Using data from the SVR study, we examined differences in long-term mortality and neurodevelopmental outcomes by SES.
Abstract
BACKGROUND:
Low socioeconomic status (SES) has emerged as an important risk factor for higher short-term mortality and neurodevelopmental outcomes in children with hypoplastic left heart syndrome and related anomalies; yet little is known about how SES affects these outcomes over the long-term.
METHODS:
We linked data from the Single Ventricle Reconstruction trial to US Census Bureau data to analyze the relationship of neighborhood SES tertiles with mortality and transplantation, neurodevelopment, quality of life, and functional status at 5 and 6 years post–Norwood procedure (N = 525). Cox proportional hazards regression and linear regression were used to assess the association of SES with mortality and neurodevelopmental outcomes, respectively.
RESULTS:
Patients in the lowest SES tertile were more likely to be racial minorities, older at stage 2 and Fontan procedures, and to have more complications and fewer cardiac catheterizations over follow-up (all P < .05) compared with patients in higher SES tertiles. Unadjusted mortality was highest for patients in the lowest SES tertile and lowest in the highest tertile (41% vs 29%, respectively; log-rank P = .027). Adjustment for patient birth and Norwood factors attenuated these differences slightly (P = .055). Patients in the lowest SES tertile reported lower functional status and lower fine motor, problem-solving, adaptive behavior, and communication skills at 6 years (all P < .05). These differences persisted after adjustment for baseline and post-Norwood factors. Quality of life did not differ by SES.
CONCLUSIONS:
Among patients with hypoplastic left heart syndrome, those with low SES have worse neurodevelopmental and functional status outcomes at 6 years. These differences were not explained by other patient or clinical characteristics.
What’s Known on This Subject:
Low neighborhood socioeconomic status (SES) is associated with worse 1-year survival after the Norwood procedure. Little is known about whether this association persists over the long-term or how SES relates to other measures of well-being.
What This Study Adds:
Patients with hypoplastic left heart syndrome and low SES have worse neurodevelopmental outcomes (adaptive behavior, problem-solving, fine motor, and communication skills) and functional status outcomes at 6 years post–Norwood procedure compared with patients with higher SES.
Low socioeconomic status (SES) has emerged as an important predictor of adverse outcomes in children with congenital heart disease (CHD). Children with CHD often require early surgical interventions and frequent medical follow-up. However, families with low SES may lack the resources to obtain high-quality specialty care or necessary follow-up. In several studies, researchers have identified associations between socioeconomic factors such as maternal education, family income, and insurance status with outcomes such as mortality, readmission, neurodevelopment, and health-related quality of life (HRQOL).1–10 However, most studies have been limited by single-center populations, missing data, or short-term follow-up. To date, there has been no comprehensive assessment of the role of SES across multiple long-term outcomes in children with CHD.
Children with hypoplastic left heart syndrome (HLHS) and other single right ventricle defects are at particularly high risk of poor long-term outcomes. Data from the Pediatric Heart Network Single Ventricle Reconstruction (SVR) study reported a 6-year transplant-free survival rate of 61%.11 Among those who survive, many have impaired long-term neurodevelopment.12 Although differences in patient comorbidities and operative care explain some of the observed differences in outcomes, there remains considerable unexplained variation. Low neighborhood SES has been associated with worse 1-year transplant-free survival after the Norwood procedure,10 yet little is known about whether this association persists over the long-term or how SES relates to other measures of well-being.
The goals of this analysis were to examine the association of neighborhood SES with 6-year outcomes after the Norwood procedure including mortality, cardiac transplant, neurodevelopment, adaptive behavior, quality of life, and functional status. Insight into the relationship between SES and long-term outcomes may reveal additional strategies for improving health and neurodevelopment in this high-risk population.
Methods
Study Design
We used data from the Pediatric Heart Network SVR trial and its follow-up studies. Details of the trial design have been previously published.13 In brief, neonates with HLHS and other single right ventricle anomalies were randomly assigned to receive either a modified Blalock-Taussig shunt or a right ventricle-to-pulmonary artery (RV-PA) shunt during the initial Norwood procedure. Neonates were enrolled between May 2005 and July 2008 at 15 centers in the United States and Canada. The SVR trial followed children for up to 14 months after the Norwood procedure. The Single Ventricle Reconstruction Extension (SVR II) study followed children up to a minimum of age 6 years (up to 9.8 years). Neurodevelopmental testing was performed at 5 or 6 years depending on the instrument. The institutional review board of all institutions approved the trial, and parents gave written informed consent.
Study Sample
A total of 555 neonates were randomly assigned in the trial. Five subjects did not undergo the Norwood procedure, and 1 subject withdrew from the trial immediately. We additionally excluded 14 children from Canada and 10 children from the United States with missing information on neighborhood SES. Therefore, a total of 525 patients were included in analyses. Of these, 330 survived to 5 years of age and 265 (80%) had at least 1 parent-completed neurodevelopmental questionnaires at 5 and 6 years as part of the SVR II study.
Variable Definitions
Data on patient demographics, birth characteristics, anatomy, perioperative details, complications, and subsequent interventions were collected by using standardized forms. Neighborhood SES was measured during the Norwood hospitalization by using a US census–based score developed by Diez Roux et al.14 Derived from 6 census-block group measures related to wealth and income, education, and occupation, scores ranged from −11 to +18 (mean 0; SD 5), with higher scores indicating higher neighborhood SES. Patients were divided into SES tertiles (≤− 2.79, > −2.79–1.96, and >1.96). We elected to use neighborhood SES in place of measures collected during the SVR trial itself because data on family SES were not collected until 14 months postoperatively.
Neurodevelopment, HRQOL, and Functional Status
Neurodevelopment was evaluated by using the Ages and Stages Questionnaire (ASQ), the Vineland Adaptive Behavior Scales (Vineland), and the Behavior Assessment System for Children, Second Edition (BASC-2) (Supplemental Table 4). Details of these scales have been previously published. Briefly, the ASQ is an interactive questionnaire used to evaluate children’s communication, gross and fine motor, problem-solving, and personal-social skills (scale 0–60).15,16 The Vineland is a psychometric instrument used to assess communication, daily living skills, socialization, motor skills, and maladaptive behavior (mean 100; SD 15).17,18 The BASC-2 is a parent-reported measure of behavioral symptoms divided into 4 composite scores (externalizing problems, internalizing problems, behavioral symptoms index, and adaptive skills) (mean 50; SD 10).19For the adaptive skills component, lower scores indicate problems, whereas for the other components, higher scores indicate problems.
HRQOL was measured by using the Pediatric Quality of Life Inventory (Peds-QL) and the Child Health Questionnaire Parent Form 50 (CHQ-PF50). The Peds-QL assesses 4 domains: physical, psychosocial, social, and school functioning (scale 0–100).20–22 The CHQ-PF50 measures 14 concepts that are aggregated into physical and psychosocial health summary scores (scale 0–100).23,24 Functional status was evaluated by using the Functional Status II(R) (FS-II[R]), which assesses normal daily, age-appropriate functions (scale 0–100).25
Statistical Analyses
Sociodemographic and clinical characteristics were compared across neighborhood SES tertiles for all patients and for patients surviving to 5 years who were eligible for neurodevelopmental testing by using χ2 tests for categorical variables and analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous variables.
Mortality
Kaplan-Meier estimation was used to model the unadjusted association of neighborhood SES with mortality. To account for the competing risk of transplant, we used the Fine and Gray26 regression model to estimate the subdistribution hazard of mortality across SES tertiles. We compared the relationship of neighborhood SES with short- (1 year) and longer-term (minimum 6 years) mortality by repeating the analyses for patients surviving transplant free the first year after Norwood (n = 365). All primary outcome analyses were repeated, adjusting for birth and Norwood characteristics including birth weight, prenatal diagnosis, genetic syndrome, and shunt type (model 1).
Neurodevelopmental, HRQOL, and Functional Status
Median (interquartile range) and mean (SD) neurodevelopment, HRQOL, and functional status scores at 5 or 6 years were compared across neighborhood SES tertiles by using Kruskal-Wallis tests and ANOVA, respectively. Linear regression was used to model the relationship between neighborhood SES tertile and patient-reported outcomes. Outcome variables that were not normally distributed were transformed. Multivariable models were built in a stepwise manner. We first adjusted for birth and Norwood characteristics including birth weight, prenatal diagnosis, the presence of a genetic syndrome, and shunt type (model 1). To examine how differences in complications or additional interventions might explain the effects of neighborhood SES, we added the total number of surgeries and total number of complications up to 6 years of age (model 2). Finally, we added race and ethnicity to the models as a sensitivity analysis. We chose not to include these race and ethnicity variables in the other models because of their high collinearity with SES but wanted to investigate the interplay between these variables. Covariates were selected by using a combination of previous literature, clinical judgment, and statistical testing (P < .1).
Missing Data
Missing covariate data varied with the study period. Fewer than 3% of patients were missing data on any baseline variables; however, 16% of patients were missing data on subsequent procedures or complications. Missing covariate data were multiply imputed (n = 10 data sets) by using a Markov chain Monte Carlo method; outcome data were not imputed and were missing in ∼20% to 25% of patients surviving to 5 years enrolled in the SVR II study (Supplemental Table 5). Compared with the 265 patients who completed at least 1 questionnaire, the 65 patients with missing outcome data were more likely to have genetic anomalies but did not differ significantly by SES, race and/or ethnicity, birth, or Norwood characteristics (Supplemental Table 6). All analyses were performed by using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Table 1 shows patient sociodemographic and clinical characteristics at baseline and post-Norwood by tertile of neighborhood SES for the entire sample (N = 525). Compared with patients in the highest SES tertile, more patients in the lowest tertile were racial minorities and of Hispanic ethnicity and fewer patients were prenatally diagnosed with CHD. There were no differences in gestational age or birth weight across SES tertiles; however, patients in the lowest SES tertile had smaller head circumferences, on average, than patients in the highest SES tertile. Over follow-up, patients in the lowest SES tertile had longer interstage periods and underwent Fontan conversion later compared with patients in the highest and middle SES tertiles. During this time, they were also more likely to experience complications and to undergo fewer cardiac catheterization procedures. Similar associations between neighborhood SES and patient characteristics were observed for those surviving to 5 years who were eligible for the parent-reported outcome assessments (Supplemental Table 7).
TABLE 1.
Sociodemographic and Clinical Characteristics by Neighborhood SES Tertile (N = 525)
| Lowest Tertile (n = 173) | Middle Tertile (n = 174) | Highest Tertile (n = 178) | Pa | |
|---|---|---|---|---|
| Demographic characteristics, n (%) | ||||
| Female | 72 (41.6) | 63 (36.2) | 67 (37.6) | .56 |
| Hispanic ethnicity | 52 (30.6) | 32 (18.7) | 15 (8.6) | <.001 |
| Racial minority | 54 (32.0) | 31 (17.9) | 19 (10.7) | <.001 |
| Neighborhood with >20% of residents living below the federal poverty level | 91 (52.6) | 11 (6.3) | 2 (1.1) | <.001 |
| Birth characteristics | ||||
| Prenatal diagnosis, n (%) | 127 (73.4) | 122 (70.1) | 151 (84.8) | .003 |
| Gestational age <37 wk, n (%) | 23 (13.3) | 21 (12.1) | 18 (10.1) | .65 |
| Birth wt, kg, mean (SD) | 3.06 (0.56) | 3.09 (0.51) | 3.15 (0.55) | .21 |
| Genetic syndrome, n (%) | 9 (8.2) | 7 (6.0) | 9 (6.7) | .80 |
| Anatomy, n (%) | ||||
| HLHS | 145 (83.8) | 141 (86.8) | 156 (87.6) | .56 |
| Norwood procedure | ||||
| Age at Norwood, d, median (IQR) | 6 (4–8) | 6 (4–8) | 6 (4–7) | .35 |
| Treatment assignment, n (%) | .44 | |||
| RV-PA conduit | 86 (49.7) | 82 (47.1) | 96 (53.9) | — |
| Modified BT shunt | 87 (50.3) | 92 (52.9) | 82 (46.1) | — |
| Sternum left open, n (%) | 135 (79.0) | 138 (79.8) | 134 (76.1) | .69 |
| Cross-clamp time, min, mean (SD) | 55.8 (23.7) | 56.8 (23.3) | 54.6 (22.7) | .68 |
| Bypass time, min, mean (SD) | 143.7 (60.0) | 143.3 (48.3) | 144.3 (55.3) | .99 |
| ECMO, n (%) | 13 (7.5) | 10 (5.8) | 10 (5.6) | .72 |
| No. complications during Norwood hospitalization, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–3) | .39 |
| Duration of intubation, d, median (IQR) | 7 (4–14) | 7 (5–12) | 7 (5–11) | .70 |
| Norwood length of stay, median (IQR) | 24.5 (16.5–42.0) | 24.0 (17.0–41.0) | 24.0 (16.0–40.0) | .70 |
| Wt z score pre-Norwood, mean (SD) | −0.51 (1.14) | −0.47 (1.12) | −0.20 (1.07) | .11 |
| Height z score pre-Norwood, mean (SD) | −0.30 (1.54) | −0.45 (1.51) | −0.53 (1.43) | .60 |
| Head circumference z score pre-Norwood, mean (SD) | −0.85 (1.40) | −0.70 (1.32) | −0.34 (1.35) | .033 |
| Post–Norwood procedure | ||||
| Age at stage 2 procedure, mo, mean (SD) | 5.6 (1.8) | 4.9 (2.3) | 5.0 (1.6) | .005 |
| Stage 2 type, n (%) | .52 | |||
| Bidirectional cavopulmonary shunt | 53 (63.1) | 59 (62.8) | 64 (59.3) | — |
| Hemi-Fontan | 19 (22.6) | 20 (21.3) | 34 (31.5) | — |
| Bidirectional cavopulmonary anastomosis | 9 (10.7) | 12 (12.8) | 9 (8.3) | — |
| Other | 3 (3.6) | 3 (3.2) | 1 (0.9) | — |
| Age at Fontan, y, mean (SD) | 3.20 (0.91) | 2.82 (0.89) | 2.76 (0.72) | <.001 |
| Fontan type, n (%) | .17 | |||
| Extracardiac | 25 (42.4) | 30 (42.9) | 52 (55.3) | — |
| Lateral tunnel | 34 (57.6) | 40 (57.1) | 42 (44.7) | — |
| Total No. surgeries up to 6 y including staged procedures, mean (SD) | 4.3 (1.7) | 4.0 (1.8) | 4.5 (1.9) | .13 |
| Total No. cardiac catheterizations up to 6 y, mean (SD) | 0.8 (1.5) | 1.2 (2.0) | 2.2 (3.3) | .002 |
| No. complications through 12 mo, median (IQR)c | 4 (2–7) | 4 (2–7) | 4 (2–7) | .86 |
| No. complications through 6 y, median (IQR)c | 7 (4–11) | 5 (3–8) | 6 (4–9) | .048 |
| Feeding issues, n (%) | 28 (44.4) | 21 (28.8) | 37 (38.5) | .16 |
| Required gastrostomy tube, n (%) | 18 (20.4) | 10 (5.8) | 20 (11.2) | .16 |
| Hospital characteristics | ||||
| Site volume (No. patients/y), n (%) | .35 | |||
| ≤15 | 34 (19.7) | 30 (17.2) | 29 (16.3) | — |
| 16–20 | 23 (13.3) | 40 (23.0) | 19 (16.3) | — |
| 21–30 | 60 (34.7) | 51 (29.3) | 62 (34.8) | — |
| >30 | 56 (32.4) | 53 (30.5) | 58 (32.6) | — |
| Mortality and transplant outcomes, n (%) | ||||
| 1-y mortality or transplant | 64 (37.0) | 54 (31.0) | 42 (23.6) | .024 |
| 1-y mortality | 60 (34.7) | 53 (30.5) | 34 (19.1) | .003 |
| 1-y transplant | 4 (2.3) | 1 (0.6) | 8 (4.5) | .054b |
| 6-y mortality or transplant | 77 (44.5) | 60 (34.5) | 61 (34.3) | .079 |
| 6-y mortality | 70 (40.5) | 59 (33.9) | 49 (27.5) | .038 |
| 6-y transplant | 7 (4.1) | 1 (0.6) | 12 (6.7) | .005b |
BT, Blalock-Taussig; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; —, not applicable.
P values are from χ2 tests for categorical variables and ANOVA or Kruskal-Wallis tests for continuous variables.
P values are from Fisher’s exact tests.
Complications included cardiac (arrhythmia, pericardial effusion, postpericardiotomy syndrome, hemopericardium, hypotension, hypertension, right ventricular dysfunction, valvar insufficiency or stenosis, superior and/or inferior vena cava stenosis or occlusion); respiratory (chronic respiratory failure, chylothorax, phrenic nerve injury, pneumothorax, vocal cord injury); neurologic (seizure, stroke, coma, hydrocephalus); gastrointestinal (liver failure, necrotizing enterocolitis, gastrointestinal bleeding); infectious (endocarditis, central line–associated bloodstream infection, pneumonia, sepsis, wound infection); renal (acute or chronic kidney injury); and hematologic (hemorrhage, thrombocytopenia).
Mortality
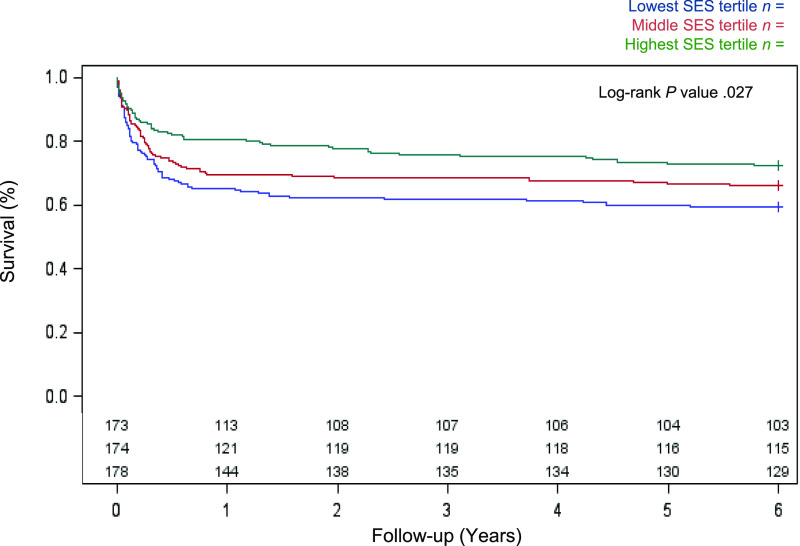
A total of 184 deaths and 20 cardiac transplants were documented during follow-up. Transplant rates were highest in the highest SES tertile (Table 1). Overall mortality was 28% at 1 year and 35% over the full follow-up period (minimum 6 years). In bivariate analyses, there was a stepwise relationship between neighborhood SES with 1-year and overall mortality, with the highest event rate observed in patients with low SES (overall log-rank P = .027; Fig 1).
FIGURE 1.
Kaplan-Meier estimates for unadjusted 6-year survival by neighborhood SES tertile.
After adjustment for birth and Norwood characteristics, the association between neighborhood SES with 1-year mortality remained significant (P = .018) but became attenuated over the full follow-up period (P = .055; Table 2). The hazard of mortality remained lower for patients in the highest SES tertile relative to the lowest tertile (hazard ratio 0.65 [95% confidence interval 0.45–0.92]). When analyses were repeated for 1-year transplant-free survivors (n = 365), the hazard of mortality was similar across SES tertiles.
TABLE 2.
Unadjusted and Adjusted Hazard Ratios for 1-Year and Overall Mortality by Neighborhood SES Tertiles
| Outcome | No. Events of N | Unadjusted | Model 1 Adjustedb | Race and/or Ethnicity–Adjusted Sensitivity Analysesc | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | Pa | HR (95% CI) | Pa | HR (95% CI) | Pa | ||
| 1-y mortality | 147 of 525 | .006 | .018 | .041 | |||
| Middle versus lowest tertile | — | 0.84 (0.59–1.21) | — | 0.85 (0.59–1.23) | — | 0.87 (0.59–1.27) | — |
| Highest versus lowest tertile | — | 0.50 (0.33–0.77) | — | 0.54 (0.35–0.83) | — | 0.56 (0.35–0.88) | — |
| Highest versus middle tertile | — | 0.60 (0.39–0.92) | — | 0.64 (0.41–0.99) | — | 0.64 (0.41–1.01) | — |
| Overall mortality | 184 of 525 | .026 | .055 | .14 | |||
| Middle versus lowest tertile | — | 0.80 (0.57–1.12) | — | 0.81 (0.57–1.14) | — | 0.83 (0.56–1.18) | — |
| Highest versus lowest tertile | — | 0.61 (0.43–0.88) | — | 0.65 (0.45–0.92) | — | 0.68 (0.46–1.00) | — |
| Highest versus middle tertile | — | 0.77 (0.53–1.11) | — | 0.80 (0.55–1.16) | — | 0.82 (0.56–1.20) | — |
| Overall mortality among 1-y survivors | 37 of 365 | .28 | .40 | .35 | |||
| Middle versus lowest tertile | — | 0.58 (0.24–1.39) | — | 0.62 (0.25–1.520) | — | 0.66 (0.26–1.67) | — |
| Highest versus lowest tertile | — | 1.12 (0.53–2.34) | — | 1.08 (0.51–2.29) | — | 1.22 ()0.54–2.76) | — |
| Highest versus middle tertile | — | 1.94 (0.85–4.46) | — | 1.75 (0.76–4.00) | — | 1.86 (0.80–4.29) | — |
CI, confidence interval; HR, hazard ratio; —, not applicable.
Global P values for SES variable (2 degrees of freedom) significance in model.
Multivariable models adjusted for prenatal diagnosis, birth weight, genetic syndrome, and treatment group.
Multivariable models adjusted for Hispanic ethnicity, person of color, prenatal diagnosis, birth weight, genetic syndrome, and treatment group.
Unadjusted Neurodevelopment, HRQOL, and Functional Status
Patient-reported outcome measures by neighborhood SES are summarized in Table 3.
TABLE 3.
Mean or Median Neurodevelopmental, Quality of Life, and Functional Status Outcomes Collected at 5 or 6 Years by Neighborhood SES Tertile
| Lowest Tertile (n = 97) | Middle Tertile (n = 115) | Highest Tertile (n = 118) | Unadjusted, Pa | Model 1 Adjusted, Pb | Model 2 Adjusted, Pc | Race and/or Ethnicity–Adjusted Sensitivity, Pd | |
|---|---|---|---|---|---|---|---|
| ASQ, median (IQR) | |||||||
| Communication | 50 (35–55) | 50 (40–60) | 55 (45–60) | .15 | .31 | .40 | .70 |
| Gross motor | 47.5 (30–55) | 45 (35–55) | 45 (35–55) | .99 | .95 | .67 | .56 |
| Fine motor | 30 (15–50) | 45 (25–55) | 47.5 (35–55) | .001 | .004 | .009 | .17 |
| Problem-solving | 50 (35–60) | 60 (47.5–60) | 60 (50–60) | <.001 | .001 | .001 | .065 |
| Personal-social | 50 (45–60) | 50 (40–60) | 55 (50–60) | .31 | .32 | .31 | .44 |
| Vineland overall, mean (SD) | |||||||
| Adaptive behavior composite | 93 (20) | 106 (22) | 102 (18) | .005 | .003 | .010 | .016 |
| Vineland subcomponents, mean (SD) | |||||||
| Communication | 97 (20) | 106 (20) | 108 (16) | .002 | .003 | .015 | .068 |
| Daily living skills | 97 (24) | 107 (24) | 103 (19) | .057 | .061 | .12 | .16 |
| Motor skills | 84 (18) | 94 (20) | 91 (15) | .014 | .014 | .041 | .062 |
| Socialization | 99 (21) | 106 (21) | 106 (17) | .069 | .065 | .12 | .33 |
| BASC-2, mean (SD) | |||||||
| Behavioral symptoms index | 51 (10) | 50 (12) | 49 (9) | .55 | .61 | .79 | .77 |
| Adaptive skills composite | 44 (12) | 47 (12) | 50 (11) | .014 | .031 | .26 | .22 |
| Externalizing problems | 49 (10) | 50 (11) | 49 (9) | .99 | .99 | .98 | .98 |
| Internalizing problems | 50 (11) | 48 (10) | 47 (8) | .29 | .28 | .33 | .29 |
| Peds-QL, mean (SD) | |||||||
| Total score | 72 (17) | 72 (16) | 75 (17) | .44 | .34 | .47 | .58 |
| Physical functioning | 69 (25) | 70 (22) | 73 (23) | .49 | .41 | .51 | .48 |
| Psychosocial functioning | 73 (15) | 74 (16) | 76 (16) | .50 | .39 | .54 | .73 |
| Social functioning | 75 (19) | 75 (19) | 78 (19) | .43 | .42 | .53 | .70 |
| Emotional functioning | 79 (19) | 76 (20) | 76 (17) | .58 | .72 | .60 | .74 |
| CHQ-PF50 | |||||||
| Physical functioning, mean (SD) | 41 (13) | 44 (12) | 44 (13) | .18 | .092 | .39 | .56 |
| Psychosocial functioning, mean (SD) | 51 (9) | 51 (9) | 52 (9) | .74 | .77 | .88 | .82 |
| FS-II(R), median (IQR)e | 94 (86–98) | 96 (90–98) | 98 (94–98) | .020 | .022 | .038 | .11 |
IQR, interquartile range.
P values represent Kruskal-Wallis test for medians and ANOVA for means.
P values represent linear regression adjusted for baseline characteristics including birth weight, prenatal diagnosis, genetic syndrome, and shunt type.
P values represent linear regression adjusted for baseline and post-Norwood characteristics from birth to 5 y including birth weight, prenatal diagnosis, genetic syndrome, shunt type, total number of surgeries including staged procedures, and total number of complications.
P values represent linear regression adjusted for baseline, post-Norwood, and race and/or ethnicity characteristics from birth to 5 y including birth weight, prenatal diagnosis, genetic syndrome, shunt type, total number of surgeries including staged procedures, total number of complications, person of color, and Hispanic ethnicity.
Variable transformed for nonlinearity by using a cubic transformation.
ASQ
Median fine motor and problem-solving scores on the ASQ were lower for patients in the lowest SES tertile compared with patients in higher tertiles. Communication, gross motor, and personal-social scores did not differ significantly by neighborhood SES.
Vineland
Patients in the middle and highest SES tertiles scored consistently higher (approximately one-half of 1 SD) on the Vineland overall and across all subcomponents than patients in the lowest tertile. These differences were most pronounced for the overall adaptive behavior composite, communication, and motor skills.
BASC-2
Patients in the lowest SES tertile scored lower on the BASC-2 adaptive skills than those in the middle and highest SES tertiles. There were no differences in behavioral symptoms, externalizing problems, or internalizing problems by neighborhood SES.
HRQOL
There were no significant differences in HRQOL by neighborhood SES as assessed by either the Peds-QL or CHQ-PF50 questionnaires.
FS-II(R)
Patients in all neighborhood SES tertiles performed highly on the FS-II(R) scale; however, patients in the lowest SES tertile scored lower than those in higher tertiles.
Adjusted Neurodevelopment, HRQOL, and Functional Status
After adjustment for birth and Norwood characteristics (model 1), all associations between neighborhood SES and neurodevelopmental outcomes persisted. Specifically, lower neighborhood SES was associated with poorer performance on the fine motor and problem-solving components of the ASQ; the adaptive behavior composite, communication skills, and motor skills on the Vineland; the adaptive behavior component on the BASC-2; and functional status on the FS-II(R) (Table 3).
After adjustment for interventions and complications that occurred during follow-up (model 2), the association between neighborhood SES and the adaptive behavior component on the BASC-2 was no longer present (P = .26) (Table 3). All other associations remained statistically significant.
Race and/or Ethnicity–Adjusted Sensitivity Analyses
Inclusion of race and ethnicity in the mortality multivariable models revealed further attenuation of the relationship between neighborhood SES and long-term mortality (P = .14; Table 2). Lower SES remained associated with poor adaptive behavior on the Vineland but became nonsignificant for all other neurodevelopmental outcomes (Table 3).
Discussion
Low SES predisposes patients with CHD to an array of adverse health outcomes. In previous reports from the SVR studies, authors have noted higher early mortality for children living in low SES neighborhoods4,7,8,10; however, these studies were limited to the first 14 months of follow-up. In addition to mortality, children with HLHS are at particularly high risk for impaired neurodevelopmental and behavioral outcomes. Goldberg et al12 found that living in a lower income area was associated with poorer problem-solving and personal-social scores on the ASQ at 3 years of age post-Norwood; however, there were only modest effects of SES on behavioral and HRQOL outcomes by 6 years of age.27 These reports provided preliminary insight into the relationship between SES and neurodevelopmental outcomes after the Norwood procedure but were not specifically designed to study SES. In the present analysis, we extend this inquiry to additional outcomes, explore potential mediators of this relationship, and expand the mortality analyses to 6 years.
In the current study, we found that children with HLHS living in areas of low neighborhood SES were at increased risk of long-term mortality and had more abnormalities in adaptive behavior and functional status. Specifically, patients in the lowest SES tertile scored lower on assessments of adaptive behavior, problem-solving, fine motor, and communication skills and reported poorer functional status at 6 years compared with those in the middle and highest SES tertiles. After adjustment for birth and Norwood characteristics, the differences in mortality across SES tertiles were no longer significant, but the differences in neurodevelopmental outcomes by SES persisted.
Adler and Newman28 have proposed a framework for understanding the pathways by which low SES affects health outcomes. Specifically, they propose 5 mechanisms: environmental exposures, social environment, health behaviors, affordability and access to health care, and chronic stress. They hypothesize that families living in low SES neighborhoods are more likely to live in lower-quality housing with greater residential crowding. These environments can increase the risk of infection, expose children to environmental pollutants, and limit opportunities for physical activity.29,30 In addition, low SES neighborhoods tend to have lower social cohesion and poorer social support, which are in turn linked to poorer self-care, lower health literacy, and elevated stress.31
Beyond environmental exposures, low SES affects access to care by limiting opportunities for health insurance. Families with low SES who do not qualify for Medicaid must often pay for more expensive plans with fewer benefits and higher deductibles, which can precipitate poor health care behaviors such as missing appointments or skipping medications. Families with low expendable income may also be forced to choose between basic needs such as nutrition, heat, or clean living conditions and medical care. This may be particularly true for families of children with CHD, for whom recurrent hospitalizations and surgeries coupled with outpatient services and medical technology create large medical expenditures. Indeed, we found that patients with low SES underwent stage 2 and Fontan procedures at older ages, which may reflect delayed follow-up due to cost. Likewise, indirect costs from lost wages and caregiver responsibilities further compound the financial burden of out-of-pocket expenses.32
Lower SES may affect neurocognitive development through environmental exposures, prenatal factors, parental stress, educational stimulation in the home, or nutrition.33 Studies in the general population have consistently demonstrated an association between higher family SES and improved cognitive development in childhood,33–35 and authors of MRI studies have provided supporting data linking changes in brain structure to SES, especially in areas related to memory and executive function.35–37 We observed a consistent association between SES and domains of problem-solving and adaptive behavior across multiple scales. Additionally, we found a relationship between SES and head circumference at birth, suggesting that these differences may begin in utero.
Interestingly, we did not find an association between neighborhood SES and HRQOL, whereas authors of other studies have documented a relationship.1,38–41 Many of those studies were performed in older CHD populations measuring individual SES and self-reported HRQOL rather than parental report. As such, it may be that differences in patient age, SES measurement, or perceived HRQOL explain some of these differences across studies.
A few of the neurodevelopmental instruments tested overlapping domains with differing results. For example, neighborhood SES was related to communication skills on the Vineland but not on the ASQ. We suspect that these differences across questionnaires are related to differences in the constructs tested and the scales used.
Authors of previous studies examining SES disparities have taken different approaches to adjusting for race and ethnicity. Whereas some have adjusted for both in their models, others have documented high collinearity and have not adjusted for these variables. Indeed, we found that SES was highly associated with race and ethnicity and removed much of the effect of SES when included in the models.
These findings highlight the importance of SES in long-term neurodevelopmental outcomes in children with HLHS and suggest possible avenues for intervention. Routine screening and surveillance of families at risk for financial stress can help connect families to local resources and facilitate appropriate follow-up care. Targeting areas such as health literacy, transportation, nutrition, medication compliance and providing safe, clean living conditions may improve outcomes for these children and alleviate parental stress.
One major limitation of this study is the inability to determine if the differences in neurodevelopmental outcomes by SES observed in our study are specific to patients with CHD or are similarly observed in the general population. Previous studies have demonstrated an association between lower SES and poorer cognitive function in kindergarten in the general population; however, these studies have been mostly focused on reading and math skills rather than behavior, motor, and language skills.42–44 Many of the same mechanisms (eg, home learning environment, parenting style and/or beliefs, early education exposure) likely contribute to the differences in cognitive development by SES. Additional limitations of the study include the use of parent-reported outcome measures, which may overestimate a child’s abilities, the measurement of SES at a single time point given the lack of long-term follow-up data on changes to residence, and the investigation of multiple outcomes that may be subject to the multiple comparisons problem.
Conclusions
We found that children with single ventricle heart disease living in low SES neighborhoods had higher crude long-term mortality after the Norwood procedure and scored lower on assessments of adaptive behavior and problem-solving. The differences in mortality were largely explained by other patient characteristics; however, the neurodevelopmental differences persisted after adjustment. Future investigations into modifiable risk factors for neurodevelopmental outcomes are needed to better understand areas of intervention to improve outcomes across the spectrum of SES.
Glossary
- ANOVA
analysis of variance
- ASQ
Ages and Stages Questionnaire
- BASC-2
Behavior Assessment System for Children, Second Edition
- CHD
congenital heart disease
- CHQ-PF50
Child Health Questionnaire Parent Form 50
- FS-II(R)
Functional Status II(R)
- HLHS
hypoplastic left heart syndrome
- HRQOL
health-related quality of life
- Peds-QL
Pediatric Quality of Life Inventory
- RV-PA
right ventricle-to-pulmonary artery
- SES
socioeconomic status
- SVR
Single Ventricle Reconstruction
- SVR II
Single Ventricle Reconstruction Extension
Footnotes
Dr Bucholz conceptualized and designed the study, performed and interpreted the data analyses, and drafted and revised the manuscript; Dr Sleeper provided analytic support, interpreted the data analyses, and revised the manuscript; Drs Goldberg, Pasquali, Anderson, Gaynor, and Cnota interpreted the data analyses and revised the manuscript; Dr Newburger conceptualized and designed the study, interpreted the data analyses, and revised the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was conducted under a Scholar Award from the Pediatric Heart Network supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number U10HL068270 (Dr Bucholz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Cassedy A, Drotar D, Ittenbach R, et al. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. 2013;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies RR, Russo MJ, Reinhartz O, et al. Lower socioeconomic status is associated with worse outcomes after both listing and transplanting children with heart failure. Pediatr Transplant. 2013;17(6):573–581 [DOI] [PubMed] [Google Scholar]
- 3.Garcia Guerra G, Robertson CMT, Alton GY, et al. ; Western Canadian Complex Pediatric Therapies Follow-up Group . Quality of life 4 years after complex heart surgery in infancy. J Thorac Cardiovasc Surg. 2013;145(2):482–488.e2 [DOI] [PubMed] [Google Scholar]
- 4.Ghanayem NS, Allen KR, Tabbutt S, et al. ; Pediatric Heart Network Investigators . Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucik JE, Nembhard WN, Donohue P, et al. Community socioeconomic disadvantage and the survival of infants with congenital heart defects. Am J Public Health. 2014;104(11):e150–e157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie AS, Gauvreau K, Newburger JW, Mayer JE, Erickson LC. Risk factors for readmission after neonatal cardiac surgery. Ann Thorac Surg. 2004;78(6):1972–1978; discussion 1978 [DOI] [PubMed] [Google Scholar]
- 7.Tabbutt S, Ghanayem N, Ravishankar C, et al. ; Pediatric Heart Network Investigators . Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor LC, Burke B, Donohue JE, et al. Risk factors for interstage mortality following the Norwood procedure: impact of sociodemographic factors. Pediatr Cardiol. 2016;37(1):68–75 [DOI] [PubMed] [Google Scholar]
- 9.Werner H, Latal B, Valsangiacomo Buechel E, Beck I, Landolt MA. Health-related quality of life after open-heart surgery. J Pediatr. 2014;164(2):254.e1-258.e1 [DOI] [PubMed] [Google Scholar]
- 10.Bucholz EM, Sleeper LA, Newburger JW. Neighborhood socioeconomic status and outcomes following the Norwood procedure: an analysis of the Pediatric Heart Network Single Ventricle Reconstruction trial public data set. J Am Heart Assoc. 2018;7(3):e007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newburger JW, Sleeper LA, Gaynor JW, et al. ; Pediatric Heart Network Investigators . Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137(21):2246–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg CS, Lu M, Sleeper LA, et al. ; Pediatric Heart Network Investigators . Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165(3):490.e8-496.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohye RG, Gaynor JW, Ghanayem NS, et al. ; Pediatric Heart Network Investigators . Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136(4):968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106 [DOI] [PubMed] [Google Scholar]
- 15.Squires J, Bricker D. Ages & Stages Questionnaires, Third Edition (ASQ-3): A Parent-Completed Child Monitoring System, 3rd ed Baltimore, MD: Brookes Publishing Company; 2009 [Google Scholar]
- 16.Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M. Validity of the Ages and Stages Questionnaires in term and preterm infants. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1468 [DOI] [PubMed] [Google Scholar]
- 17.Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol. 1985;10(2):215–225 [DOI] [PubMed] [Google Scholar]
- 18.Sparrow SS, Ciccheti DV, Saulnier CA. Vineland Adaptive Behavior Scales, 3rd ed Bloomington, MN: Pearson; 2016 [Google Scholar]
- 19.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children (BASC). Circle Pines, MN: American Guidance Services; 1992 [Google Scholar]
- 20.Varni JW, Burwinkle TM, Seid M. The PedsQL as a pediatric patient-reported outcome: reliability and validity of the PedsQL Measurement Model in 25,000 children. Expert Rev Pharmacoecon Outcomes Res. 2005;5(6):705–719 [DOI] [PubMed] [Google Scholar]
- 21.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341 [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812 [DOI] [PubMed] [Google Scholar]
- 23.HealthActCHQ. CHQ: Child Health Questionnaire. 2018. Available at: https://www.healthactchq.com/survey/chq. Accessed January 2, 2019
- 24.Raat H, Bonsel GJ, Essink-Bot ML, Landgraf JM, Gemke RJBJ. Reliability and validity of comprehensive health status measures in children: the Child Health Questionnaire in relation to the Health Utilities Index. J Clin Epidemiol. 2002;55(1):67–76 [DOI] [PubMed] [Google Scholar]
- 25.Stein REK, Jessop DJ. Functional Status II(R). A measure of child health status. Med Care. 1990;28(11):1041–1055 [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509 [Google Scholar]
- 27.Goldberg CS, Hu C, Brosig C, et al. ; PHN Investigators . Behavior and quality of life at 6 years for children with hypoplastic left heart syndrome. Pediatrics. 2019;144(5):e20191010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76 [DOI] [PubMed] [Google Scholar]
- 29.Fuller-Thomson E, Hulchanski JD, Hwang S. The housing/health relationship: what do we know? Rev Environ Health. 2000;15(1–2):109–133 [DOI] [PubMed] [Google Scholar]
- 30.Krieger J, Higgins DL. Housing and health: time again for public health action. Am J Public Health. 2002;92(5):758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192–2217 [DOI] [PubMed] [Google Scholar]
- 32.Connor JA, Kline NE, Mott S, Harris SK, Jenkins KJ. The meaning of cost for families of children with congenital heart disease. J Pediatr Health Care. 2010;24(5):318–325 [DOI] [PubMed] [Google Scholar]
- 33.Ronfani L, Vecchi Brumatti L, Mariuz M, et al. The complex interaction between home environment, socioeconomic status, maternal IQ and early child neurocognitive development: a multivariate analysis of data collected in a newborn cohort study. PLoS One. 2015;10(5):e0127052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399 [DOI] [PubMed] [Google Scholar]
- 35.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brito NH, Noble KG. Socioeconomic status and structural brain development. Front Neurosci. 2014;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang L, Su Z, Liu Y, et al. Impact of family socioeconomic status on health-related quality of life in children with critical congenital heart disease. J Am Heart Assoc. 2019;8(1):e010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrindle BW, Williams RV, Mitchell PD, et al. ; Pediatric Heart Network Investigators . Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113(8):1123–1129 [DOI] [PubMed] [Google Scholar]
- 40.Diller GP, Inuzuka R, Kempny A, et al. Detrimental impact of socioeconomic status on exercise capacity in adults with congenital heart disease. Int J Cardiol. 2013;165(1):80–86 [DOI] [PubMed] [Google Scholar]
- 41.Landolt MA, Valsangiacomo Buechel ER, Latal B. Health-related quality of life in children and adolescents after open-heart surgery. J Pediatr. 2008;152(3):349–355 [DOI] [PubMed] [Google Scholar]
- 42.Larson K, Russ SA, Nelson BB, Olson LM, Halfon N. Cognitive ability at kindergarten entry and socioeconomic status. Pediatrics. 2015;135(2). Available at: www.pediatrics.org/cgi/content/full/135/2/e440 [DOI] [PubMed] [Google Scholar]
- 43.Dearden L, Sibieta L, Sylva K. The socio-economic gradient in early child outcomes: evidence from the Millennium Cohort Study. Longit Life Course Stud. 2011;2(1):19–40 [Google Scholar]
- 44.Feinstein L. Inequality in the early cognitive development of British children in the 1970 cohort. Economic. 2003;70(277):73–97 [Google Scholar]