Abstract
Background:
Immune checkpoint inhibitors (ICIs) are standard therapies for patients with advanced non-small-cell lung cancer (NSCLC) and a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) ≥50%. Tumor mutation burden (TMB) also predicts response to ICIs but is often not available in real time for decision making in the first-line setting. Smoking exposure can be a proxy for TMB in NSCLC. The impact of smoking status on efficacy of PD-1 blockade in NSCLC patients with PD-L1 TPS ≥50% has not been well defined.
Patients and methods:
To investigate the relationship between smoking and activity of ICIs in NSCLC, we retrospectively studied 315 patients with NSCLC and PD-L1 TPS ≥50% at five USA academic medical centers. Objective response rates (ORRs), progression-free survival (PFS), and duration of response (DOR) were compared between never (<100 lifetime cigarettes), light (≤10 pack-years), and heavy (>10 pack-years) smokers. A subset of patients underwent next-generation sequencing to estimate TMB.
Results:
We identified 36 (11%) never, 42 (13%) light, and 237 (75%) heavy smokers with NSCLC and PD-L1 TPS ≥50% treated with ICIs. Objective responses were observed in 27%, 40%, and 40% of never, light, and heavy smokers, respectively (P = 0.180 never versus heavy; P = 1.000 light versus heavy). Median PFS and median DOR were numerically shorter in never and light smokers compared with heavy smokers (PFS 3.0 versus 4.0 versus 5.4 months; median DOR 6.9 versus 10.8 versus 17.8 months), but were not statistically different [PFS: hazard ratio (HR) 1.37, P = 0.135 and HR 1.24, P = 0.272; DOR: HR 1.92, P = 0.217 and HR 1.79, P = 0.141].
Conclusions:
PD-(L)1 inhibitors are associated with antitumor activity in NSCLC with PD-L1 TPS ≥50% regardless of smoking status. Nevertheless, there is a signal of potentially decreased durability among never and light smokers that should be further evaluated. Distinct immunobiologic features may affect initial response versus durability of antitumor immunity to programmed cell death 1 (PD-1) blockade.
Keywords: NSCLC, PD-1 inhibitor, PD-L1 expression, tumor mutation burden
INTRODUCTION
Immune checkpoint inhibitors (ICIs) targeting the programmed cell death 1 (PD-1) axis have transformed the management of non-small-cell lung cancer (NSCLC).1 Despite the impressive and sometimes durable activity of these agents, most NSCLC patients do not respond to therapy.2 This pattern of uncommon but profound responses has spurred ongoing efforts to identify clinical, pathologic, and molecular features associated with response to ICIs.3,4
Programmed death-ligand 1 (PD-L1) expression is an important predictive biomarker in NSCLC.5 In patients with a PD-L1 tumor proportion score (TPS) ≥50%, the PD-1 inhibitor pembrolizumab significantly improved progression-free survival (PFS) and overall survival (OS) compared with platinum-doublet chemotherapy.1 Furthermore, the combination of pembrolizumab and platinum-doublet chemotherapy led to significant improvements in OS compared with platinum-doublet chemotherapy alone, with the greatest benefits observed in the PD-L1 TPS ≥50% subgroups.6,7 Based upon these data, pembrolizumab with or without chemotherapy has become standard first-line therapy for patients with NSCLC and PD-L1 TPS ≥50%.
Beyond PD-L1 expression, tumor mutation burden (TMB) is an independent biomarker of ICI response.3,4 One challenge in assessing TMB is that prolonged turnaround times currently limit its impact on first-line treatment decisions. Although imperfect, smoking exposure may be a proxy for TMB. Never or light smokers generally have low TMB and are typically less responsive to PD-(L)1 inhibitors.8,9 Whether never or light smokers similarly have diminished efficacy within the subgroup of patients with PD-L1 ≥50% has not been well explored. Indeed, only five never smokers with PD-L1 ≥50% were randomized to pembrolizumab in the pivotal KEYNOTE 024 study.1 Differential efficacy among these patients could have important implications for clinicians considering use of pembrolizumab monotherapy versus chemotherapy plus PD-(L)1 inhibitor combinations in these patients.
To investigate the impact of smoking on PD-(L)1 inhibitor efficacy in NSCLC with PD-L1 TPS ≥50%, we performed a large, retrospective analysis across five academic centers.
METHODS
Patients
Never (<100 lifetime cigarettes), light (≤10 pack-years), and heavy (>10 pack-years) smokers with advanced NSCLC and PD-L1 TPS ≥50% were identified at participating institutions: Massachusetts General Hospital (n = 54), Memorial Sloan Kettering Cancer Center (n = 110), Dana Farber Cancer Institute (DFCI; n = 85), MD Anderson Cancer Center (n = 41), and Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (n = 25). All patients received PD-(L)1 inhibitors between July 2012 and December 2017. Patients receiving chemotherapy plus ICIs were excluded. This study was approved by the Institutional Review Board at each participating institution.
Medical records were retrospectively reviewed. Objective responses were assessed at each site using RECIST version 1.1. PFS was measured from the time of PD-(L)1 treatment initiation to clinical/radiographic progression or death. Patients without documented disease progression were censored on the date of last follow-up. Duration of response (DOR) was defined as the time between the date of first response and the date of first documented event of progression or death. All data were updated as of August 2018.
PD-L1 and TMB
PD-L1 TPS ≥50% was defined as membranous staining of tumor cells of any intensity on 50% or more tumor cells. Several different antibodies were used across sites, including: 22C3 (Dako, Santa Clara, CA; n = 104), E1L3N (Cell Signaling Technologies, Danvers, MA, n = 200), 28-8 (Dako, n = 6), and SP263 (Ventana, Oro Valley, AZ, n = 3). Recent harmonization efforts have demonstrated that these antibodies are largely similar.10 Specific antibody clone information was not available in one patient; testing was performed using SP142 (Ventana) in another patient.
Estimation of TMB was performed using two assays: MSK-IMPACT™ and DFCI OncoPanel, as previously described.4,11 Given methodologic differences between assays, TMB estimates across institutions were analyzed separately (supplementary Methods, available at Annals of Oncology online).
Statistical analysis
The Fisher’s exact test was used to compare categorical variables by smoking exposure. Age, TPS, and TMB were compared using the Wilcoxon rank-sum test. PFS and DOR were estimated by the Kaplan–Meier method; median follow-up was calculated by the reverse Kaplan–Meier method. Differences in PFS and DOR between groups were estimated as a hazard ratio (HR) by proportional hazards regression and assessed using the score test (supplementary Methods, available at Annals of Oncology online).
RESULTS
Study population
We identified 315 NSCLC patients with PD-L1 ≥50% who were treated with PD-(L)1 inhibitors: 36 (11%) never smokers, 42 (13%) light smokers [median 5 pack-years (range 0.15–10)], and 237 (75%) heavy smokers [median 35 pack-years (range 11–208)]. Aside from tobacco exposure, baseline clinicopathologic features between groups were generally well balanced (Table 1, supplementary Table S1, available at Annals of Oncology online). Never, light, and heavy smokers had similar distributions of PD-L1 expression (median TPS 72.5%, 90%, and 80%, respectively; P = 0.189; Figure 1A). Most patients (66%) received ICIs as first-line therapy, and pembrolizumab was the most common agent received (81%). In total, nine (3%) patients received dual PD-1/CTLA-4 inhibitors. Duration of follow-up since initiation of ICIs was similar across groups (median 14.9, 14.8, and 14.6 months).
Table 1.
Baseline clinical and pathologic characteristics
| Characteristica | Never smokers n = 36 |
Light Smokers n = 42 |
Heavy Smokers n = 237 |
P |
|---|---|---|---|---|
| Age at treatment initiation, years | 0.607 | |||
| Median | 70 | 66 | 67 | |
| Range | 35–87 | 30–88 | 38–92 | |
| Sex, n (%) | 0.599 | |||
| Male | 13 (36) | 17 (40) | 106 (45) | |
| Female | 23 (64) | 25 (60) | 131 (55) | |
| Histology, n (%) | 0.639 | |||
| Adenocarcinoma | 31 (86) | 31 (74) | 184 (78) | |
| Squamous cell | 2 (6) | 6 (14) | 32 (13) | |
| Other | 3 (8)b | 5 (12) | 21 (9) | |
| ECOG performance status, n (%) | 0.192 | |||
| 0 | 6 (17) | 14 (33) | 42 (18)c | |
| 1 | 26 (72) | 25 (60) | 58 (67) | |
| ≥2 | 4 (11) | 3 (7) | 35 (15) | |
| Brain metastases, n (%) | 0.783 | |||
| Yes | 9 (25) | 12 (29) | 73 (31) | |
| No | 27 (75) | 30 (71) | 164 (69) | |
| Prior lines of therapy, n (%) | 0.182 | |||
| 0 | 22 (61) | 29 (69) | 156 (66) | |
| 1 | 6 (17) | 11 (26) | 57 (24) | |
| ≥2 | 8 (22) | 2 (5) | 24 (10) | |
| PD-(L)1 inhibitor received, n (%) | 0.464 | |||
| Pembrolizumab | 29 (81) | 32 (76) | 193 (81) | |
| Nivolumab | 6 (17) | 5 (12) | 33 (14) | |
| Atezolizumab | 0 (0) | 2 (5) | 6 (3) | |
| Nivolumab/ipilimumab | 1 (3) | 2 (5) | 3 (1) | |
| Durvalumab/tremelimumab | 0 (0) | 1 (2) | 2 (1) |
ECOG, Eastern Cooperative Oncology Group.
Percentages may not add up to 100% due to rounding.
Includes one patient with adenosquamous histology.
Not available in two patients.
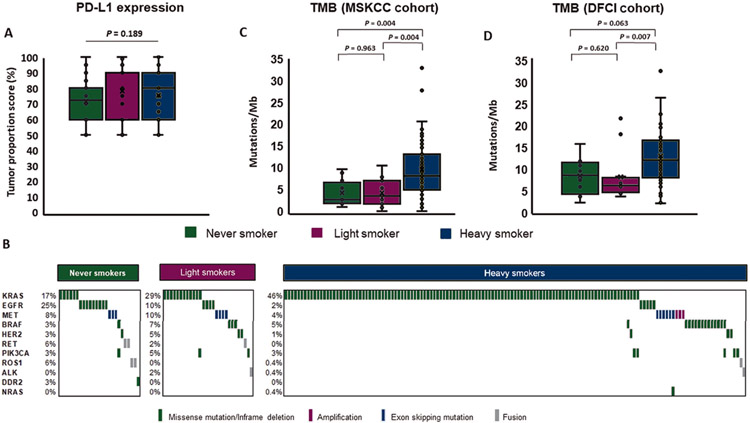
Figure 1.
(A) Comparison of programmed death-ligand 1 (PD-L1) expression tumor proportion scores (TPS) between never/light smokers and heavy smokers. (B) Frequency and distribution of genetic alterations in select oncogenic drivers within never, light, and heavy smoker cohorts. (C) and (D) Comparison of tumor mutation burden (TMB) estimates in never/light smokers and heavy smokers with MSK-IMPACT platform (n = 81) and DFCI OncoPanel (n = 78) testing available.
Molecular characteristics
We first compared baseline molecular characteristics across cohorts (Figure 1B, supplementary Table S2, available at Annals of Oncology online). As expected, genetic alterations in targetable oncogenes (EGFR, ALK/ROS1/RET fusions, BRAFV600E, and MET amplification/exon 14 skipping) were more common in the never and light smoking cohorts (47% and 29% versus 8%, respectively; P < 0.001 for both comparisons), while KRAS mutations were more common in heavy smokers [46% versus 17% (never) and 29% (light); P < 0.001 for both comparisons]. Among patients with sensitizing alterations in EGFR, ALK, and ROS1, 62% and 71% of never/light smokers and heavy smokers, respectively, received tyrosine kinase inhibitors (TKIs) before PD-(L)1 inhibitors. Conversely, only one patient (4%) with MET-altered or BRAFV600E-positive tumors received targeted therapies before ICIs.
We next compared TMB estimates across smoking cohorts, restricting our analysis to patients who underwent targeted next-generation sequencing (NGS) using MSK-IMPACT™ (n = 81; 26%) or DFCI OncoPanel (n = 78; 26%). TMB was lower among never and light smokers compared with heavy smokers in both the Memorial Sloan Kettering Cancer Center cohort (median 2.6 and 3.5 versus 8.2 mutations/Mb; P = 0.003 for both comparisons, respectively; Figure 1C and supplementary Figure S1, available at Annals of Oncology online) and DFCI cohort (median 8.6 and 6.4 versus 12.1 mutations/Mb; P = 0.064 and P = 0.007, respectively; Figure 1D). The distribution of TMB was similar between never and light smokers in both cohorts (P = 0.963 and P = 0.638, respectively).
Clinical outcomes
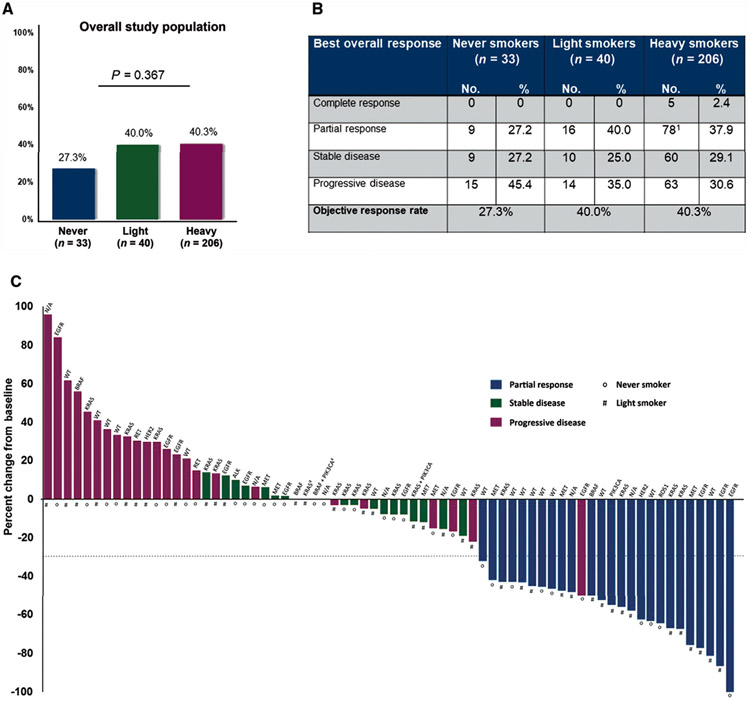
Of 315 patients in the study population, 279 patients had measurable disease by RECIST v1.1 criteria. Objective responses were observed in 27%, 40%, and 40% of never, light, and heavy smokers, respectively (P = 0.180 never versus heavy, P = 1.000 light versus heavy, Figure 2A and B). Similar objective response rates (ORRs) across smoking groups were observed among patients treated in the first-line setting (supplementary Figure S2, available at Annals of Oncology online) and among patients treated with PD-(L)1 monotherapy (supplementary Table S3, available at Annals of Oncology online).
Figure 2.
(A) Objective response rates according to smoking status among patients with baseline measurable disease (n = 279) according to RECIST, version 1.1. (B) Best overall responses according to RECIST version 1.1 among never, light, and heavy smokers. The heavy smoker cohort includes one unconfirmed partial response. (C) Antitumor responses to PD-(L)1 inhibitors in never and light smokers with programmed death-ligand 1 (PD-L1) tumor proportion scores (TPS) ≥50% and measurable disease at baseline according to RECIST version 1.1. The bars represent the best percent change in target tumor burden from baseline. Three patients with baseline measurable disease died before repeat response assessment and therefore are not depicted. The gene name above each bar indicates the genetic alterations present in each patient.
a Two patients had a best percentage change of 0%, but were characterized as having progressive disease based upon the development of new lesions.
The activity of PD-(L)1 inhibitors was not enriched within any one molecular genotype (Figure 2C and supplementary Figures S3 and S4, available at Annals of Oncology online). Among PD-L1high never or light smokers, responses were observed among patients with EGFR [n = 3/13 (23%)] and MET exon 14 alterations [n = 3/7 (43%); supplementary Tables S4 and S5, available at Annals of Oncology online], among others. Of five never or light smokers with ALK, ROS1, or RET fusions, one (20%) achieved an objective response. The lone responder was a never smoker with a ROS1 rearrangement; however, this patient’s response duration was limited (2.2 months). There was no difference in ORRs in never or light smokers treated with or without prior kinase inhibitors (ORR 22% versus 32%; P = 1.000). To account for the uneven distribution of EGFR and KRAS mutations across smoking groups, we next examined antitumor activity among non-EGFR/non-KRAS patients, finding comparable responses rates across all smoking cohorts [ORR 42% (never), 42% (light), and 40% (heavy); P = 1.000]. Beyond oncogenic driver status, we also examined the impact of co-mutations in TP53, STK11/LKB1, and KEAP1 on clinical activity of PD-(L)1 inhibitors within this cohort (supplementary Table S6, available at Annals of Oncology online). Consistent with prior studies,12 STK11/LKB1 mutations were associated with lower response rates to PD-1 pathway blockade (ORRs 22% versus 41% for STK11 mutant and wild-type, respectively; P = 0.141).
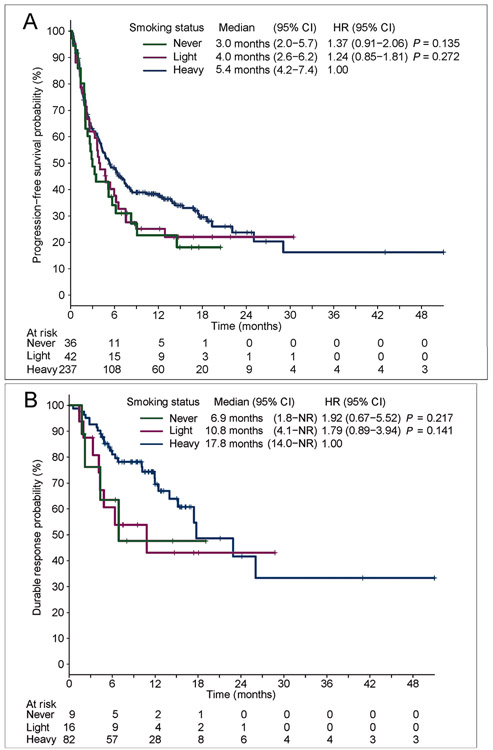
We next evaluated the relationship between smoking and PFS on PD-(L)1 inhibitors. In the overall population, median PFS was numerically shorter in never and light smokers {3.0 [95% confidence interval (CI) 2.0–5.7] and 4.0 months [95% CI 2.6–6.2]} compared with heavy smokers [5.4 months (95% CI 4.2–7.4); HR 1.37, P = 0.135 and HR 1.24, P = 0.272, respectively; Figures 3 and 4], but the differences were not statistically significant. One-year PFS rates were 22.6% (95% CI 9.7% to 38.7%), 25.1% (95% CI 13.2% to 39.0%), and 37.8% (95% CI 31.4% to 44.1%) among never, light, and heavy smokers, respectively. To adjust for patients receiving ICIs at various points, we next analyzed patients treated in the first-line setting (n = 207). Baseline clinical, pathologic, and molecular features are summarized in supplementary Table S7, available at Annals of Oncology online. In the first-line subgroup, there was no significant difference in PFS between never and light smokers (median PFS 4.6 and 4.7 months) versus heavy smokers (5.5 months; HR 1.13, P = 0.668 and HR 1.32, P = 0.231, respectively; supplementary Figure S5, available at Annals of Oncology online). Interestingly, the frequency of genetic alterations in EGFR, ALK, and ROS1 were generally lower in the first-line cohorts, particularly within the never smoker group. This may have partly offset differences in PFS between smoking subgroups in the first-line setting.
Figure 3.
(A) Progression-free survival and (B) duration of response for patients with non-small-cell lung cancer (NSCLC) and programmed death-ligand 1 (PD-L1) tumor proportion scores (TPS) ≥50% according to smoking status. NR indicates upper limit of the 95% confidence interval (CI) for median duration of response (DOR) has not been reached. HR, hazard ratio.
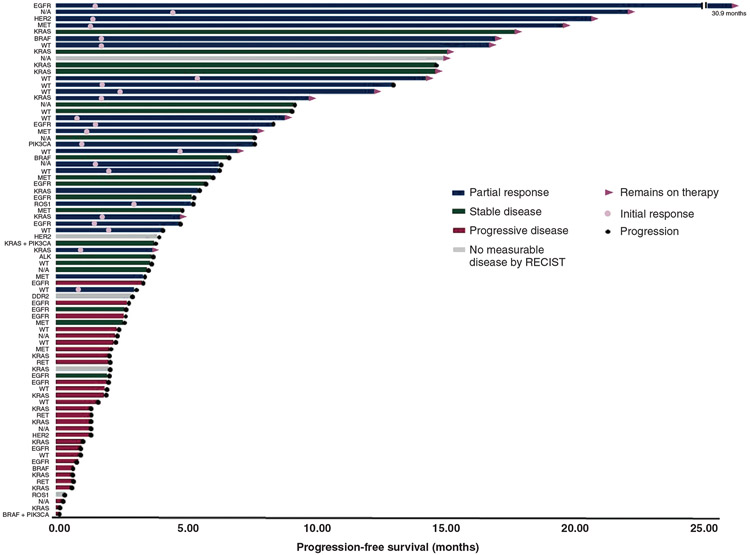
Figure 4.
Treatment response: Swimmer’s plot of the 78 patients in the never and light smoker cohorts with best objective response, time to first response, and progression-free-survival for each patient.
Finally, we evaluated DOR to PD-(L)1 inhibitors according to smoking exposure. Never and light smokers had numerically but not significantly shorter median DOR [6.9 (95% CI 1.8–not reached) and 10.8 months (95% CI 4.1–not reached) versus 17.8 months (95% CI 14.0–not reached), HR 1.92, P = 0.217 and HR 1.79, P = 0.141; Figure 3B]. The proportion of patients with DOR >1 year was also lower in the never and light smoker cohorts (47.6%, 43.1%) than the heavy smokers (69.6%). Thus, while PD-(L)1 inhibitors are active in a subset of never and light smokers with PD-L1 TPS ≥50%, there was a trend towards shorter DOR among these patients.
DISCUSSION
The management of metastatic NSCLC has evolved rapidly. For patients without targetable driver mutations, the mainstay of therapy is now PD-(L)1 inhibition with or without chemotherapy based upon PD-L1 score.1,6,7,13 Nonetheless, as PD-L1 expression is an imperfect biomarker, clinicians are commonly faced with how best to incorporate additional predictors into treatment decisions.
Here, we retrospectively examined the relationship between smoking and efficacy of PD-(L)1 inhibitors among 315 patients with PD-L1 TPS ≥50%. Based upon retrospective studies in unselected patient populations,9,14 we had originally hypothesized that never/light smokers would have limited clinical benefit from PD-(L)1 inhibition even in the setting of high PD-L1 expression. Instead, we observed fewer differences than initially anticipated. Roughly onequarter of never smokers and 40% of light smokers experienced objective responses to PD-(L)1 inhibitors. Thus, a significant fraction of never/light smokers with PD-L1 TPS ≥50% can derive initial clinical benefit from PD-(L)1 blockade.
Despite this antitumor activity, however, our data also suggest that the durability of these responses may differ from that of heavy smokers. Specifically, we observed trends towards shorter PFS and DOR and fewer patients with >1 year of ongoing benefit among never and light smokers compared with the heavy smoker group. While these differences were not statistically significant, likely owing to our sample size, these findings suggest that there may be distinct immunobiologic features determining initial response versus durability of response to ICIs. Additional, larger studies are needed to confirm these findings.
Our findings also underscore the need for additional analysis of key subgroups in prospective trials. For example, in the recently reported KEYNOTE 042 trial, pembrolizumab failed to improve OS compared with chemotherapy among never smokers with TPS ≥1%, ≥20%, and ≥50%, but data on ORR, PFS, and DOR in the never smoker subgroup has not been reported.15 These data will be important in validating our observations.
Collectively, our data suggest that PD-(L)1 inhibitors should be considered a standard treatment option for patients with TPS ≥50% even in the absence of significant tobacco exposure, but the timing of administration and whether to use these agents in combination may vary based upon other factors. For example, in patients with wellestablished, targetable oncogenic driver mutations (e.g. EGFR, ALK, and ROS1), targeted therapies should continue to be prioritized. Indeed, these recommendations are reinforced by findings from the IMMUNOTARGET registry16 and from a prospective study of pembrolizumab in TKInaive, EGFR-mutant NSCLC (n = 8/11 with TPS ≥50%), which demonstrated minimal activity with upfront ICIs in this population.17 Several recent reports have also described enhanced toxicities when ICIs were administered immediately before TKIs.18,19 Thus, targeted therapies should remain the preferred first-line therapies for patients with specific genetic alterations (e.g. EGFR, ALK, and ROS1) even in the setting of high PD-L1 expression.
Currently, there is no consensus on whether pembrolizumab monotherapy or pembrolizumab plus chemotherapy is optimal for NSCLC with TPS ≥50%, although many clinicians favor the use of monotherapy. Given the trend we observed of a shorter PFS/DOR with PD-(L)1 monotherapy among never/light smokers with TPS ≥50%, smoking status may be considered an additional factor in deciding between pembrolizumab monotherapy versus pembrolizumab plus chemotherapy. While the latter was not examined here given the timing of regulatory approvals, KEYNOTE 189 recently demonstrated an OS benefit with pembrolizumab and platinum-pemetrexed compared with chemotherapy alone, including in never smokers.6 Likewise, significant improvements in PFS were observed in EGFR-mutant/ALK-rearranged patients receiving the combination of atezolizumab, chemotherapy, and bevacizumab compared with chemotherapy and bevacizumab alone.13
Our study has several notable limitations. First, this was a retrospective study with a limited sample size. Larger studies will therefore be necessary to confirm the trends we observed regarding differences in DOR between smoking groups. Another limitation is that certain genetic subsets (e.g. EGFR, ALK) were underrepresented, likely reflecting alternative therapeutic options for these patients and prior reports suggesting diminished benefit with ICIs in these subgroups.20 This may have contributed to a higher than expected ORR with PD-(L)1 inhibitors in this study. Nonetheless, this represents the largest cohort of never/light smokers treated with PD-(L)1 inhibitors to date, and the distribution of other molecular alterations reflects real-world practice. Another limitation of this analysis is that while smoking status may be a proxy for TMB estimation, tobacco exposure alone cannot fully capture the biological complexities surrounding neoantigen generation and presentation. Finally, while we observed comparable ORRs to those seen in prospective studies in NSCLC with TPS ≥50%, the PFS in our cohort was shorter.1,15 Such differences may reflect the inclusion of patients with Eastern Cooperative Oncology Group (ECOG) PS ≥2 or brain metastasis, treatment with various PD-(L)1 inhibitors, and inclusion of patients treated in the second-line setting and beyond.
In summary, this study provides important new insights into the activity of PD-(L)1 inhibitors in never and light smokers with NSCLC and PD-L1 TPS ≥50%. Interestingly, we observed moderate antitumor activity in these subgroups, but also a signal of potentially decreased durability of response compared with patients with more significant tobacco exposure. Additional studies are needed to validate these findings and determine the optimal treatment regimen for never/light smokers with PD-L1 TPS ≥50%. Finally, efforts are needed to elucidate potentially distinct determinants of initial versus durable response to PD-(L)1 blockade in NSCLC.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Andrew Do (MGH) for his excellent technical assistance.
FUNDING
This research was supported by a Stand Up To Cancer—American Cancer Society Lung Cancer Dream Team Translational Research Grant (grant number: SU2CAACR-DT17-15). Stand Up To Cancer (SU2C) is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, a scientific partner of SU2C. JFG also received support from an ALK Positive/LUNGevity award. MN was supported by R01CA203636 (NCI). Supported by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748) and the Druckenmiller Center for Lung Cancer Research at MSKCC. MDH is a Damon Runyon Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (CI-98-18) and is a member of the Parker Institute for Cancer Immunotherapy.
Footnotes
DISCLOSURE
JFG: consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Ariad/Takeda, Loxo, Pfizer, Incyte, Novartis, Merck, Agios, Amgen, Regeneron, Oncorus, Array, Jounce, and Clovis Oncology, research support from Novartis, Genentech/Roche, and Ariad/Takeda, and institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo. JAR: consultant and equity in Genprex. BPL: Elsevier textbook author and editor, and receives royalties for his work. KA: consultant or received honoraria from AstraZeneca and Jackson Laboratory. JA: owns stock in Merck, Pfizer, and Thermo Fischer Scientific. MMK: consultant for Merimack Pharmaceuticals and H3 Biomedicine. ATS: consultant or received honoraria from Pfizer, Novartis, Chugai, Genentech/Roche, Ariad/Takeda, Ignyta, LOXO, Blueprint Medicines, KSQ Therapeutics, Daiichi Sankyo, EMD Serono, Taiho Pharmaceutical, TP Therapeutics, Bayer, Foundation Medicine, Guardant, and Natera. Institutional research funding from: Pfizer, Novartis, Roche/Genentech, Ariad, Ignyta, and TP Therapeutics. MMA: consultant or received honoraria from Merck, Bristol-Myers Squibb, Genentech, AstraZeneca, Nektar, Blueprint, Maverick, and Syndax and research support from Bristol-Myers Squibb, AstraZeneca, Lilly, and Genentech. MDH: has received research funding from Bristol-Myers Squibb; is a paid consultant to Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, and Immunai; has received travel support/honoraria from AztraZeneca and BMS; and a patent has been filed by MSK related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J Clin Oncol. 2018;36:1675–1684. [DOI] [PubMed] [Google Scholar]
- 3.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 8.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricciuti B, Kravets S, Dahlberg SE, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Huang X, Fu L. Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: a meta-analysis. Onco Targets Ther. 2018;11:3691–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 16.Mazières J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JJ, Chin E, Yeap BY, et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small cell lung cancer. J Thorac Oncol. 2019;14:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4:1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.