Abstract
Background
There is limited information describing the characteristics and outcomes of hospitalized older patients with confirmed coronavirus disease 2019 (COVID-19).
Method
We conducted a multicentric retrospective cohort study in 13 acute COVID-19 geriatric wards, from March 13 to April 15, 2020, in Paris area. All consecutive patients aged 70 years and older, with confirmed COVID-19, were enrolled.
Results
Of the 821 patients included in the study, the mean (SD) age was 86 (7) years; 58% were female; 85% had ≥2 comorbidities; 29% lived in an institution; and the median [interquartile range] Activities of Daily Living scale (ADL) score was 4 [2–6]. The most common symptoms at COVID-19 onset were asthenia (63%), fever (55%), dyspnea (45%), dry cough (45%), and delirium (25%). The in-hospital mortality was 31% (95% confidence interval [CI] 27–33). On multivariate analysis, at COVID-19 onset, the probability of in-hospital mortality was increased with male gender (odds ratio [OR] 1.85; 95% CI 1.30–2.63), ADL score <4 (OR 1.84; 95% CI 1.25–2.70), asthenia (OR 1.59; 95% CI 1.08–2.32), quick Sequential Organ Failure Assessment score ≥2 (OR 2.63; 95% CI 1.64–4.22), and specific COVID-19 anomalies on chest computerized tomography (OR 2.60; 95% CI 1.07–6.46).
Conclusions
This study provides new information about older patients with COVID-19 who are hospitalized. A quick bedside evaluation at admission of sex, functional status, systolic arterial pressure, consciousness, respiratory rate, and asthenia can identify older patients at risk of unfavorable outcomes.
Keywords: COVID-19, Geriatrics, In-hospital mortality
On January 30, 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19), a public health emergency of international concern (1). This outbreak began in Wuhan (China) in December 2019, and has since spread worldwide (2). As of July 2, 2020, there had been 10 533 779 confirmed cases and 512 842 deaths worldwide (3).
The SARS-Cov-2 virus affects all age groups, particularly adults (4–8). Clinical presentation varies widely among individuals, with most (81%) showing a mild form and a minority (19%) presenting severe and critical forms (6). The mortality rate has been reported to be greater in patients with comorbidities, particularly diabetes, hypertension, obesity, and cardiovascular disease, and in older patients (5–10). Wu and McGoogan reported a case-fatality rate of 2.3% in all confirmed cases, 8% in patients aged 70–79 years, and 14.8% in those aged 80 years and older (6).
Although age has been widely reported as a major risk factor for severe COVID-19 and death, no large geriatric cohort of older patients hospitalized in a geriatric ward has been described. Only 2 cohort studies of hospitalized patients, defined as geriatric, have been published but they included patients younger than 70 years and with few very old patients (11,12). More than half of these patients were still hospitalized at the time of the analysis, which could bias the results and conclusions.
Yet, questions arise about this frail population, who is hospitalized in geriatric wards, with limited access to intensive care units (ICUs). The objective of this multicentric French retrospective study was to describe the clinical characteristics and outcomes of a large cohort of older patients with confirmed COVID-19 who were admitted to acute care geriatric wards and to determine the prognostic factors of in-hospital mortality.
Materials and Methods
This multicentric retrospective cohort study was approved by the COVID-19 Assistance Publique-Hôpitaux de Paris (APHP) research committee on April 17, 2020 and by the institutional ethics board of Sorbonne University on May 11, 2020 (no. 2020-CER-2020-43). All included patients or their legal representative gave their consent for use of their medical data collected during their hospital stay. This report follows the STROBE recommendations (Supplementary Method 1) (13).
Study Design, Setting, and Participants
This study was conducted in 13 acute COVID-19 geriatric wards in Paris area. All physicians in these wards were geriatricians. A bi-weekly telephone meeting took place since the opening of the first COVID-19 geriatric ward until May 6, 2020, to share standardized medical practices, experiences, and the cohort project (14). Patient care was left to the discretion of the referring physicians.
All consecutive patients aged 70 years and older, with confirmed COVID-19 and admitted to one of these geriatric wards from March 13 to April 15, 2020, were enrolled. Patients without social security or who refused the use of their medical data were excluded. COVID-19 was diagnosed by real-time reverse polymerase chain reaction (RT-PCR) for SARS-CoV-2 or chest computerized tomography (CT), according to WHO interim guidance (15). The clinical outcomes (ie, discharge, in-hospital mortality, length of stay) were monitored up to May 7, 2020, the final date of follow-up (discharge of the last patient included). We had no missing data on death or destination at discharge.
Data Collection Methods and Data Management
Patients’ medical records were carefully reviewed and analyzed by 2 trained physicians per geriatric ward. The physicians reviewed clinical charts, nursing records, laboratory findings, chest X-rays or chest CTs, and treatments for all enrolled patients by using a standardized electronic data collection form. Information recorded included baseline characteristics before COVID-19: age, gender, home or nursing home residence, previous medical history, and chronic medications. Comorbidity severity was assessed with the Charlson index (16), frailty was assessed with the adjusted Rockwood score (17), and functional status was assessed with the Activities of Daily Living (ADL) scale (6 basic human functions: bathing, dressing, toileting, transfer, continence, and feeding; 1 point for each function (18)). From these data and the wishes of patients and families, advance care planning and decisions about do not resuscitate were systematically made on admission to geriatric wards by multidisciplinary consultation (geriatricians, intensivists).
The date of COVID-19 onset was defined as the day when the first symptoms were noticed; symptoms were fever, hypotension, fatigue, dyspnea, respiratory rate, dry cough, chest pain, myalgia, pharyngalgia, diarrhea, nausea, vomiting, abdominal pain, dizziness, delirium according to the Confusion Assessment Method (19), headache, anosmia, and ageusia. A quick Sequential Organ Failure Assessment (qSOFA) score (range 0–3, with 1 point each for systolic hypotension [≤100 mm Hg], tachypnea [≥22/min], or altered consciousness [Glasgow Coma Score <14]) was retrospectively calculated for all patients (20). Its use is simple (bedside clinical score) and its predictive validity for in-hospital mortality has been validated among encounters with suspected infection outside of the ICU (20).
The interval from symptom onset to admission in a geriatric ward and length of hospital stay were also recorded. Presumed hospital-related disease transmission (nosocomial COVID-19) was suspected if a cluster of medical professionals or hospitalized patients in the same wards became infected in a certain time period (≤14 days). We recorded the date of the initial chest CT and its results (specific COVID-19 anomalies: yes or no) and the date of the initial nasopharyngeal swab tested by RT-PCR.
During the hospitalization, all complications (respiratory rate ≥22/min, dyspnea, thromboembolic events, hemorrhagic events, acute cardiac injury, acute atrial fibrillation, stroke, acute kidney injury [AKI], delirium, secondary infection, stool impaction, urinary retention requiring drainage, pressure sore, admission to an ICU, and deaths) were recorded (with their start date). Acute kidney injury was identified according to the Kidney Disease Improving Global Outcomes (KDIGO) definition (21). Cardiac injury was diagnosed with serum level of troponin above the 99th percentile upper reference limit or new abnormalities observed on electrocardiography and echocardiography routinely performed (7).
Only routine blood tests were performed and results were recorded, including complete blood count, renal and liver function, C-reactive protein (CRP), and albumin levels.
Data were collected on specific treatments (ie, antiviral therapy, corticosteroid therapy, hydroxychloroquine, and anticytokine or immunomodulatory agents), respiratory support, hydration, blood transfusion, antibiotic therapy, proton pump inhibitor, prophylactic anticoagulation, and midazolam and morphine therapies.
Finally, the status (alive or dead) and the destination at discharge (home, including nursing home, rehabilitation center, or other, including palliative care center) were also recorded.
Statistical Analysis
The statistical plan of the study was established before the statistical analysis (Supplementary Method 2). The study was based on all available patients during the study period and thus no a priori power calculation was conducted.
Data are presented as mean (SD) or median (interquartile range [IQR]) for continuous variables and number (percentage) for categorical variables. Normality was assessed with the Kolmogorov–Smirnov test and a graphical representation of the distribution. Mann–Whitney test was used for continuous variables and chi-squared test or Fisher’s exact test for categorical variables.
A logistic mixed model with a center effect as a random effect was performed to assess independent variables, present at COVID-19 onset, associated with in-hospital mortality, and adjusted odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated. To avoid overestimation, a conservative approach was used: all variables with p <.10 on univariate analysis and all clinically relevant variables from the literature were included. Correlation between variables was assessed by a focused principal analysis. The choice between 2 correlated variables was based on their respective clinical relevance. Continuous variables were dichotomized by receiver operating characteristic curve analysis to determine the best threshold (maximization of the Youden index) for multivariable analysis.
Missing data were not imputed. We assessed missing values and their distribution in the dead and alive groups (Supplementary Method 3). Because missing values represented <3% of the data and were balanced between the 2 groups, no specific treatment strategy was necessary.
All tests were 2-sided, and p <.05 was considered statistically significant. Statistical analyses involved using R v4.0.0.
Results
Demographic Data and Patient Baseline Characteristics
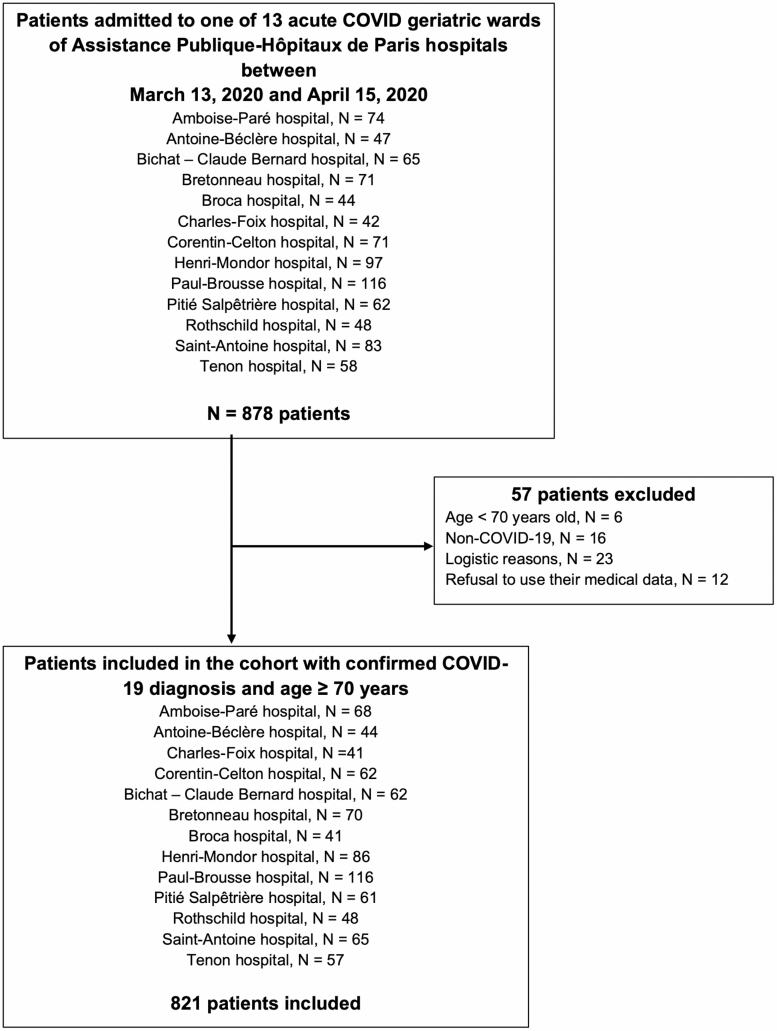
During the study period, 821 admissions for confirmed COVID-19 in people 70 years and older were recorded in the 13 geriatric COVID wards of APHP (Figure 1). COVID-19 was diagnosed by RT-PCR in 758 (92%) patients and chest CT in 63 (8%) (Table 1) with a median interval from symptom onset to admission in a geriatric ward at 4 [1–8] days.
Figure 1.
Study flow chart.
Table 1.
Demographic Data and Baseline Characteristics of Older Patients With COVID-19 Overall and Stratified by Living Status at the End of Hospitalization (N = 821)
| Characteristics | Total N = 821 | Dead n = 250 | Alive n = 571 | Missing Values | p Value |
|---|---|---|---|---|---|
| Age, mean (SD), years, n (%) | 86 (7) | 87 (7) | 86 (7) | 0 | .51 |
| 70–79 | 160 (19) | 48 (19) | 112 (20) | ||
| 80–85 | 229 (28) | 63 (30) | 166 (29) | ||
| 86–90 | 218 (27) | 66 (27) | 152 (27) | ||
| >90 | 214 (26) | 73 (25) | 141 (25) | ||
| Female sex, n (%) | 473 (58) | 126 (50) | 347 (61) | 0 | .004 |
| Comorbidities, n (%) | |||||
| Dementia | 443 (54) | 129 (52) | 314 (55) | 2 | .40 |
| Depression | 241 (29) | 68 (27) | 173 (30) | 1 | .38 |
| Stroke | 180 (22) | 60 (24) | 120 (21) | 0 | .30 |
| Parkinson disease | 42 (5) | 14 (6) | 28 (5) | 2 | .64 |
| Hypertension | 547 (67) | 160 (64) | 387 (68) | 0 | .27 |
| Diabetes | 204 (25) | 57 (23) | 147 (26) | 2 | .41 |
| Atrial fibrillation | 247 (30) | 82 (33) | 165 (29) | 0 | .23 |
| Obesity | 83 (10) | 29 (12) | 54 (10) | 8 | .29 |
| Coronary artery disease | 181 (22) | 54 (22) | 127 (22) | 0 | .95 |
| Congestive heart failure | 188 (23) | 67 (27) | 121 (21) | 0 | .06 |
| Chronic renal failure | 297 (36) | 98 (39) | 199 (35) | 1 | .25 |
| Thromboembolic disease | 82 (10) | 24 (10) | 58 (10) | 4 | .79 |
| Cancer | 87 (11) | 31 (12) | 56 (10) | 3 | .24 |
| COPD | 96 (12) | 24 (10) | 72 (13) | 3 | .26 |
| Gastroduodenal ulcer | 62 (8) | 20 (8) | 42 (7) | 2 | .70 |
| Adjusted Charlson index, median [IQR] | 7 [6–8] | 7 [6–9] | 7 [6–8] | 12 | .28 |
| Comedications, n (%) | |||||
| Anticoagulants | 211 (26) | 68 (27) | 143 (25) | 2 | .46 |
| Antiplatelets | 273 (33) | 81 (32) | 192 (34) | 2 | .75 |
| ACE inhibitors | 151 (18) | 41 (16) | 110 (19) | 2 | .37 |
| ARBs | 157 (19) | 46 (18) | 111 (19) | 4 | .69 |
| Beta-blockers | 278 (34) | 86 (34) | 192 (34) | 2 | .70 |
| Proton pump inhibitors | 296 (36) | 101 (40) | 195 (34) | 2 | .07 |
| Benzodiazepine | 197 (24) | 53 (21) | 144 (25) | 24 | .18 |
| Neuroleptics | 92 (11) | 26 (10) | 66 (12) | 3 | .65 |
| Number of drugs, median [IQR] | 6 [4–9] | 6 [4–8] | 6 [4–9] | 3 | .40 |
| Autonomy and frailty | |||||
| Living in nursing home, n (%) | 239 (29) | 83 (33) | 156 (27) | 4 | .09 |
| ADL, median [IQR] | 4 [2–6] | 3 [1–6] | 4.5 [2–6] | 70 | .003 |
| Rockwood, median [IQR] | 6 [4–6] | 6 [4–7] | 5 [4–6] | 32 | .005 |
| Symptoms at onset, n (%) | |||||
| Fever | 452 (55) | 150 (60) | 302 (53) | 22 | .008 |
| Hypotension | 118 (14) | 39 (16) | 79 (14) | 7 | .44 |
| Asthenia | 518 (63) | 174 (70) | 344 (60) | 14 | .007 |
| Dyspnea | 368 (45) | 155 (62) | 213 (37) | 1 | <.001 |
| Dry cough | 367 (45) | 111 (44) | 256 (45) | 3 | .84 |
| Delirium | 205 (25) | 81 (32) | 124 (22) | 7 | <.001 |
| Chest pain | 36 (4) | 10 (4) | 26 (5) | 1 | .69 |
| Myalgia | 97 (12) | 27 (11) | 70 (12) | 80 | .62 |
| Pharyngalgia | 20 (2) | 7 (3) | 13 (2) | 110 | .58 |
| Diarrhea | 120 (15) | 34 (14) | 86 (15) | 0 | .66 |
| Nausea/vomiting | 61 (7) | 8 (3) | 53 (9) | 1 | .003 |
| Abdominal pain | 67 (8) | 23 (9) | 44 (8) | 3 | .43 |
| Headache | 33 (4) | 5 (2) | 28 (5) | 51 | .04 |
| Anosmia | 31 (4) | 10 (4) | 21 (4) | 151 | .85 |
| Ageusia | 34 (4) | 11 (4) | 23 (4) | 154 | .84 |
| qSOFA score ≥ 2 | 107 (13) | 55 (22) | 52 (9) | 14 | <.001 |
| Nosocomial COVID-19a, n (%) | 430 (52) | 115 (46) | 315 (55) | 0 | .02 |
| RT-PCR positive, n (%) | 758 (92) | 233 (93) | 525 (92) | 15 | 0.79 |
| Chest CT, n (%) | |||||
| COVID-19 anomalies | 464 (56) | 140 (56) | 324 (57) | 0 | .007 |
| No COVID-19 anomalies | 55 (7) | 7 (3) | 48 (8) | ||
| No chest CT | 302 (37) | 103 (41) | 199 (35) | ||
| Median interval from symptom onset to admission in a geriatric ward [IQR], days | 4 [1–8] | 4 [1–7] | 4 [1–8] | 56 | .78 |
Notes: ACE = angiotensin-converting enzyme; ADL = activities of daily living; ARBs = angiotensin II receptor blockers; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; CT = computed tomography; qSOFA = quick Sequential Organ Failure Assessment; RT-PCR = reverse transcriptase–polymerase chain reaction. Data are mean (SD), median (interquartile range [IQR]), or number (percentage). Comparison between the 2 groups by Mann–Whitney U test for quantitative variables and chi-squared test or Fisher’s exact test for qualitative variables.
aHospital-related disease transmission.
The mean (SD) age was 86 (7) years; 473 (58%) were female; 699 (85%) had ≥2 comorbidities; 544 (66%) had ≥5 daily medications; and the median [IQR] ADL score was 4 [2–6] (Table 1). All demographic and patient baseline characteristics are described in Table 1.
Clinical, Biological, and Imaging Features
The most common symptoms at COVID-19 onset were asthenia (n = 518, 63%), fever (n = 452, 55%), dyspnea (n = 368, 45%), dry cough (n = 367, 45%), and delirium (n = 205, 25%) (Table 1). All other symptoms at COVID-19 onset are described in Table 1. A significant proportion of patients had severe disease on admission, with qSOFA score ≥2 for 107 (13%).
Before admission, 519 (63%) patients had had a chest CT. Specific COVID-19 anomalies were found in 464 patients who had a CT scan (89%): 140 in the group of patients who died during hospitalization (95%) and 372 in the group of patients discharged alive from hospital (87%), p = .007 (Table 1).
The most common laboratory anomalies during the acute stay were lymphocytopenia (lymphocyte count < 1.5 × 109/L, n = 563, 68%), high CRP level (CRP ≥ 6 mg/L, n = 761, 93%), AKI, and cytolysis (Table 2).
Table 2.
Evolution and Complications During the Acute Care Stay of 821 Hospitalized Older Patients With COVID-19 Overall and by Living Status at the End of Hospitalization
| Evolution and Complications | Total N = 821 | Dead N = 250 | Alive N = 571 | Missing Values | p Value |
|---|---|---|---|---|---|
| Complications, n (%) | |||||
| Heart failure | 113 (14) | 43 (17) | 70 (12) | 3 | .04 |
| Chest pain | 39 (5) | 11 (4) | 28 (5) | 1 | .66 |
| Arrhythmia | 49 (7) | 16 (6) | 33 (6) | 2 | .66 |
| Dyspnea | 441 (54) | 210 (84) | 231 (40) | 6 | <.001 |
| AKIa | 321 (39) | 110 (44) | 211 (37) | 101 | .002 |
| Deliriuma | 330 (40) | 200 (80) | 130 (23) | 4 | <.001 |
| Stroke | 3 (0.4) | 0 (0) | 3 (0.5) | 5 | <.001 |
| Thromboembolic event | 25 (3) | 5 (2) | 20 (4) | 7 | .28 |
| Hemorrhagic event | 34 (4) | 15 (6) | 19 (3) | 5 | .09 |
| Fever | 704 (86) | 233 (93) | 471 (82) | 35 | <.001 |
| Diarrhea | 136 (17) | 26 (10) | 110 (19) | 6 | .003 |
| Asthenia | 490 (60) | 172 (69) | 318 (56) | 22 | <.001 |
| Urinary retention | 76 (9) | 24 (10) | 52 (9) | 5 | .78 |
| Pressure sore | 77 (9) | 27 (11) | 50 (9) | 5 | .36 |
| qSOFA score ≥ 2 | 452 (55) | 205 (82) | 247 (43) | 60 | <.001 |
| Admission to ICU | 14 (2) | 5 (2) | 9 (2) | 0 | .670 |
| Palliative care decision | 205 (25) | 183 (73) | 22 (4) | 0 | <.001 |
| Laboratory findings | |||||
| WBC count, ×109/L | 9.2 [6–12] | 9.5 [7–13] | 8.0 [5.8–11] | 12 | <.001 |
| Lymphocyte count, ×109/L | 0.9 [0.6–1.7] | 0.7 [0.4–1.6] | 0.9 [0.6–1.7] | 24 | <.001 |
| Platelet count, ×109/L | 207 [146–293] | 197 [131–323] | 208 [151–289] | 17 | .27 |
| Creatinine, μmol/L | 91 [71–133] | 116 [82–170] | 84.5 [67–119] | 15 | <.001 |
| Albumin, g/L | 30 [27–34] | 29 [24–32] | 31 [27–34] | 174 | <.001 |
| Cytolyse: yes, n (%) | 225 (27) | 90 (36) | 135 (24) | 79 | <.001 |
| Cholestasis: yes, n (%) | 183 (22) | 61 (24) | 122 (21) | 79 | .09 |
| CRP, mg/L | 113 [54–190] | 189 [106–272] | 86 [38–152] | 39 | <.001 |
| Specific treatment, n (%) | |||||
| Antiviral therapy | 24 (3) | 7 (3) | 17 (3) | 0 | .93 |
| Corticosteroid therapy | 119 (14) | 50 (20) | 69 (12) | 0 | .003 |
| Hydroxychloroquine | 50 (6) | 17 (7) | 33 (6) | 0 | .53 |
| Immunomodulatory or anticytokine or agents | 26 (3) | 10 (4) | 16 (3) | 3 | .35 |
| Symptomatic treatment, n (%) | |||||
| Oxygen ≥ 6 L | 294 (36) | 182 (73) | 112 (20) | 0 | <.001 |
| Non-invasive ventilation | 23 (3) | 11 (4) | 12 (2) | 2 | .06 |
| Antibiotics | 583 (71) | 201 (80) | 382 (67) | 0 | <.001 |
| Proton pump inhibitor | 461 (56) | 113 (45) | 348 (61) | 1 | <.001 |
| Prophylactic anticoagulation | 529 (65) | 148 (59) | 381 (67) | 3 | .02 |
| Blood transfusion | 28 (3) | 8 (3) | 20 (4) | 3 | .88 |
| Midazolam | 253 (31) | 202 (81) | 51 (9) | 2 | <.001 |
| Morphine | 338 (41) | 229 (92) | 109 (19) | 0 | <.001 |
Notes: AKI = acute kidney injury; CRP = C-reactive protein; ICU = intensive care unit; qSOFA = quick Sequential Organ Failure Assessment; WBC = white blood cell. Cytolyse: liver transaminases (aspartate aminotransferase or alanine aminotransferase) ≥2 times normal level. Cholestase: alkaline phosphatase or gamma-GT ≥2 times normal level. Data are median [IQR] or number (percentage). Comparison between the 2 groups by Mann–Whitney U test for quantitative variables and chi-squared test or Fisher’s exact test for qualitative variables.
aAKI was identified according to the Kidney Disease Improving Global Outcomes (KDIGO) definition. Delirium was defined according to the Confusion Assessment Method (CAM).
Treatments
The treatments used during hospitalization are described in Table 2; very few patients received specific treatment for COVID-19. However, a large proportion received symptomatic treatments, including oxygen therapy, antibiotics, proton pump inhibitors, prophylactic anticoagulation, morphine, and midazolam.
Complications
All complications that occurred during hospitalization are described in Table 2. The most common complications were dyspnea (n = 441, 54%), delirium (n = 330, 40%), AKI (n = 321, 39%), and heart failure (n = 113, 14%). Very few patients experienced a hemorrhagic or thromboembolic event.
On univariate analysis, the incidence of complications, particularly dyspnea, heart failure, AKI, and delirium, was significantly higher for patients who died than those who survived (Table 2).
Fourteen (2%) patients were transferred to an ICU, and palliative care was decided for 205 (25%).
Outcomes and Prognostic Factors at COVID-19 Onset
In total, 250 patients (31%; 95% CI 27–33) died during the hospital stay, and for those who died, the median [IQR] time to death from symptom onset was 10 [7–14] days. In the final multivariate logistic mixed model (N = 720 patients), at COVID-19 onset, probability of in-hospital mortality was increased with male sex, functional status (ADL score < 4), asthenia, qSOFA score ≥2, and specific COVID-19 anomalies on chest CT (Table 3).
Table 3.
Multivariate Analysis of Risk of In-Hospital Mortality in Hospitalized Older Patients With COVID-19
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Gender, reference value = female | <.001 | |
| Gender = male | 1.85 (1.30–2.63) | |
| Adjusted Charlson index, reference value < 7 | .65 | |
| Adjusted Charlson index ≥ 7 | 1.02 (0.94–1.11) | |
| ADL, reference value ≥ 4 | .002 | |
| ADL < 4 | 1.84 (1.25–2.70) | |
| Asthenia, reference value = no | .02 | |
| Asthenia = yes | 1.59 (1.08–2.32) | |
| qSOFA, reference value < 2 | <.001 | |
| qSOFA ≥ 2 | 2.63 (1.64–4.22) | |
| Specific COVID-19 anomalies on chest CT, reference value = No | .04 | |
| 2.60 (1.07–6.46) | ||
| Specific COVID-19 anomalies: yes | .005 | |
| No chest CT | 3.83 (1.50–9.80) |
Note: ADL = activities of daily living; AIC = Akaike’s information criterion; CI = confidence interval; CT = computed tomography; ICC = intraclass correlation; OR = odds ratio; qSOFA = quick Sequential Organ Failure Assessment. N = 720; Charlson index = 0.69; 95% CI = 0.69–0.76; ICC = 0.049; AIC = 837.7.
At discharge, 365 (45%) patients were transferred to a rehabilitation center, 159 (19%) returned home, and 47 (5%) were transferred to another ward (including 5 [1%] to a palliative care center). The median [IQR] length of stay was 9 [5–14] days, with a significant difference between the patients who died and those who survived (5 [3–8] vs 11 [8–16] days, p < .001).
Discussion
This report, to our knowledge, is the first to date to describe a large cohort of older, frail patients (mean age 86 years) with COVID-19 admitted in acute care geriatric wards. We observed a mortality rate of 31% and identified several risk factors for death in this population. Probability of in-hospital mortality was increased with male gender, functional status, asthenia, high qSOFA score, and specific COVID-19 anomalies on chest CT. Moreover, unlike most published cohorts (7,8,11,12), we followed all patients in our cohort until their discharge (no censored data).
The reported in-hospital mortality (31%) is higher than that reported by the Chinese Center for Disease Control (6), but the baseline characteristics, disease severity, and status (hospitalized or not) of older patients in that cohort were not specified. Conversely, the reported rate (in all age groups) was lower than those in a cohort of hospitalized patients in the New York city area (32% with age 70–79 years, 54.3% with age 80–89 years, and 52.3% with age 90 years and older) (8). These differences may be related to differences in the severity of COVID-19, comorbidity patterns, treatments used, and characteristics of health care systems. It may also be related to hospitalization in geriatric wards in our study, where the entire team (physicians and paramedics) is trained to treat these frail and comorbid patients.
Unlike for other cohorts (5–12), we did not find any association between older age, comorbidities, and in-hospital mortality, probably because we had a relatively homogeneous cohort, with an average age and prevalence of comorbidities much higher than those in the literature. However, frailty and dependence, 2 key elements in geriatrics (22,23), were associated with in-hospital mortality. Because there was significant correlation between the ADL and Rockwood scores, the ADL score was retained as the variable assessing autonomy (most used in current clinical practice). These elements are related to severe conditions, such as dementia reported in more than half of our cohort, and are indicators of high patient vulnerability. To our knowledge, these variables have never been reported in COVID-19, probably because they are rarely evaluated outside of geriatric wards. They represent 2 powerful variables, easily measured and clinically relevant.
The main clinical manifestations included asthenia, fever, dyspnea, and dry cough, which are consistent with the clinical manifestations previously reported (4,7,8,11). As recently reported in a published national French survey including 353 COVID-19 patients aged 70 and older, based on declarative data from physicians, we found that older adults with COVID-19 less often exhibited fever at COVID-19 onset than younger adults (24). These results are consistent with previous literature reporting that fever, a cardinal sign of infection, may be absent or blunted 20%–30% of the time in older patients (25). Of note, delirium was a frequent pattern, which may be related to multimorbidity, high proportion of dementia, and severity of COVID-19. We found that anosmia and ageusia were rare, which is consistent with previous literature finding that older age was negatively associated with having olfactory dysfunction (24,26). This may be related to preexisting olfactory and gustatory dysfunctions associated with advanced age (27).
COVID-19 might be harder to identify in the elderly, whose symptoms could be masked, leading to misdiagnosis (28). Diagnostic delay has serious consequences for older people, including nosocomial transmission (52% in our cohort) (29). This underscores the importance of proactive steps to identify and exclude potentially infected staff and visitors, early recognition of potentially infected patients (isolation room/area if available), and implementation of infection prevention and control measures (contact and droplet or airborne precautions), particularly in this fragile population (15,29).
As previously reported (5–8,10,11), we found that COVID-19 can cause both pulmonary and systemic inflammation, leading to multiorgan dysfunction associated with in-hospital mortality in these older patients: conditions included dyspnea, AKI, delirium, heart failure, lymphopenia, liver dysfunction, and elevated levels of inflammatory markers. Moreover, specific COVID-19 anomalies on chest CT at COVID-19 onset were significantly associated with in-hospital mortality. Not having a chest CT was also associated with in-hospital mortality, in the multivariate analysis, probably because serious cases did not have this imaging at COVID-19 onset.
SARS-CoV-2 infection was reported to predispose patients to thrombotic disease, both in venous and arterial circulation, due to excessive inflammation, platelet activation, endothelial dysfunction, and stasis (30). Because of this, 791 (96%) of our patients received anticoagulation therapy (curatively for conditions other than COVID-19 or prophylactically) during the whole hospital stay, as proposed in our bi-weekly phone meetings. This situation could explain the low prevalence of thrombotic disease in our cohort. Conversely, at the beginning of the COVID geriatric ward setup, we noticed several cases of gastrointestinal hemorrhage, which led to proposing the systematic introduction of a proton pump inhibitor in all hospitalized patients (used in 529 [65%] patients) limited to the hospital stay.
No specific treatment, including antiviral therapy, hydroxychloroquine, and anticytokine or immunomodulatory agents, was significantly associated with survival or death in our cohort, which agreed with the literature (31,32). The low prevalence of hydroxychloroquine use was related to the warning in older patients with cardiac disease and/or arrhythmia in the geriatric community, lack of scientific data, and shared and regular phone meetings between geriatric wards (33). The significant association between corticosteroid therapy and in hospital-mortality (Table 2) should be interpreted with caution (retrospective data, few patients, patients with potentially different profiles, especially used during respiratory worsening). The significant association between midazolam and morphine therapies and in-hospital mortality (Table 2) was probably due to these therapeutics usually introduced at the end of life.
For patients discharged alive, the proportion discharged to rehabilitation versus home (including nursing home) was high (45% vs 19%) and much higher than the U.S. rate (14%) probably due to different health care systems (8). Hospital administrators, governments, and policymakers must be prepared for a substantial increase in rehabilitation need for older patients weakened by COVID-19.
Limitations
The study included only patients within the Paris area, but this is one of the 2 most affected areas in France. Although this study was retrospective, its observational nature allowed us to include more patients with severe baseline characteristics than what we could observe in a randomized controlled trial. We were able to obtain a substantial amount of information concerning baseline characteristics of our patients, in line with a geriatric point of view that tries to assess all variables that characterize the geriatric patient in comparison to healthy aging. However, because of the retrospective design, some values were missing, particularly laboratory results. Finally, our results may relate in part to certain characteristics of the French health care system and may not be extrapolated to other countries.
Conclusion
In this multicentric retrospective cohort study of 821 hospitalized older patients with confirmed COVID-19 in the Paris area, in-hospital morality was high (31%; 95% CI 27–33). At COVID-19 onset, probably in-hospital mortality was associated with male gender, functional status (ADL score < 4), qSOFA score ≥2, asthenia, and specific COVID-19 anomalies on chest CT. Thus, a quick bedside evaluation may identify older patients with COVID-19 at risk of unfavorable outcomes.
Funding
None declared.
Conflict of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. J.B. reported personal fees for lectures from VIFOR Pharma and Baxter companies outside the submitted work. O.H. reported personal fees for lectures from Bayer, Novartis, BMS, Pfizer, Servier, Boehringer, Vifor, and Astra Zeneca outside the submitted work. All other authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
Special thanks to all health care professionals and all physicians who took care of patients with COVID-19 in acute COVID geriatric wards:
Ambroise-Paré Hospital: Prof. Laurent Teillet, Dr. Tristan Cudennec, Dr. Sophie Moulias, Dr. Cyril Sellier, Dr. Lucie Aubert, Dr. Delphine Casciari, and Dr. Soline de la Roche Saint-André. Antoine-Béclère Hospital: Dr. Mathieu Mion, Alix Dussourt, Anne-Solène Peric, Dr. Marie-Christelle Gulczynski, and Dr. Hamat Sall. Bichat – Claude Bernard Hospital: Prof. Agathe Raynaud-Simon, Dr. Nathalie Faucher, Dr. Angèle Néouze, and Dr. Hélène Esnault. Bretonneau Hospital: Dr. Amélie Aregui, Dr. Camille Loiseau-Breton, Dr. Maguelone Mistral, Dr. Charlotte Golstein, Dr. Olivier Drunat, and Clothilde Riquier. Broca Hospital: Dr. Adrien Cohen, Dr. Bastien Genet, Dr. Maelle Beunardeau, Dr. Louise Harle, and Dr. Anna Goncalves. Charles – Foix Hospital: Prof. Eric Pautas. Paul brousse Hospital: Dr. Morgane Jacques, Dr. Nicoletta Brunetti, Dr. Marie Neiss, Dr. Pauline Simon, Dr. Marion Colas, Dr. Magali Guichardon, Dr. Christophe Trivalle, Dr. Narges Toussi, Dr. Jorge Sanchez-Tamayo, and Jill Kosowski. Pitié Salpêtrière Hospital: Dr. Zina Barrou, Dr. Elise Poumier, Dr. Luca Royer, and Dr. Maurine Palaric. Rothschild Hospital: Dr. Romain Veber, Dr. Anne-Gaelle Andrieu, Dr. Anne-Sophie Grancher, Dr. Vania Leclercq, and Dr. Virginie Loffler. Saint Antoine Hospital: Dr. Sirine Dahmani, Dr. Valérie Bellamy, and Dr. Laura Moïsi.
Author Contributions
J.B. had full access to all the data in the study and takes responsibility for the integrity and the accuracy of the data. Study concept and design: L.Z., H.V., and J.B. Acquisition of the data: L.Z., E.B., M.P., M.M., S.K., C.B., S.R., A.G., C.T., N.L., A.D., S.B., F.K., C.B., C.d.V., M.R., M.-A.D., E.D., J.-P.D., C.T., E.P., P.d.M., E.B., M.L., E.M., A.M., O.H., V.F.-D., L.B., H.V., and J.B. Statistical analysis: E.B. Drafting of the manuscript: L.Z., B.R., and J.B. Critical revision of the manuscript for important intellectual content: all authors. English editing: Laura Smales from BioMedEditing (Toronto, Canada).
Transparency statement: The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Ethics approval: The institutional ethics board of Sorbonne University reviewed and approved our research project on May 11, 2020 (N°2020-CER-2020-43).
Data sharing: Data can be obtained on request from J.B. (jacques.boddaert@aphp.fr).
References
- 1. World Health Organization. Corona virus disease (COVID-19) outbreak www.who.int/fr/emergencies/diseases/novel-coronavirus-2019. Accessed July 3, 2020.
- 2. Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Novel coronavirus (2019-nCoV) situation reports www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed July 3, 2020.
- 4. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323(13):1239. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu C, Chen X, Cai Y, et al. . Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;e200994. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T, Wu D, Chen H, et al. . Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, He W, Yu X, et al. . Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niu S, Tian S, Lou J, et al. . Clinical characteristics of older patients infected with COVID-19: a descriptive study. Arch Gerontol Geriatr. 2020;89:104058. doi: 10.1016/j.archger.2020.104058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID19-APHP Group. Assistance Publique-Hôpitaux de Paris’ response to the COVID-19 pandemic. Lancet 2020;395(10239):1760–1761. doi: 10.1016/S0140-6736(20)31210-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Clinical management of COVID-19 www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed July 3, 2020.
- 16. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 17. Rockwood K, Song X, MacKnight C, et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14(Suppl. 5):116–118. doi: 10.1097/00005650-197605001-00018 [DOI] [PubMed] [Google Scholar]
- 19. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seymour CW, Liu VX, Iwashyna TJ, et al. . Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). J Am Med Assoc. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kellum JA, Lameire N; KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Millán-Calenti JC, Tubío J, Pita-Fernández S, et al. . Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50(3):306–310. doi: 10.1016/j.archger.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 23. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. ; Gerontopole Brussels Study group Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 24. Annweiler C, Sacco G, Salles N, et al. . National French survey of COVID-19 symptoms in people aged 70 and over. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norman DC. Fever in the elderly. Clin Infect Dis. 2000;31(1):148–151. doi: 10.1086/313896 [DOI] [PubMed] [Google Scholar]
- 26. Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114–120. doi: 10.1177/0194599820929185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 28. Vetter P, Vu DL, L’Huillier AG, Schibler M, Kaiser L, Jacquerioz F. Clinical features of covid-19. Br Med J. 2020;369:m1470. doi: 10.1136/bmj.m1470 [DOI] [PubMed] [Google Scholar]
- 29. McMichael TM, Currie DW, Clark S, et al. ; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bikdeli B, Madhavan MV, Jimenez D, et al. . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;S0735-1097(20):35008-35007. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg ES, Dufort EM, Udo T, et al. . Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. J Am Med Assoc. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fihn SD, Perencevich E, Bradley SM. Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Netw Open. 2020;3(4):e209035. doi: 10.1001/jamanetworkopen.2020.9035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.