Abstract
Waterpipe smoking is becoming more popular worldwide and there is a pressing need to better characterize the exposure of smokers to chemical compounds present in the mainstream smoke. We report real-time measurements of mainstream smoke for carbon monoxide, volatile organic compounds and nanoparticle size distribution and chemical composition using a custom dilution flow tube. A conventional tobacco mixture, a dark leaf unwashed tobacco and a nicotine-free herbal tobacco were studied. Results show that carbon monoxide is present in the mainstream smoke and originates primarily from the charcoal used to heat the tobacco. Online measurements of volatile organic compounds in mainstream smoke showed an overwhelming contribution from glycerol. Gas phase analysis also showed that very little filtration of the gas phase products is provided by the percolation of mainstream smoke through water. Waterpipe smoking generated high concentrations of 4–100 nm nanoparticles, which were mainly composed of sugar derivatives and especially abundant in the first 10 min of the smoking session. These measured emissions of volatiles and particles are compared with those from a reference cigarette (3R4F) and represent the equivalent of the emission of one or more entire cigarettes for a single puff of hookah smoke. Considerations related to the health impacts of waterpipe smoking are discussed.
1. Introduction
Waterpipe smoking (also known as narghile or hookah depending on cultural traditions) is a way of smoking tobacco in which air is passed over heated charcoal, which transfers its thermal energy to the tobacco located in the head of the hookah underneath the charcoal, producing smoke. The smoke, composed of both charcoal burning products and compounds released from the heated tobacco, is entrained down the stem of the waterpipe and bubbles through water by the action of puffing on the waterpipe hose before being inhaled by the smoker. The attraction for this mode of smoking tobacco is driven by the variety of available tobacco flavors, the absence of visible side-stream smoke, the social aspect of smoking in group, the misconception that waterpipe smoking is less harmful than smoking cigarettes due to the possible filtration effects provided by the water, and the prevalence of advertised nicotine-free tobacco (Akl et al. 2013; Aljarrah K., Ababneh and Al-Delaimy 2009; Salloum et al. 2015).
The number of waterpipe smokers is significant and on the rise in the United States (Cobb et al. 2010; Sutfin et al. 2014) and worldwide (Martinasek, McDermott and Martini 2011; Maziak 2011). Waterpipe use is growing most dramatically among university students and other young adults (Aslam et al. 2014; Grekin and Ayna 2008; Primack et al. 2008). Smith et al. (2011) reported that the usage of waterpipe by all adults (ages > 18) had increased by 41.8% (men) and 47.4% (women) from 2005 to 2008 in California, and by young adults (ages 18–24) by about 24%. In a different study, Grekin and Ayna (2012) showed that 1 out of 5 college students in the United States and Europe had smoked waterpipes in 2011, and 1 out of 4 college students in the Middle East had smoked waterpipes in the last month prior the survey. Furthermore, data from a recent National Youth Tobacco Survey (NYTS) (Jamal, Getzke and Hu 2017) reported an increase in the usage of waterpipe among high school (4.1% to 4.8%) and middle school students (1.0% to 2.0%) in the United States from 2011 to 2016. As a consequence, there is a growing concern for understanding impacts of waterpipe smoking on human health and needs for regulations (Maziak 2011; World Health Organization 2015).
Numerous carcinogens and toxic pollutants have been previously identified in mainstream hookah smoke (smoke directly inhaled by the smoker) including nicotine (Katurji et al. 2010; Schubert et al. 2011a; Shihadeh 2003; Shihadeh and Saleh 2005; Shihadeh et al. 2012), nitrosamines (Schubert et al. 2011a), aromatic amines (Schubert et al. 2011b), polycyclic aromatic hydrocarbons (PAHs) (Hammal et al. 2015; Monzer et al. 2008; Schubert et al. 2011a; Sepetdjian, Shihadeh and Saliba 2008; Shihadeh and Saleh 2005; Shihadeh et al. 2012), volatile carbonyl compounds (Al Rashidi, Shihadeh and Saliba 2008; Hammal et al. 2015; Schubert et al. 2012b; Shihadeh et al. 2012), benzene (Schubert et al. 2015), phenols (Schubert et al. 2015; Sepetdjian et al. 2013), furans (Schubert et al. 2012a), carbon monoxide (CO) (Hammal et al. 2015; Katurji et al. 2010; Monn et al. 2007; Monzer et al. 2008; Schubert et al. 2011a; Shihadeh et al. 2014; Shihadeh and Saleh 2005; Shihadeh et al. 2012), nitric oxide (Hammal et al. 2015; Shihadeh et al. 2014; Shihadeh et al. 2012), and heavy metals (Schubert et al. 2015; Shihadeh 2003). In addition, waterpipe tobacco smoking has been associated with negative impacts on the respiratory and cardiovascular systems, periodontal diseases, low birth weight, cancers, and a higher risk for infection due to sharing the mouthpiece (Akl et al. 2010; Aslam et al. 2014; El-Zaatari, Chami and Zaatari 2015; Fakhreddine, Kanj and Kanj 2014; Kim, Kabir and Jahan 2016; Rezk-Hanna and Benowitz 2018).
Carbon monoxide is well-known for binding to the hemoglobin to form carboxyhemoglobin (CO-Hb) and thus inhibiting the hemoglobin’s ability to bind with vital oxygen. Several acute CO poisoning cases have been previously reported for waterpipe smokers where levels of CO-Hb were almost always higher than 20% of total hemoglobin (Cavus et al. 2010; Eichorn et al. 2018; La Fauci et al. 2012; Lim, Lim and Seow 2009; Retzky 2017; Veen 2016). CO levels are influenced by the choice of heat source, and the design of the waterpipe. For example, Monzer et al. (2008) reported that CO levels in mainstream hookah smoke can be reduced by a factor of 10 when using a custom-built electrical heater instead of charcoal, and Shaleh and Shihadeh (2008) reported that switching from plastic to more permeable leather hose would reduce the CO concentration ultimately inhaled by the smoker by about half; however, the CO emitted directly from the charcoal and that escaping from the mainstream smoke will still be present in the air surrounding the smoker (sidestream smoke).
All previously reported chemical investigations relied on off-line analysis techniques, which are often time-consuming, lack sensitivity towards low-volatility gases and small particles, and have primarily focused on total toxic pollutant and particle yields. Additionally, off-line analyses often integrate over an entire smoking session, so examinations of the time evolution of the concentration or composition in the mainstream smoke during session were not possible. The present work introduces a new approach to the study of waterpipe mainstream smoke by allowing online measurements of CO, volatile organic compounds (VOCs) and particle size distributions and chemical composition. Thermal Desorption Chemical Ionization Mass Spectrometry, TDCIMS (Smith et al. 2004), is applied for the first time for characterizing waterpipe mainstream smoke chemical composition, and this is the first report of real-time size distributions of particles in mainstream waterpipe smoke. Although on-line measurements of VOCs using proton-transfer-reaction mass spectrometry (PTR-MS) has been previously applied to smoke from cigarettes (Brinkman et al. 2015; Gordon et al. 2011) and e-cigarettes (Blair et al. 2015; Breiev et al. 2016), this is the first application of this technique to mainstream waterpipe smoke.
Our study compared the emissions from a glass waterpipe using three different tobacco mixtures, including one unwashed dark leaf tobacco and a nicotine-free herbal tobacco, following a modified Beirut protocol smoking pattern (4 s puff duration, 2 min−1 puff frequency, 860 ml puff volume). These conditions were well within the range of conditions recorded from a cohort of waterpipe smokers in Beirut, Lebanon (Shihadeh 2003; Shihadeh et al. 2004) from which the Beirut protocol was established, and very similar to recent studies performed in the US involving established waterpipe users (Brinkman et al. 2018; Kim et al. 2016). Typical waterpipe smoking duration recorded in natural and laboratory settings range between ~20 min up to > 60 min (Jawad et al. 2019; Maziak et al. 2009; Shihadeh 2003). In addition, acute health effects studies are oftentimes reported for a 30 min smoking duration (El-Zaatari, Chami and Zaatari 2015). As a result, a representative 30 min smoking session duration was selected for this study. All reported concentrations are given hereafter as the concentrations of each toxic pollutant directly emitted from the waterpipe during smoking, i.e., after accounting for the dilution necessitated by the measurement devices. The emissions from waterpipe experiments were directly compared with emissions from a reference cigarette (3R4F), also measured in this work, and potential risks and impacts on health are discussed.
2. Material and Methods
A brief description of the experimental procedure is provided below, but further details can be found in the online Supplementary Information (SI), including the complete waterpipe smoking protocol and the details of the instrument operations. All smoking sessions were performed using the same waterpipe (Anahi Smoke, model “Fantasy”) which was constructed entirely of glass for ease in cleaning (Figure S1). At the beginning of each experiment, 10 g of tobacco were packed loosely in the head of the waterpipe and wrapped with a pre-punched aluminum foil (Starbuzz Tobacco Inc., Starbuzz Premium foil). Three different tobacco mixtures were tested in this study, including a conventional tobacco (Al Fakher, apple flavor), a nicotine-free herbal tobacco (Hydro Herbal, banana flavor) and an unwashed dark leaf tobacco (Vintage by Starbuzz, Dark Mist, blackberry flavor). Then, three charcoal cubes (100% natural coconut husks; Black Diamond) were lighted and placed atop the head to uniformly heat the tobacco (Figure S1). The smoking session lasted for 30 min, using the following smoking regimen: puff frequency, 2 puff/min; puff duration, 4 s; puff volume, 860 mL. Control experiments including no charcoal (or charcoal only), no water (or water only), or no tobacco, were also performed to identify the sources of both the organic gases and particles. Lastly, as glycerol is a major component of the tobacco formulations, a control experiment with pure glycerol (EMD Millipore, ACS grade) instead of the tobacco was conducted.
A fast flow dilution system was developed to dilute the mainstream smoke emission of the waterpipe to levels manageable by our analytical instruments as well as to provide continuous flow conditions that are needed for stable instrument operation. A diagram of the system is presented in Figure 1. Additional details on the dilution system can be found in SI, including the characterization of the transmission efficiency of the particles through the dilution system (Figures S2, S3 and S4). The total dilution factor of the waterpipe emission sampled through the entire system ranged from 241 to 325. An average value of 276 ± 34 (1σ) is used hereafter for all calculations.
Figure 1.
Diagram of the fast flow dilution system used in the waterpipe smoking experiments. A fraction of the total puff flow is sampled through the dilution system (F2, ~3 L min−1) while the rest is pumped off (F1, ~10 L min−1). Dilution air from a purge air generator is provided in the two stages of dilution and the flows in and out are balanced (F4 = F4’ and F5 = F5’). Flows F2 and F3 are equal and governed by the sum of the analytical instruments connected at the end of the mixing section as F2 = F3 = Ftotal = FTDCIMS + FSMPS + FPTR-ToF-MS + FCO.
The mainstream smoke sampling train was composed of a series of dedicated instruments including a scanning mobility scanning mobility particle sizer (SMPS; TSI) to measure size distribution of particles, a CO monitor (Thermo Fisher Scientific, model 48i) and two mass spectrometers measuring the volatiles (PTR-ToF-MS, model 8000, Ionicon Analytik) (Jordan et al. 2009) and the particle chemical composition (TDCIMS) (Lawler et al. 2018; Smith et al. 2004). All details of the instrumentation operation are given in SI. Additionally, two relative humidity and temperature probes (Vaisala Corp., model HMP110) were placed at the entrance and exit of the dilution system.
Although this study focused on the mainstream emissions from waterpipe, a comparative study with one type of reference cigarette (3R4F, University of Kentucky) was conducted using the same fast flow dilution system. This type of cigarette has a filter ventilation of 20%, high tar (9.40 mg/cig.) and a nicotine content of 0.73 mg/cig (Sampson et al. 2014). Details of the smoking regimen and protocol are provided in SI.
3. Results and Discussion
For all the waterpipe experiments, an optically dense white aerosol formed within the waterpipe at the first puff, and was maintained throughout the 30-min smoking session. No side stream smoke was observed in any experiments. The tobacco mixtures were all weighed at the end of the sessions, and an average loss of 2.2 ± 0.4 g (out of the initial 10 g) was measured. Control experiments performed in absence of water showed a similar behavior. Experiments carried out with ~4 g of pure liquid glycerol instead of the tobacco only yielded a loss of ~0.2 g of glycerol over the 30-min smoking session.
In a separate experiment, the temperature in the head of the hookah (i.e., the place where the tobacco resides) was measured using a thermocouple (Omega, probe type K). The temperature ranged from 265°C to 318°C during the 30-min smoking session. Although three charcoal cubes were necessary to uniformly heat the tobacco mixture, the temperature recorded in this study was in agreement with a previous measurement using 1 easy-lighting briquette (Monzer et al. 2008). Measurements indicated that at every puff, the temperature inside the head of the hookah decreased by ~50°C for the duration of the puff (as sample air was drawn into the head of the hookah) and then rose up between puffs. For comparison, the temperature (and RH) of the mainstream smoking exiting the waterpipe and at the outlet of the dilution system is presented in Figure S5.
3.1. Gas phase measurements
3.1.1. Carbon monoxide
Due to instrument malfunction, the CO monitor was available for only selected experiments with the conventional tobacco and for the 3R4F reference cigarettes. No significant differences were observed for the full waterpipe experiment (tobacco + water + charcoal) and the controls (no water; charcoal only). A separate experiment was carried out without charcoal (no heat) showing no detectable CO, confirming that CO mainly came from the combustion of the charcoal, consistent with previous reports (Monzer et al. 2008). After correcting for calibration bias and dilution, an average mixing ratio of 2.1 (± 0.6) × 103 ppm of CO was observed from the waterpipe mainstream smoke, compared to 3.3 (± 0.3) × 104 ppm from the cigarette smoke. The estimated mass of CO inhaled in 1 puff of mainstream smoke was 2.0 (± 0.6) mg and 1.4 (± 0.1) mg respectively for the hookah and cigarette smoke. Those results are in agreement with previously reported CO dose from mainstream smoke by Monn et al. (2007). If this dose is integrated over the entire waterpipe smoking session (i.e., 60 puffs), a total amount of 121 mg of CO is expected to be inhaled per session. For comparison with literature values summarized in Shihadeh et al. (2015), our measurement was multiplied by a factor of 2 to account for the difference in smoking time (all reported studies were for ~1hr smoking session), and yield a total of 242 mg CO emitted, which is well within the range reported by previous studies (e.g., 57–367 mg/session). Likewise, the integrated dose of CO from one cigarette was 9.7 mg, which is also in agreement with previously reported values (Eldridge et al. 2015; Roemer et al. 2012). Therefore, the dose of CO from a single waterpipe smoking session was equivalent to a dose of CO from 12 references cigarettes.
3.1.2. Volatile organic compound measurements
Figure 2 (a–b) represents typical background subtracted mass spectra obtained from the conventional tobacco mixture and the 3R4F reference cigarette, respectively (the background corresponds to the baseline signal intensity before the smoking session started). For comparison, the mass spectra for the other tobacco mixtures are presented in Figure S6.
Figure 2.
Typical unit mass resolution PTR-ToF-MS mass spectra from (a) the waterpipe mainstream smoke of the conventional tobacco and (b) the 3R4F reference cigarette. All spectra were collected at the end of the smoking session (the last 10 puffs for the waterpipe sample, and the last puff for the cigarette sample) and the background signal has been subtracted out.
All spectra derived from hookah mainstream smoke show the distribution of VOC-derived ions observed during the last 5 min (10 puffs) of the smoking session. Ions at nominal m/z 41, 43, 45, 57, 59, 61, 75 and 93 were the major common ions observed for all three tobacco mixtures (Figure 2a and S6). Table 1 reports the likely attributions for the ions observed in this study. These attributions are consistent with previously reported assignments from PTR-MS measurements (de Gouw and Warneke 2007; Yuan et al. 2017), and in addition, each m/z observed in our experimental mass spectra matched the exact mass of the assigned chemical to within 3 mDa, which is an acceptable error on accurate mass determination for m/z up to 1000 Da (Greaves and Roboz 2014).
Table 1.
Ions observed in the PTR-ToF-MS mass spectra from the waterpipe mainstream smoke samples (conventional tobacco; nicotine-free herbal tobacco; dark leaf unwashed tobacco) and the 3R4F reference cigarette.
| Nominal m/z | Exact m/z | Empirical Formula | Assigned Compound |
|---|---|---|---|
| 31 | 31.018 | [CH2O + H]+ | formaldehyde |
| 33 | 33.033 | [CH4O + H]+ | methanol |
| 41 | 41.039 | [C3H4 + H]+ | propadienea |
| 42 | 42.034 | [C2H3N + H]+ | acetonitrileb |
| 43 | 43.018 | [C2H2O + H]+ | acetic acid fragment,c glycolaldehyde fragment,c hexyl acetate fragmentd |
| 43.054 | [C3H6 + H]+ | propylenea | |
| 45 | 45.033 | [C2H4O + H]+ | acetaldehyde |
| 47 | 47.013 | [CH2O2 + H]+ | formic acid |
| 47.049 | [C2H6O + H]+ | ethanol | |
| 57 | 57.033 | [C3H4O + H]+ | acrolein, hexyl acetate fragmentd |
| 57.070 | [C4H8 + H]+ | butenea | |
| 59 | 59.049 | [C3H6O + H]+ | acetone, propionaldehyde, methyl vinyl ether |
| 61 | 61.028 | [C2H4O2 + H]+ | acetic acid, hydroxyacetaldehyde (glycolaldehyde) |
| 69 | 69.033 | [C4H4O + H]+ | furan |
| 69.070 | [C5H8 + H]+ | isoprenea | |
| 75 | 75.044 | [C3H6O2 + H]+ | hydroxyacetone (acetol), 3-hydroxypropanal, 1,3-dihydroxypropene, glycerol fragment (−H2O) |
| 79 | 79.054 | [C6H6+H]+ | benzenee |
| 91 | 90.948 | FeOH(H2O)+ | intrinsic ion from the PTR-ToF-MS ion sourcef |
| 91.039 | [C3H6O3+H]+ | glyceraldehyde, lactic acid | |
| 91.054 | [C7H6+H]+ | fragment from benzyl compoundsg | |
| 93 | 93.055 | [C3H8O3 + H]+ | glycerol |
| 93.070 | [C7H8 + H]+ | toluene | |
| 117 | 117.055 | [C5H8O3 + H]+ | 1-acetoxyacetoneh |
| 163 | 163.123 | [C10H14N2 + H]+ | nicotine |
Correspond to compounds often found in biomass burning samples (refer to Brilli et al. (2014) and references therein); significant in the cigarette samples.
Not present in the waterpipe mainstream smoke.
Refer to Basssandorj et al. (2015) and Haase et al. (2012).
Hexyl acetate was found in biomass burning (Brilli et al., 2014) as well as in numerous waterpipe tobacco mixtures acting as a flavoring agent (Schubert et al., 2013).
Significant in the cigarette samples; ethylbenzene fragment can also contribute to m/z 79 (Rogers et al., 2006), however, ethylbenzene (m/z 107) was only observed in the cigarette sample (see Figure S7).
Refer to Schroder et al. (2008).
No clear source for the tropylium ion (C7H7+) could be found here, although Sovova et al. (2011), reported that phytogenic compounds such as benzyl acetate or benzyl benzoate can produce efficiently this ion upon SIFT-MS ionization. Benzyl acetate was in numerous apple flavor waterpipe tobacco mixtures (Schubert et al., 2013).
Previously identified in pine wood combustion samples (Fitzpatrick et al., 2007) and African grass (Stockwell et al., 2015)
Figure S7 illustrates the advantage of using a high resolution time-of-flight mass spectrometer that allows us to differentiate between molecular formulas having the same nominal mass. With the exception of m/z 91 (glyceraldehyde), 93 (glycerol) and 163 (nicotine), all compounds have been previously reported in biomass burning samples (Brilli et al. 2014; Bruns et al. 2017; Fitzpatrick et al. 2007; Karl et al. 2007; Muller et al. 2016; Stockwell et al. 2015; Warneke et al. 2011), so it is not surprising to observe them in this study.
It is interesting to note that all three tobaccos show similar patterns in the relative intensity of the detected ions (Table S1), with the exception of the conventional tobacco which exhibits a larger contribution from m/z 59.049, 41.039 and 31.018 than the other two tobaccos. The differences between tobaccos are likely due to the differences in flavoring. The nicotine-free herbal tobacco shows, in general, a higher ion intensity at every major ion, including m/z 117.055 ([C5H8O3 + H]+) that is clearly visible in the spectra. We assigned this ion to 1-acetoxyacetone (Table 1) because it was previously found in pine wood combustion samples (Fitzpatrick et al. 2007).
Mass spectra from control experiments, which include experiments performed without any tobacco (charcoal + water only), with the conventional tobacco but no charcoal (no heat) and only glycerol (replacing the tobacco) are given in Figure S8. The major common ions (nominal m/z 43, 45, 57, 61 and 75) observed in the waterpipe mainstream smoke were common to all spectra, including that of glycerol (propane-1,2,3-triol). Upon thermal decomposition, glycerol has been reported to form not only two major products, namely acetaldehyde (C2H4O, MW = 44 g mol−1) and acrolein (C3H4O, MW = 56 g mol−1), but also 3-hydroxypropanal (C3H6O2, MW = 74 g mol−1), 1-hydroxypropan-2-one (C3H6O2, MW = 74 g mol−1), hydroxyacetone (or acetol; C3H6O2, MW = 74 g mol−1), glycolaldehyde (C2H4O2, MW = 60 g mol−1), and acetic acid (C2H4O2, MW = 60 g mol−1) (Corma et al. 2008; Hemings et al. 2012; Jensen, Strongin and Peyton 2017; Katryniok et al. 2010; Martinuzzi et al. 2014; Nimlos et al. 2006). Ionization of these compounds in the PTR-ToF-MS ion source would give the observed [M + H]+ ions. Both acetic acid and glycolaldehyde are known to fragment under typical PTR-MS conditions such as the ones applied here, to give an additional fragment at m/z 43.018 (C2H3O+) (Baasandorj et al. 2015). Though, acetic acid is thought to be formed from secondary oxidation process (Jensen, Strongin and Peyton 2017; Katryniok et al. 2010), it is more likely that the peaks at m/z 61/43 are due to glycolaldehyde instead. Further evidence for this assignment is the presence of a minor peak at m/z 91.039 attributed to glyceraldehyde (Figure S7), which was previously proposed as an intermediate in the decomposition process of glycerol leading to glycolaldehyde. (Jensen, Strongin and Peyton 2017)
In addition, experiments and theoretical calculations demonstrated that the protonated [M+H]+ ion of glycerol at nominal m/z 93 can itself undergo dehydration to yield ions at m/z 75, 61, 57, 45 and 43 (Dass 1994; Nimlos et al. 2006). Note, although the glycerol parent ion at m/z 93.055 was observed in the waterpipe mainstream smoke of all three tobacco, it was not detectable in the glycerol experiment due to a very low concentration of glycerol in the gas phase sampled (but was observed for pure glycerol particles measured in separate experiments; Figure S8d). Alcohols are known to readily fragment by losing a molecule of water upon proton transfer reaction ionization process via reaction with H3O+ (Spanel and Smith 1997) and only at high concentration the parent peak was observable. In brief, glycerol related peaks overwhelm the mass spectra of the gas phase products emitted by the waterpipe during smoking, and this is not surprising as it is used as a wetting agent or humectant for the tobacco (Rainey et al. 2013; Schubert et al. 2012a; Schubert et al. 2011a; Schubert et al. 2012b). Our results are also consistent with a previous study by Schubert et al. (2011a) in which significant amount of glycerol in waterpipe mainstream smoke was measured (up to 423 mg/session).
As seen in Figure 2b, the PTR-MS spectrum of mainstream smoke from the waterpipe experiment is very different from that of the 3R4F reference cigarette smoke. The latter shows a different distribution of ions, including significant signal intensities at m/z 42.034 (acetonitrile), 33.034 (methanol), 69.070 (isoprene) and 79.054 (benzene). While methanol is visible in the conventional tobacco mass spectra, acetonitrile is not present in any of the waterpipe samples as illustrated by the high resolution mass spectra of nominal m/z 42 (Figure S7). A peak at m/z 69.034 ([C4H4O + H]+) is also observed as a minor peak in the MS spectra for the waterpipe mainstream smoke, attributed to a furan compound, while in the cigarette smoke, it is attributed to isoprene (m/z 69.0704, [C5H8+H]+) (Table 1, Figure S7). Present in cigarette smoke, a peak at m/z 79.054 attributed to benzene was also identified as a minor contributor of hookah mainstream smoke (Figure S9) consistent with previous studies (Schubert et al. 2015; Shihadeh et al. 2015). Although a small fraction of benzene found in the hookah smoke was emitted from the charcoal only, most of the benzene measured in the conventional and nicotine-free herbal tobacco originated from the tobacco itself upon heating (Figure S9). Lastly, an ion at m/z 163.123 indicative of nicotine (C10H14N2) was observed in the cigarette mainstream smoke sample. Nicotine was also detected in the waterpipe mainstream smoke of both the conventional and dark leaf unwashed tobacco as a minor product, and none was measured for the nicotine-free herbal tobacco (Figure S7). Instead, a peak at m/z 163.097 was observed for the herbal tobacco, and assigned to [C7H14O4+H]+ ion.
Figure S10 represents the evolution of the PTR-ToF-MS mass spectra as a function of smoking time (each mass spectrum corresponding to an average over 10 puffs) for the conventional tobacco runs performed under ‘normal’ conditions (charcoal + tobacco + water) and for control experiments without water present in the waterpipe. The time evolution shows that in both cases, the signal intensity of each ion increases as a function of smoking time (number of puffs) to reach a plateau, and no significant differences exists with and without water present in terms of ion distribution or intensities. However, care should be taken to interpret the increase of the signal as a function of time as a true emission profile from the waterpipe, as instrument delays have been observed previously to happen with sampling tubes (Mikoviny, Kaser and Wisthaler 2010; Pagonis et al. 2017).
Two exceptions exist when comparing experiments ran with and without water in the waterpipe bowl, which are the ions observed at m/z 33.034 and 47.049 (attributed to methanol and ethanol respectively). Those ions appear to be particularly sensitive to the presence of water, for which a strong filtration effect is observed as illustrated in the expanded mass spectra in Figure S11. This observation is consistent with their respective Henry’s law constant (KH), which are 2.03 and 1.7 mol m−3 Pa−1 respectively (Sander 2015). It is not surprising to observe no filtration effect for carbonyls compounds measured in this study as their KH values by comparison is much smaller with average values of, for example, 0.14 mol m−3 Pa−1 for acetaldehyde and 0.10 mol m−2 Pa−1 for acrolein (Sander 2015). Following a similar analysis, Al Rashidi et al. (2008) previously demonstrated that acetaldehyde, acrolein, propionaldehyde and methacrolein all remained in the gas phase, while formaldehyde, the most soluble of the carbonyl compounds measured, was found in the particle phase due to its much higher solubility, KH ~ 43 mol m−3 Pa−1 (Sander 2015).
Although extremely soluble with KH ~ 7.2 × 103 mol m−3 Pa−1 (Sander 2015), the small nicotine signal observed at m/z 163.125 was not noticeably affected by the presence of water (Figure S11) suggesting a very small trapping effect. Overall, our observations show that water does not act as a filter for volatile organic compounds under our smoking conditions.
The efficiency for water to filter out pollutants can be influenced by different factors affecting the bubble properties (controlling the exchange with the gas phase), including the puff topography, the design of the waterpipe (specifically the body stem), the amount of water present and the depth of the body stem immersed in the water that could be different from one study to the other (Oladhosseini and Karimi 2016). As described earlier, our experiments were performed with 800 mL of deionized water and the body stem (25.4 cm in length; 10–12 mm inner diameter; equipped with 6 slits, 1 × 15 mm) placed 39 mm under the water surface. Based on calculations from Oladhosseini and Karimi (2016), the partitioning of VOCs under our experimental conditions should not reach equilibrium, and could be potentially enhanced by reducing the diameter of the body stem, increasing the immersion depth of the body stem, increasing the total water volume placed in the bowl and increasing the volume of the puff. However, based on a limited number of published studies, lack of complete water filtration is a common phenomenon among various waterpipe designs. For example, Schubert et al. (2011b) showed that aromatic amines are not efficiently filtered by the water (amount not filtered by the water ranging from 57 to 90%). In another recent study, Schubert et al. (2012b) reported that carbonyl compounds seemed to be filtered to a greater extent, although a large fraction was still measurable in the mainstream smoke (amount not filtered by the water ranging from 20 to 42%). Similarly, Shihadeh (2003) showed that nicotine was not completely removed due to water absorption, and ~23% remained present in the mainstream smoke. Our work combined with previous studies suggest that significant amount of toxic compounds will still reach the smoker’s mouth when water is present in the bowl.
3.1. Size distribution of particles in mainstream smoke
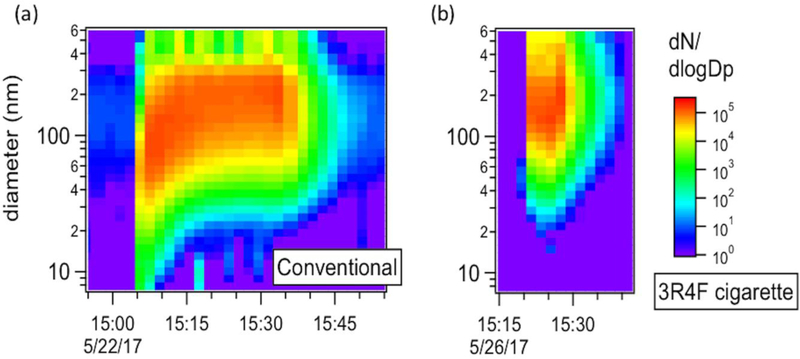
Figure 3a shows a typical particle size distribution of mainstream smoke from the conventional tobacco mixture, corrected for dilution, as function of time during a full smoking session with the conventional tobacco (charcoal + tobacco + water). This is the first report of real-time size distribution measurement of particles from hookah mainstream smoke. Each scan represents an average over 1 min, which did not make it possible to capture the individual isolated puffs, but provided an average over 2 puffs (2 puffs/min smoking frequency) which still captured the general dynamics of the evolution of the size distribution over time.
Figure 3.
Typical size distributions for individual smoking sessions measured for (a) the conventional tobacco and (b) the 3R4F reference cigarette. Concentrations are corrected for dilution and size-dependent sampling losses through the experimental system. The start of the smoking session corresponds to the sudden increase on the particle number concentration.
Ultrafine particles with diameters ranging from 4 to 100 nm are clearly visible at the beginning of the session (first 10 min), during which they dominate the size distribution. Particles were observed to grow quickly to larger diameters as the smoking session proceeded, likely due to the stagnation of the large concentration of particles within the waterpipe bowl. The particle mode diameter was ~80 nm during the first 10 min of the smoking session but shifted to ~200 nm for the remaining of the session.
The general trend observed for the conventional tobacco is not unique; indeed, as can be seen in Figure S12, the other two investigated tobacco mixtures show the same significant contribution of ultrafine particles early on in the smoking session. In the case of the dark leaf unwashed tobacco, a persistent mode around 10 nm was observed throughout the experiments, distinct from the other tobaccos. This mode indicates that there was nucleation of new particles from the mainstream smoke throughout the smoking session. This observation is consistent with the TDCIMS measurements described in detail below in that organic compounds from the dark leaf unwashed tobacco desorbed from the Pt wire at a later time compared to the other two tobaccos, which suggests lower volatility compounds and thus a greater potential to nucleate particles.
Control experiments performed in the absence of the water in the waterpipe bowl (Figure S13a) showed particle number concentration and size distribution that were similar to those when water was present, suggesting that the water does not act as a filter for those particles. This phenomenon was briefly mentioned in a previous study (Monn et al. 2007). This observation is also consistent with previous studies (Hogan et al. 2005; Spanne, Grzybowski and Bohgard 1999; Wei, Rosario and Montoya 2010) that demonstrated that the collection efficiency for particles with diameter ranging from 10 to ~700 nm using the commonly used impinger technique (where the air sample is bubbled through a reservoir containing water, similar to the waterpipe principle) is very small (less than 20% at 1–12 L min−1).
For comparison, Figure 3b presents a typical size distribution of mainstream smoke from the 3R4F reference cigarette and shows that the mode of the distribution is centered around 150 nm for the entire duration of the session. Some ultrafine particles are also present in the cigarette mainstream smoke, but the majority of the particles have diameters larger than 100 nm. It is important to note that the particles from the hookah mainstream smoke likely underwent a higher degree of evaporation during their transport through the dilution system, as it has been reported for e-cigarettes particles compared to reference cigarette (Ingebrethsen, Cole and Alderman 2012). However, comparison between conditions can still be made.
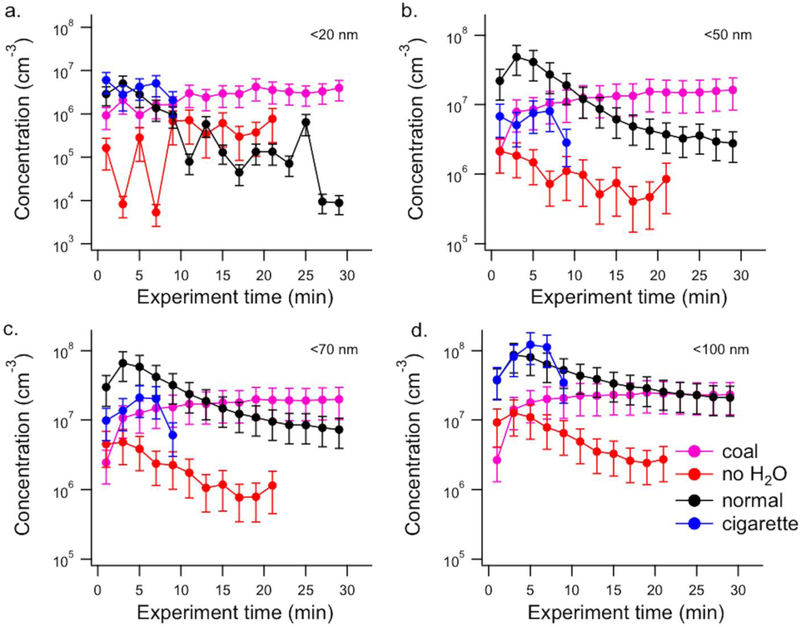
Ultrafine particles are expected to be deposited in the deepest part of the respiratory tract (Chow and Watson 2007; Hinds 1999). The smallest nanoparticles (<50 nm) can even cross the blood-brain barrier (Betzer et al. 2017; Oberdorster et al. 2004). To compare the potential health impact of ultrafine particles measured for hookah and cigarette mainstream smoke inhaled into the respiratory tract, the particle concentration for different size ranges was determined. Figure 4 (a–d) shows the time profile of particles concentrations with diameter d < 100nm, d < 70 nm, d < 50 nm and d < 20 nm for the conventional tobacco, charcoal only, the conventional tobacco run without water in the bowl and the reference 3R4F cigarette.
Figure 4.
Concentration profile of nanoparticles with diameters d < 20 nm (a), d < 50 nm (b), d < 70 nm (c) and d < 100 nm (d) measured in the mainstream smoke as a function of smoking time for the conventional tobacco (black trace; n = 4), the coal only experiment (pink trace; n = 3), the conventional tobacco with no water (red trace; n = 2) and the 3R4F reference cigarette (blue cigarette; n = 3). Concentrations are corrected for dilution and for size-dependent transmission losses through the experimental system. Error bars are one standard deviation of the mean.
It can be seen that the charcoal itself generated a substantial number of particles of all diameters at a relatively constant concentration during the smoking session. The mainstream smoke from the conventional tobacco experiment showed an enhancement of nanoparticles of all size ranges at the beginning of the smoking session (first 10 min). The increase in the observed particle number concentration is likely due to nucleation from the vapors emitted from the tobacco. As the smoking session proceeds, the particles grow and no new particles are formed, as seen by the decrease in the < 20 nm and < 50 nm particles concentration. This is likely due to the increase in condensation sink caused by the higher concentrations of large particles, which effectively scavenge smaller particles. At the end of the experiments, concentrations of < 70 nm and <100 nm particles for the conventional tobacco and charcoal-only are similar.
The experiment performed without water in the bowl shows relatively low particle concentrations at d < 20 nm in the first 10 min of the smoking session, but also much lower ultrafine particle concentrations in general. It appears that, rather than acting as a protective filter for the user, the water vapor actually appears to support new particle formation in the hookah mainstream smoke, likely due to the cooling effect of the water, leading to higher concentrations of ultrafine particles in general and the smallest size classes in particular.
By comparison, cigarette smoke produces fewer particles with diameter < 70 nm compared to the hookah mainstream smoke, as shown in Figure 4c (first 10 min). However, a similar concentration of particles with diameter < 100 nm is observed for both the hookah smoke and the 3R4F reference cigarette.
An averaged dose of ultrafine particles received by one smoker (defined in this study as the total number of particles inhaled in one puff of mainstream smoke) can be estimated from the size distribution data for both the hookah and cigarette samples as follows:
where C is the total particle concentration (particles per cm3) measured by the SMPS averaged across all three tobaccos at a time t, dil. factor is the averaged dilution factor provided by the sampling system, and volpuff is the volume of one puff of hookah smoke (860 mL) or cigarette smoke (36 mL). Table 2 shows the comparison between hookah and cigarette total ultrafine particle number received by a smoker for one puff of smoke for each size range. Results show that the dose of particles received by one smoker is much higher for the hookah mainstream smoke at the beginning of a session than the cigarette mainstream smoke, and the difference increases as the diameter of the particles decreases. Taking the average values for total ultrafine particles inhaled in one puff (d <100 nm), the dose received by one smoker per hookah puff corresponds to smoking 2.4 cigarettes (assuming 7 puffs/cigarette). However, at the very beginning of the hookah session, when the concentration of the particles is the highest, the total particles with diameter < 100 nm and < 20 nm inhaled by one smoker in one puff would be the equivalent of smoking 5 and 25 cigarettes (assuming 7 puffs/cigarette) respectively. After 10 min, the dose of hookah mainstream smoke particles received by one smoker in one puff is somewhat comparable to the dose from one 3R4F reference cigarette.
Table 2.
Averaged dose of particles inhaled by a smoker determined from size distribution measurements as a function of particle diameters defined as the total number concentration of particles contained in 1 puff (hookah puff volume = 860 mL; cigarette puff volume = 36 mL).
| Time after starting the session | < 100 nm | < 70 nm | < 50 nm | < 20 nm |
|---|---|---|---|---|
| Normal (n = 8)a | ||||
| 4 min | 9.92 (± 3.5) × 1010 | 6.07 (± 2.2) × 1010 | 3.73 (± 1.3) × 1010 | 2.09 (± 0.8) × 109 |
| 15 min | 3.55 (± 1.3) × 1010 | 1.34 (± 0.5) × 1010 | 4.83 (± 1.7) × 109 | 2.63 (± 0.9) × 107 |
| average | 4.44 (± 0.8) × 1010 | 2.13 (± 0.5) × 1010 | 1.08 (± 0.4) × 1010 | 3.38 (± 1.5) × 108 |
| No water (n = 2)b | ||||
| 4 min | 1.05 (± 0.6) × 1010 | 3.71 (± 2.0) × 109 | 1.29 (± 0.7) × 109 | 2.98 (± 1.5) × 106 |
| 15 min | 2.28 (± 1.2) × 109 | 4.87 (± 2.5) × 108 | 1.36 (± 0.8) × 108 | 4.21 (± 3.0) × 107 |
| average | 4.76 (± 1.0) × 109 | 1.45 (± 0.4) × 109 | 4.75 (± 1.4) × 108 | 2.76 (± 0.6) × 107 |
| No tobacco (n = 3)c | ||||
| 4 min | 9.39 (± 4.6) × 109 | 6.46 (± 3.2) × 109 | 4.14 (± 2.0) × 109 | 3.33 (± 1.7) × 108 |
| 15 min | 1.54 (± 0.8) × 1010 | 1.09 (± 0.5) × 1010 | 7.22 (± 3.5) × 109 | 5.32 (± 2.7) × 108 |
| average | 1.37 (± 0.2) × 1010 | 9.92 (± 1.4) × 109 | 6.75 (± 1.0) × 109 | 5.37 (± 0.9) × 108 |
| Reference cigarette (n = 3) | ||||
| average | 2.68 (± 0.7) × 109 | 3.56 (± 1.0) × 108 | 7.31 (± 1.8) × 107 | 1.17 (± 0.2) × 107 |
Include all the tobacco (n = 4 for the conventional tobacco; n = 2 for the nicotine-free herbal tobacco; n = 2 for the dark leaf unwashed tobacco)
Conventional tobacco (n = 2)
Includes the run with glycerol in the place of the tobacco.
3.2. Chemical composition of ultrafine particles in mainstream smoke
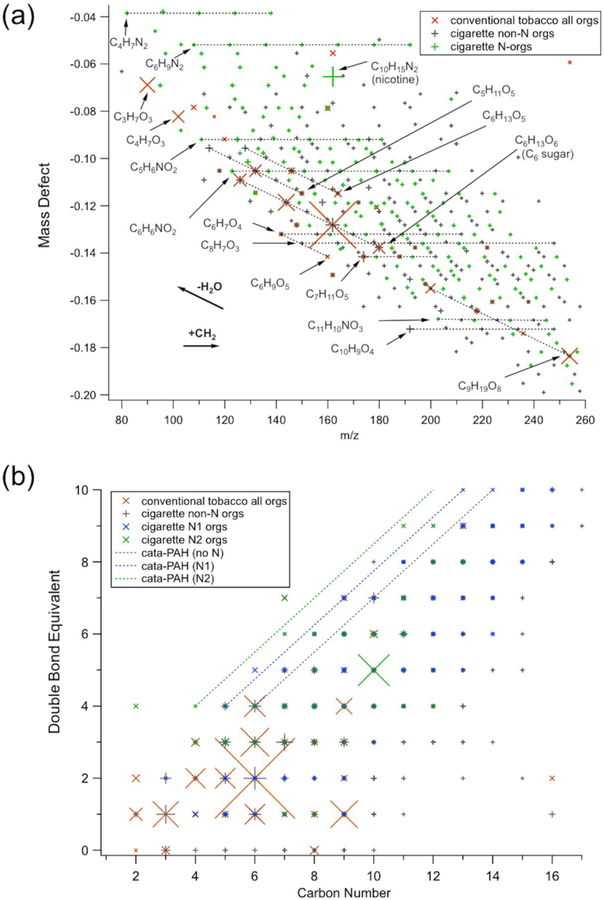
The chemical composition of ultrafine particles was measured by TDCIMS. The size-resolved particulate mass distribution collected by the instrument at early times of a hookah smoking session is shown in Figure S14, and indicates that the total mass collected on the wire was dominated by particles with diameters ranging from 40–80 nm. The mass spectra obtained for each tobacco evaluated are presented in Figure S15 (a-c), and the one corresponding the reference cigarette in Figure S15d. The upper limit for the m/z range in Figures S15 is restricted by the resolution of the mass spectrometer, as it is difficult to unambiguously assign molecular formulas to peaks above m/z 260. The lower limit of m/z 120 was chosen to show primarily unfragmented species rather than ionic fragments. Most ions smaller than this size can have a major thermal decomposition component (as evidenced by their late evolution in the TDCIMS temperature ramp). Figure 5a represents the resulting Kendrick mass defect plot based on CH2 units derived from the mass spectra obtained for the conventional tobacco mixture compared with the 3R4F reference cigarette sample.
Figure 5.
(a) Kendrick Mass Defect plot based on CH2 units from TDCIMS measurements and (b) Double bond equivalent plot for TDCIMS-detected ions for respectively two characteristic experiments one with the conventional tobacco using the waterpipe, and one experiment using the 3R4F reference cigarette. In (a), formulas are given in their detected ionic form (as an H+ adduct) with the charge symbol dropped to avoid clutter. The orange X markers are data from the tobacco, which had almost no major contributions from N-containing molecules. The + markers are from the cigarette experiment, where grey points are non-N organics and green points are N-containing organics. The symbol sizes are scaled to the relative intensity of the peaks in the mass spectra, and only peaks contributing at least 0.1% to the total signal were included. Vectors indicating shifts of CH2 addition and loss of H2O are indicated with labeled bold arrows. In (b), the lines correspond to the region where cata-condensed (i.e. least compact) PAHs with 0, 1 or 2 heterocyclic nitrogen respectively would appear. Thus, if a molecule falls in the line or is above the line, then it is most likely a condensed-aromatic.
Major observed positive ions can be attributed to sugar or polysacharide derivatives. One of the major peaks observed in the mainstream smoke from the conventional tobacco was at m/z 163.060 corresponding to a chemical composition of [C6H11O5]+ (Figure 5a and S15a), most likely indicating particulate levoglucosan, a major combustion product of cellulose and also likely arising from the sweetener added to the tobacco. This peak is part of a series of dehydrated compounds that arise from simple monosaccharide, C6H12O6 (detected as C6H13O6+, Figure 5a). The tobacco additive glycerol was likely the dominant semivolatile component of the hookah smoke, evidenced by very large signals in the gas phase background, indicating adsorption from the gas phase onto the filament. Glycerol was sometimes detected as a particle phase component, but the strong gas phase background signal made detection equivocal in many cases and for this reason it was excluded from plots. However, glycerol appeared to form a condensation product (C9H18O8) with a C6 monosaccharide in the particle phase, and this product was a major component of the hookah smoke aerosol.
Nicotine (C10H14N2) was also identified in the hookah mainstream particles for both the conventional tobacco and the dark leaf unwashed tobacco, with a protonated molecular ion peak at m/z 163.123 ([C10H15N2]+). The resolving power of the instrument was sufficient to distinguish it from the adjacent levoglucosan [C6H11O5]+ peak t m/z 163.060 (Figure S16). A higher fraction of nicotine was measured in the dark leaf unwashed tobacco (Figure S15c), which is often observed in unwashed dark leaf tobacco products (Salloum et al. 2015). However, the particle phase nicotine fraction of the cigarette smoke was much higher than for any of the tobacco-generated aerosol (Figure S15d). Nicotine was the major identified peak in the cigarette aerosol mass spectrum. Presence of relatively high amount of nicotine in the particle phase by comparison to gas phase (PTR-ToF-MS measurement described above) is consistent with established gas-particle partitioning of nicotine that favored particle phase in cigarette smoke, and its partitioning can be highly influenced by the temperature and RH of the mainstream smoke (John et al. 2018; Pankow 2001; Pankow et al. 2003).
Overall, the hookah smoke aerosol positive ion TDCIMS mass spectra were very simple, largely containing cyclic saccharide-derived polyols, with very little influence of nitrogen-containing species (Figure 5). This suggests that the actual tobacco was not very efficiently aerosolized and that the additives may actually dominate the composition of generated particles. There were, in general, significant gas phase background signals from many compounds, consistent with the view that a large component of the aerosol was semi-volatile and was likely subjected to evaporation and condensation. The observed homogeneous nucleation of new particles in the hookah mainstream smoke is also consistent with vapor condensation playing a major role. In contrast, the cigarette smoke ultrafine aerosol was much more chemically complicated, with a wide range of oxidation states and degrees of aromaticity (Figure 5b). The detected species in the cigarette smoke had lower gas phase backgrounds, suggesting that they were primarily low-volatility species confined to the particles or that the aerosol was more viscous and retained semivolatile species more effectively than the hookah smoke. The cigarette smoke ultrafine aerosol composition was broadly consistent with that for other types of biomass burning aerosol, for example showing homologous series of heterocyclic reduced-nitrogen species, oxygenated aromatics, alkanoic acids, and polycyclic aromatic hydrocarbons (Laskin, Smith and Laskin 2009). The major differences between the hookah smoke and cigarette smoke nanoparticles are likely due to the difference in the combustion and volatilization process, involving much lower temperature for the waterpipe than the cigarette (Shihadeh 2003).
4. Conclusions
The results presented in this study advance our understanding of the properties and potential impacts of hookah mainstream smoke. Major components of the measured hookah smoke included glycerol and its decomposition products, dehydrated sugars, and carbon monoxide. We observed large concentrations of ultrafine particles (<100 nm in diameter), especially during the first 10 min of the smoking session. It is remarkable that the number of ultrafine particles inhaled in a single puff of hookah mainstream smoke is often equivalent to smoking more than one entire cigarette. While the dose-response relationship for the hookah smoke and cigarette smoke are likely to be different (reflecting the different volume of mainstream smoke inhaled by the smoker; 860 mL for the hookah vs 36 mL for the cigarette), the large ultrafine particle number concentration in the former is cause for concern and highlights the potential danger of regular waterpipe smoking.
The chemical composition of the hookah aerosol is also remarkably different from that of a cigarette. The former is dominated by the glycerol and its thermal decomposition products in the gas phase, and by sugar-related molecules in the particle phase. The latter is far more complex reflecting the higher combustion temperature in the cigarette, which generates a huge diversity of toxic compounds, while the low temperature waterpipe process and the practice of adding sugared flavoring and glycerol results in particles that are less concentrated in many toxic compounds. Nevertheless, aside from the significant exposure to carbon monoxide, hookah mainstream smoke is not without health hazard, considering the presence of large amount of glycerol decomposition products in the hookah mainstream smoke, such as acrolein and acetaldehyde, or benzene. Previous studies (Baker and Dixon 2006; Chaouachi 2009; Moldoveannu, Coleman and Wilkins 2007) showed that the respiratory tract absorbs more than 90% of the mainstream smoke inhaled carbonyl compounds, which have been associated with severe irritations and potential risks for cancer (Feng et al. 2006; IARC 1999). We must also point out that our work only describes relative abundances of the measured chemical compounds and does not quantify doses of specific hookah smoke toxic compounds, which may still be present at levels relevant for human health. Ongoing work using biological models will more directly address health outcomes related to waterpipe smoking.
Supplementary Material
Acknowledgements
This work was supported by NIH under Award Number R01ES027232. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge the NSF Major Research Instrumentation (MRI) program (grant number 0923323) for the PTR-ToF-MS.
Footnotes
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Akl EA, Gaddam S, Gunukula SK, Honeine R, Abou Jaoude P, Irani J. 2010. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int. J. Epidemiol 39:834–857. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- Akl EA, Jawad M, Lam WY, Co CN, Obeid R, Irani J. 2013. Motives, beliefs and attitudes towards waterpipe tobacco smoking: a systematic review. Harm Reduct. J 10:19. doi: 10.1186/1477-7517-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rashidi M, Shihadeh A, Saliba NA. 2008. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem. Toxicol 46:3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljarrah K, Ababneh ZQ, Al-Delaimy WK. 2009. Perceptions of hookah smoking harmfulness: predictors and characteristics among current hookah users. Tob. Induc. Dis 5:16–22. doi: 10.1186/1617-9625-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam HM, Saleem S, German S, Qureshi WA. 2014. Harmful effects of shisha: literature review. Int. Arch. Med 7:16–24. doi: 10.1186/1755-7682-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasandorj M, Millet DB, Hu L, Mitroo D, Williams BJ. 2015. Measuring acetic and formic acid by proton-transfer-reaction mass spectrometry: sensitivity, humidity dependence, and quantifying interferences. Atmos. Meas. Tech 8:1303–1321. doi: 10.5194/amt-8-1303-2015. [DOI] [Google Scholar]
- Baker RR and Dixon M. 2006. The retention of tobacco smoke constituents in the human respiratory tract. Inhal. Toxicol 18:255–294. doi: 10.1080/08958370500444163. [DOI] [PubMed] [Google Scholar]
- Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, Okun E, Yadid G, Popovtzer R. 2017. The effect of nanoparticle size on the ability to cross the blood-brain barrier: an in vivo study. Nanomedicine-Uk 12:1533–1546. doi: 10.2217/nnm-2017-0022. [DOI] [PubMed] [Google Scholar]
- Blair SL, Epstein SA, Nizkorodov SA, Staimer N. 2015. A real-time fast-flow tube study of VOC and particulate emissions from electronic, potentially reduced-harm, conventional, and reference cigarettes. Aerosol Sci. Tech 49:816–827. doi: 10.1080/02786826.2015.1076156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiev K, Burseg KMM, O’Connell G, Hartungen E, Biel SS, Cahours X, Colard S, Mark TD, Sulzer P. 2016. An online method for the analysis of volatile organic compounds in electronic cigarette aerosol based on proton transfer reaction mass spectrometry. Rapid Commun. Mass Spectrom 30:691–697. doi: 10.1002/rcm.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Gioli B, Ciccioli P, Zona D, Loreto F, Janssens IA, Ceulemans R. 2014. Proton Transfer Reaction Time-of-Flight Mass Spectrometric (PTR-TOF-MS) determination of volatile organic compounds (VOCs) emitted from a biomass fire developed under stable nocturnal conditions. Atmos. Environ 97:54–67. doi: 10.1016/j.atmosenv.2014.08.007. [DOI] [Google Scholar]
- Brinkman MC, Kim H, Buehler SS, Adetona a. M., Gordon SM, Clark PI. 2018. Evidence of compensation among waterpipe smokers using harm reduction components. Tob. Control: doi: 10.1136/tobaccocontrol-2018-054502. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman MC, Kim H, Chuang JC, Kroeger RR, Deojay D, Clark PI, Gordon SM. 2015. Comparison of true and smoothed puff profile replication on smoking behavior and mainstream smoke emissions. Chem. Res. Toxicol 28:182–190. doi: 10.1021/tx500318h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns EA, Slowik JG, El Haddad I, Kilic D, Klein F, Dommen J, Temime-Roussel B, Marchand N, Baltensperger U, Prevot ASH. 2017. Characterization of gas-phase organics using proton transfer reaction time-of-flight mass spectrometry: fresh and aged residential wood combustion emissions. Atmos. Chem. Phys 17:705–720. doi: 10.5194/acp-17-705-2017. [DOI] [Google Scholar]
- Cavus UY, Rehber ZH, Ozeke O, Ilkay E. 2010. Carbon monoxide poisoning associated with Narghile use. Emerg. Med. J 27:406–406. doi: 10.1136/emj.2009.077214. [DOI] [PubMed] [Google Scholar]
- Chaouachi K 2009. Hookah (shisha, narghile) smoking and environmental tobacco smoke (ETS). A critical review of the relevant literature and the public health consequences. Int. J. Env. Res. Pub. He 6:798–843. doi: 10.3390/ijerph6020798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JC and Watson JG. 2007. Overview of ultrafine particles and human health. WIT Trans. Ecol. Environ 99:619–632. doi: 10.2495/Rav060601. [DOI] [Google Scholar]
- Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. 2010. Waterpipe tobacco smoking: An emerging health crisis in the United States. Am. J. Health Behav 34:275–285. 10.5993/Ajhb.34.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corma A, Huber GW, Sauvanauda L, O’Connor P. 2008. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal 257:163–171. doi: 10.1016/j.jcat.2008.04.016. [DOI] [Google Scholar]
- Dass C 1994. Gas-phase fragmentation reactions of protonated glycerol and its oligomers - Metastable and collision-induced dissociation reactions, associated deuterium-isotope effects and the structure of [C3H5O]+, [C2H5O]+, [C2H4O]+. and [C2H3O]+ ions. Org. Mass Spectrom 29:475–482. doi: 10.1002/oms.1210290906. [DOI] [Google Scholar]
- de Gouw J and Warneke C. 2007. Measurements of volatile organic compounds in the earths atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev 26:223–257. doi: 10.1002/mas.20119. [DOI] [PubMed] [Google Scholar]
- Eichorn L, Michaelis D, Kemmerer M, Juttner B, Tetzlaff K. 2018. Carbon Monoxide poisoning from waterpipe smoking: a retrospective cohort study. Clin. Toxicol 56:264–272. doi. [DOI] [PubMed] [Google Scholar]
- El-Zaatari ZM, Chami HA, Zaatari GS. 2015. Health effects associated with waterpipe smoking. Tob. Control 24:31–43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge A, Betson TR, Gama MV, McAdam K. 2015. Variation in tobacco and mainstream smoke toxicant yields from selected commercial cigarette products. Regul. Toxicol. Pharm 71:409–427. doi: 10.1016/j.yrtph.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Fakhreddine HMB, Kanj AN, Kanj NA. 2014. The growing epidemic of water pipe smoking: Health effects and future needs. Resp. Med 108:1241–1253. doi: 10.1016/j.rmed.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Feng ZH, Hu WW, Hu Y, Tang MS. 2006. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. P. Natl. Acad. Sci. USA 103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick EM, Ross AB, Bates J, Andrews G, Jones JM, Phylaktou H, Pourkashanian M, Williams A. 2007. Emission of oxygenated species from the combustion of pine wood and its relation to soot formation. Process Saf Environ 85:430–440. doi: 10.1205/psep07020. [DOI] [Google Scholar]
- Gordon SM, Brinikman MC, Meng RQ, Anderson GM, Chuang JC, Kroeger RR, Reyes IL, Clark PI. 2011. Effect of cigarette menthol content on mainstream smoke emissions. Chem. Res. Toxicol 24:1744–1753. doi: 10.1021/tx200285s. [DOI] [PubMed] [Google Scholar]
- Greaves J and Roboz J. 2014. Mass spectromtry for the novice: CRC Press, Boca Raton, FL, USA. [Google Scholar]
- Grekin ER and Ayna D. 2008. Argileh use among college students in the United States: An emerging trend. J. Stud. Alcohol Drugs 69:472–475. doi: 10.15288/jsad.2008.69.472. [DOI] [PubMed] [Google Scholar]
- Grekin ER and Ayna D. 2012. Waterpipe smoking among college students in the United States: A review of the literature. J. Am. Coll. Health 60:244–249. doi: 10.1080/07448481.2011.589419. [DOI] [PubMed] [Google Scholar]
- Hammal F, Chappell A, Wild TC, Kindzierski W, Shihadeh A, Vanderhoek A, Huynh CK, Plateel G, Finegan BA. 2015. ‘Herbal’ but potentially hazardous: an analysis of the constituents and smoke emissions of tobacco-free waterpipe products and the air quality in the cafes where they are served. Tob. Control 24:290–297. doi: 10.1136/tobaccocontrol-2013-051169. [DOI] [PubMed] [Google Scholar]
- Hemings EB, Cavallotti C, Cuoci A, Faravelli T, Ranzi E. 2012. A detailed kinetic study of pyrolysis and oxidation of glycerol (propane-1,2,3-triol). Combust. Sci. Technol 184:1164–1178. doi: 10.1080/00102202.2012.664006. [DOI] [Google Scholar]
- Hinds WC 1999. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd Edition: Wiley. [Google Scholar]
- Hogan CJ, Kettleson EM, Lee MH, Ramaswami B, Angenent LT, Biswas P. 2005. Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. J. Appl. Microbiol 99:1422–1434. doi: 10.1111/j.1365-2672.2005.02720.x. [DOI] [PubMed] [Google Scholar]
- IARC. 1999. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans - Re-Evaluation of some Organic Chemicals, Hydrazine and Hydrogen Peroxide: IARC Press. [PMC free article] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. 2012. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol 24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Jamal A, Getzke A, Hu SS. 2017. Tobacco use among middle and high school students - United States, 2011–2016. MMWR Morb. Mortal. Wkly Rep 66:597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M, Eissenberg T, Salman R, Soule E, Alzoubi KH, Khabour O. f., Karaoghlanian N, Baalbaki R, El Hage R, Saliba N, Shihadeh A. 2019. Toxicant inhalation among singleton waterpipe tobacco users in natural settings. Tob. Control 28:181–188. doi: 10.1136/tobaccocontrol-2017-054230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Strongin RM, Peyton DH. 2017. Solvent chemistry in the electronic cigarette reaction vessel. Sci Rep-Uk 7. doi: ARTN 42549 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John E, Coburn S, Liu C, McAughey J, Mariner D, McAdam KG, Sebestyen Z, Bakos I, Dobe S. 2018. Effect of temperature and humidity on the gas-particle partitioning of nicotine in mainstream cigarette smoke: A diffusion denuder study. J. Aerosol Sci 117:100–117. doi: 10.1016/j.jaerosci.2017.12.015. [DOI] [Google Scholar]
- Jordan A, Haidacher S, Hanel G, Hartungen E, Mark L, Seehauser H, Schottkowsky R, Sulzer P, Mark TD. 2009. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass spectrom 286:122–128. doi: 10.1016/j.ijms.2009.07.005. [DOI] [Google Scholar]
- Karl TG, C. TJ, Yokelson RJ, Artaxo P, Hao WH, Guenther A. 2007. The tropical forest and fire emissions experiment: method evaluation of volatile organic compound emissions measured by PTR-MS, FTIR, and GC from tropical biomass burning. Atmos. Chem. Phys 7:5883–5897. doi: 10.5194/acp-7-5883-2007. [DOI] [Google Scholar]
- Katryniok B, Paul S, Belliere-Baca V, Rey P, Dumeignil F. 2010. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem 12:2079–2098. doi: 10.1039/c0gc00307g. [DOI] [Google Scholar]
- Katurji M, Daher N, Sheheitli H, Saleh R, Shihadeh A. 2010. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhal. Toxicol 22:1101–1109. doi: 10.3109/08958378.2010.524265. [DOI] [PubMed] [Google Scholar]
- Kim H, Brinkman MC, Sharma E, Gordon SM, Clark PI. 2016. Variability in puff topography and exhaled CO in waterpipe tobacco smoking. Tobacco Regulatory Science 2:301–308. doi: 10.18001/TRS.2.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kabir E, Jahan SA. 2016. Waterpipe tobacco smoking and its human health impacts. J. Hazard. Mater 317:229–236. doi: 10.1016/j.jhazmat.2016.05.075. [DOI] [PubMed] [Google Scholar]
- La Fauci G, Weiser G, Steiner IP, Shavit I. 2012. Carbon monoxide poisoning in narghile (waterpipe) tobacco smokers. Can. J. Emerg. Med 14:57–59. doi: 10.2310/8000.2011.110431. [DOI] [PubMed] [Google Scholar]
- Laskin A, Smith JS, Laskin J. 2009. Molecular characterization of nitrogen-containing organic compounds in biomass burning aerosols using high-resolution mass spectrometry. Environ. Sci. Technol 43:3764–3771. doi: 10.1021/es803456n. [DOI] [PubMed] [Google Scholar]
- Lawler MJ, Rissanen MP, Ehn M, Mauldin RL, Sarnela N, Sipila M, Smith JN. 2018. Evidence for diverse biogeochemical drivers of boreal forest new particle formation. Geophys. Res. Lett 45:2038–2046. doi: 10.1002/2017gl076394. [DOI] [Google Scholar]
- Lim BL, Lim GH, Seow E. 2009. Case of carbon monoxide poisoning after smoking shisha. Int. J. Emerg. Med 2:121–122. doi: 10.1007/s12245-009-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinasek MP, McDermott RJ, Martini L. 2011. Waterpipe (hookah) tobacco smoking among youth. Curr. Prob. Pediatr. Ad 41:34–57. doi: 10.1016/j.cppeds.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Martinuzzi I, Azizi Y, Devaux JF, Tretjak S, Zahraa O, Leclerc JP. 2014. Reaction mechanism for glycerol dehydration in the gas phase over a solid acid catalyst determined with on-line gas chromatography. Chem. Eng. Sci 116:118–127. doi: 10.1016/j.ces.2014.04.030. [DOI] [Google Scholar]
- Maziak W 2011. The global epidemic of waterpipe smoking. Addictive Behaviors 36:1–5. doi: 10.1016/j.addbeh.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Rastam S, Ibrahim I, Ward KD, Shihadeh A, Eissenberg T. 2009. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob. Res 11:806–811. doi: 10.1093/ntr/ntp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoviny T, Kaser L, Wisthaler A. 2010. Development and characterization of a high-temperature proton-transfer-reaction mass spectrometer (HT-PTR-MS). Atmos. Meas. Tech 3:537–544. doi: 10.5194/amt-3-537-2010. [DOI] [Google Scholar]
- Moldoveannu S, Coleman WI, Wilkins J. 2007. Determination of carbonyl compounds in exhaled cigarette smoke. Contrib. Tob. Res 22:346–357. doi: 10.2478/cttr-2013-0841. [DOI] [Google Scholar]
- Monn C, Kindler P, Meile A, Brandli O. 2007. Ultrafine particle emissions from waterpipes. Tob. Control 16:390–393. doi: 10.1136/tc.2007.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. 2008. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem. Toxicol 46:2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Muller M, Anderson BE, Beyersdorf AJ, Crawford JH, Diskin GS, Eichler P, Fried A, Keutsch FN, Mikoviny T, Thornhill KL, Walega JG, Weinheimer AJ, Yang M, Yokelson RJ, Wisthaler A. 2016. In situ measurements and modeling of reactive trace gases in a small biomass burning plume. Atmos. Chem. Phys 16:3813–3824. doi: 10.5194/acp-16-3813-2016. [DOI] [Google Scholar]
- Nimlos MR, Blanksby SJ, Qian XH, Himmel ME, Johnson DK. 2006. Mechanisms of glycerol dehydration. J. Phys. Chem. A 110:6145–6156. doi: 10.1021/jp060597q. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. 2004. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol 16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oladhosseini S and Karimi G. 2016. Mathematical modeling of transport phenomena during waterpipe smoking-a parametric study. Int. J. Multiphase Flow 85:314–325. doi: 10.1016/j.ijmultiphaseflow.2016.06.022. [DOI] [Google Scholar]
- Pagonis D, Krechmer JE, de Gouw J, Jimenez JL, Ziemann PJ. 2017. Effect of gas-wall partitioning in Teflon tubing and instrumentation on time-resolved measurements of gas-phase organic compounds. Atmos. Meas. Tech 10:4687–4696. doi: 10.5194/amt-10-4687-2017. [DOI] [Google Scholar]
- Pankow JF 2001. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol 14:1465–1481. doi: 10.1021/tx0100901. [DOI] [PubMed] [Google Scholar]
- Pankow JF, Tavakoli AD, Luo WT, Isabelle LM. 2003. Percent free base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes. Chem. Res. Toxicol 16:1014–1018. doi: 10.1021/tx0340596. [DOI] [PubMed] [Google Scholar]
- Primack BA, Sidani J, Agarwal AA, Shadel WG, Donny EC, Eissenberg TE. 2008. Prevalence of and associations with waterpipe tobacco smoking among US university students. Ann. Behav. Med 36:81–86. doi: 10.1007/s12160-008-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey CL, Shifflett JR, Goodpaster JV, Bezabeh DZ. 2013. Quantitative analysis of humectants in tobacco products using gas chromatography (GC) with simultaneous mass spectrometry (MSD) and flame ionization detection (FID). Contrib. Tob. Res 25:576–585. doi: 10.2478/cttr-2013-0934. [DOI] [Google Scholar]
- Retzky S 2017. Carbon monoxide poisoning from hookah smoking: an emerging public health problem. J. Med. Toxicol 13:193–194. doi: 10.1007/s13181-017-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk-Hanna M and Benowitz NL. 2018. Cardiovascular effects of hookah smoking: potential implications for cardiovascular risk. Nicotine Tob. Res 00:1–11. doi: 10.1093/ntr/nty065. [DOI] [PubMed] [Google Scholar]
- Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, Berges A, Stueber M, Muench M, Trelles-Sticken E, Pype J, Kohlgrueber K, Voelkel H, Wittke S. 2012. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 3R4F. Beitr. Tabakforsch. Int 25:316–335. doi: 10.2478/cttr-2013-0912. [DOI] [Google Scholar]
- Saleh R and Shihadeh A. 2008. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food Chem. Toxicol 46:1461–1466. doi: 10.1016/j.fct.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Salloum RG, Maziak W, Hammond D, Nakkash R, Islam F, Cheng X, Thrasher JF. 2015. Eliciting preferences for waterpipe tobacco smoking using a discrete choice experiment: implications for product regulation. Bmj Open 5:1–7. doi: ARTN e009497 10.1136/bmjopen-2015-009497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MM, Chambers DM, Pazo DY, Moliere F, Blount BC, Watson CH. 2014. Simultaneous analysis of 22 volatile organic compounds in cigarette smoke using gas sampling bags for high-throughput solid-phase microextraction. Anal. Chem 86:7088–7095. doi: 10.1021/ac5015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander R 2015. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys 15:4399–4981. doi: 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- Schubert J, Bewersdorff J, Luch A, Schulz TG. 2012a. Waterpipe smoke: A considerable source of human exposure against furanic compounds. Anal. Chim. Acta 709:105–112. doi: 10.1016/j.aca.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz TG. 2011a. Mainstream smoke of the waterpipe: Does this environmental matrix reveal as significant source of toxic compounds? Toxicol. Lett 205:279–284. doi: 10.1016/j.toxlet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Schubert J, Heinke V, Bewersdorff J, Luch A, Schulz TG. 2012b. Waterpipe smoking: the role of humectants in the release of toxic carbonyls. Arch. Toxicol 86:1309–1316. doi: 10.1007/s00204-012-0884-5. [DOI] [PubMed] [Google Scholar]
- Schubert J, Kappenstein O, Luch A, Schulz TG. 2011b. Analysis of primary aromatic amines in the mainstream waterpipe smoke using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 1218:5628–5637. doi: 10.1016/j.chroma.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Schubert J, Muller FD, Schmidt R, Luch A, Schulz TG. 2015. Waterpipe smoke: source of toxic and carcinogenic VOCs, phenols and heavy metals? Arch. Toxicol 89:2129–2139. doi: 10.1007/s00204-014-1372-x. [DOI] [PubMed] [Google Scholar]
- Sepetdjian E, Halim RA, Salman R, Jaroudi E, Shihadeh A, Saliba NA. 2013. Phenolic compounds in particles of mainstream waterpipe smoke. Nicotine Tob. Res 15:1107–1112. doi: 10.1093/ntr/nts255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepetdjian E, Shihadeh A, Saliba NA. 2008. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem. Toxicol 46:1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Shihadeh A 2003. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem. Toxicol 41:143–152. doi: 10.1016/S0278-6915(02)00220-X. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonios C, Haddad A. 2004. Towards a topographical model of narghile water-pipe cafe smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol. Biochem. Behav 79:75–82. doi: 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Eissenberg T, Rammah M, Salman R, Jaroudi E, El-Sabban M. 2014. Comparison of tobacco-containing and tobacco-free waterpipe products: Effects on human alveolar cells. Nicotine Tob. Res 16:496–499. doi: 10.1093/ntr/ntt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A and Saleh R. 2005. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem. Toxicol 43:655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Salman R, Jaroudi E, Saliba N, Sepetdjian E, Blank MD, Cobb CO, Eissenberg T. 2012. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “tar” and nicotine yields. Food Chem. Toxicol 50:1494–1498. doi: 10.1016/j.fct.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA. 2015. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob. Control 24:22–30. doi: 10.1136/tobaccocontrol-2014-051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Moore KF, McMurry PH, Eisele FL. 2004. Atmospheric measurements of sub-20 nm diameter particle chemical composition by thermal desorption chemical ionization mass spectrometry. Aerosol Sci. Tech 38:100–110. doi: 10.1080/02786820490249036. [DOI] [Google Scholar]
- Smith JR, Edland SD, Novotny TE, Hofstetter CR, White MM, Lindsay SP, Al-Delaimy WK. 2011. Increasing hookah use in California. Am. J. Public Health 101:1876–1879. doi: 10.2105/Ajph.2011.300196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanel P and Smith D. 1997. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of alcohols. Int. J. Mass spectrom 167:375–388. doi: 10.1016/S0168-1176(97)00085-2. [DOI] [Google Scholar]
- Spanne M, Grzybowski P, Bohgard M. 1999. Collection efficiency for submicron particles of a commonly used impinger. Am. Ind. Hyg. Assoc. J 60:540–544. doi: . [DOI] [Google Scholar]
- Stockwell CE, Veres PR, Williams J, Yokelson RJ. 2015. Characterization of biomass burning emissions from cooking fires, peat, crop residue, and other fuels with high-resolution proton-transfer-reaction time-of-flight mass spectrometry. Atmos. Chem. Phys 15:845–865. doi: 10.5194/acp-15-845-2015. [DOI] [Google Scholar]
- Sutfin EL, Song EY, Reboussin BA, Wolfson M. 2014. What are young adults smoking in their hookahs? A latent class analysis of subtances smoked. Addictive behaviors 39:1191–1196. doi: 10.1016/j.addbeh.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen M 2016. Carbon monoxide poisoning caused by waterpipe smoking: A case series. J Emerg Med 51:E41–E44. doi: 10.1016/j.jemermed.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Warneke C, Roberts JM, Veres P, Gilman J, Kuster WC, Burling I, Yokelson RJ, de Gouw J. 2011. VOC identification and inter-comparison from laboratory biomass burning using PTR-MS and PIT-MS. Int. J. Mass spectrom 303:6–14. doi: 10.1016/j.ijms.2010.12.002. [DOI] [Google Scholar]
- Wei ZC, Rosario RC, Montoya LD. 2010. Collection efficiency of a midget impinger for nanoparticles in the range of 3–100 nm. Atmos. Environ 44:872–876. doi: 10.1016/j.atmosenv.2009.11.037. [DOI] [Google Scholar]
- World Health Organization. 2015. Advisory Note - Waterpipe tobacco smoking: health effects, resaerch needs and recommended actions for regulators: WHO document Production services, Geneva, switzerland. [Google Scholar]
- Yuan B, Koss AR, Warneke C, Coggon M, Sekimoto K, de Gouw JA. 2017. Proton-transfer-reaction mass spectrometry: Applications in atmospheric sciences. Chem. Rev 117:13187–13229. doi: 10.1021/acs.chemrev.7b00325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.