In this secondary analysis of a randomized clinical trial, we tested the effects of DHA + AA supplementation versus placebo for 180 days on caregiver-reported socioemotional development of toddlers born preterm.
Abstract
BACKGROUND AND OBJECTIVES:
Children born preterm experience socioemotional difficulties, including increased risk of autism spectrum disorder (ASD). In this secondary analysis, we tested the effect of combined docosahexaenoic acid (DHA) and arachidonic acid (AA) supplementation during toddlerhood on caregiver-reported socioemotional outcomes of children born preterm. We hypothesized that children randomly assigned to DHA + AA would display better socioemotional outcomes compared with those randomly assigned to a placebo.
METHODS:
Omega Tots was a single-site randomized, fully masked, parallel-group, placebo-controlled trial. Children (N = 377) were 10 to 16 months at enrollment, born at <35 weeks’ gestation, and assigned to 180 days of daily 200-mg DHA + 200-mg AA supplementation or a placebo (400 mg corn oil). Caregivers completed the Brief Infant-Toddler Social and Emotional Assessment and the Pervasive Developmental Disorders Screening Test–II, Stage 2 at the end of the trial. Liner mixed models and log-binomial regression compared socioemotional outcomes between the DHA + AA and placebo groups.
RESULTS:
Outcome data were available for 83% of children (ntreatment = 161; nplacebo = 153). Differences between DHA + AA and placebo groups on Brief Infant-Toddler Social and Emotional Assessment scores were of small magnitude (Cohen’s d ≤ 0.15) and not statistically significant. Children randomly assigned to DHA + AA had a decreased risk of scoring at-risk for ASD on the Pervasive Developmental Disorders Screening Test–II, Stage 2 (21% vs 32%; risk ratio = 0.66 [95% confidence interval: 0.45 to 0.97]; risk difference = −0.11 [95% confidence interval: −0.21 to −0.01]) compared with children randomly assigned to a placebo.
CONCLUSIONS:
No evidence of benefit of DHA + AA supplementation on caregiver-reported outcomes of broad socioemotional development was observed. Supplementation resulted in decreased risk of clinical concern for ASD. Further exploration in larger samples of preterm children and continued follow-up of children who received DHA + AA supplementation as they approach school age is warranted.
What’s Known on This Subject:
Preterm children are at increased risk for socioemotional difficulties, including autism spectrum disorder (ASD). Docosahexaenoic acid (DHA) supplementation may reduce ASD behaviors, but effects on socioemotional development more broadly are less clear.
What This Study Adds:
Differences between the DHA + arachidonic acid and placebo groups on socioemotional development were not statistically significant, and effects were small. Children randomly assigned to DHA + arachidonic acid had a decreased risk of clinical concern for ASD compared with children randomly assigned to a placebo.
In the United States, 1 of every 10 infants is born preterm (<37 completed weeks’ gestation).1 Children born preterm experience higher rates of cognitive impairments, behavioral problems, and socioemotional difficulties, including higher incidence of autism spectrum disorder (ASD), compared with term children.2–7
Animal and cell studies suggest that docosahexaenoic acid (DHA) supports neurotransmitter function, signal transduction, gene expression, neurogenesis, and anti-inflammation, which may impact socioemotional development.8–14 DHA with the addition of arachidonic acid (AA) has been found to support physical growth and visual acuity in preterm infants.15–18 Preterm infants miss some or all of the last trimester of pregnancy when rapid acquisition of DHA and AA occurs in the developing brain.19–21 Insufficient DHA may contribute to the developmental delays that children born preterm experience.
DHA supplementation may enhance aspects of cognitive ability and reduce behaviors commonly associated with ASD.22,23 Effects on broader aspects of socioemotional development are mixed. Trials that supplemented neonates reported no benefit on socioemotional outcomes to age 5 years.6,24,25 However, negative long-term effects have been reported at 7 years for girls born preterm.26
In this secondary analysis, we sought to test the effect of DHA + AA supplementation for 180 days on caregiver-reported socioemotional outcomes, including behaviors commonly associated with ASD, in a sample of toddlers born at <35 completed weeks’ gestation. Because authors of previous research reported negative effects of DHA supplementation on multiple aspects of socioemotional outcomes at 7 years for girls born preterm, we used exploratory subgroup analyses to examine treatment effects by child sex.26
Methods
Study Design and Setting
Omega Tots was a single-site, randomized, fully masked, parallel-group, placebo-controlled trial (identifier NCT01576783) conducted at Nationwide Children’s Hospital (NCH) (Columbus, OH) and approved by the NCH Institutional Review Board. Caregivers provided written informed consent for participation. Enrollment in the 180-day trial took place between April 2012 and September 2016. Socioemotional development was not a prespecified primary outcome of the trial, but measures of the child’s socioemotional development were included in the protocol from the beginning as other prespecified outcomes. Analysis of the primary outcomes revealed that supplementation resulted in no improvement in cognitive development or executive function and may have resulted in negative effects on language development and effortful control in certain subgroups of children. These findings and the full protocol are presented elsewhere.27,28
Participants and Sample Size
Participants were children 10 to 16 months of prematurity-adjusted age at enrollment who were born at <35 completed weeks’ gestation and admitted to a Columbus, Ohio, NICU post birth or had a neonatology clinic follow-up visit scheduled at NCH.
Eligible children weighed between the fifth and 95th percentiles for corrected age and sex and had discontinued human milk and formula consumption, and English was the family’s primary language. Children were excluded for consuming fatty acid supplements, fatty fish, or nutritional-support beverages with DHA >2 times per week; for having fish, corn, or soy allergy; for planning to relocate; or for having a major malformation or feeding, metabolic, or digestive disorder precluding participation or nutrient absorption. Three hundred seventy-seven children enrolled and were randomly assigned (Fig 1). All were included in the analysis. The study was powered on the basis of the primary outcome.27
FIGURE 1.
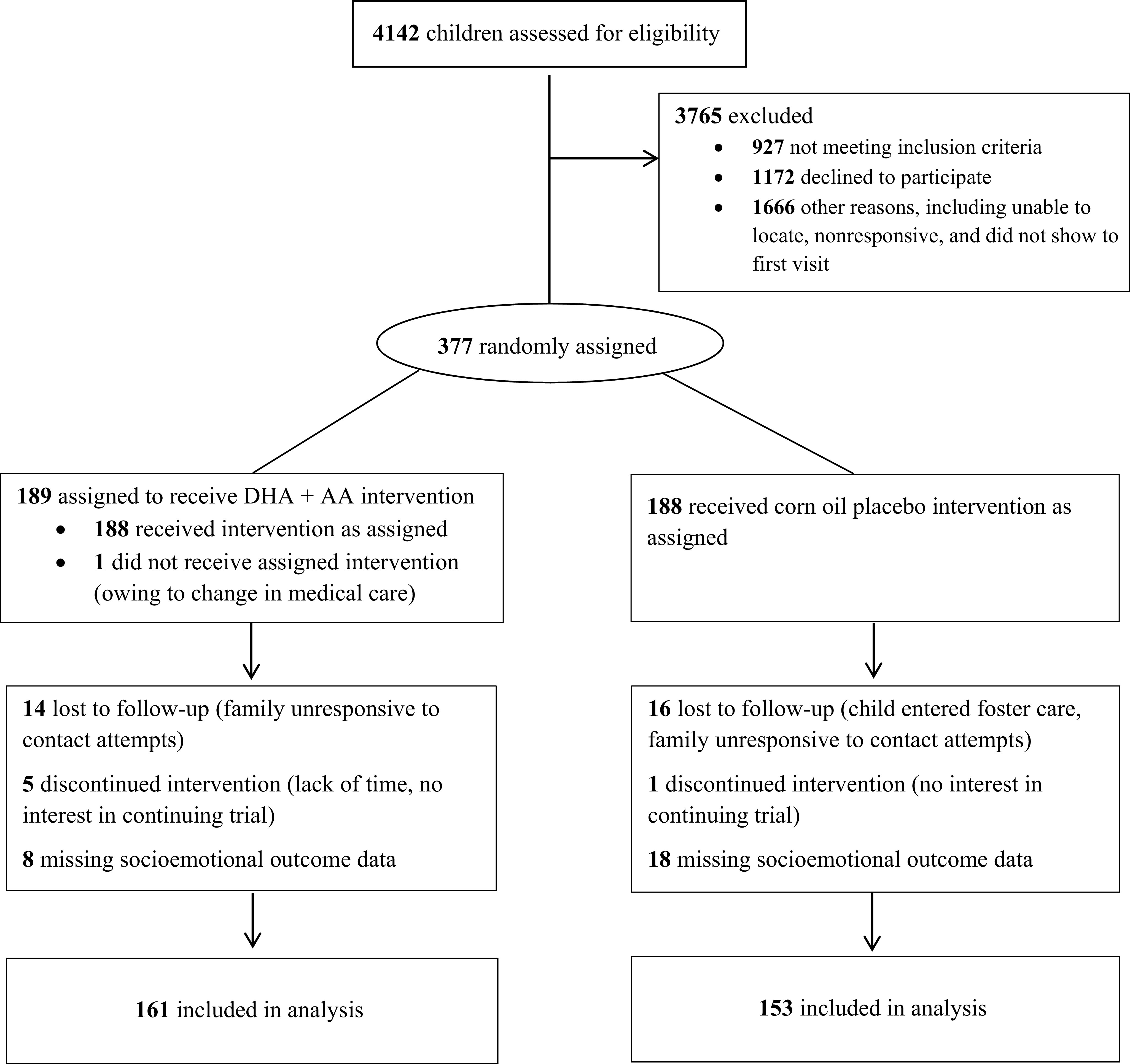
Participant Consolidated Standards of Reporting Trials flow diagram, Omega Tots trial (N = 377), 2012–2017.
Randomization, Blinding, and Intervention
Children were allocated to treatment or placebo by using a randomization scheme with block sizes of 4 and 8, with 1:1 allocation to treatment (DHA + AA) or placebo. A statistician with no participant contact prepared the randomization scheme, assigned identification numbers, and prepared opaque, sealed, tamper-resistant envelopes before the study began. After enrollment, study staff opened the next sequentially numbered envelope to assign children to DHA + AA or placebo. All investigators, families, and staff remained blinded throughout the trial. Sets of twins and triplets were randomly assigned together.29
The treatment group was assigned to 180 days of daily oral supplementation with dissolvable, 200-mg microencapsulated DHA (from Schizochytrium species algal oil), and 200-mg AA (from fungal Mortierella alpina oil) powder (DSM Nutritional Products, Heerlen, Netherlands). The dose was selected to approximate the peak daily DHA consumed per body weight during infancy (ie, for a typical 4-month-old infant, 20 mg/kg per day). DHA and AA were both included because earlier trials revealed that this supported neonatal growth.17,30 A 1:1 balance was selected because this is in line with international formula standards and was recently reinforced by expert opinion.31,32 The 180-day period was selected to ensure significant incorporation of DHA into neuronal cell membranes while maximizing enrollment and retention because behavioral changes have been reported among young children after 90 days of supplementation in other fatty acid trials.22,33 Because of the dearth of trials involving toddlers born preterm, the 180-day period was conservatively selected. The placebo group was assigned to 180 days of a matching placebo: 400 mg of a daily microencapsulated corn oil powder. DSM Nutritional Products packaged both products identically in foil packets. The NCH Investigational Drug Services distributed the packets directly to families and advised families to dissolve 2 packets daily in the child’s milk or food for the duration of the trial.
Compliance and Adverse Events
Caregivers recorded the number of packets given each day to measure compliance. The study physician reviewed adverse events and assessed whether they were related to the intervention.
Data Collection
The baseline study visit (day 0) included a caregiver-completed questionnaire that collected demographics and child diet information. At the end of the trial (day 180, prespecified time point of interest), child socioemotional development was assessed and caregivers were asked to guess their child’s treatment assignment. Children’s age, gestational age, and birth weight were obtained from electronic medical records.
DHA Food Frequency Questionnaire
Using the DHA Food Frequency Questionnaire, caregivers reported the number of servings per month of fatty fish, moderately fatty fish, and white fish and/or shellfish the child consumed.34 Results provided an estimated DHA and eicosapentaenoic acid (EPA) (a precursor to DHA) intake (milligrams per day).34Intake as reported on this questionnaire has been positively correlated with plasma phospholipid and erythrocyte fatty acid levels in previous research.
Brief Infant-Toddler Social and Emotional Assessment
The Brief Infant-Toddler Social and Emotional Assessment (BITSEA) is composed of 42 items for assessing socioemotional and behavioral problems or delays in toddlerhood. Caregivers answered each item as not true or rarely (0), somewhat true or sometimes (1), or very true or often (2) of the child’s behavior in the previous month. The BITSEA has established scales: competence (11 items) and problem (31 items). Item scores were summed to provide competence (possible range: 0–22) and problem (possible range: 0–62) scores, respectively. The scales have excellent test-retest reliability (competence = 0.82; problem = 0.92) and have been well validated.35 The problem scale is further divided into subscales: externalizing (6 items; possible range: 0–12), internalizing (8 items; possible range: 0–16), and dysregulation (8 items; possible range: 0–16). Fourteen items comprise a red flag scale (possible range: 0–28). The BITSEA developers identified 8 competence items and 9 problem items that are behaviors often seen in children with ASD. Each ASD item was dichotomized to illustrate the presence (1) (ie, competence items absent, problem items present) or absence (0) (ie, competence items present, problem items absent) of each behavior.35 Items were summed to derive an ASD score (possible range: 0–17). Higher competence scores represented better functioning; higher problem, red flag, and ASD scores represented poorer functioning. All summed BITSEA scores were calculated and interpreted on the basis of standardized procedures.
Per manual recommendations, children scoring in the ≤14th percentile on the competence scale and the ≤24th percentile on the problem scale were categorized as having a possible delay in that domain.35 The competence, problem, and ASD scales were also dichotomized at cut scores (competence = ≤14; problem = ≥13; ASD = ≥6) previously reported to be predictive of ASD diagnoses for children born preterm.36 The cut scores have acceptable sensitivity (competence = 0.74; problem = 0.78; ASD = 0.70) and specificity (competence = 0.76; problem = 0.68; ASD = 0.73) for predicting ASD in children born preterm.36
Pervasive Developmental Disorders Screening Test–II, Stage 2
The Pervasive Developmental Disorders Screening Test–II, Stage 2 (PDDST-II) is a clinically derived, caregiver-completed screener to assist in differentiating an ASD diagnosis from other disorders in children with developmental concerns, including those born preterm. The PDDST-II is composed of 14 yes or no items that indicate the presence (1) or absence (0) of developmental concerns. Items were summed (possible range: 0–14), and higher scores represented greater developmental concern; a cut score ≥5 signified concern for ASD (sensitivity = 0.73; specificity = 0.49).37
Statistical Analysis
For all analyses, we used SAS software (version 9.4; SAS Institute, Inc, Cary, NC) and analyses were conducted according to intent-to-treat methods. No interim analyses were conducted. In analyses of treatment effects, we compared BITSEA and PDDST-II continuous scores at the end of the trial between groups using a linear mixed model. Analyses included a random effect for the family to address statistical nonindependence because of the inclusion of multiple-gestation births. Group mean differences divided by the SD were calculated as standardized effect sizes (Cohen’s d). Log-binomial regression was used to calculate risk ratios (RRs) for binary outcomes, and identity link with binomial error structure was used to calculate risk differences (RDs).38 The within-family correlation was accounted for by using generalized estimating equations. Interactions with child sex were tested by using 2-way interaction terms. Exploratory post hoc subgroup analyses based on child sex were performed. Adjustments for multiple comparisons were not made.39,40
Results
Participant Characteristics
The median age of children was 15.7 (interquartile range [IQR] = 2.9) months at enrollment; 15.4% were born at ≤28 completed weeks’ gestation. On average, children weighed 1727 g at birth, and 48.3% were female. All but 1 child received their assigned intervention. Most respondents were mothers (96.0%) with a median age of 30.8 (IQR = 9.3) years at enrollment; 67.5% were married or living with a partner, 49.7% reported having public or no insurance, 46.5% reported an annual household income of <$35 000 US dollars, and 27.7% had a high school or general equivalency diploma (GED) or lower level of education. Baseline characteristics were similar between treatment groups (Table 1). Eighty-three percent of enrolled children (ntreatment = 161; nplacebo = 153) had socioemotional outcome data. Children with socioemotional outcome data were more likely to be white and less likely to be in receipt of public or no insurance or live in a household with income <$35 000 US dollars compared with children without socioemotional outcome data (Supplemental Table 4). Their caregivers were also more likely to be married or living with a partner and more likely to have a higher level of education.
TABLE 1.
Participant Characteristics at Baseline, Omega Tots Trial (N = 377), 2012–2017
| Baseline Characteristics | Full Sample (N = 377) | DHA + AA (n = 189) | Placebo (n = 188) | No. Missing Observations |
|---|---|---|---|---|
| Child characteristics | ||||
| Age at randomization, mo, adjusted for prematurity, median (IQR) | 15.7 (2.9) | 15.7 (2.8) | 15.6 (3.4) | 0 |
| Gestational age at delivery, completed wk, median (IQR) | 32.0 (4.0) | 32.0 (4.0) | 32.0 (4.0) | 0 |
| Birth wt, g, mean (SD) | 1727.4 (552.3) | 1705.4 (534.3) | 1749.5 (570.4) | 1 |
| Birth wt <1250 g, n (%) | 81 (21.5) | 45 (23.9) | 36 (19.2) | 1 |
| Sets of multiple births (eg, twins, triplets), n | 55 | 29 | 26 | 0 |
| Child sex female, n (%) | 182 (48.3) | 84 (44.4) | 98 (52.1) | 0 |
| Race, n (%) | ||||
| White | 236 (62.6) | 114 (60.3) | 122 (64.9) | 0 |
| African American or Black | 105 (27.9) | 52 (27.5) | 53 (28.2) | — |
| Other or multiple races | 36 (9.6) | 23 (12.2) | 13 (6.9) | — |
| Hispanic or Latino ethnicity, n (%) | 17 (4.5) | 7 (3.7) | 10 (5.3) | 0 |
| Dietary DHA + EPA intake before randomization, mg/d, median (IQR) | 48.0 (63.6) | 44.3 (53.8) | 55.3 (68.7) | 0 |
| Caregiver and household characteristics | ||||
| Relationship to child, n (%) | ||||
| Mother | 362 (96.0) | 186 (98.4) | 176 (93.6) | 0 |
| Age at enrollment, y, median (IQR) | 30.8 (9.3) | 30.8 (8.7) | 30.8 (10.2) | 0 |
| Marital status, n (%) | ||||
| Married or living with partner | 253 (67.5) | 129 (68.6) | 124 (66.3) | 2 |
| Separated or divorced | 18 (4.8) | 7 (3.7) | 11 (5.9) | — |
| Single, never married, or not living with partner | 104 (27.7) | 52 (27.7) | 52 (27.8) | — |
| Public or no health insurance, n (%) | 187 (49.7) | 96 (51.1) | 91 (48.4) | 1 |
| Annual household income <$35 000, n (%) | 174 (46.5) | 85 (45.2) | 89 (47.9) | 3 |
| Education, n (%) | ||||
| High school or GED or less | 104 (27.7) | 47 (25.0) | 57 (30.3) | 1 |
| Some college or associate’s degree | 136 (36.2) | 73 (38.8) | 63 (33.5) | — |
| Bachelor’s degree or higher | 136 (36.2) | 68 (36.2) | 68 (36.2) | — |
—, not applicable.
Compliance, Blinding, and Adverse Events
Children consumed 81% of the prescribed study product (of children with adherence data). At trial end, 55% of caregivers in the treatment group and 60% in the placebo group guessed their child was assigned to the treatment group, suggesting that blinding remained intact (χ2 = 0.65; P = .42). The proportion of participants reporting adverse events was similar between groups (1.7 per child in the DHA + AA group; 2.0 per child in the placebo group; difference = −0.37 [95% confidence interval (CI): −0.07 to 0.80]; P = .10). No adverse events were judged to be serious and related to the intervention.
Treatment Effect on Caregiver-Reported Socioemotional Outcomes, Full Sample
Competence did not differ between the DHA + AA and placebo groups (Cohen’s d = 0.10; P = .42; Table 2). The problem scale (Cohen’s d = 0.05; P = .68), and the externalizing (Cohen’s d = 0.03; P = .78), internalizing (Cohen’s d = 0.12; P = .32), and dysregulation (Cohen’s d = −0.005; P = .97) subscales also did not differ by treatment group at the end of the trial, nor did the red flag (Cohen’s d = 0.06; P = .62) or continuous ASD (Cohen’s d = 0.05; P = .69) BITSEA scales. Similarly, PDDST-II continuous scores did not differ between groups (Cohen’s d = 0.15; P = .22).
TABLE 2.
Difference in Continuous Socioemotional Outcomes at End of 180-Day Trial, Omega Tots Trial, 2012–2017
| Outcome | DHA + AA/Placebo, No. | Score at End of Trial, Mean (SD) | Difference, β (95% CI)a | Effect Size | P | Interaction Term P, Child Sex | |
|---|---|---|---|---|---|---|---|
| DHA + AA | Placebo | ||||||
| Full sample | |||||||
| BITSEA competence | 161/152 | 16.50 (3.09) | 16.73 (2.94) | .29 (−0.42 to 0.99) | 0.10 | .42 | .24 |
| BITSEA problem | 161/153 | 9.81 (6.01) | 10.18 (6.84) | .31 (−1.19 to 1.81) | 0.05 | .68 | .22 |
| BITSEA externalizing | 161/153 | 2.29 (2.06) | 2.30 (1.98) | .07 (−0.40 to 0.54) | 0.03 | .78 | .70 |
| BITSEA internalizing | 161/153 | 2.04 (1.58) | 2.31 (2.10) | .22 (−0.21 to 0.65) | 0.12 | .32 | .17 |
| BITSEA dysregulation | 161/153 | 3.25 (2.57) | 3.29 (2.59) | −.01 (−0.62 to 0.60) | −0.005 | .97 | .43 |
| BITSEA red flag | 161/153 | 2.93 (2.48) | 3.12 (2.85) | .16 (−0.46 to 0.78) | 0.06 | .62 | .59 |
| BITSEA ASD | 161/152 | 4.11 (2.29) | 4.36 (2.98) | .13 (−0.49 to 0.74) | 0.05 | .69 | .14 |
| PDDST-II | 160/153 | 2.91 (2.35) | 3.37 (3.02) | .39 (−0.24 to 1.02) | 0.15 | .22 | .80 |
| Female sex | |||||||
| BITSEA competence | 69/81 | 17.29 (3.03) | 16.91 (3.02) | −.19 (−1.19 to 0.82) | −0.06 | .71 | — |
| BITSEA problem | 69/81 | 8.68 (5.19) | 9.90 (6.67) | 1.13 (−0.88 to 3.14) | 0.18 | .27 | — |
| BITSEA externalizing | 69/81 | 1.71 (1.61) | 1.86 (1.84) | .15 (−0.43 to 0.74) | 0.09 | .61 | — |
| BITSEA internalizing | 69/81 | 1.99 (1.55) | 2.44 (2.22) | .41 (−0.23 to 1.06) | 0.21 | .21 | — |
| BITSEA dysregulation | 69/81 | 2.91 (2.42) | 3.17 (2.32) | .26 (−0.53 to 1.06) | 0.11 | .51 | — |
| BITSEA red flag | 69/81 | 2.58 (2.29) | 3.23 (2.90) | .60 (−0.29 to 1.50) | 0.22 | .18 | — |
| BITSEA ASD | 69/81 | 3.55 (2.13) | 4.41 (3.02) | .72 (−0.16 to 1.61) | 0.27 | .11 | — |
| PDDST-II | 69/81 | 2.84 (2.29) | 3.35 (3.01) | .36 (−0.55 to 1.27) | 0.13 | .44 | — |
| Male sex | |||||||
| BITSEA competence | 92/71 | 15.90 (3.01) | 16.52 (2.86) | .63 (−0.32 to 1.58) | 0.21 | .19 | — |
| BITSEA problem | 92/72 | 10.66 (6.45) | 10.49 (7.06) | −.20 (−2.40 to 1.99) | −0.03 | .86 | — |
| BITSEA externalizing | 92/72 | 2.73 (2.26) | 2.79 (2.03) | .14 (−0.56 to 0.84) | 0.07 | .69 | — |
| BITSEA internalizing | 92/72 | 2.08 (1.61) | 2.15 (1.97) | .01 (−0.57 to 0.60) | 0.01 | .96 | — |
| BITSEA dysregulation | 92/72 | 3.50 (2.65) | 3.43 (2.86) | −.15 (−1.05 to 0.75) | −0.05 | .75 | — |
| BITSEA red flag | 92/72 | 3.20 (2.59) | 3.00 (2.81) | −.16 (−1.05 to 0.72) | −0.06 | .72 | — |
| BITSEA ASD | 92/71 | 4.52 (3.34) | 4.30 (2.94) | −.26 (−1.10 to 0.58) | −0.10 | .54 | — |
| PDDST-II | 91/72 | 2.96 (2.41) | 3.50 (3.04) | .52 (−0.35 to 1.40) | 0.19 | .24 | — |
—, not applicable.
The difference column is based on a mixed-effect model and includes random effects for the family, and thus the difference in score on each outcome may differ from the results obtained when calculating the raw difference between treatment and placebo. The values are represented in terms of the original metric.
The percentage of children scoring in at-risk ranges did not differ between groups for any of the dichotomized BITSEA scores (Table 3). Children randomly assigned to DHA + AA had a decreased risk of scoring above the PDDST-II cut score (indicating clinical concern) at trial end compared with those randomly assigned to placebo (RR = 0.66 [95% CI: 0.45 to 0.97]; RD = −0.11 [95% CI: −0.21 to −0.01]).
TABLE 3.
Risk of Dichotomized Socioemotional Outcomes at End of 180-Day Trial, Omega Tots Trial, 2012–2017
| DHA + AA/Placebo, No. at End of Trial | Caregiver Endorsed at End of 180-d Trial, n (%) | RR (95% CI) | RD (95% CI) | Interaction Term P, Child Sex | ||
|---|---|---|---|---|---|---|
| DHA + AA | Placebo | |||||
| Full sample | ||||||
| BITSEA competence, delayed | 161/152 | 22 (14) | 25 (16) | 0.87 (0.50 to 1.50) | −0.02 (−0.10 to 0.06) | .15 |
| BITSEA competence, ASD risk cutoff | 161/152 | 32 (20) | 35 (23) | 0.91 (0.59 to 1.42) | −0.02 (−0.11 to 0.07) | .05 |
| BITSEA problem, delayed | 161/153 | 35 (22) | 35 (23) | 0.97 (0.64 to 1.48) | −0.01 (−0.10 to 0.09) | .45 |
| BITSEA problem, ASD risk cutoff | 161/153 | 42 (26) | 39 (25) | 1.07 (0.73 to 1.55) | 0.02 (−0.08 to 0.12) | .56 |
| BITSEA ASD, ASD risk cutoff | 161/152 | 39 (24) | 45 (30) | 0.88 (0.60 to 1.28) | −0.03 (−0.14 to 0.07) | .003 |
| PDDST-II, fail | 160/153 | 33 (21) | 49 (32) | 0.66 (0.45 to 0.97) | −0.11 (−0.21 to −0.01) | .29 |
| Female sex | ||||||
| BITSEA competence, delayed | 69/81 | 6 (9) | 15 (19) | 0.52 (0.21 to 1.28) | −0.08 (0.05 to −0.18) | — |
| BITSEA competence, ASD risk cutoff | 69/81 | 8 (12) | 21 (26) | 0.57 (0.30 to 1.07) | −0.09 (−0.20 to 0.01) | — |
| BITSEA problem, delayed | 69/81 | 13 (19) | 19 (23) | 0.86 (0.47 to 1.57) | −0.03 (−0.16 to 0.10) | — |
| BITSEA problem, ASD risk cutoff | 69/81 | 15 (22) | 19 (23) | 0.99 (0.56 to 1.74) | 0.00 (−0.14 to 0.13) | — |
| BITSEA ASD, ASD risk cutoff | 69/81 | 9 (13) | 28 (35) | 0.43 (0.22 to 0.85) | −0.18 (−0.31 to −0.05) | — |
| PDDST-II, fail | 69/81 | 17 (25) | 25 (31) | 0.81 (0.48 to 1.36) | −0.06 (−0.20 to 0.08) | — |
| Male sex | ||||||
| BITSEA competence, delayed | 92/71 | 16 (17) | 10 (14) | 1.19 (0.55 to 2.56) | 0.03 (−0.09 to 0.14) | — |
| BITSEA competence, ASD risk cutoff | 92/71 | 24 (26) | 14 (20) | 1.31 (0.72 to 2.38) | 0.06 (−0.07 to 0.19) | — |
| BITSEA problem, delayed | 92/72 | 22 (24) | 16 (22) | 1.08 (0.61 to 1.91) | 0.02 (−0.11 to 0.15) | — |
| BITSEA problem, ASD risk cutoff | 92/72 | 27 (29) | 20 (28) | 1.11 (0.66 to 1.85) | 0.03 (−0.12 to 0.18) | — |
| BITSEA ASD, ASD risk cutoff | 92/71 | 30 (33) | 17 (24) | 1.37 (0.81 to 2.31) | 0.09 (−0.05 to 0.23) | — |
| PDDST-II, fail | 91/72 | 16 (18) | 24 (33) | 0.54 (0.31 to 0.95) | −0.15 (−0.30 to −0.01) | — |
—, not applicable.
Exploratory Assessment of Treatment Effect on Caregiver-Reported Socioemotional Outcomes, by Sex
Girls randomly assigned to DHA + AA had a decreased risk of scoring above the BITSEA ASD cutoff compared with girls randomized to placebo (RR = 0.43 [95% CI: 0.22 to 0.85]; RD = −0.18 [95% CI: −0.31 to −0.05]; Table 3), and boys randomly assigned to DHA + AA had a decreased risk of scoring above the PDDST-II cut score compared with boys randomly assigned to placebo (RR = 0.54 [95% CI: 0.31 to 0.95]; RD = −0.15 [95% CI: −0.30 to −0.01]). No other subgroup effects were statistically significant.
Discussion
In this secondary analysis of a randomized, fully masked, placebo-controlled trial, we explored the effect of DHA + AA supplementation during toddlerhood on caregiver-reported socioemotional development in toddlers born preterm. Supplementation did not affect measures of competence or problem behaviors, but it did result in 0.66 times the risk of clinical concern for ASD in the full cohort, per the PDDST-II. Exploratory subgroup analyses revealed a decreased risk of clinical concern for ASD in girls (per the BITSEA ASD cut score) and boys (per the PDDST-II cut score) randomly assigned to DHA + AA compared with placebo. The magnitude of all effects was small.
Authors of previous experimental work in which neonates were supplemented with varying levels of DHA, alone or in combination with AA or EPA, reported inconsistent findings on socioemotional outcomes throughout early childhood. Additionally, in studies in which the authors reported no effect of supplementation on socioemotional development in the short-term, they found negative effects on these outcomes as children entered school.26,41
In the DHA for the Improvement of Neurodevelopmental Outcome in Preterm Infants trial, the milk of preterm (<33 weeks) neonates was supplemented with DHA. High-DHA milk had no effect on socioemotional development at 3 to 5 years; however, at 7 years, girls randomly assigned to high-DHA milk had more conduct problems and overall socioemotional difficulties than girls randomly assigned to standard-DHA milk.6,26 In the Preemie Tots trial, the diets of preterm (≤29 weeks) toddlers (aged 18–36 months) were supplemented with fish and borage oil or a placebo for 90 days. The authors reported no treatment effects for competence or problem behaviors but did report a large reduction in parent-reported ASD behaviors (Cohen’s d = 0.71; P = .03) after supplementation.22 The authors of a Norwegian trial in which the diets of low birth weight infants were supplemented with DHA + AA reported no effect on socioemotional outcomes at 6 or 20 months.24,25 Finally, in a trial in Ethiopia, DHA + EPA was provided to lactating mothers, directly to their breastfed children, or to both the mother and the child, and the authors reported no effect of supplementation on socioemotional development from 6 to 24 months.42
The timing, dose, and duration of supplementation may help explain the inconsistencies in findings. For example, the supplementation period for the DINO trial and the trial in Norway was from within 1 week of birth for 8 to 9 weeks, on average. In comparison, in the Omega Tots trial, supplementation started later (ie, closer to age 1 year) and continued for a longer period of time (ie, 6 months). Starting at 1 year, the average DHA intake for children not receiving breast milk or formula is ∼20 mg/day, an estimated 48% to 71% reduction compared to when the child is breastfed and/or formula fed.43 Supplementation that begins in toddlerhood, after a child is weaned from DHA-rich breast milk or formula and begins a diet low in DHA, may have different relationships with developmental outcomes because it provides DHA at a time when the child would otherwise consume low amounts of DHA. Although in the Ethiopian trial, a 12-month supplementation period began at 6 to 12 months, only infants who were still breastfeeding were eligible. This criterion and dietary differences between the United States and Ethiopia may explain the difference in findings reported here. Given the negative long-term effects of early supplementation reported previously and the large volume of infant and toddler products high in DHA readily available in the United States, assessing the long-term effects of DHA on children is crucial.
Children born preterm are at an increased risk for ASD, a risk that increases with the degree of prematurity.44–47 The prevalence of ASD in children born preterm is significantly higher than in children born term (1.78% vs 1.22%; P < .001), with children of the lowest gestational ages demonstrating the highest prevalence.44 Furthermore, late or moderately preterm children have a nearly 60% higher risk of having a positive ASD screen result at age 2 years compared with their term peers.48 ASD symptomology may be detectable earlier than general socioemotional delays, making ASD behaviors particularly susceptible to identification and DHA support in toddlerhood. Although in the current study, we did not find a treatment effect of DHA supplementation on competence and problem behaviors at 2 years, effects on socioemotional development may emerge as children approach school age, as has been reported previously.26,41,49
This study has limitations. The study relied on caregiver-reported behaviors; therefore, caregiver bias cannot be ruled out. However, caregivers were blinded and poor at guessing their child’s treatment assignment. Some of our measures may not have been sensitive to treatment effects, especially at this young age. We relied on caregiver report of compliance with assigned treatment. Although caregivers reported mixing >80% of the prescribed dose in the child’s food, whether the child ate the food is unknown. Outcomes were collected at the final study visit; therefore, we could not examine incremental changes from the beginning to the end of the trial or as a function of treatment assignment, nor could we include a baseline value in the analytical model. However, given that children were randomly assigned to DHA + AA or a placebo, we expected that baseline socioemotional development would be similar across groups and that any differences in outcomes at the end of the trial would be due to DHA + AA supplementation. The lower ASD risk for children randomly assigned to DHA + AA compared with placebo may be a chance effect due to multiple comparisons.
This trial has several strengths. Treatment compliance was very good, indicating that the approach was feasible for families with young children born preterm. Because multiple-gestation births are common among preterm births, they are often included in clinical trials related to prematurity, as in this trial. Additionally, we included children up to 35 weeks’ completed gestation, as opposed to more limited gestational ages included in previous studies.22,26 Because we drew participants from a roster of all children who required NICU care, the source population was not subject to some of the participation biases in studies that relied on volunteers or patients of neonatal follow-up clinics. Collectively, these methodologic strengths increase the generalizability of the findings to the larger preterm population. Finally, because caregivers were poor at correctly guessing their child’s treatment assignment, we can rule out accidental unblinding or caregiver suspicions about treatment assignment as influences on caregiver reports of child behavior.
Conclusions
Although no overall treatment effect of DHA + AA supplementation was observed on caregiver-reported outcomes of child competence and problem behaviors, children randomly assigned to DHA + AA had a decreased risk of clinical concern for ASD compared with those assigned to a placebo. Given the study’s short time frame and the post hoc design, these findings should be interpreted cautiously. Future research in larger samples of children born preterm and longer follow-up are warranted to explore the long-term effects of DHA + AA supplementation for socioemotional development, including ASD diagnosis, as children enter school.
Acknowledgments
We thank the entire Omega Tots research team: Dkeama Alexis, Seanceray Bellinger, Holly Blei, Ashlea Braun, Anne Brown, Lautaro Cabrera, Chelsea Dillon, Eva Fabian, Connor Grannis, Rachel Haeuptle, Nathan Hanna, Chenali Jayadeva, Justin Jackson, Sarah Landry, Julia Less, Cara Lucke, Melissa Kwitowski, Joseph Macklin, Krista McManus, Emily Messick, Yvette Noah, Grace Pelak, Evan Plunkett, John Rissell, Rachel Ronau, Ashley Ronay, Kamma Smith, Katie Smith, and Sarah Snyder.
We also thank the participants, the NCH Division of Neonatology and NCH Investigational Drug Services, and DSM Nutritional Products.
Glossary
- AA
arachidonic acid
- ASD
autism spectrum disorder
- BITSEA
Brief Infant-Toddler Social and Emotional Assessment
- CI
confidence interval
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- GED
general equivalency diploma
- IQR
interquartile range
- NCH
Nationwide Children’s Hospital
- PDDST-II
Pervasive Developmental Disorders Screening Test–II, Stage 2
- RD
risk difference
- RR
risk ratio
Footnotes
Ms Boone participated in the study concept and design, study supervision, analysis and interpretation of data, initial drafting of the manuscript, and review and revision of the manuscript; Dr Parrott participated in the analysis and interpretation of data, initial drafting of the manuscript, and review and revision of the manuscript; Drs Rausch, Yeates, Klebanoff, and Norris Turner participated in the study concept and design, analysis and interpretation of data, and critical revision of the manuscript; Dr Keim conceptualized and designed the study, obtained grant funding for the study, supervised the study, and participated in the analysis and interpretation of data and critical revision of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Deidentified individual participant data will not be made available.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Health Resources and Services Administration of the US Department of Health and Human Services (grant R40MC28316), the March of Dimes (grant 12-FY14-171), the Allen Foundation, Cures Within Reach, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01HD100493), and the National Center for Advancing Translational Sciences of the National Institutes of Health (grant UL1TR001070), and internal support was received from the Abigail Wexner Research Institute at Nationwide Children’s Hospital. DSM Nutritional Products provided the investigational products at no cost and had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The information, content, and/or conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the Health Resources and Services Administration, the US Department of Health and Human Services, or the US Government. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention. Preterm birth. 2019. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Accessed December 11, 2019
- 2.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161(4):326–333 [DOI] [PubMed] [Google Scholar]
- 3.Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CS, van Elburg RM, Oosterlaan J. Academic performance of children born preterm: a meta-analysis and meta-regression. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F322–F330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luu TM, Rehman Mian MO, Nuyt AM. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol. 2017;44(2):305–314 [DOI] [PubMed] [Google Scholar]
- 5.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737 [DOI] [PubMed] [Google Scholar]
- 6.Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. Am J Clin Nutr. 2010;91(3):628–634 [DOI] [PubMed] [Google Scholar]
- 7.Spittle AJ, Treyvaud K, Doyle LW, et al. Early emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009;48(9):909–918 [DOI] [PubMed] [Google Scholar]
- 8.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):259–269 [DOI] [PubMed] [Google Scholar]
- 9.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–271 [DOI] [PubMed] [Google Scholar]
- 10.Kitajka K, Puskás LG, Zvara A, et al. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc Natl Acad Sci U S A. 2002;99(5):2619–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136(6):1570–1575 [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505 [DOI] [PubMed] [Google Scholar]
- 13.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RA, Lewis MD, Calkins SD. Reassessing emotion regulation. Child Dev Perspect. 2008;2(3):124–131 [Google Scholar]
- 15.Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45(3):205–229 [DOI] [PubMed] [Google Scholar]
- 16.Clandinin MT, Van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146(4):461–468 [DOI] [PubMed] [Google Scholar]
- 17.Innis SM, Adamkin DH, Hall RT, et al. Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J Pediatr. 2002;140(5):547–554 [DOI] [PubMed] [Google Scholar]
- 18.O’Connor DL, Hall R, Adamkin D, et al. ; Ross Preterm Lipid Study . Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108(2):359–371 [DOI] [PubMed] [Google Scholar]
- 19.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(suppl A):S70–S75 [DOI] [PubMed] [Google Scholar]
- 20.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6(5):437–449 [DOI] [PubMed] [Google Scholar]
- 21.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4(2):121–129 [DOI] [PubMed] [Google Scholar]
- 22.Keim SA, Gracious B, Boone KM, et al. ω-3 and ω-6 fatty acid supplementation may reduce autism symptoms based on parent report in preterm toddlers. J Nutr. 2018;148(2):227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulkin M, Pimpin L, Bellinger D, et al. n-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: a systematic review and meta-analysis. J Nutr. 2018;148(3):409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henriksen C, Haugholt K, Lindgren M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121(6):1137–1145 [DOI] [PubMed] [Google Scholar]
- 25.Westerberg AC, Schei R, Henriksen C, et al. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011;100(1):47–52 [DOI] [PubMed] [Google Scholar]
- 26.Collins CT, Gibson RA, Anderson PJ, et al. Neurodevelopmental outcomes at 7 years’ corrected age in preterm infants who were fed high-dose docosahexaenoic acid to term equivalent: a follow-up of a randomised controlled trial. BMJ Open. 2015;5(3):e007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keim SA, Boone KM, Klebanoff MA, et al. Effect of docosahexaenoic acid supplementation vs placebo on developmental outcomes of toddlers born preterm: a randomized clinical trial. JAMA Pediatr. 2018;172(12):1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keim S. Omega Tots: a randomized, controlled trial of long-chain polyunsaturated fatty acid supplementation of toddler diets and developmental outcomes. Available at: https://clinicaltrials.gov/ct2/show/NCT01576783. Accessed March 18, 2020
- 29.Bernardo J, Nowacki A, Martin R, Fanaroff JM, Hibbs AM. Multiples and parents of multiples prefer same arm randomization of siblings in neonatal trials. [published correction appears in J Perinatol. 2015;35(4):310]. J Perinatol. 2015;35(3):208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A. 1993;90(3):1073–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Codex Alimentarius Commission Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. Rome, Italy: Codex Alimentarius Commission; 2007 [Google Scholar]
- 32.Koletzko B, Bergmann K, Brenna JT, et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am J Clin Nutr. 2020;111(1):10–16 [DOI] [PubMed] [Google Scholar]
- 33.Puri BK, Martins JG. Which polyunsaturated fatty acids are active in children with attention-deficit hyperactivity disorder receiving PUFA supplementation? A fatty acid validated meta-regression analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2014;90(5):179–189 [DOI] [PubMed] [Google Scholar]
- 34.Kuratko C. Food-frequency questionnaire for assessing long-chain ω-3 fatty-acid intake: Re: assessing long-chain ω-3 polyunsaturated fatty acids: a tailored food-frequency questionnaire is better. Nutrition. 2013;29(5):807–808 [DOI] [PubMed] [Google Scholar]
- 35.Briggs-Gowan MJ, Carter AS. The Brief Infant-Toddler Social and Emotional Assessment (BITSEA) Examiner’s Manual. San Antonio, TX: Harcourt Assessment, Inc; 2006 [Google Scholar]
- 36.Boone KM, Brown AK, Keim SA. Screening accuracy of the Brief Infant Toddler Social-Emotional Assessment to identify autism spectrum disorder in toddlers born at less than 30 weeks’ gestation. Child Psychiatry Hum Dev. 2018;49(4):493–504 [DOI] [PubMed] [Google Scholar]
- 37.Siegel B. Pervasive Developmental Disorders Screening Test-II (PDDST-II): Early Childhood Screener for Autistic Spectrum Disorders. San Antonio, TX: Harcourt Assessment, Inc; 2004 [Google Scholar]
- 38.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200 [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46 [PubMed] [Google Scholar]
- 41.Gould JF, Treyvaud K, Yelland LN, et al. Seven-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2017;317(11):1173–1175 [DOI] [PubMed] [Google Scholar]
- 42.Argaw A, Huybregts L, Wondafrash M, et al. Neither n-3 long-chain PUFA supplementation of mothers through lactation nor of offspring in a complementary food affects child overall or social-emotional development: a 2 × 2 factorial randomized controlled trial in rural Ethiopia. J Nutr. 2019;149(3):505–512 [DOI] [PubMed] [Google Scholar]
- 43.Keim SA, Branum AM. Dietary intake of polyunsaturated fatty acids and fish among US children 12-60 months of age. Matern Child Nutr. 2015;11(4):987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr. 2014;164(1):20–25 [DOI] [PubMed] [Google Scholar]
- 45.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156(4):525–531.e2 [DOI] [PubMed] [Google Scholar]
- 46.Verhaeghe L, Dereu M, Warreyn P, De Groote I, Vanhaesebrouck P, Roeyers H. Extremely preterm born children at very high risk for developing autism spectrum disorder. [published correction appears in Child Psychiatry Hum Dev. 2016;47(6):1009]. Child Psychiatry Hum Dev. 2016;47(5):729–739 [DOI] [PubMed] [Google Scholar]
- 47.Pritchard MA, de Dassel T, Beller E, et al. Autism in toddlers born very preterm. Pediatrics. 2016;137(2):e20151949. [DOI] [PubMed] [Google Scholar]
- 48.Guy A, Seaton SE, Boyle EM, et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J Pediatr. 2015;166(2):269–275.e3 [DOI] [PubMed] [Google Scholar]
- 49.Makrides M, Gould JF, Gawlik NR, et al. Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2014;311(17):1802–1804 [DOI] [PubMed] [Google Scholar]


