Abstract
Breast cancer remains one of the leading causes of cancer-associated death in women. MiR-27a is highly expressed in breast cancer tissue. However, the underlying mechanisms that promote breast cancer progression are unknown. In this study, we investigated the regulatory mechanisms of miR-27a and its target glycogen Synthase Kinase 3-β (GSK-3β) in breast cancer cells. We found that miR-27a was highly expressed in breast cancer tissues, which downregulated GSK-3β expression. We further identified GSK-3β as a direct target of miR-27a, and found that the miR-27a mediated suppression of GSK-3β activated Wnt/β-catenin-associated proliferative and invasive factor in breast cancer. The cell transfection assay demonstrated the overexpression of miR-27a also enhanced cell proliferation and invasion, and reduced cell apoptosis through GSK-3β. Finally, we demonstrated that the overexpression of miR-27a facilitated breast cancer progression through its ability to down-regulate the phosphorylation of GSK-3β both in vivo and vitro. These findings highlighted miR-27a as a novel therapeutic target in breast cancer.
Keywords: miR-27a, GSK-3β, breast cancer, therapeutic target, phosphorylation
Introduction
Breast cancer is a malignant tumor that occurs most commonly in women.1,2 The morbidity and mortality of breast cancer have remained high due to uncontrolled cell proliferation and metastasis.3,4 Despite the advances in the early diagnosis and treatment of breast cancer patients, survival rates remained low primarily due to distant metastasis.5-7 Therefore, exploring novel molecular biomarkers to improve breast cancer therapeutics are of utmost importance.
MicroRNAs (miRNAs) are endogenous small RNAs of 18-24 nucleotides that act as regulators of cancer progression.8-10 Ectopically regulated miRNAs and promote tumorigenesis and metastasis in various cancers. For example, the miR-302b/EphA2 axis prevents tumorigenesis in gastric cancer through its regulation of Wnt/β-catenin/ epithelial-mesenchymal transition (EMT) signaling.11 MiR-195 blocked the tumorigenesis of non-small cell lung cancer via targeting cyclin D3 and surviving.12 The downregulation of miR-133a-3p activated PI3K/AKT signaling to accelerate prostate cancer bone metastasis.13 In triple-negative breast cancer, miR-124 targeted ZEB2 to repress cell invasion and metastasis.14 Furthermore, miR-27a has been proposed as an onco-microRNA in human cancers and facilitated EMT procession in ovarian cancer through the regulation of Wnt/β-catenin signaling through FOXO1 targeting.15 The increased expression of miR-27a also reduced the efficacy of cisplatin for the treatment of liver cancer.16 MiR-27a-3p modulated colon cancer cell proliferation and apoptosis through the regulation of BTG1.17 MiR-27a was also implicated in the development of breast cancer18 where it promoted the EMT and cell migration in a FBXW7-dependent manner.19 MiR-27a also targeted CDC27 to regulate radiosensitivity in triple-negative breast cancer (TNBC).20 Despite this knowledge, a deep understanding of the molecular role(s) of miR-27a in breast cancer cells was lacking.
In this study, we described a link between miR-27a and GSK-3β in breast cancer progression. GSK-3β was one of the two isoforms described for GSK-3 and in addition to modulating glycogen synthesis, it regulated many cellular processes, such as protein synthesis, cell proliferation, cell differentiation, neuronal signaling, immune function, and inflammation.21 GSK-3β promoted the degradation of several proteins involved in proliferation, such as β-catenin, c-Myc, or cyclin D,22,23 all of which related to Wnt/β-catenin signaling. Therefore, we hypothesized a relationship between miR-27a, GSK-3β, and Wnt/β-catenin-related factor.
In this study, we investigated the specific effects of miR-27a in breast cancer cells. We showed that the miR-27a/GSK-3β axis facilitated breast cancer progression via Wnt/β-catenin-associated factor. These data highlighted the promise of miR-27a as a novel target for much-needed breast cancer therapeutics.
Materials and Methods
Collection of Tissue Samples
Breast cancer tissues and para-carcinoma tissues were collected from breast cancer patients at the ShengLi Oilfield Central Hospital (Dongying, China). The Para-carcinoma tissue meant the corresponding noncancerous tissue specimen. After resection, samples were stored at −80°C prior to further analysis. Subjects had not received any anti-cancer therapy prior to surgery. Informed consent was provided by all patients and the study was approved by the Ethics Committee of ShengLi Oilfield Central Hospital (approval number: 2019-44).
Cell Culture
The human TNBC cell lines (BT-20, MDA-MB-231), HER2+ breast cancer cell (MCF-7, T-47D), and normal breast epithelial cell line (MCF-10A) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37°C with 5% CO2.
Cell Transfection
MiR-27a mimics and negative controls (NC mimics) were obtained from GenePharma (Shanghai, China), both of which were transfected into BT-20 or MDA-MB-231 cells at 70% to 80% confluence using Lipofectamine 3000 (Invitrogen). The pcDNA3.1 vector targeting GSK-3β was synthesized by GenePharma to generate pcDNA3.1/GSK-3β for overexpression studies. Transfections were performed using Lipofectamine 3000. The GSK-3β overexpression cells were named GSK-3β, and the negative control was named vector in the experimental group.
Quantitative Real Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated from breast cancer tissues and cells using TRIzol reagent (Invitrogen). Extracted RNA was reverse transcribed into cDNA using the Taqman Advanced miRNA cDNA Synthesis Kit (Takara, Japan). Based on ABI7500 real-time qPCR system (Thermo Fisher, Waltham, MA, USA), real-time qPCRs were performed using SYBR Green kit (Takara, Dalian, China). The 2−ΔΔCt method was used to calculate the relative expression of miR-27a and GSK-3β. Values were normalized to U6 or GAPDH. Primer sequences are shown in Table 1
Table 1.
The Primer Sequence.
| GENE | Primer sequence(5’-3’) |
|---|---|
| MiR-27a | F: UUCACAGUGGCUAAGUUCCGC R: UUAAUGCUAAUCGUGAUAGGGGU |
| U6 | F: CTCGCTTCGGCAGCACA R: AACGCTTCACGAATTTGCGT |
| GAPDH | F: TATGATGATATCAAGAGGGTAGT R: TATGATGATATCAAGAGGGTAGT |
MTT Assays
BT-20 cells were grown on 96-well plates for 0, 1, 2, 3, 4, 5, 6, 7 days. At each time point, 20 µL of MTT reagent was added to each well for 4 h. The resulting formazan product was dissolved in DMSO and the absorbance was read using a microplate reader (BioRad, Carlsbad, CA, USA) at 570 nm.
Flow Cytometry Assays
Apoptosis assays were performed using fluorescein isothiocyanate (FITC) Annexin V apoptosis detection kits (BD Biosciences, Ashland, OR, USA). Briefly, BT-20 cells (2 × 104) seeded into 6-well plates were resuspended in PBS and fixed with precooled 70% ethanol. Treated cells were stained with FITC Annexin V and propidium iodide (PI), and the population of apoptotic cells was determined by flow cytometry (BD Biosciences, Ashland, OR, USA).
Transwell Assays
Transfected BT-20 cells (1 × 105) were supplemented with serum-free DMEM and plated onto upper chambers coated with Matrigel. Medium (600 µL) supplemented with 10% FBS was added to the lower chambers and after 48 h, invading cells were fixed in methanol and stained using crystal violet. Invading cells were counted from 5 random fields.
Western Blot Analysis
Total protein was isolated breast cancer tissues or cells harvested in RIPA buffer (Beyotime, Nanjing, China). Protein concentrations were determined via the BCA assay (Beyotime) and equal concentrations of proteins (40 μg) were resolved on 10% SDS-PAGE gels. Proteins were then transferred to PVDF membranes (Millipore, MA, USA) and blocked in 5% skimmed milk. Membranes were probed with primary antibodies overnight at 4°C and labeled with horseradish peroxidase-coupled secondary antibodies for 2 h. Protein bands were visualized using the ECL Detection System (Thermo Fisher Scientific, Waltham, MA, USA). Primary antibodies used were as follows: anti-p-GSK-3β (phospho Y216) (ab75745); anti-p-GSK-3β (ab131356); anti-β-catenin (ab32572); anti-c-myc (ab32072); anti-CyclinD1 (ab16663); and anti-GAPDH (ab181602).
Luciferase Reporter Assays
Wild-type or mutant GSK-3β reporters (pmirGLO-GSK-3 beta WT or pmirGLO-GSK-3 beta Mut) were co-transfected with miR-27a or NC mimics into BT-20 cells using Lipofectamine 3000 (Invitrogen). Luciferase activity was assessed using luciferase reporter assays (Promega, Madison, WI, USA).
In Vivo Assessments
Six-week-old nude BALB/c male mice (about 20 g) were provided by Vital River Co. Ltd. (Beijing, China). BT-20 cells (5 × 106) were subcutaneously injected into the right flank of the axilla and mice were sacrificed upon tumor development 25 days later. Total body weight and tumor weights were measured in each treatment group (n = 5 per group). All animal procedures were approved by the Animal Experiment Center of the Institute of Radiation Medicine of the Chinese Academy of Medical Science.
Statistical Analysis
All data were analyzed by SPSS version 26.0 statistical software (IBM, Armonke, NY, USA). The comparison between 2 groups was analyzed by independent sample T test. Data among 3 or more groups were analyzed by using 1-way or 2-way analysis of variance, followed by Tukey’s multiple comparisons test. Pearson correlation test was used to analyze the correlation between p-GSK-3β/GSK-3β and miR-27a levels. P < 0.05 was considered statistically significant difference.
Results
Expression of miR-27a and GSK-3β in Breast Cancer Tissues
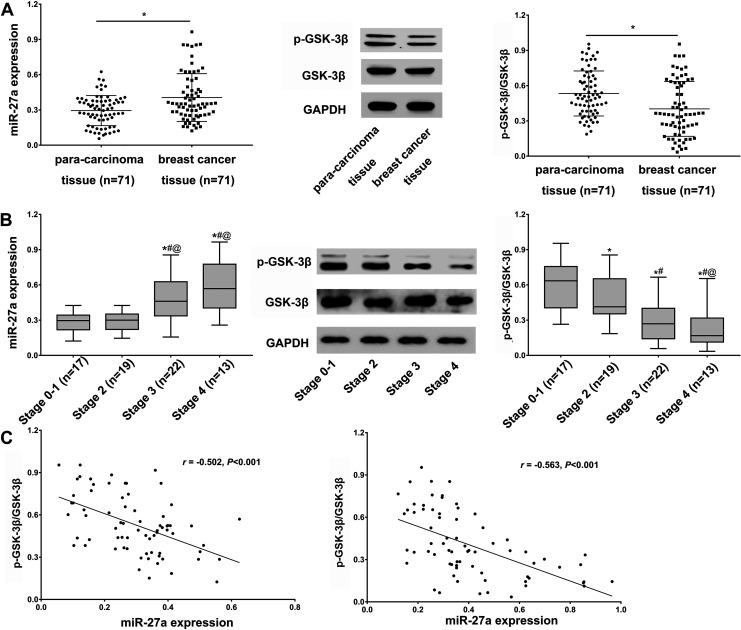
It has previously been shown that miR-27a promotes various cancers, including breast cancer. Consistent with these studies, we showed that in comparison to para-carcinoma tissues, miR-27a was highly expressed in breast cancer tissue (Figure 1A, left panel). As GSK-3β and its phosphorylation (reflected by the ratio of p-GSK-3β to GSK-3β) related to Wnt/β-catenin signaling, which regulated cancer progression, we further assessed its expression in breast cancer tissue. We observed low levels of GSK-3β and the ratio of p-GSK-3β to GSK-3β in breast cancer tissue compared to para-carcinoma tissues (Figure 1A, middle and right panel). Based on the TNM stage, we found that miR-27a expression was higher in advanced stages, and inversely correlated with p-GSK-3β/GSK-3β expression (Figure 1B, middle and right panel). The negative correlation between miR-27a and p-GSK-3β/GSK-3β in either para-carcinoma or breast cancer tissue was confirmed by the Pearson correlation test (Figure 1C, left: para-carcinoma tissue, right: breast cancer tissue). Taken together, these data demonstrated that miR-27a expression was increased in breast cancer cells, and inversely correlated with the phosphorylation of GSK-3β.
Figure 1.
Expression of miR-27a and GSK-3β in breast cancer tissue (A) MiR-27a and GSK-3β expression in breast cancer tissues and para-carcinoma tissues detected by RT-qPCR and Western blot analysis, respectively. *P < 0.05 vs. para-carcinoma tissues. Statistical data were analyzed using independent-samples T test. (B) Expression of miR-27a and GSK-3β in breast cancer tissues according to TNM stage determined through RT-qPCR and Western blot analysis, respectively. *p < 0.05 vs. Stage 1 groups, #p < 0.05 vs. Stage 2 groups, @p < 0.05 vs. Stage 3 groups. Statistical data were analyzed using one-way ANOVA test. (C) Correlation between miR-27a and p-GSK-3β/GSK-3β expression in para-carcinoma and breast cancer tissues (Left, para-carcinoma tissues; Right, breast cancer tissues) assessed by the Pearson correlation test which demonstrated that miR-27a expression inversely correlated with the expression of GSK-3β in breast cancer tissues. ***P < 0.001.
Expression of miR-27a and GSK-3β in Breast Cancer Cell Lines
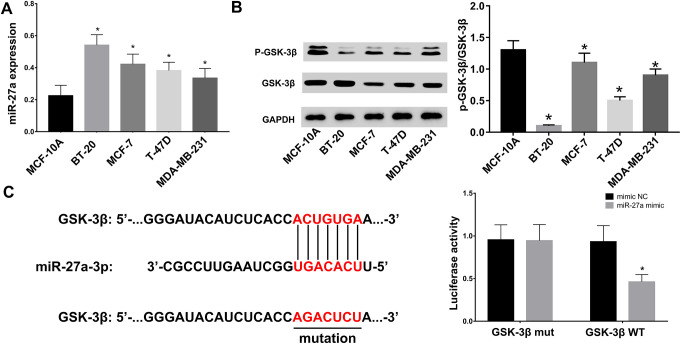
We next explored the expression of miR-27a and GSK-3β in breast cancer cell lines (BT-20, MCF-7, T-47D, MDA-MB-231 cell line). According to RT-qPCR assays, we showed that, compared to the normal breast epithelial MCF-10A cell line, miR-27a levels markedly increased in breast cancer cell lines (Figure 2A). Correspondingly, Western blot analysis showed that the phosphorylation of GSK-3β in BT-20, MCF-7, T-47D, MDA-MB-231 cell line was decreased compared with MCF-10A cells (Figure 2B). We next predicted the binding sites between miR-27a and GSK-3β through bioinformatics analysis. As shown in Figure 2C, luciferase reporter assays showed that the luciferase activity of GSK-3β WT reporters was weakened by miR-27a mimics, while GSK-3β Mut reporters were unaffected. Collectively, these data showed that miR-27a directly bound to GSK-3β and regulated its phosphorylation in breast cancer cells.
Figure 2.
Expression of miR-27a and GSK-3β in breast cancer cell lines. (A-B) Expression of miR-27a and GSK-3β in breast cancer cell lines evaluated by RT-qPCR and Western blot analysis, respectively. *P < 0.05 vs. the MCF-10A group. Statistical data were analyzed using one-way ANOVA test. (C) Binding sequences between miR-27a and GSK-3β assessed by luciferase reporter assays. Statistical data were analyzed using 2-way ANOVA test *p < 0.05 vs. mimic NC groups.
MiR-27a Enhances Cell Proliferation Through the Regulation of GSK-3β in BT-20 Cells and MDA-MB-231 Cells
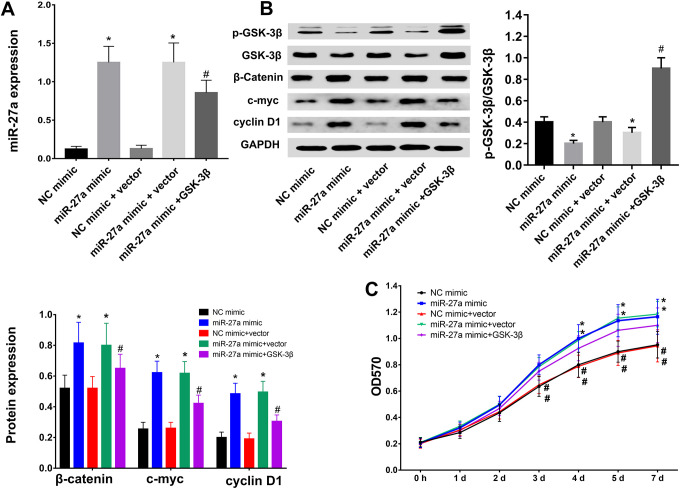
The above-mentioned results demonstrated that GSK-3β and its phosphorylation related to breast cancer cells, therefore, we further investigated the regulatory role(s) of the miR-27a/GSK-3β axis in breast cancer cells (both BT-20 cells and MDA-MB-231 cells), the effects of GSK-3β overexpression were assessed (Figure 3A and S1A). We found that the increased levels of GSK-3β counteracted the inhibitory effect of miR-27a mimics on p-GSK-β/GSK-3β, and alleviated the effects of miR-27a on the expression of β-catenin, c-myc, and cyclin D1 (Figure 3B) that was in accordance with MDA-MB-231 cells (Figure S1B). These data suggested that miR-27a activated Wnt/β-catenin-associated factor through the inhibition of p-GSK-3β. In addition, MTT assays revealed that GSK-3β overexpression abolished the miR-27a overexpression-mediated increase in TNBC cell proliferation (Figure 3C and S1C). In summary, miR-27a promoted cell proliferation through the regulation of GSK-3β in BT-20 cells and MDA-MB-231 cells.
Figure 3.
MiR-27a overexpression promotes cell proliferation through the regulation of GSK-3β in BT-20 cells. (A) Expression of miR-27a in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups confirmed by RT-qPCR. (B) Expression of p-GSK-3β, GSK-3β, β-catenin, c-myc, and cyclin D1 in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups measured by Western blot analysis. The corresponding statistic graphics are shown (left bottom and top right corner). (C) Proliferative ability of BT-20 cells in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups examined via MTT assays. *p < 0.05 vs. the NC mimic group, #p < 0.05 vs. the miR-27a mimic group. Statistical data from (A), (B), (C) were analyzed using one-way ANOVA test.
MiR-27a Suppresses Cell Apoptosis and Enhances Cell Invasion Through the Regulation of GSK-3β in BT-20 Cells and MDA-MB-231 Cells
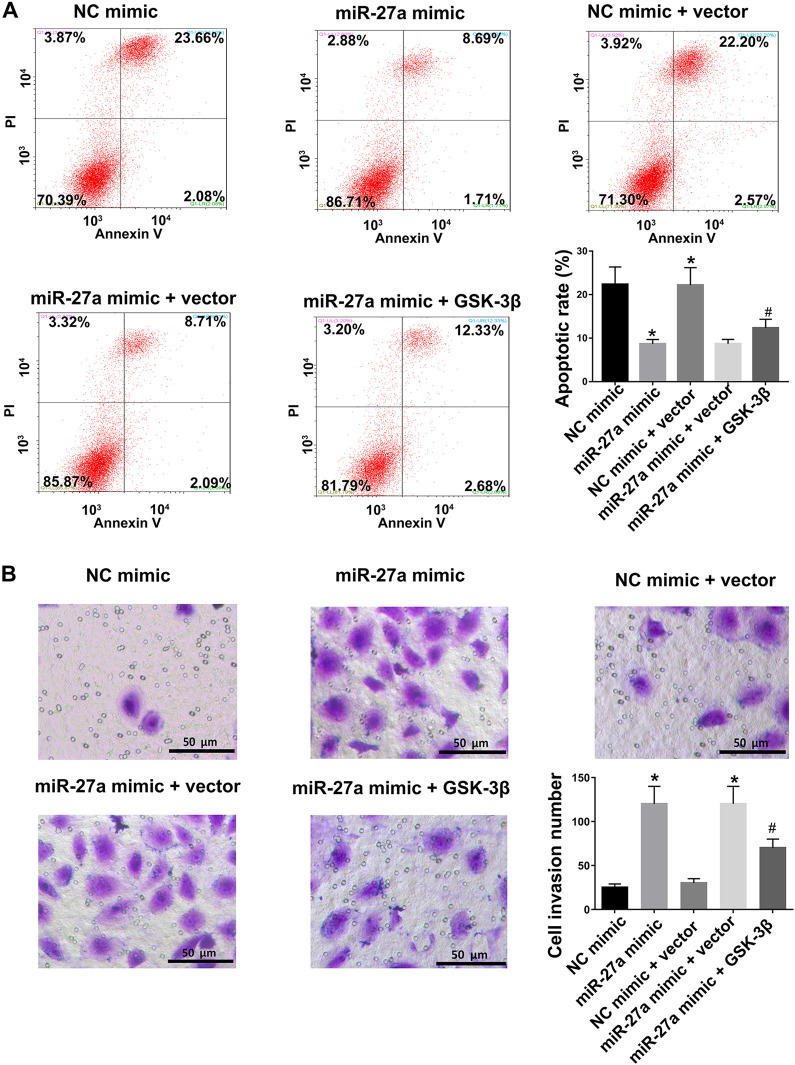
To verify the role of the miR-27a/GSK-3β axis in BT-20 cells survival and apoptosis, flow cytometry and transwell assays were performed. We showed that the inhibitory effects of miR-27a mimics on cell apoptosis could be reversed by GSK-3β overexpression (Figure 4A and S2A). As shown by transwell assays, the upregulation of GSK-3β co-incubation with miR-27 mimic further decreased the proliferative and invasive effect of BT-20 cells and MDA-MB-231 cells, compared to miR-27a mimic alone (Figure 4B and S2B). Taken together, these data demonstrated that the up-regulation of miR-27a suppressed apoptosis and enhanced cell invasion through the regulation of GSK-3β beta in BT-20 cells and MDA-MB-231 cells.
Figure 4.
MiR-27a overexpression suppresses cell apoptosis and promotes cell invasion through the regulation of GSK-3β in BT-20 cells. (A) Apoptosis rates of BT-20 cells in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups determined by flow cytometry. (B) Invasion ability of BT-20 cells in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups assessed via transwell assays. Statistical data were analyzed using one-way ANOVA test. *p < 0.05 vs. NC mimic group, #p < 0.05 vs. miR-27a mimic group, Scale bar = 50μm.
MiR-27a Facilitates Tumor Growth Through Targeting GSK-3β In Vivo
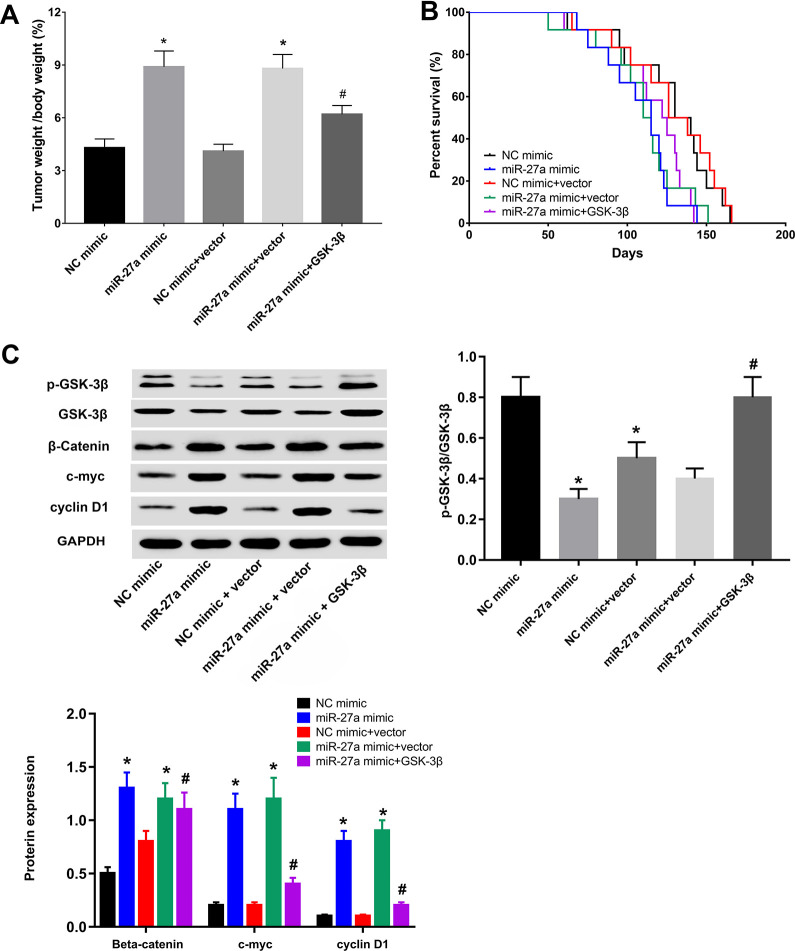
Finally, we explored the role of the miR-27a/GSK-3β axis in vivo. The ratio of tumor weight to body weight was increased in BT-20 or MDA-MB-231 cells transfected with miR-27a mimics, which could be abrogated by GSK-3β overexpression (Figure 5A and S3A). In addition, we found that the survival rates of mice increased following GSK-3β overexpression combined with miR-27a mimic ((Figure 5B and S3B), compared to miR-27a mimic alone. Similar to the in vitro data, the effect of miR-27a mimic on the expression of p-GSK-3β, GSK-3β, β-catenin, c-myc, and cyclin D1 was alleviated by GSK-3β overexpression in vivo (Figure 5C and S3C). Thus, these findings demonstrated that the upregulation of miR-27a facilitated tumor growth through its ability to target GSK-3β in vivo.
Figure 5.
Up-regulated miR-27a transfected in BT-20 cells promotes tumor growth through the regulation of GSK-3β in vivo. (A) Tumor weights were measured in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups. Statistical data were analyzed using one-way ANOVA test. (B) Survival rates of mice in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups through Kaplan-Meier analysis, which demonstrated that GSK-3β overexpression combined with miR-27a mimic improved the survival rate, compared with miR-27a mimic treatment alone. The relationship between different treatment groups and survival percentage analyzed by Kaplan-Meier. (C) p-GSK-3β, GSK-3β, β-catenin, c-myc, and cyclin D1 levels in NC mimic, miR-27a mimic, NC mimic+vector, and miR-27a+GSK-3β groups assessed by Western blot analysis. Statistical data were analyzed using one-way ANOVA test. The graphics closed to panel C were corresponding statistic graphics. *p < 0.05 vs. NC mimic groups, #p < 0.05 vs. miR-27a mimic groups.
Discussion
Advances have been made in the identification of effective biological markers for human cancer. Among them, miRNAs have attracted intense research interest due to their emerging roles in the tumorigenesis of gastric cancer, non-small cell lung cancer, prostate cancer, and TNBC cells.11-14 Current evidence suggested that miR-27a had a pro-oncogenic effect in breast cancer.18-20 However, the biological roles and regulatory mechanism of miR-27a have not yet been investigated at the molecular level. In this study, we showed that miR-27a expression was up-regulated in both breast cancer tissues and cell lines.
In the ceRNA network, lncRNAs regulated mRNAs via binding to miRNAs, thereby affecting the development of breast cancer.24-27 MiRNAs modulated the translation of complementary sequences through binding to 3′-untranslated regions (3′-UTRs) of target mRNAs.28,29 Herein, miR-27a was predicted to target GSK-3β, a serine/threonine kinase that regulated an array of cellular processes.30,31 Previously, GSK3-β demonstrated to induce cell proliferation and survival in cancer, a function that was related to its ability to regulate the transcriptional regulator NF-κB.31,32 In addition, Wnt/beta-catenin, MEK/ERK, PI3K/Akt, and Notch pathways exhibited vital regulatory effects on tumor initiation, EMT processes, and other pro-oncogenic phenotypes of cancer cells.33-35 The suppression of GSK-3β enhanced drug resistance in cancer cells through its ability to regulate Raf/MEK/ERK and Wnt/β-catenin signaling.36,37 The lncRNA OGFRP1/miR-124-3p/SIRT1 axis facilitated endometrial cancer progression through activation of the PI3K/AKT/GSK-3β signaling.38 GSK-3β affected cell proliferation in vascular smooth muscle cells by positively regulating Notch signaling. 39 DAX1 activated Wnt/β-catenin signaling via GSK-3β to accelerate cervical cancer tumorigenesis.40 In the nasal polyps of chronic rhinosinusitis, 1,8-cineol modulated GSK-3β dephosphorylation to repress Wnt/β-catenin signaling.41
In this study, we showed that miR-27a and p-GSK-3β/GSK-3β was highly expressed in breast cancer tissue compared with para-carcinoma tissue, and that there was a negative correlation between p-GSK-3β/GSK-3β and miR-27a. Different breast cancer cell lines were utilized to testify miR-27a and p-GSK-3β/GSK-3β levels, and there was a significant difference in the BT-20 cell line compared with other cell lines. Next, a luciferase reporter assay was used to show that miR-27a directly bound to GSK-3β and regulated its phosphorylation in breast cancer cells. Additionally, the Western blot analysis, MTT assays, flow cytometry, and transwell assays further confirmed that the overexpression of miR-27 increased the cell proliferation and invasion ability, while this effect was reversed by GSK-3β in BT-20 and MDA-MB-231 cells. The survival rate curve showed that overexpression of miR-27 decreased survival rate, while this effect was aborted by GSK-3β overexpression.
In summary, we found that GSK-3β levels were decreased in breast cancer tissues and TNBC cells through miR-27a mediated repression, leading to a loss of p-GSK-3β and GSK-3β, and increased Wnt/β-catenin-associated proliferative factor. Moreover, the overexpression of miR-27a enhanced breast cancer cell proliferation and invasion, and suppressed apoptosis through its regulation of GSK-3β. This study provided evidence that miR-27a targeted GSK-3β to facilitate breast cancer progression via the Wnt/β-catenin-associated proliferative factor. Taken together, these findings will offer new insight into the diagnosis and treatment of breast cancer.
Supplemental Material
Supplemental Material, Figure_S2 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment
Supplemental Material, revised_Figure_S1 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment
Supplemental Material, revised_Figure_S3 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment
Acknowledgments
We thank all participants of this study.
Authors’ Note: Huijin Chen and Yuanyuan Zhang contributed equally to this work. Informed consent was provided by all patients and the study was approved by the Ethics Committee of ShengLi Oilfield Central Hospital.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peipei Mou  https://orcid.org/0000-0001-9317-8016
https://orcid.org/0000-0001-9317-8016
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ennour-Idrissi K, Ayotte P, Diorio C. Persistent organic pollutants and breast cancer: a systematic review and critical appraisal of the literature. Cancers (Basel). 2019;11(8):1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395–406. [DOI] [PubMed] [Google Scholar]
- 4. Libson S, Lippman M. A review of clinical aspects of breast cancer. Int Rev Psychiatry. 2014;26(1):4–15. [DOI] [PubMed] [Google Scholar]
- 5. De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11(7):1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradley JA, Mendenhall NP. Novel radiotherapy techniques for breast cancer. Annu Rev Med. 2018;69:277–288. [DOI] [PubMed] [Google Scholar]
- 7. Jia H, Truica CI, Wang B, et al. Immunotherapy for triple-negative breast cancer: existing challenges and exciting prospects. Drug Resist Updat. 2017;32:1–15. [DOI] [PubMed] [Google Scholar]
- 8. Goh JN, Loo SY, Datta A, et al. microRNAs in breast cancer: regulatory roles governing the hallmarks of cancer. Biol Rev Camb Philos Soc. 2016;91(2):409–428. [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan S, Selvan ST, Archunan G, Gulyas B, Padmanabhan P. MicroRNAs—the next generation therapeutic targets in human diseases. Theranostics. 2013;3(12):930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Beijnum JR, Giovannetti E, Poel D, Nowak-Sliwinska P, Griffioen AW. miRNAs: micro-managers of anticancer combination therapies. Angiogenesis. 2017;20(2):269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, He Y, McLeod HL, et al. miR-302b inhibits tumorigenesis by targeting EphA2 via Wnt/ beta-catenin/EMT signaling cascade in gastric cancer. BMC Cancer. 2017;17(1):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu X, Zhang Y, Cavazos D, et al. miR-195 targets cyclin D3 and survivin to modulate the tumorigenesis of non-small cell lung cancer. Cell Death Dis. 2018;9(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang Y, Pan J, Huang S, et al. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji H, Sang M, Liu F, Ai N, Geng C. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol Res Pract. 2019;215(4):697–704. [DOI] [PubMed] [Google Scholar]
- 15. Zhang LY, Chen Y, Jia J, Zhu X, He Y, Wu LM. MiR-27a promotes EMT in ovarian cancer through active Wnt/-catenin signalling by targeting FOXO1. Cancer Biomark. 2019;24(1):31–42. [DOI] [PubMed] [Google Scholar]
- 16. Li W, Yu ZX, Ma BF. The increase of miR-27a affects the role of cisplatin on proliferation and migration capacities of liver cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(17):5490–5498. [DOI] [PubMed] [Google Scholar]
- 17. Su C, Huang DP, Liu JW, Liu WY, Cao YO. miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1. Oncol Lett. 2019;18(3):2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang W, Zhu J, Su S, et al. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One. 2012;7(12):e51702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang G, Shi W, Fang H, , Zhang X . miR27a promotes human breast cancer cell migration by inducing EMT in a FBXW7dependent manner. Mol Med Rep. 2018;18(6):5417–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren YQ, Fu F, Han J. MiR-27a modulates radiosensitivity of triple-negative breast cancer (TNBC) cells by targeting CDC27. Med Sci Monit. 2015;21:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–1026. [DOI] [PubMed] [Google Scholar]
- 23. Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14(19):2501–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li LJ, Zhao W, Tao SS, et al. Competitive endogenous RNA network: potential implication for systemic lupus erythematosus. Expert Opin Ther Targets. 2017;21(6):639–648. [DOI] [PubMed] [Google Scholar]
- 25. Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou S, Wang L, Yang Q, et al. Systematical analysis of lncRNA-mRNA competing endogenous RNA network in breast cancer subtypes. Breast Cancer Res Treat. 2018;169(2):267–275. [DOI] [PubMed] [Google Scholar]
- 27. Fan CN, Ma L, Liu N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J Transl Med. 2018;16(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang W, Ma J, Zhou W, et al. Reciprocal regulations between miRNAs and HIF-1alpha in human cancers. Cell Mol Life Sci. 2019;76(3):453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Y, Liu X, Zhong L, et al. The combined use of miRNAs and mRNAs as biomarkers for the diagnosis of papillary thyroid carcinoma. Int J Mol Med. 2015;36(4):1097–1103. [DOI] [PubMed] [Google Scholar]
- 30. Golpich M, Amini E, Hemmati F, et al. Glycogen synthase kinase-3 beta (GSK-3beta) signaling: implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26. [DOI] [PubMed] [Google Scholar]
- 31. Walz A, Ugolkov A, Chandra S, et al. Molecular pathways: revisiting glycogen synthase kinase-3β as a target for the treatment of cancer. Clin Cancer Res. 2017;23(8):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Wu J, Ling MT, Zhao L, Zhao KN. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol Cancer. 2015;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teoh SL, Das S. Notch Signalling pathways and their importance in the treatment of cancers. Curr Drug Targets. 2018;19(2):128–143. [DOI] [PubMed] [Google Scholar]
- 35. Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/beta-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39(7):648–658. [DOI] [PubMed] [Google Scholar]
- 36. Sokolosky M, Chappell WH, Stadelman K, et al. Inhibition of GSK-3beta activity can result in drug and hormonal resistance and alter sensitivity to targeted therapy in MCF-7 breast cancer cells. Cell Cycle. 2014;13(5):820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X-F, Li X-Y, Zheng P-S, Yang W-T. DAX1 promotes cervical cancer cell growth and tumorigenicity through activation of Wnt/β-catenin pathway via GSK3β. Cell Death Dis. 2018;9(3):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lv Y, Chen S, Wu J, et al. Upregulation of long non-coding RNA OGFRP1 facilitates endometrial cancer by regulating miR-124-3p/SIRT1 axis and by activating PI3K/AKT/GSK-3beta pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):2083–2090. [DOI] [PubMed] [Google Scholar]
- 39. Guha S, Cullen JP, Morrow D, et al. Glycogen synthase kinase 3 beta positively regulates Notch signaling in vascular smooth muscle cells: role in cell proliferation and survival. Basic Res Cardiol. 2011;106(5):773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu XF, Li XY, Zheng PS, Yang WT. DAX1 promotes cervical cancer cell growth and tumorigenicity through activation of Wnt/beta-catenin pathway via GSK3beta. Cell Death Dis. 2018;9(3):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruchhage KL, Koennecke M, Drenckhan M, Plotze-Martin K, Pries R, Wollenberg B. 1,8-cineol inhibits the Wnt/beta-catenin signaling pathway through GSK-3 dephosphorylation in nasal polyps of chronic rhinosinusitis patients. Eur J Pharmacol. 2018;835:140–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Figure_S2 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment
Supplemental Material, revised_Figure_S1 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment
Supplemental Material, revised_Figure_S3 for MiR-27a Facilitates Breast Cancer Progression via GSK-3β by Huijin Chen, Yuanyuan Zhang, Xin Cao and Peipei Mou in Technology in Cancer Research & Treatment