Abstract
Background:
Persistent anemia has been described in kidney transplant (KTx) recipients with parvovirus B19 virus infection. However, the epidemiology of parvovirus B19 and parvovirus B19-related anemia after KTx remains unclear. We conducted this systematic review (1) to investigate the incidence of parvovirus B19 infection after KTx and (2) to assess the incidence of parvovirus B19 among KTx patients with anemia.
Materials and Methods:
A systematic review was conducted in EMBASE, MEDLINE, and Cochrane databases from inception to March 2019 to identify studies that reported the incidence rate of parvovirus B19 infection and/or seroprevalence of parvovirus B19 in KTx recipients. Effect estimates from the individual studies were extracted and combined using random-effects, generic inverse variance method of DerSimonian and Laird. The protocol for this systematic review is registered with PROSPERO (no. CRD42019125716).
Results:
Nineteen observational studies with a total of 2108 KTx patients were enrolled. Overall, the pooled estimated seroprevalence of parvovirus B19 immunoglobulin G was 62.2% (95% confidence interval [CI]: 45.8%–76.1%). The pooled estimated incidence rate of positive parvovirus B19 DNA in the 1st year after KTx was 10.3% (95% CI: 5.5%–18.4%). After sensitivity analysis excluded a study that solely included KTx patients with anemia, the pooled estimated incidence rate of positive parvovirus B19 DNA after KTx was 7.6% (95% CI: 3.7%–15.0%). Among KTx with anemia, the pooled estimated incidence rate of positive parvovirus B19 DNA was 27.4% (95% CI: 16.6%–41.7%). Meta-regression analysis demonstrated no significant correlations between the year of study and the incidence rate of positive parvovirus B19 DNA (P = 0.33). Egger's regression asymmetry test was performed and demonstrated no publication bias in all analyses.
Conclusion:
The overall estimated incidence of positive parvovirus B19 DNA after KTX is 10.3%. Among KTx with anemia, the incidence rate of positive parvovirus B19 DNA is 27.4%. The incidence of positive parvovirus B19 DNA does not seem to decrease overtime.
Keywords: Kidney transplantation, meta-analysis, parvovirus B19, renal transplantation, systematic reviews
INTRODUCTION
Parvovirus B19, first discovered in 1975, is one of the smallest viruses known to infect humans. It is a nonenveloped, single-stranded, linear DNA virus that belongs to the Parvoviridae family.[1,2] Parvovirus B19, as the prototype of the genus erythrovirus, has a unique erythroid progenitor cell tropism with the only known host being humans.[2] Infection from parvovirus B19 is common across the world both in sporadic and in cluster cases. Infection is most common among children so that half of the population have detectable levels of immunoglobulin G (IgG) antibody by age 15, and 70% of adults have positive parvovirus B19-IgG antibody.[3,4,5]
Parvovirus B19 can transmit through infected respiratory secretions, close person-to-person contact, transplacental, blood transfusion, and organ transplantation.[6,7] In immunosuppressed patients, especially kidney transplant (KTx) recipients, parvovirus B19 infection can occur from reactivation of the endogenous latent virus or persistent viremia.[8] It is directly cytotoxic to the erythroid series and replicates in the precursor erythroid cells in the bone marrow and peripheral blood, ultimately leading to the cessation of erythropoiesis and destruction of red blood cells.[2,9] Acute and chronic persistent anemia have been reported in patients with parvovirus B19 infection.[2,9] Pure red cell aplasia, a life-threatening complication, has also been described in immunosuppressed patients, including hematopoietic stem cell transplant and solid organ transplant recipients.[2] The epidemiology of parvovirus B19 and parvovirus B19-related anemia after KTx remains unclear.[5,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]
Therefore, we conducted this systematic review and meta-analysis (1) to investigate the frequency of parvovirus B19 infection and (2) to assess the incidence of parvovirus B19 among KTx with anemia.
MATERIALS AND METHODS
Search strategy
The protocol for this systematic review is registered with PROSPERO (no. CRD42019125716). PRISMA statement guidelines were followed for conducting and reporting meta-analysis data,[30] Online Supplementary Data 1. A systematic review was performed in EMBASE, MEDLINE, and Cochrane databases from inception to March 2019 to identify studies that evaluated (1) the incidence of parvovirus B19 infection or (2) the incidence of parvovirus B19 among KTx recipients with anemia using the search terms “kidney transplant” and “parvovirus” without any language restrictions, as described in Online Supplementary Data 1. References of selected articles were also manually searched for additional studies.
Inclusion criteria
Studies were eligible for this meta-analysis if the following inclusion criteria were met: (1) observational, cohort (either prospective or retrospective), case–control, or cross-sectional studies published as original studies to evaluate the epidemiology of parvovirus B19 in KTx patients. Included studies must have provided data to estimate the incidence of parvovirus B19 with 95% confidence intervals (CIs). Retrieved articles were individually reviewed for eligibility by two investigators (C. T. and W. C.). Discrepancies were addressed and solved by mutual consensus. Inclusion was not limited by the size of the study.
Review process and data extraction
Two study investigators (C. T. and W. C.) independently reviewed the titles and abstracts of all retrieved articles. Articles that did not fulfill inclusion criteria were excluded. Only potentially relevant articles underwent full-text review to determine eligibility. A structured data collecting form was utilized to obtain the following data from each study including title, name of the first author, year of the study and publication, country where the study was conducted, overall incidence rate of positive parvovirus B19 DNA, incidence rate of positive parvovirus B19 DNA among KTx patients with anemia, and seroprevalence of parvovirus B19 IgG.
Statistical analysis
Analyses were conducted utilizing the Comprehensive Meta-Analysis 3.3 software (Biostat Inc., Englewood, NJ, USA). Adjusted point estimates from each study were consolidated by the generic inverse variance approach of DerSimonian and Laird, which designated the weight of each study based on its variance.[31] Due to the probability of between-study variance, we used a random-effects model rather than a fixed-effects model. Cochran's Q test and I2 statistic were applied to ascertain the between-study heterogeneity. A value of I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity, and 76%–100% high heterogeneity.[32] The presence of publication bias was evaluated by the Egger test.[33]
RESULTS
A total of 285 potentially eligible articles were identified using our search strategy. After exclusion of 188 articles based on title and abstract for clearly not fulfilling inclusion criteria due to the type of article, patient population, study design, or outcome of interest, and an additional 40 excluded due to duplication, 57 articles were left for full-length review. Twenty-six of these were excluded from full-length review as they did not report the outcome of interest, whereas 12 articles were excluded because they were not observational studies. Thus, 19 observational studies with a total of 2108 KTx patients were enrolled. The literature retrieval, review, and selection process are demonstrated in Figure 1. The characteristics of the included studies are presented in Table 1.
Figure 1.
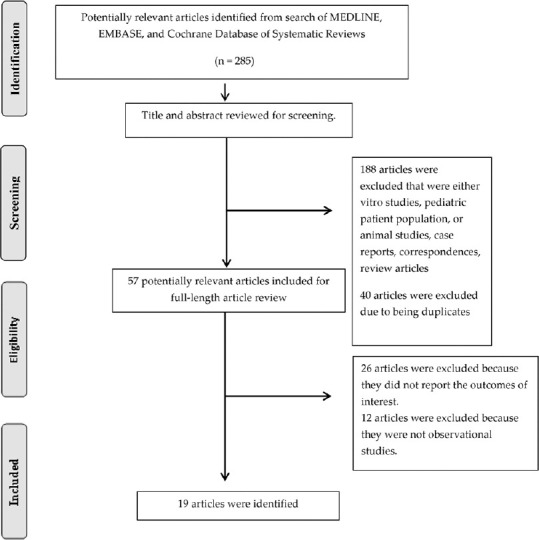
Study selection
Table 1.
| Study | Year | Country | Patients | n | Parvovirus B19 detection | Incidence of parvovirus B19 infection (%) | Incidence of anemia among parvovirus B19 infection (%) |
|---|---|---|---|---|---|---|---|
| Bertoni et al.[10] | 1997 | Italy | Deceased kidney transplant patients with severe hypoproliferative anemia | 9 | PCR | Severe anemia - 4/9 (44.4) | NR |
| Gallinella et al.[11] | 1999 | Italy | Kidney transplant patients within 6 months after transplant | 59 | PCR | 0/59 (0) | NR |
| Zolnourian et al.[12] | 2000 | Ireland | Kidney transplant patients within 4 months after transplant | 110 | PCR | 2/110 (1.8) | 0/2 (0) |
| Malyszko et al.[13] | 2002 | Poland | Kidney transplant patients | 47 | IgM, IgG | IgM - 11/47 (23.4) IgG - 31/47 (66) | NR |
| Cavallo et al.[14] | 2003 | Italy | Kidney transplant patients | 48 with anemia 21 without anemia | PCR | With anemia - 11/48 (22.9) Without anemia - 1/21 (4.8) | NR |
| Ki et al.[15] | 2005 | South Korea | Kidney transplant patients | 167 | PCR | 52/167 (31.1) | Acute rejection - 7/52 (13) Graft failure - 1/52 (2) Pure red cell aplasia - 2/52 (4) |
| Eid et al.[16] | 2006 | US | Kidney transplant patients within 1 year after transplant | 9 | PCR | 0/9 (0) | NR |
| Egbuna et al.[17] | 2006 | US | Kidney transplant patients with anemia and erythropoietin resistance | 8 | PCR, IgM, IgG | PCR - 3/8 (37.5) IgM - 1/8 (12.5) IgG - 2/8 (25) | NR |
| Ardalan et al.[18] | 2008 | Iran | Kidney transplant patients | 100 | PCR, IgM, IgG | PCR - 6/100 (6) IgM - 6/100 (6) | NR |
| Park et al.[19] | 2009 | South Korea | Kidney transplant patients | 143 | PCR | Positive B19 PCR - 84/143 (58.7) High titer >106 copies/5 µL - 13/143 (9.1) | Persistent severe anemia Positive B19 PCR - 13/84 (15) High titer - 11/13 (85) Anemia at 6 months Positive B19 PCR - 13/84 (15) High titer - 3/13 (23) Anemia at 12 months Positive B19 PCR - 9/84 (11) High titer - 1/13 (8) |
| Čapenko et al.[20] | 2012 | Latvia | Deceased kidney transplant patients | 63 (38 with anemia, 25 without anemia) | PCR and IgM | All - 12/63 (19) With anemia - 12/38 (31.6) Without anemia - 0/25 (0) | Anemia - 12/12 (100) EPO-resistant severe anemia - 7/12 (58) |
| Carraturo et al.[21] | 2012 | Italy | Kidney transplant patients | 64 (14 with anemia, 50 without anemia) | PCR | All - 2/64 (3) With anemia - 0/14 (0) Without anemia - 2/50 (4) | 0/2 (0) |
| Plentz et al.[5] | 2013 | Germany | Kidney transplant patients within 6 months after transplant | 147 | PCR | 2/147 (1.4) | NR |
| Porignaux et al.[22] | 2013 | France | Kidney transplant patients within 1 year after transplant | 60 | PCR | 6/60 (10) | NR |
| Khameneh et al.[23] | 2014 | Iran | Kidney transplant patients with anemia | 91 | IgG | 63/91 (69.2) | Anemia - 63/63 (100) Severe anemia - 47/63 (75) |
| Pambrun et al.[24] | 2014 | France | Kidney transplant patients undergoing bone marrow aspiration for cytopenia | 72 | PCR | Bone marrow - 11/72 (15.3) Blood - 4/72 (5.5) | NR |
| Krishnan et al.[25] | 2015 | USA | Kidney transplant patients | 144 | PCR or IgM | 3/144 (2.1) | 3/3 (100) |
| Baek et al.[26] | 2017 | South Korea | Kidney transplant patients | 602 | PCR | 39/602 (6.5) | Severe anemia (Hb <7) - 27/39 (69) |
| Xiao et al.[27] | 2013 | China | Living related kidney transplant patients | 144 | PCR | 27/144 (18.8) | PRCA - 2/27 (7.4) |
EPO: Erythropoietin, IgG: Immunoglobulin G, NR: Not reported, IgM: Immunoglobulin M, PCR: Polymerase chain reaction, PRCA: Pure red cell aplasia, Hb: Hemoglobin
Incidence of parvovirus B19 infection among kidney transplant patients
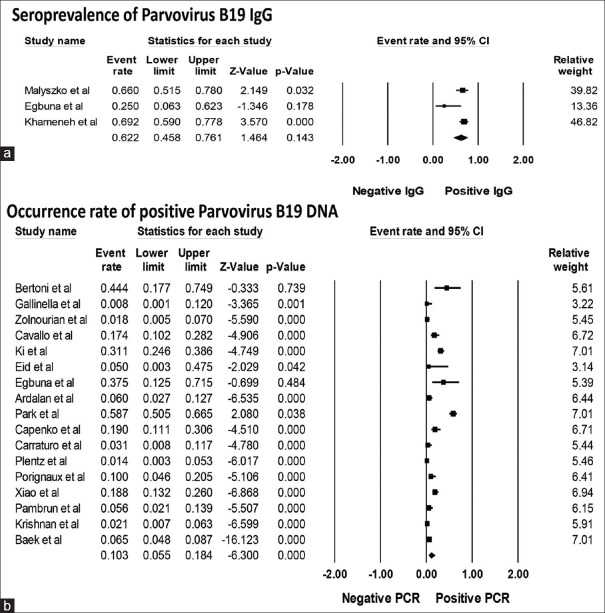
Overall, the pooled estimated seroprevalence of parvovirus B19 IgG was 62.2% in KTx recipients (95% CI: 45.8%–76.1%, I2= 60%) [Figure 2a]. The pooled estimated incidence rate of positive parvovirus B19 DNA in the 1st year after KTx was 10.3% (95% CI: 5.5%–18.4%, I2= 94%) [Figure 2b]. Sensitivity analysis excluding a study[10](that solely included KTx patients with anemia) was performed and showed the pooled estimated incidence rate of positive parvovirus B19 DNA after KTx of 7.6% (95% CI: 3.7%–15.0%, I2= 94%).
Figure 2.
Forest plots of the included studies assessing. (a) Seroprevalence of parvovirus B19 immunoglobulin G and (b) incidence rate of positive parvovirus B19 DNA in kidney transplant patients. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval
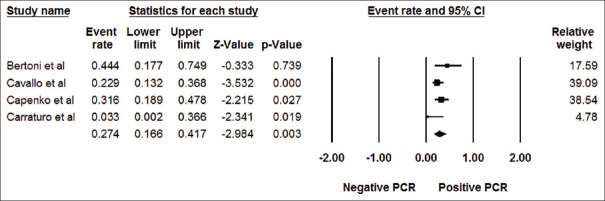
Among KTx patients with anemia, the pooled estimated incidence rate of positive parvovirus B19 DNA was 27.4% (95% CI: 16.6%–41.7%, I2= 38%) [Figure 3]. Meta-regression analysis demonstrated no significant correlations between the year of study and the incidence rate of positive parvovirus B19 DNA (P = 0.33) [Figure 4].
Figure 3.
Forest plots of the included studies assessing incidence rate of positive parvovirus B19 DNA among kidney transplant patients with anemia. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval
Figure 4.
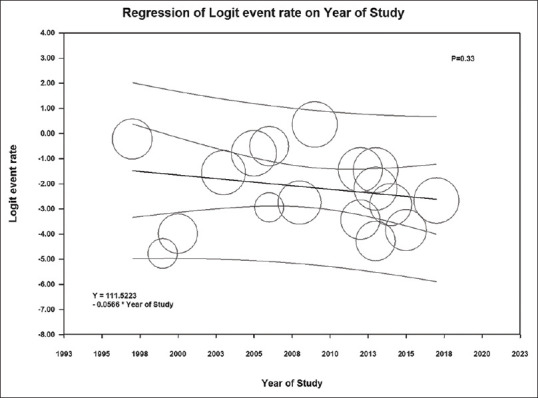
Meta-regression analysis demonstrated no significant correlations between the year of study and the incidence rate of positive parvovirus B19 DNA (P = 0.33). The solid black line represents the weighted regression line based on variance-weighted least squares. The inner and outer lines show the 95% confidence interval and prediction interval around the regression line. The circles indicate log event rates in each study
Evaluation for publication bias
Funnel plot [Supplementary Figure 1] and Egger's regression asymmetry test were performed to evaluate for publication bias in the analyses evaluating the pooled estimated incidence rate of positive parvovirus B19 DNA in KTx recipients. There was no significant publication bias, P = 0.08.
DISCUSSION
In this systematic review and meta-analysis, we revealed that the overall estimated incidence of positive parvovirus B19 DNA among KTx recipients is 10.3%. Among KTx patients with anemia, the incidence rate of positive parvovirus B19 DNA is 27.4%. The incidence of positive parvovirus B19 DNA has not seemed to decrease over time.
Parvovirus B19 attaches to erythrocytePantigen, a cellular receptor found on erythroid cells, erythroid precursor cells, red cells of the fetal myocardium, liver, and placental tissues.[2] It is directly cytotoxic to the erythroid series and replicates in the precursor erythroid cells in the bone marrow and peripheral blood, ultimately leading to the cessation of erythropoiesis and red blood cell destruction. Common causes of post-KTx anemia include bone marrow suppression from iatrogenic immunosuppressants, iron deficiency related to acute and chronic gastrointestinal losses, angiotensin-converting enzyme inhibitors, viral infections such as cytomegalovirus (CMV), reduced erythropoietin synthesis from renal dysfunction, and other etiologies.[21] However, our study demonstrated that among anemic KTx recipients, the incidence rate of positive parvovirus B19 DNA is common and as high as 27.4%. Therefore, clinical suspicion for parvovirus B19 should be high in KTx recipients with anemia, especially those with profound recurrent anemia that is resistant to erythropoietic-stimulating agents, requires multiple blood transfusions, or persists despite immunosuppressive reduction and/or withdrawal.[4,17,34,35]
In addition to chronic or refractory anemia, studies have reported that parvovirus B19 infection may lead to significant complications among KTx patients such as acute rejection (following immunosuppression reduction for parvovirus B19 infection), collapsing glomerulopathy, hemophagocytic lymphohistiocytosis, and thrombotic microangiopathy.[25,36] Although intravenous immunoglobulin has been reported as an effective treatment for parvovirus B19 infection after solid organ transplantation,[28,37] its use for parvovirus B19 and other viral infections posttransplantation is usually in combination with immunosuppression reduction. Acute rejection following immunosuppression reduction has been reported in KTx patients with CMV, BK polyomavirus, and parvovirus B19 infections, which can lead to allograft failure.[25,38]
Although ubiquitous across the globe, the frequency of the parvovirus B19 infection among immunocompromised patients may be underestimated as they do not typically manifest the characteristic immune-mediated symptoms, such as fever, arthralgia, and rash among others.[22] The lack of universal screening for parvovirus B19 among KTx recipients may have also underestimated the exact incidence among KTx patent population. With this in mind, our meta-analysis found the overall estimated incidence of positive parvovirus B19 DNA among KTx recipients of 10.3%. Porignaux et al.[22] performed a study of sixty patients in the 1st year post-KTx who had nucleic acid testing for various opportunistic viruses. They showed that 10% of KTx recipients had detectable parvovirus B19 virus, which was nearly high as the detectable rates of CMV and Epstein–Barr virus reactivation (13% and 12%, respectively) in the same cohort of KTx patients.[22] Our study's findings further support the need for future studies to identify a cost-effective surveillance and treatment strategy for parvovirus B19 among KTx recipients.
Several limitations in our meta-analysis are worth mentioning. First, there are statistical heterogeneities in our study. Potential sources for heterogeneities were the variations in the KTx-recipient screening methods, patient characteristics, and differences in the immunosuppressive regimens used at various transplant centers, which may have affected the incidence of parvovirus B19 infection. Second, the included studies lacked data on the genotype of parvovirus B19. Parvovirus B19 genotypes 1 and 2 are typical to Western countries, whereas genotype 3 is seen in South America and Sub-Saharan nations.[39] Although there are no definitive differences in genotype-specific clinical manifestations in the general population,[2] the incidence and effect of different parvovirus B19 genotypes on the risk of anemia among KTx recipients remain unclear. Thus, future studies evaluating the incidence of different genotypes of parvovirus B19 infection after KTx are needed. Finally, this study is a meta-analysis of retrospective cohort studies, and the data from population-based studies were limited. Thus, future large population-based studies evaluating the incidence of parvovirus B19 among KTx recipients are needed.
CONCLUSION
The overall incidence rate of positive parvovirus B19 DNA is 10.3%. In addition, the incidence rate of positive parvovirus B19 DNA among KTx patients with anemia is high as 27.4% and does not seem to decrease over time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1:72–3. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- 2.Eid AJ, Chen SF. AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):201–5. doi: 10.1111/ajt.12111. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LJ, Tsou C, Parker RA, Chorba TL, Wulff H, Tattersall P, et al. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:522–6. doi: 10.1128/jcm.24.4.522-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razonable RR. Not the usual viral suspects: Parvovirus B19, West Nile virus, and human T-cell lymphotrophic virus infections after kidney transplantation. Semin Nephrol. 2016;36:428–34. doi: 10.1016/j.semnephrol.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Plentz A, Würdinger M, Kudlich M, Modrow S. Low-level DNAemia of parvovirus B19 (genotypes 1-3) in adult transplant recipients is not associated with anaemia. J Clin Virol. 2013;58:443–8. doi: 10.1016/j.jcv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Pattison JR, Jones SE, Hodgson J, Davis LR, White JM, Stroud CE, et al. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981;1:664–5. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- 7.Jordan J, Tiangco B, Kiss J, Koch W. Human parvovirus B19: Prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients. Vox Sang. 1998;75:97–102. [PubMed] [Google Scholar]
- 8.Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2(Suppl 1):S47–56. doi: 10.2215/CJN.01060307. [DOI] [PubMed] [Google Scholar]
- 9.Woolf AD, Campion GV, Chishick A, Wise S, Cohen BJ, Klouda PT, et al. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989;149:1153–6. [PubMed] [Google Scholar]
- 10.Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrzewska K, Guidi S, et al. Aplastic anemia due to B19 parvovirus infection in cadaveric renal transplant recipients: An underestimated infectious disease in the immunocompromised host. J Nephrol. 1997;10:152–6. [PubMed] [Google Scholar]
- 11.Gallinella G, Manaresi E, Venturoli S, Grazi GL, Musiani M, Zerbini M. Occurrence and clinical role of active parvovirus B19 infection in transplant recipients. Eur J Clin Microbiol Infect Dis. 1999;18:811–3. doi: 10.1007/s100960050406. [DOI] [PubMed] [Google Scholar]
- 12.Zolnourian ZR, Curran MD, Rima BK, Coyle PV, O’Neill HJ, Middleton D. Parvovirus B19 in kidney transplant patients. Transplantation. 2000;69:2198–202. doi: 10.1097/00007890-200005270-00043. [DOI] [PubMed] [Google Scholar]
- 13.Malyszko J, Hryszko T, Malyszko JS, Wolczyński S, Myśliwiec M. Parvovirus B19 infection and IGF system components in relation to erythropoiesis in dialyzed patients and kidney transplant recipients. Transplant Proc. 2002;34:3211–4. doi: 10.1016/s0041-1345(02)03653-9. [DOI] [PubMed] [Google Scholar]
- 14.Cavallo R, Merlino C, Re D, Bollero C, Bergallo M, Lembo D, et al. B19 virus infection in renal transplant recipients. J Clin Virol. 2003;26:361–8. doi: 10.1016/s1386-6532(02)00104-x. [DOI] [PubMed] [Google Scholar]
- 15.Ki CS, Kim IS, Kim JW, Lee NY, Kim SH, Lee KW, et al. Incidence and clinical significance of human parvovirus B19 infection in kidney transplant recipients. Clin Transplant. 2005;19:751–5. doi: 10.1111/j.1399-0012.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 16.Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: A review of 98 cases. Clin Infect Dis. 2006;43:40–8. doi: 10.1086/504812. [DOI] [PubMed] [Google Scholar]
- 17.Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J. A cluster of parvovirus B19 infections in renal transplant recipients: A prospective case series and review of the literature. Am J Transplant. 2006;6:225–31. doi: 10.1111/j.1600-6143.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- 18.Ardalan MR, Shoja MM, Tubbs RS, Jayne D. Parvovirus B19 microepidemic in renal transplant recipients with thrombotic microangiopathy and allograft vasculitis. Exp Clin Transplant. 2008;6:137–43. [PubMed] [Google Scholar]
- 19.Park JB, Kim DJ, Woo SY, Choi GS, Chun JM, Jung GO, et al. Clinical implications of quantitative real time-polymerase chain reaction of parvovirus B19 in kidney transplant recipients – A prospective study. Transpl Int. 2009;22:455–62. doi: 10.1111/j.1432-2277.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 20.Čapenko S, Kozireva S, Folkmane I, Bernarde K, Rozentāls R, Murovska M. Anemia as a complication of parvovirus b19 infection in renal transplant recipients. Medicina (Kaunas) 2012;48:299–304. [PubMed] [Google Scholar]
- 21.Carraturo A, Catalani V, Ottaviani D, Menichelli P, Rossini M, Terella D, et al. Parvovirus B19 infection and severe anemia in renal transplant recipients. Scientific World Journal 2012. 2012 doi: 10.1100/2012/102829. 102829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porignaux R, Vuiblet V, Barbe C, Nguyen Y, Lavaud S, Toupance O, et al. Frequent occurrence of parvovirus B19 DNAemia in the first year after kidney transplantation. J Med Virol. 2013;85:1115–21. doi: 10.1002/jmv.23557. [DOI] [PubMed] [Google Scholar]
- 23.Khameneh ZR, Sepehrvand N, Sohrabi V, Ghasemzadeh N. The seroprevalence of parvovirus B19 among kidney transplant recipients: A single-center study. Saudi J Kidney Dis Transpl. 2014;25:16–21. doi: 10.4103/1319-2442.124465. [DOI] [PubMed] [Google Scholar]
- 24.Pambrun E, Mengelle C, Fillola G, Laharrague P, Esposito L, Cardeau-Desangles I, et al. An association between BK virus replication in bone marrow and cytopenia in kidney-transplant recipients. J Transplant 2014. 2014 doi: 10.1155/2014/252914. 252914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan P, Ramadas P, Rajendran PP, Madhavan P, Alex A, Jayaschandran V, et al. Effects of parvovirus B19 infection in renal transplant recipients: A retrospective review of three cases. Int J Angiol. 2015;24:87–92. doi: 10.1055/s-0034-1371759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baek CH, Kim H, Yang WS, Han DJ, Park SK. Risk factors and long-term outcomes of parvovirus B19 infection in kidney transplant patients. Transpl Infect Dis. 2017;19:e12754. doi: 10.1111/tid.12754. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C, Wang CX, Liu LS, Fu Q. Clinical investigation of human parvovirus B19 infection after renal transplantation in China. Transplant Proc. 2013;45:1593–9. doi: 10.1016/j.transproceed.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant. 2011;11:196–202. doi: 10.1111/j.1600-6143.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 29.Barsoum NR, Bunnapradist S, Mougdil A, Toyoda M, Vo A, Jordan SC. Treatment of parvovirus B-19 (PV B-19) infection allows for successful kidney transplantation without disease recurrence. Am J Transplant. 2002;2:425–8. doi: 10.1034/j.1600-6143.2002.20505.x. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clinic Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring in consistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 34.Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrewska K, Guidi S, et al. Unusual incidence of aplastic anaemia due to B-19 parvovirus infection in renal transplant recipients. Transplant Proc. 1997;29:818–9. doi: 10.1016/s0041-1345(96)00147-9. [DOI] [PubMed] [Google Scholar]
- 35.Vanrenterghem Y. Anaemia after renal transplantation. Nephrol Dial Transplant. 2004;19(Suppl 5):V54–58. doi: 10.1093/ndt/gfh1057. [DOI] [PubMed] [Google Scholar]
- 36.Ardalan MR, Shoja MM, Tubbs RS, Esmaili H, Keyvani H. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant. 2008;8:1340–4. doi: 10.1111/j.1600-6143.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 37.Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17:856–79. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 38.Cheungpasitporn W, Kremers WK, Lorenz E, Amer H, Cosio FG, Stegall MD, et al. De novo donor-specific antibody following BK nephropathy: The incidence and association with antibody-mediated rejection. Clin Transplant. 2018;32:e13194. doi: 10.1111/ctr.13194. [DOI] [PubMed] [Google Scholar]
- 39.Parsyan A, Szmaragd C, Allain JP, Candotti D. Identification and genetic diversity of two human parvovirus B19 genotype 3 subtypes. J Gen Virol. 2007;88:428–31. doi: 10.1099/vir.0.82496-0. [DOI] [PubMed] [Google Scholar]