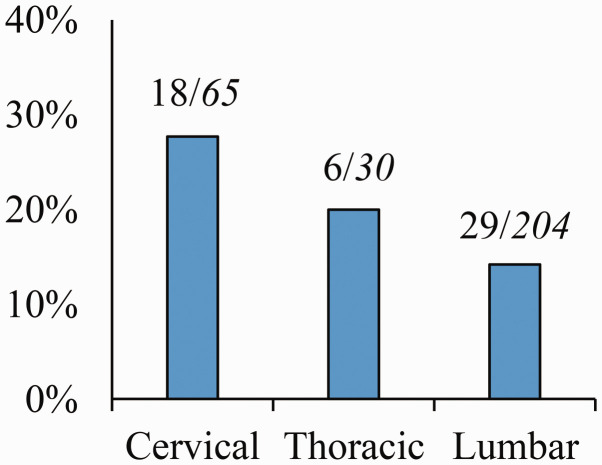
The patients’ preoperative demographic data are summarized in Table 1. The 299 patients included in this study comprised 137 (45.8%) men and 162 (54.2%) women. Their mean age at the time of surgery was 78.9 years (range, 75–94 years). Of the 299 patients, 65, 30, and 204 patients underwent cervical, thoracic, and lumbar spinal surgery, respectively (Figure 1). Around 26.4% of the patients had a history of diabetes mellitus, and >20% used benzodiazepines, antithrombogenic drugs, and H2 blockers.
Table 1.
Preoperative demographic data.
| Total patients | 299 |
|---|---|
| Age, years | 78.9 (75–94) |
| Sex, M/ F (M%) | 137/162 (45.8) |
| Diabetes mellitus | 79 (26.4) |
| Renal failure with dialysis | 11 (3.7) |
| Stroke | 25 (8.4) |
| Cardiovascular disease | 50 (16.7) |
| Mental disorder | 8 (2.7) |
| Medical history | |
| Benzodiazepines | 95 (31.8) |
| Non-benzodiazepines | 37 (12.4) |
| Suvorexant and ramelteon | 4 (1.3) |
| Mood stabilizers | 7 (2.3) |
| Antipsychotic drugs | 12 (3.0) |
| Antidepressants | 7 (2.3) |
| Antithrombogenic drugs | 80 (26.8) |
| H2 blockers | 67 (22.4) |
| Beta blockers | 40 (13.4) |
| Anticholinergic drugs | 19 (6.4) |
| Dopamine agonists | 12 (4.0) |
Data are presented as average (range) or n (%).
M, male; F, female.
The clinical data in the delirium and non-delirium groups are summarized in Table 2. Postoperative delirium occurred in 53 (17.7%) patients. The average onset of postoperative delirium was 1.6 days (range, 0–14 days) postoperatively; 88.7% of the patients developed delirium within 48 hours. Patients who developed delirium more than 1 week after surgery were those with postoperative insomnia or heart failure. Furthermore, the average period of recovery from postoperative delirium was 4.3 days (range, 1–14 days); 5.7% of the patients experienced prolonged postoperative delirium for more than 1 week. There were no significant differences in the age-to-sex ratio between the two groups. Postoperative delirium was more frequent in patients with a history of stroke (P = 0.016); patients with mental disorders (P = 0.001); and patients taking drugs including benzodiazepines, non-benzodiazepines, antipsychotic drugs (P = 0.001), and antidepressants (P = 0.015). We also investigated the patients’ history of mental disorders excluding sleep disorders and dementia, and we found that schizophrenia, bipolar disorder, and depression were present among the patients in this study. Furthermore, when the proportion of each of these three mental disorders in both groups was examined, the proportion of depression was significantly higher in the delirium than non-delirium group (P = 0.007). There were no significant differences in the use of suvorexant and ramelteon between the delirium and non-delirium groups. The preoperative percentage vital capacity (P < 0.001), hemoglobin concentration (P = 0.010), hematocrit (P = 0.003), sodium concentration (P < 0.001), albumin concentration (P < 0.001), and total lymphocyte count (P = 0.001) were significantly lower in the delirium than non-delirium group. With respect to operative factors, the incidence of postoperative delirium (27.7%) was significantly higher in patients who underwent cervical spine surgery (P = 0.024) (Figure 1). Compared with posterior cervical spine surgery, anterior cervical spine surgery was significantly more closely associated with delirium (OR, 6.43; 95% CI, 1.06–38.90; P = 0.036) (Table 3). There were no significant differences in the operative time or intraoperative blood loss volume between the two groups. In terms of health scale factors, the SF-36 PCS score (P = 0.005) and the PNI (P < 0.001) were significantly lower and the CONUT score (P < 0.001) was significantly higher in the delirium group. Thus, the preoperative risk factors identified were a history of stroke and mental disorders, medication history of benzodiazepines and non-benzodiazepines, malnutrition, hyponatremia, anemia, respiratory dysfunction, and cervical surgery. In the multiple logistic regression analysis, five variables were identified as significant independent risk factors for postoperative delirium: medication history of non-benzodiazepines (OR, 5.68; 95% CI, 2.16–14.97; P < 0.001), history of stroke (OR, 6.31; 95% CI, 1.71–23.21; P = 0.008), preoperative sodium concentration (OR, 0.83; 95% CI, 0.73–0.94; P = 0.003), PNI (OR, 0.91; 95% CI, 0.84–0.98; P = 0.014), and SF-36 PCS score (OR, 0.95; 95% CI, 0.91–0.99; P = 0.011) (Table 4).
Table 2.
Univariate analysis of postoperative delirium between delirium and non-delirium groups.
| Delirium group(n = 53) | Non-delirium group (n = 246) | OR (95% CI) | P | |
|---|---|---|---|---|
| Background factors | ||||
| Age, years | 79.1 ± 3.3 | 78.9 ± 3.4 | 1.02 (0.93–1.11) | 0.639 |
| Sex, M/F (M%) | 21/32 (39.6) | 116/130 (47.2) | 0.74 (0.40–1.34) | 0.316 |
| Diabetes mellitus | 13 (24.5) | 66 (26.8) | 1.12 (0.57–2.24) | 0.731 |
| Renal failure with dialysis | 3 (5.7) | 8 (3.3) | 1.79 (0.46–6.97) | 0.404 |
| Stroke | 9 (17.0) | 16 (6.5) | 2.94 (1.22–7.07) | 0.016* |
| Cardiovascular disease | 11 (20.8) | 39 (15.9) | 1.39 (0.63–2.86) | 0.397 |
| Mental disorder | 6 (11.3) | 2 (0.8) | 15.57 (3.05–79.53) | 0.001** |
| Medication factors | ||||
| Benzodiazepines | 27 (50.9) | 68 (27.6) | 2.72 (1.48–4.99) | 0.001** |
| Non-benzodiazepines | 14 (26.4) | 23 (9.3) | 3.48 (1.65–7.34) | 0.001** |
| Suvorexant and ramelteon | 1 (1.9) | 3 (1.2) | 1.56 (0.16–15.27) | 0.704 |
| Mood stabilizers | 1 (1.9) | 6 (2.4) | 0.77 (0.09–6.53) | 0.809 |
| Antipsychotic drugs | 7 (13.2) | 5 (2.0) | 7.33 (2.23–24.11) | 0.001** |
| Antidepressants | 4 (7.5) | 3 (1.2) | 6.61 (1.43–30.48) | 0.015* |
| Antithrombogenic drugs | 17 (32.1) | 63 (25.6) | 1.37 (0.72–2.61) | 0.336 |
| H2 blockers | 14 (26.4) | 53 (21.5) | 1.31 (0.66–2.59) | 0.441 |
| Beta blockers | 10 (18.9) | 30 (12.2) | 1.67 (0.76–3.68) | 0.199 |
| Anticholinergic drugs | 6 (11.3) | 13 (5.3) | 2.29 (0.83–6.33) | 0.110 |
| Dopamine agonists | 3 (5.7) | 9 (3.7) | 1.58 (0.41–6.04) | 0.504 |
| Preoperative examination factors | ||||
| %VC, % | 85.3 ± 16.9 | 95.6 ± 18.2 | 0.97 (0.94–0.98) | <0.001** |
| FEV 1.0%, % | 77.1 ± 10.2 | 74.3 ± 9.2 | 1.03 (0.99–1.08) | 0.076 |
| Hb, mEq/L | 12.1 ± 1.7 | 12.7 ± 1.6 | 0.78 (0.65–0.95) | 0.010* |
| Ht, % | 36.1 ± 4.7 | 38.1 ± 4.4 | 0.90 (0.84–0.97) | 0.003** |
| Na, g/dL | 139.2 ± 3.6 | 141.1 ± 3.0 | 0.84 (0.77–0.92) | <0.001** |
| Alb, g/dL | 3.7 ± 0.5 | 4.0 ± 0.5 | 0.35 (0.20–0.61) | <0.001** |
| TC, mg/dL | 186.4 ± 39.7 | 196.7 ± 38.2 | 0.99 (1.00–1.01) | 0.086 |
| TLC, cells/µL | 1360.5 ± 575.3 | 1656.8 ± 590.5 | 0.99 (0.99–0.99) | 0.001** |
| BUN, mg/dL | 19.6 ± 9.2 | 18.0 ± 7.4 | 1.02 (0.99–1.06) | 0.177 |
| Cre, mg/dL | 1.2 ± 1.7 | 0.96 ± 0.9 | 1.17 (0.94–1.45) | 0.163 |
| Operative factors | ||||
| Cervical surgery | 18 (34.0) | 47 (19.1) | 2.08 (1.10–3.95) | 0.024* |
| Operative time, minutes | 199.9 ± 106.3 | 202.2 ± 100.3 | 0.99 (0.99–1.00) | 0.883 |
| Intraoperative blood loss, mL | 265.7 ± 316.8 | 298.4 ± 343.8 | 0.99 (0.99–1.00) | 0.524 |
| Health scale factors | ||||
| SF-36 PCS score | 16.4 ± 10.3 | 22.7 ± 12.2 | 0.95 (0.92–0.99) | 0.005** |
| SF-36 MCS score | 49.6 ± 9.9 | 50.3 ± 9.8 | 0.99 (0.96–1.03) | 0.659 |
| SF-36 RCS score | 27.2 ± 15.5 | 28.6 ± 16.8 | 0.99 (0.97–1.02) | 0.625 |
| CONUT score | 2.7 ± 2.5 | 1.4 ± 1.8 | 1.29 (1.13–1.48) | <0.001** |
| PNI | 43.8 ± 7.1 | 48.1 ± 6.0 | 0.91 (0.87–0.95) | <0.001** |
Data shown as mean ± standard deviation or n (%). P < 0.05 is considered statistically significant. *P < 0.05, **P < 0.01.
OR, odds ratio; CI, confidence interval; M, male; F, female; %VC, percentage vital capacity; FEV, forced expiratory volume; Hb, hemoglobin; Ht, hematocrit; Na, sodium; Alb, albumin; TC, total cholesterol; TLC, total lymphocyte count; BUN, blood urea nitrogen; Cre, creatinine; SF-36, Medical Outcomes Study Short-Form 36-Item Health Survey; PCS, physical component summary; MCS, mental component summary; RCS, role/social component summary; CONUT, controlling nutritional status; PNI, prognostic nutritional index.
Figure 1.
Relationship between surgical lesion and postoperative delirium. The number above the bar represents the ratio of the number of patients with postoperative delirium to the number of patients with a surgical lesion.
Table 3.
Incidence of postoperative delirium by type of spinal surgery.
| Total number of patients | Number of patients with postoperative delirium | |
|---|---|---|
| Cervical (n = 65) | ||
| Anterior surgery | 6 | 4 (66.7) |
| Posterior surgery | 59 | 14 (23.7) |
| Thoracic (n = 30) | ||
| Posterior surgery | 29 | 6 (20.7) |
| Anterior–posterior surgery | 1 | 0 (0.0) |
| Lumbar (n = 204) | ||
| Posterior surgery | 202 | 28 (13.9) |
| Anterior–posterior surgery | 2 | 1 (50.0) |
Data are presented as n or n (%).
Table 4.
Independent risk factors for postoperative delirium: logistic regression analysis.
| Risk factors | OR (95% CI) | P |
|---|---|---|
| Non-benzodiazepines | 5.68 (2.16–14.97) | <0.001 |
| Stroke | 6.31 (1.71–23.21) | 0.008 |
| Na, g/dL | 0.83 (0.73–0.94) | 0.003 |
| PNI | 0.91 (0.84–0.98) | 0.014 |
| SF-36 PCS score | 0.95 (0.91–0.99) | 0.011 |
P < 0.05 is considered statistically significant.
OR, odds ratio; CI, confidence interval; Na, sodium; PNI, prognostic nutritional index; SF-36, Medical Outcomes Study Short-Form 36-Item Health Survey; PCS, physical component summary.
The length of hospital stay and the perioperative complications are summarized in Table 5. The delirium group had a significantly longer hospital stay and a higher incidence of surgical site infection, urinary tract infection, and heart failure. Furthermore, the adverse events that occurred for up to 1 year postoperatively are summarized in Table 6. The causes of death in the delirium group were pneumonia (n = 2) and heart failure (n = 1), and the causes of death in the non-delirium group were pneumonia (n = 1) and cancer (n = 2). Compared with the non-delirium group, significantly higher numbers of patients in the delirium group developed progressive dementia (OR, 31.95; 95% CI, 6.84–149.31; P < 0.001). Furthermore, 15.1% of patients in the delirium group but only 6.1% of those in the non-delirium group were lost to follow-up at 1 year postoperatively (OR, 2.74; 95% CI, 1.10–6.84; P = 0.031), representing a significant loss.
Table 5.
Hospital stay and perioperative complications.
| Delirium group(n = 53) | Non-delirium group (n = 246) | OR (95% CI) | P | |
|---|---|---|---|---|
| Hospital stay, days | 33.1 (14–139) | 23.3 (12–86) | 1.04 (1.02–1.06) | <0.001** |
| Epidural hematoma | 1 (1.9) | 6 (2.4) | 0.77 (0.09–6.53) | 0.804 |
| Surgical site infection | 3 (5.7) | 1 (0.4) | 14.70 (1.50–144.22) | 0.011* |
| Wound complication | 3 (5.7) | 3 (1.2) | 4.86 (0.95–24.78) | 0.068 |
| Nerve root palsy | 1 (1.9) | 6 (2.4) | 0.77 (0.09–6.53) | 0.804 |
| Pneumonia | 1 (1.9) | 0 (0.0) | 0.062 | |
| Urinary tract infection | 4 (7.5) | 3 (1.2) | 6.61 (1.43–30.48) | 0.018* |
| Heart failure | 3 (5.7) | 2 (0.8) | 7.32 (1.19–44.94) | 0.033* |
| Deep venous thrombosis | 1 (1.9) | 1 (0.4) | 4.71 (0.29–76.55) | 0.297 |
| Pulmonary embolism | 1 (1.9) | 0 (0.0) | 0.062 | |
| Rectal ulcer | 0 (0) | 1 (0.4) | 0.532 |
Data are presented as average (range) or n (%). P < 0.05 is considered statistically significant. *P < 0.05, **P < 0.01.
OR, odds ratio; CI, confidence interval.
Table 6.
Adverse events up to 1 year postoperatively.
| Delirium group(n = 53) | Non-delirium group (n = 246) | OR (95% CI) | P | |
|---|---|---|---|---|
| Lost to follow-up | 8 (15.1) | 15 (6.1) | 2.74 (1.10–6.84) | 0.031* |
| Fall and fracture | 2 (3.8) | 11 (4.5) | 0.84 (0.18–3.90) | 0.821 |
| Dementia progression | 11 (20.8) | 2 (0.8) | 31.95 (6.84–149.31) | <0.001** |
| Bedridden state | 5 (9.4) | 10 (4.1) | 2.03 (0.68–6.03) | 0.202 |
| Pneumonia | 3 (5.7) | 4 (1.6) | 3.63 (0.79–16.72) | 0.098 |
| Death | 3 (5.7) | 3 (1.2) | 4.86 (0.95–24.77) | 0.068 |
Data are presented as n (%). P < 0.05 is considered statistically significant. *P < 0.05, **P < 0.01.
OR, odds ratio; CI, confidence interval.
Discussion
In the present study, we investigated the preoperative risk factors for postoperative delirium in elderly patients undergoing spinal surgery with the goal of facilitating early management in these patients. Independent predictors of delirium were a history of stroke, non-benzodiazepine hypnotic drug use, a lower preoperative serum sodium concentration, the PNI, and the SF-36 PCS score.
In this study, the incidence of postoperative delirium was 17.7% among patients aged ≥75 years who underwent spinal surgery. Lee and Park2 reported that 13.6% of 217 patients aged >60 years who underwent lumbar spine surgery developed postoperative delirium. Kobayashi et al.4 reported that 5.7% of 262 patients aged >80 years who underwent spinal surgery developed postoperative delirium. The incidence of postoperative delirium was higher in our study than in previous reports that targeted elderly patients undergoing spinal surgery. This difference may be attributed to the inclusion of a liaison psychiatry team, resulting in a more accurate and inductive diagnosis of postoperative delirium (including hypoactive delirium, which may be underestimated by non-experts).20 Furthermore, postoperative delirium was significantly prevalent in patients who underwent cervical spine surgery, especially anterior cervical surgery (Figure 1 and Table 3). Although cervical spine surgery may reportedly be associated with postoperative delirium,4 few reports have specified whether delirium is more prevalent in the anterior or posterior cervical approach. Therefore, this needs to be examined in detail in future studies.
The causes of delirium are multifactorial and include preoperative, intraoperative, and postoperative factors.5,13 Because we can only control preoperative risk factors, it is important to identify these factors. We found that a history of stroke was an independent predictor of delirium. It is speculated that direct brain damage from stroke may increase the risk of postoperative delirium13,21; however, this factor cannot be controlled preoperatively. We also found that preoperative hyponatremia was a significant risk factor for postoperative delirium, and Morino et al.22 reported the same finding in an age-matched analysis. Moreover, Halladay et al.23 identified the following delirium-screening targets: preexisting cognitive impairment, infection, and a sodium concentration of <135 or >145 mEq/L. Electrolyte disorders are thought to directly cause neuronal injury, induce the production of neurotransmitters, and induce delirium.21,24 Thus, the preoperative sodium balance may require strict control.
The two other predictive risk factors for delirium were the SF-36 PCS score and the PNI. Because the brain has a high nutritional requirement, malnutrition may play an important role in the development of delirium.25 Micronutrients and other vitamin deficiencies such as niacin or thiamine have an impact on delirium onset because of impaired neurotransmission.26 The PNI and the CONUT score are simple malnutrition assessment tools and are often used for the nutritional assessment of patients in either the terminal phase of a disease or in rehabilitation.18,19 Oe et al.6 assessed the preoperative PNI and CONUT score and reported that a PNI of <49.7 was a risk factor for postoperative delirium in adults with spinal deformities. Kin et al.27 reported that the preoperative general health perception score of the SF-36 was an independent risk factor for postoperative delirium in patients with cervical spondylotic myelopathy. Collectively, undernutrition is an important risk factor for postoperative delirium, and these simple scores are useful when assessing the nutritional status of patients before spinal surgery.
In this study, non-benzodiazepine use (as opposed to benzodiazepine use) was identified as an independent risk factor for delirium; however, there was no difference in the proportion of patients (though small) using suvorexant or ramelteon between the delirium and non-delirium groups. Although the use of benzodiazepines, which directly affect the central nervous system, is thought to be a risk factor for delirium, these drugs are widely used to treat anxiety and insomnia in the clinical setting.28 Whereas benzodiazepines nonselectively target the α1, α2, α3, or α5 subunits of the GABAA receptors, non-benzodiazepines selectively target the α2 and α3 subunits, causing sedation, anxiolysis, and muscle relaxation. Non-benzodiazepines also partially bind to the α1 and α5 subunits with adverse effects of amnesia and intoxication, resulting in central cholinergic deficits and neurotransmitter hyperactivity and leading to delirium.29–31 In this study, benzodiazepines and non-benzodiazepines were prescribed in half the patients; thus, their social impact seems to be a large risk factor for delirium. Therefore, preoperative administration of both benzodiazepines non-benzodiazepines to the elderly should be carefully considered, and it would be more desirable to switch to suvorexant or ramelteon, neither of which has an effect on GABAA receptors.
The data in Table 5 suggest that postoperative delirium increased the length of hospital stay and the incidence of perioperative complications, which is consistent with previous reports.5,6,9–11 Furthermore, the data in Table 6 show that the health status of the patients in the delirium group was not necessarily better than that of the patients in the non-delirium group at 1 year postoperatively. This included a significantly higher rate of dementia progression in the delirium group, although the direct association of dementia progression with postoperative delirium is not known. However, dementia progression can certainly worsen elderly patients’ postoperative quality of life.1,32 Malnutrition and hyponatremia are considered risk factors for delirium,6,22,23 and preventive interventions for these factors also reduce the incidence of other complications such as postoperative surgical site infection and mortality.33–35 Therefore, a prospective study of postoperative delirium in elderly patients who have undergone spinal surgery with preoperative correction of malnutrition and hyponatremia may be important.36
This study has several limitations. First, this was a retrospective study and the sample size was relatively small. Second, because it was a single-center study, bias during patient selection cannot be ruled out. Third, we examined the incidence of postoperative delirium in people aged ≥75 years. If we had included patients of all ages, we may have been able to establish, for example, that dementia occurs more frequently in people aged >75 than <75 years. Fourth, we did not examine the role of the type of anesthesia or the effects of intraoperative hemodynamics and all types of mental disorders in the development of postoperative delirium. Because the differences between inhalation anesthesia and total intravenous anesthesia, intraoperative hypertension and hypotension, and the effects of various mental disorders may affect the incidence of postoperative delirium,37,38 further study is required to elucidate the effects of general anesthesia, intraoperative hemodynamics, and mental disorders. Finally, the problems associated with an accurate diagnosis of low-activity delirium may be easily overlooked. Delirium is a complex disease involving multiple factors; therefore, prospective studies are needed to strengthen the collaboration between physicians and the liaison psychiatry team and to develop delirium prevention techniques. Despite these limitations, we believe that our results are of clinical value for designing prophylactic measures against postoperative delirium.
In conclusion, we identified five independent preoperative risk factors for postoperative delirium in patients aged ≥75 years undergoing spinal surgery: a history of stroke, a history of using non-benzodiazepine medication, hyponatremia, the PNI, and the SF-36 PCS score. These predictors may help to identify patients at a higher risk of delirium after spinal surgery and, based on the assessment of the preoperative status of elderly patients, may facilitate the designing of appropriate prophylactic measures for delirium.
Acknowledgment
The authors thank Ms. Nobuko Nakajima for her administrative support.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Hiroyuki Inose https://orcid.org/0000-0003-4195-2545
References
- 1.Kawaguchi Y, Kanamori M, Ishihara H, et al. Postoperative delirium in spine surgery. Spine J 2006; 6: 164–169. [DOI] [PubMed] [Google Scholar]
- 2.Lee JK, Park YS. Delirium after spinal surgery in Korean population. Spine 2010; 35: 1729–1732. [DOI] [PubMed] [Google Scholar]
- 3.Fineberg SJ, Nandyala SV, Marquez-Lara A, et al. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine 2013; 38: 1790–1796. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi K, Imagama S, Ando K, et al. Risk factors for delirium after spine surgery in extremely elderly patients aged 80 years or older and review of the literature: Japan Association of Spine Surgeons with Ambition multicenter study. Global Spine J 2017; 7: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C, Yang C, Gao R, et al. Risk factors for delirium after spinal surgery: a meta-analysis. World Neurosurg 2015; 84: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 6.Oe S, Togawa D, Yamato Y, et al. Preoperative age and prognostic nutritional index are useful factors for evaluating postoperative delirium among patients with adult spinal deformity. Spine 2019; 44: 472–478. [DOI] [PubMed] [Google Scholar]
- 7.Nazemi AK, Gowd AK, Carmouche JJ, et al. Prevention and management of postoperative delirium in elderly patients following elective spinal surgery. Clin Spine Surg 2017; 30: 112–119. [DOI] [PubMed] [Google Scholar]
- 8.European Delirium Association . The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014; 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol 2020: e202273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph JL, Marcantonio ER. Postoperative delirium: acute change with long-term implications. Anesth Analg 2011; 112: 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 2017; 17: 1045–1047. [DOI] [PubMed] [Google Scholar]
- 15.Umekawa M, Takai K, Taniguchi M. Complications of spine surgery in elderly Japanese patients: implications for future of world population aging. Neurospine 2019; 16: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MY, Green BA, Shah S, et al. Complications associated with lumbar stenosis surgery in patients older than 75 years of age. Neurosurg Focus 2003; 14: 1–4. [DOI] [PubMed] [Google Scholar]
- 17.Sciubba DM, Scheer JK, Yurter A, et al. Patients with spinal deformity over the age of 75: a retrospective analysis of operative versus non-operative management. Eur Spine J 2016; 25: 2433–2441. [DOI] [PubMed] [Google Scholar]
- 18.De Ulíbarri JI, Gonzalez-Madrono A, De Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38–45. [PubMed] [Google Scholar]
- 19.Mullen JL, Buzby GP, Matthews DC, et al. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann Surg 1980; 192: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosker C, Ward D. Hypoactive delirium. BMJ 2017; 357: j2047. [DOI] [PubMed] [Google Scholar]
- 21.Maclullich AM, Ferguson KJ, Miller T, et al. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res 2008; 65: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morino T, Hino M, Yamaoka S, et al. Risk factors for delirium after spine surgery: an age-matched analysis. Asian Spine J 2018; 12: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halladay CW, Sillner AY, Rudolph JL. Performance of electronic prediction rules for prevalent delirium at hospital admission. JAMA Netw Open 2018; 1: e181405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LH, Xu DJ, Wei XJ, et al. Electrolyte disorders and aging: risk factors for delirium in patients undergoing orthopedic surgeries. BMC Psychiatry 2016; 16: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosted E, Prokofieva T, Sanders S, et al. Serious consequences of malnutrition and delirium in frail older patients. J Nutr Gerontol Geriatr 2018; 37: 105–116. [DOI] [PubMed] [Google Scholar]
- 26.Ringaitienė D, Gineitytė D, Vicka V, et al. Impact of malnutrition on postoperative delirium development after on pump coronary artery bypass grafting. J Cardiothorac Surg 2015; 10: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kin K, Yasuhara T, Tomita Y, et al. SF-36 scores predict postoperative delirium after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 2019; 30: 777–782. [DOI] [PubMed] [Google Scholar]
- 28.International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs: ensuring adequate access for medical and scientific purposes. Report, United Nations, New York, January 2011.
- 29.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 2011; 10: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan KR, Rudolph U, Lüscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 2011; 34: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singler K, Hafner M, Sieber C. Delirium is a very common and life-threatening condition. Ther Umsch 2010; 67: 63–67. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe Y, Yoshida G, Hasegawa T, et al. Effect of perioperative mental status on health-related quality of life in patients with adult spinal deformities. Spine 2020; 45: E76–E82. [DOI] [PubMed] [Google Scholar]
- 33.Vassalotti JA, DuPree E. Preoperative hyponatremia: an opportunity for intervention? Arch Med Sci 2012; 172: 1482–1483. [DOI] [PubMed] [Google Scholar]
- 34.Zheng HL, Lu J, Li P, et al. Effects of preoperative malnutrition on short- and long-term outcomes of patients with gastric cancer: can we do better? Ann Surg Oncol 2017; 24: 3376–3385. [DOI] [PubMed] [Google Scholar]
- 35.Cuesta M, Thompson C. The relevance of hyponatraemia to perioperative care of surgical patients. Surgeon 2015; 13: 163–169. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med 2000; 32: 257–263. [DOI] [PubMed] [Google Scholar]
- 37.Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev 2018; 8: Cd012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Sun DF, Han J, et al. Effects of intraoperative hemodynamics on incidence of postoperative delirium in elderly patients: a retrospective study. Int J Clin Exp Med 2016; 22: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]