Primary care–based child obesity prevention from pregnancy through early childhood may reduce child weight trajectories among socioeconomic and ethnic groups with known disparities.
Abstract
OBJECTIVES:
To determine impact of a primary care–based child obesity prevention intervention beginning during pregnancy on early childhood weight outcomes in low-income Hispanic families.
METHODS:
A randomized controlled trial comparing mother–infant pairs receiving either standard care or the Starting Early Program providing prenatal and postpartum nutrition counseling and nutrition parenting support groups targeting key obesity-related feeding practices in low-income groups. Primary outcomes were reduction in weight-for-age z-scores (WFAzs) from clinical anthropometric measures, obesity prevalence (weight for age ≥95th percentile), and excess weight gain (WFAz trajectory) from birth to age 3 years. Secondary outcomes included dose effects.
RESULTS:
Pregnant women (n = 566) were enrolled in the third trimester; 533 randomized to intervention (n = 266) or control (n = 267). Also, 358 children had their weight measured at age 2 years; 285 children had weight measured at age 3 years. Intervention infants had lower mean WFAz at 18 months (0.49 vs 0.73, P = .04) and 2 years (0.56 vs 0.81, P = .03) but not at 3 years (0.63 vs 0.59, P = .76). No group differences in obesity prevalence were found. When generalized estimating equations were used, significant average treatment effects were detected between 10-26 months (B = −0.19, P = .047), although not through age 3 years. In within group dose analyses at 3 years, obesity rates (26.4%, 22.5%, 8.0%, P = .02) decreased as attendance increased with low, medium, and high attendance.
CONCLUSIONS:
Mean WFAz and growth trajectories were lower for the intervention group through age 2 years, but there were no group differences at age 3. Further study is needed to enhance sustainability of effects beyond age 2.
What’s Known on This Subject:
Elevated weight in infancy contributes to disparities in later obesity, yet study of primary care–based preventive models during pregnancy and early childhood for high-risk groups is limited.
What This Study Adds:
In this randomized trial of low-income Hispanic mother–infant pairs, the Starting Early Program led to lower weight trajectories and weight-for-age z-scores through age 2 years, although not sustained at age 3 years. Increased intervention exposure was associated with greater impacts.
Obesity prevalence in the United States remains high, with widespread socioeconomic, racial, and ethnic disparities.1,2 Groups with lower education and income and racial and ethnic minority groups have the highest rates.3 Elevated weight, even during infancy, significantly contributes to later obesity4 and decreased socioeconomic achievement across the life-course.5–7
The high cost and disease burden of obesity,8 and its resistance to treatment once established, underscores the need for effective primary prevention for high-risk groups before obesity develops to prevent disparities.9,10 Although childhood obesity prevention trials beginning in pregnancy or infancy have reported weight impacts, most were conducted either outside the United States11–13 or in middle-income US communities.14,15 These trials have been limited to first-time mothers and used home visiting platforms16 not available to the majority of US children at high risk of obesity.17 Scalable models with broader reach for at-risk communities are needed.
Primary health care during pregnancy and infancy has great potential to serve as a platform for the primary prevention of adverse health outcomes because visits are frequent and widely attended. There is also potential to lower cost by using existing administrative infrastructure and decreasing needs for additional transportation, missed work, and child care.18 Although prenatal and pediatric primary care can reduce adverse maternal and infant outcomes, including maternal and neonatal morbidity,19 prematurity, and low birth weight,20 it has not significantly impacted the rising rates of obesity, particularly among low-income, Hispanic children.21 Currently, there are no comprehensive child obesity prevention programs in the United States designed for integration into prenatal and pediatric primary care.
We developed the Starting Early Program (StEP), a primary health care–based child obesity prevention intervention, to address these limitations. StEP was developed to capitalize on the reach and scalability of primary care during pregnancy and early childhood and was specifically designed for low-income, Hispanic communities. Previous reports documented StEP impacts on feeding and activity at ages 3 and 10 months.22–24 Primary aims were to examine intervention effects on child weight outcomes compared with controls receiving standard care. We hypothesized that the intervention group would have reduced weight-for-age z-scores (WFAzs), obesity prevalence, and weight gain trajectories from birth through 3 years compared with the controls. Secondary analyses examined how intervention dose related to child weight outcomes.
Methods
Study Design
We conducted a randomized controlled trial to test the efficacy of StEP compared with standard care. The study was conducted in prenatal and pediatric clinics of an urban public hospital and an affiliated health center in New York City. Pregnant women were enrolled in a prenatal clinic and were followed until their child reached the age of 3 years. The New York University Grossman School of Medicine and New York City Health + Hospitals Institutional Review Board approved this study and it was registered on clinicaltrials.gov (NCT01541761).
Participants
We included pregnant women who were ≥18 years old, Latina or Hispanic, English- or Spanish-speaking, with a singleton uncomplicated pregnancy, who planned pediatric care at our study sites. We excluded women with severe medical or psychiatric illness or fetal anomalies. Our 3-step eligibility screening process was previously described.22,23 Interested eligible women signed informed written consent, and completed baseline assessments. Enrollment occurred between August 2012 and December 2014. Women were randomly assigned after 32 weeks’ gestational age by using computer-generated random numbers, with a block size of 10, stratified by site. Group assignments were concealed from research assistants conducting follow-up.
StEP
StEP is a family-centered, strengths-based program using social cognitive theory25 to promote healthy behaviors. StEP is delivered by bilingual English- and Spanish-speaking registered dietitians who are certified lactation counselors. Its main components are prenatal nutrition counseling, postpartum lactation support, and nutrition and parenting support groups coordinated with pediatric visits. Fifteen sessions were delivered, including 2 individual (third trimester; postpartum) and 13 group sessions at 1, 2, 4, 6, 9, 12, 15, 18, 21, 24, 27, 30, and 33 months.
Intervention content was based on guidelines from National Academy of Medicine,9,26,27 American Academy of Pediatrics,28,29 and the US Department of Agriculture.30 Prenatal and postpartum sessions assessed feeding intentions, barriers, lactation, and bottle-feeding. Group sessions with 4 to 8 mother–child pairs addressed feeding, activity, and parenting and included a family meal to model healthy behaviors and practice skills (Supplemental Table 5). We assessed fidelity during 74% of sessions and found that 97% provided all curriculum components.
The intervention used 4 strategies. First, sessions used a multimodal approach, with didactics, interactive demonstrations, and hands-on practicing. Second, individualized counseling used motivational interviewing and goal setting. Third, group structure facilitated interaction, peer modeling, and social support. Last, the ecologically informed curriculum was sensitive to poverty-related risks, health literacy, and cultural differences. The curriculum, developed by dietitians and pediatricians with extensive experience working with low-income Hispanic families, targeted common behaviors.31,32 Plain-language, picture-based handouts and bilingual videos33,34 reinforced the curriculum.35–37 Health literacy experts provided feedback on language, literacy, numeracy, and cultural appropriateness.38
Both groups were offered standard prenatal, postpartum, and pediatric primary care, including 1 prenatal nutrition consultation, 1 childbirth or breastfeeding class, as-needed lactation support, and pediatric visits according to American Academy of Pediatrics guidelines.39 To enhance retention, families received frequent reminder mailings and calls for intervention and assessment sessions and were reimbursed for time spent in assessment activities.
Assessments
Primary outcomes were reduction in WFAz from clinical anthropometric measures, obesity prevalence (weight for age [WFA] ≥95th percentile), and excess weight gain (WFAz trajectory) from birth to age 3 years.
Child Anthropometrics
Anthropometrics were obtained from birth through age 3 years on the basis of clinical measurements, with supplemental research measurements for the final assessment at age 3. Both clinical and research measurements were obtained by using Seca scales and infantometers from birth through 23 months and stadiometers for children aged ≥2 years. Weight and recumbent length (<2 years) or height (≥2 years) were measured.
Clinical anthropometrics from birth to 3 years were collected from medical record review. Data were obtained from weights and lengths or heights performed by medical assistants. Clinical anthropometrics were used for 3 reasons: (1) practical difficulties in collecting research measures for families randomized to the intervention, because the sessions were integrated into well-child visits, potentially leading to bias in ascertainment across groups; (2) high frequency of clinical measures (every 1–3 months) increased power to evaluate trajectories; and (3) feasibility of studying obesity prevention in real-world health care settings. In addition, research staff measured weight and height at the final 3-year assessment, to the nearest 10th of a kilogram and centimeter.
Infant birth weight, sex, and gestational age were used to generate birth weight z-scores with Fenton growth curves.40 Although our original intent was to use z-scores based on weight and length or height, we identified biologically implausible variations in clinically obtained lengths or heights.41 Among 3-year-olds for whom both clinical and research-based anthropometrics were collected (n = 219), the mean (SD) height-for-age z-scores generated from clinically measured and research-measured heights were significantly different (−0.10 [1.06] vs −0.21 [0.93], P = .01), suggesting decreased validity for clinically-measured heights. In contrast, there was no significant difference between clinically-measured and research-measured mean WFAz (0.60 [1.12] vs 0.61 [1.17], P = .65).
Between birth and 3 years, WFAz were calculated by the World Health Organization Anthro macro.42 For ages 2 and 3 years, weight status was defined by WFA percentiles, with ≥85th and ≥95th designated as overweight and obese. Trajectory analyses used all clinically measured weights from birth to 3 years. Cross-sectional group comparisons were performed at 6 month intervals from birth to 3 years, by using clinically measured WFAz obtained within 60 days of the given age.43 At age 3 years, when research-based anthropometrics were available, analyses were performed by using World Health Organization WFA percentiles ≥85th and ≥95th and BMI z-scores, based on the Centers for Disease Control and Prevention 2000 growth charts.44
Family Characteristics
Baseline characteristics, collected by survey, included age, parity, education, employment, Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and Supplemental Nutrition Assistance Program (SNAP) participation, marital status, country of origin, depressive symptoms,45 food insecurity,46 and social support.47 Prepregnancy BMI was obtained from the medical record; BMI ≥30 was classified as obese.48
Statistical Analyses
The study was powered to detect impacts on obesity prevalence reduction. To achieve 80% power to detect 15% obesity reduction with 30% loss to follow-up (α = .05), we needed ≥500 participants.49 Analyses were based on intent-to-treat, by using SPSS version 23 (SPSS Inc, Chicago, IL), Stata 15, and R. We examined bivariate relationships with mean WFAz and weight status at 6-month intervals using independent samples t tests and χ2 analyses for continuous and categorical variables. At age 3 years, we repeated analyses using WFAz and BMIz generated from research measurements. Using nonparametric regression techniques,36 we generated growth trajectories, including 95% confidence intervals at each time point, which allows for cross-sectional comparison of groups. We used multiple endpoint adjustment methods50,51 to allow for multiple comparisons at different time-points, accounting for repeated measures within subjects. Using generalized estimating equations (GEEs) with an exchangeable working correlation matrix, we then estimated an average treatment effect (ATE), with baseline growth allowing for a quadratic or cubic response, depending on the time interval. Because baseline education differed between groups, we performed sensitivity analyses adjusting for education with comparable results (Supplemental Tables 6, 7).
We performed within-intervention group analyses based on tertile of attendance. Cutoffs reflected the sample distribution, and high dose corresponded to attending ≥70% of activities. For these analyses, we examined unadjusted and adjusted relationships between dose and interval mean WFAz and weight status using ANOVA and χ2 analyses. Because of variability in intervention dose (number of sessions attended), and to explore dose effects using the entire sample, we also used instrumental variable (IV) analysis.52 An IV analysis identifies an instrument (measured variable), which is correlated with a variable of primary interest (dose) that has no direct path from the instrument to the outcome, operating exclusively through the variable of primary interest. Our instrument was randomization, satisfying both requirements, which allows us to correct for differential receipt of the intervention using a 2-stage least squares approach.53 To allow comparison of dose analyses with ATE estimates, we report an IV effect size scaled by multiplying the IV dose effect by the average number of intervention sessions in the period.
Results
Study Sample
All pregnant women at their first prenatal visit between August 2012 and December 2014 were screened for eligibility; follow-up was completed in June 2018 when all children had reached 3 years of age. Of 933 eligible pregnant women, 566 (61%) consented and 533 were randomized to intervention (n = 266) or control (n = 267) groups, with 529 live births (Fig 1). Our primary anthropometric outcomes from clinical measurements were available for 358 (67.7%) children at 2 years and 285 (53.9%) children at 3 years. Research assistants measured children’s weights (research weight measurements) if the 3-year survey was done in person (versus telephone). Research measures were obtained on 254 children (134 control; 120 intervention). No adverse events were reported. For trajectory analyses, there was at least 1 recorded clinical weight on 525 children between birth and age 3, ranging from 1 to 26 per child, with an average of 12.8 per child. Of these 525 children, 99% had at least 1 weight recorded in year 1, 88% in year 2, and 81% in year 3.
FIGURE 1.
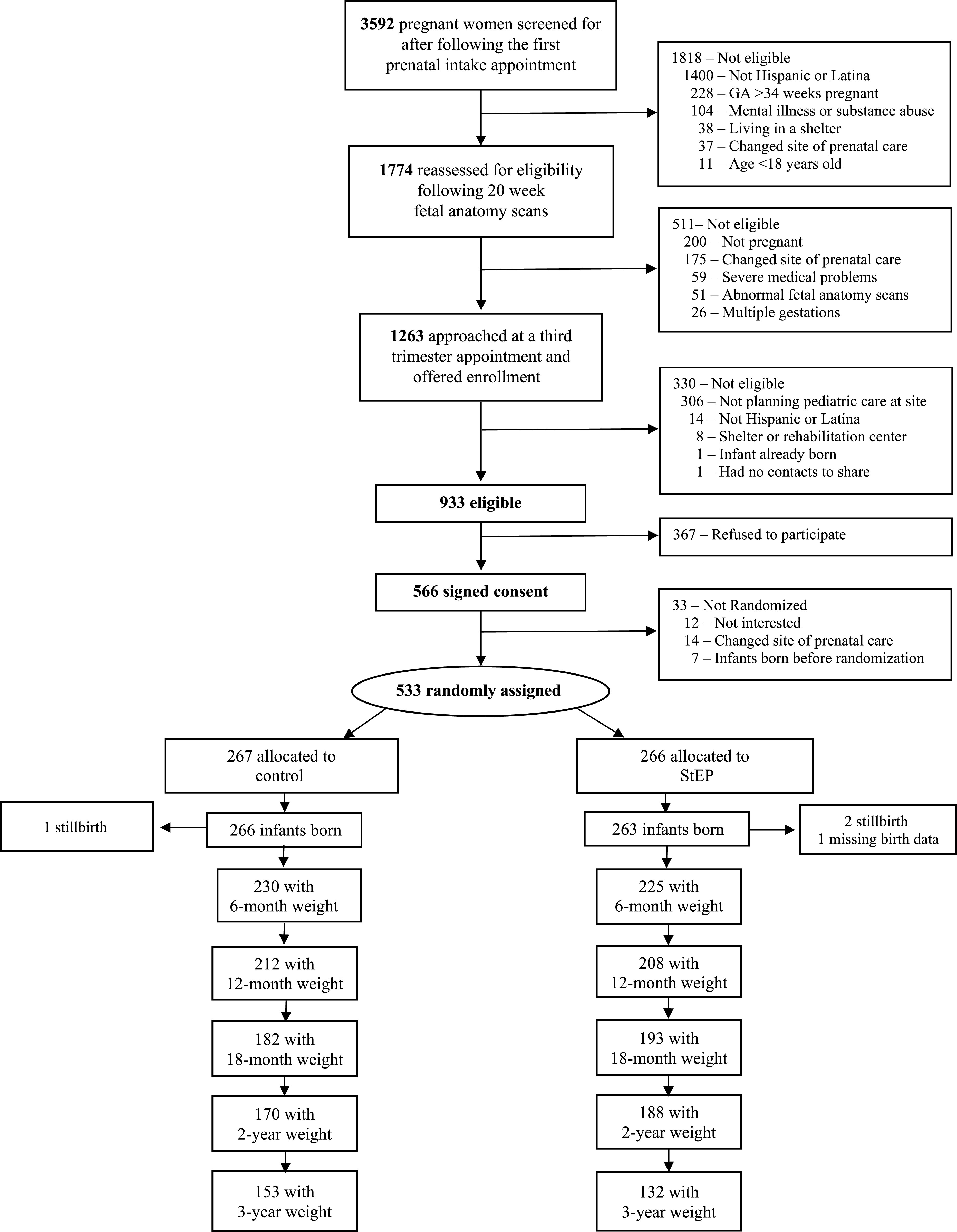
Participant enrollment and assessment.
Groups did not differ at baseline, except intervention mothers had less education (Table 1). Birth weight z-score was not significantly different between intervention and control groups (−0.05 vs 0.02, P = .36). Intervention children had slightly more weight measurements than controls (14.89 vs 14.11; P = .04). Children with weight measurements at 3 years had higher mean birth weight (3.43 vs 3.32, P = .01) and mothers who were older (28.9 vs 27.5 years, P = .01), less likely to be US born (15.4% vs 25.4%, P ≤ .01), and more likely to receive SNAP benefits (43.2% vs 28.2%, P ≤ .01) compared with those without a 3-year weight. Among participants with a 3-year weight, intervention mothers were older (29.7 vs 28.2 years, P = .03), less likely to have graduated high school (56.8% vs 68.8% P = .04), and more likely to receive WIC (92.4% vs 84.3%, P = .04), compared with controls.
TABLE 1.
Baseline Characteristics of Sample Randomized to Receive StEP Versus Standard Care Control (n = 533)
| Control, n (%) | Intervention, n (%) | |
|---|---|---|
| Mother (prenatal) | 267 | 266 |
| Age, mean (SD) | 27.9 (5.7) | 28.6 (6.1) |
| Primiparous | 107 (40.1) | 92 (34.6) |
| Education (less than high school)a | 77 (28.8) | 100 (37.6) |
| Working | 67 (25.1) | 67 (25.2) |
| Married or living as married | 191 (71.5) | 198 (70.7) |
| US born | 51 (19.1) | 56 (21.1) |
| Prenatal depressive symptomsb | 90 (33.7) | 91 (34.3) |
| Low social support | 53 (19.8) | 53 (19.9) |
| Household food insecurity | 88 (33.0) | 77 (29.0) |
| WIC participant | 228 (85.4) | 237 (89.1) |
| SNAP participant | 95 (35.6) | 98 (36.8) |
| Prepregnancy obese status | 82 (30.7) | 81 (30.5) |
| Child (birth) | 266 | 263 |
| Girls | 139 (52.3) | 131 (49.8) |
| Cesarean birth | 58 (21.7) | 46 (17.9) |
| Premature <37 wk gestational agec | 6 (2.3) | 10 (3.6) |
| Birth wt, mean (SD)c | 3.40 (0.49) | 3.36 (0.45) |
| Birth wt z-scorec | 0.02 (0.91) | −0.05 (0.81) |
Significant at P ≤ .05.
n = 532.
n = 521.
Child Weight Outcomes
Intervention infants had significantly lower mean WFAz at 18 months (0.49 vs 0.73, P = .04) and 2 years (0.56 vs 0.81, P = .03) but not at 3 years (0.63 vs 0.59, P = .76) (Table 2). Obesity prevalence was not significantly different between groups at any age point, including 2 years (WFA ≥85th percentile, 33.5% vs 39.4%, P = .11; WFA ≥95th percentile, 17.0% vs 22.9%, P = .16) and age 3 years (WFA ≥85th percentile, 36.4% vs 30.1%, P = .26; WFA ≥95th percentile, 16.7% vs 15.7%, P = .82)(Table 3).
TABLE 2.
StEP Impacts on WFAz at 6-Month Intervals
| Age | N | WFAz,a Mean (SD) | P |
|---|---|---|---|
| 6 mo | .14 | ||
| Control | 230 | 0.46 (0.98) | — |
| Intervention | 225 | 0.33 (0.95) | — |
| 12 mo | .08 | ||
| Control | 212 | 0.57 (1.02) | — |
| Intervention | 208 | 0.39 (1.05) | — |
| 18 mo | .04 | ||
| Control | 182 | 0.73 (1.16) | — |
| Intervention | 193 | 0.49 (1.04) | — |
| 2 y | .03 | ||
| Control | 170 | 0.81 (1.03) | — |
| Intervention | 188 | 0.56 (1.09) | — |
| 2 1/2 y | .50 | ||
| Control | 134 | 0.76 (1.21) | — |
| Intervention | 170 | 0.67 (1.21) | — |
| 3 y | .76 | ||
| Control | 153 | 0.59 (1.09) | — |
| Intervention | 132 | 0.63 (1.17) | — |
—, not applicable.
Anthropometric data used for these analyses were obtained by medical record review.
TABLE 3.
StEP Impacts on Obesity Prevalence at Age 2 and 3 Years
| Age | n | WFA ≥85th percentile, n (%) | P | WFA ≥95th percentile, n (%) | P |
|---|---|---|---|---|---|
| 2 y | .11 | .16 | |||
| Control | 170 | 67 (39.4) | — | 39 (22.9) | — |
| Intervention | 188 | 59 (33.5) | — | 32 (17.0) | — |
| 3 y | .26 | .82 | |||
| Control | 153 | 46 (30.1) | — | 24 (15.7) | — |
| Intervention | 132 | 48 (36.4) | — | 22 (16.7) | — |
—, not applicable.
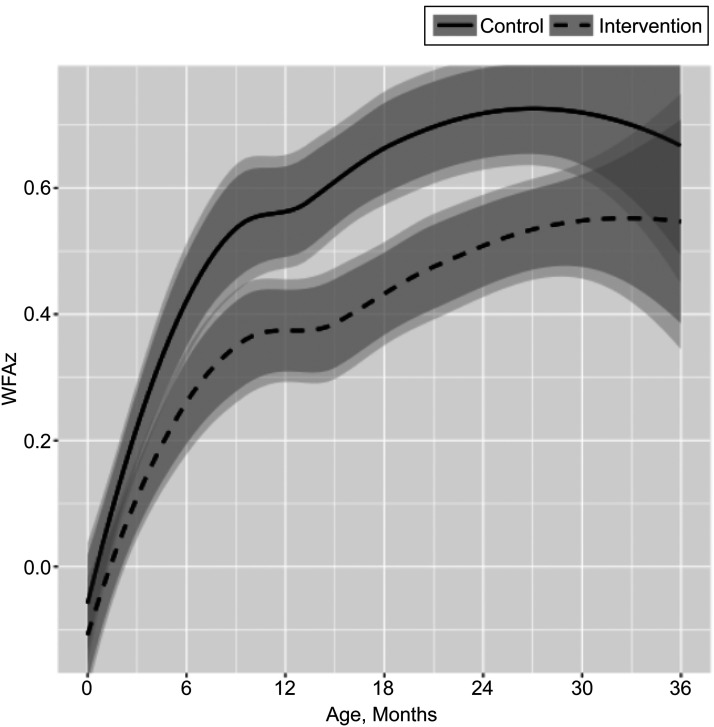
Trajectory analyses demonstrated that by age 6 months, the groups began to become distinct, with lower WFAz for the intervention group, with the difference diminishing by 30 months (Fig 2). ATE analysis using GEE and cubic baseline growth restricted to the 10 to 26 month interval, which corresponds to the interval endpoints used in the 12 month and 2 year analyses above, yielded an ATE of B = −0.19, P = .047.
FIGURE 2.
Impact of StEP on WFAz trajectory from birth to age 3 years. Lines represent nonparametric growth trajectory estimates for the intervention (dashed line) and control (solid line) groups ages 0 to 36 months. The darker bands represent 95% confidence intervals at each time point; the lighter bands are 95% confidence intervals adjusted for correlated repeated measures within subjects.
Among the subset with research-measured anthropometrics at 3 years, the intervention group had similar mean WFAz (0.62 vs 0.63, P = .91) and rates of overweight (33.3% vs 35.8%, P = .68) and obesity (16.7% vs 15.7%, P = .83) as controls. Similar analyses conducted by using mean BMIz and weight status based on Centers for Disease Control and Prevention standards did not show group differences.
Intervention Dose
Of 15 possible sessions, the mean number attended was 7.75 (4.5), with highest tertile ≥10 by age 2 years and ≥11 by age 3 years. In within group analyses (Table 4), the 2-year mean WFAz decreased as attendance increased with low, medium and high attendance (0.92, 0.59, 0.37, P < .01), and obesity rates decreased (25.9%, 22.6%, 9.8%, P = .01). At 3 years, the mean WFAz decreased as attendance increased (0.90, 0.74, 0.41, P = .03), and obesity rates decreased (26.4%, 22.5%, 8.0%, P = .02). When using the interval of 10 to 26 months, the IV effect of −0.033 (P = .037 [asym.]; P = .047 [boot.]) multiplied by 6.1 (mean number of doses in range) yields −0.20, consistent with GEE findings.
TABLE 4.
Within Intervention Group Dose Effects on Mean WFAz and WFA Percentile Categories
| Within Intervention Group Dose Effects | |||||
|---|---|---|---|---|---|
| Low Attendance (0–4 Sessions) | Medium Attendance (5–9 sessions) | High Attendance (10–12 sessions) | P | Adjusted Pa | |
| Age 2 y | |||||
| Sample No. | 58 | 84 | 61 | — | — |
| Continuous, mean (SD) | |||||
| WFAz | 0.92 (1.17) | 0.59 (1.13) | 0.37 (0.97) | .02 | <.01 |
| Categorical, n (%) | |||||
| WFA ≥85th percentile | 26 (44.8) | 28 (33.3) | 14 (23.0) | .04 | <.01 |
| WFA ≥95th percentile | 15 (25.9) | 19 (22.6) | 6 (9.8) | .06 | .01 |
| Age 3 y | |||||
| Sample No. | 53 | 71 | 72 | — | — |
| Continuous, mean (SD) | |||||
| WFAz | 0.90 (1.27) | 0.74 (1.32) | 0.41 (1.06) | .07 | .03 |
| Categorical, n (%) | |||||
| WFA ≥85th percentile | 21 (39.6) | 28 (39.4) | 21 (29.2) | .35 | .66 |
| WFA ≥95th percentile | 14 (26.4) | 16 (22.5) | 6 (8.0) | .02 | .02 |
—, not applicable.
Adjusted P value obtained using multiple linear and logistic regression controlling for maternal education (did not complete high school versus completed high school), country of origin (not born in the United States versus born in the United States), parity (first child versus not first child), and prepregnancy obesity (BMI <30 vs BMI ≥30).
Discussion
StEP, a prenatal and pediatric primary care-based early child obesity prevention intervention targeting low-income Hispanic families, resulted in lower standardized weights and trajectories between ages 1 and 2 years, although these findings were not significant at 3 years. No significant group differences were found in obesity prevalence. The absolute z-score difference among intervention infants was 0.24 at 18 months and 0.25 at 2 years, close to that achieved by home visiting interventions, in populations with less obesity. These effect sizes are similar to those referenced by the 2017 US Preventive Services Task Force report, which concluded that for children ≥6 years with obesity, a BMI z-score reduction of 0.20 to 0.25 was a suitable threshold for clinically important change associated with improved cardiovascular and metabolic risk factors.54 In dose analyses, increased StEP exposure was associated with lower mean WFAz and obesity prevalence. Children receiving high dose had obesity rates at 2 and 3 years that were ∼60% lower than those receiving low dose.
Achieving weight impacts in child obesity prevention trials, particularly those designed for at-risk groups, has proved difficult.55,56 A recent series of well-designed trials focusing on low-income minority children failed to impact weight.57,58 Several trials beginning during pregnancy or infancy, demonstrated only behavioral improvements.11,12,59 Researchers of 2 trials of home visiting interventions enrolling first-time mothers reported significant weight reductions. Healthy Beginnings in Australia reported a BMI reduction of 0.29 at age 2,13 although not sustained at age 5.60 Researchers of Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT), conducted in primarily middle-income white U.S. families with low rates (∼8%) of obesity, found that intervention infants had lower weight-for-length percentiles (57.5% vs 64.4%) and overweight status (5.5% vs 12.7%) at 12 months15 and lower BMI z-scores at 3 years (−0.28).14 Notably, StEP achieved comparable weight reductions through age 2 years among U.S. children at highest risk of obesity, with low-income, Hispanic ethnicity, and high maternal obesity. Furthermore, this effect was achieved by using the underused health care platform11,12,61,62 and without limiting to first-time mothers.
Research to establish the optimum dose is limited. Authors of a recent US Preventive Services Task Force report found that at least 26 hours was required to achieve clinically meaningful weight reductions, with greater contact associated with larger effects.63 However, the dose needed for obesity prevention remains unknown. A recent systematic review was unable to detect clear relationships between dose and weight, and recommended further study of dose.64 Our findings exploring the dose effects of a preventive intervention during pregnancy and early childhood begin to fill these gaps. The StEP dose received was >50%, equivalent to similar health care-based programs.11,65 Although our findings demonstrate that weight impacts are detected in those receiving at least 5 sessions, maximum impacts are noted in those receiving at least 10, informing recommendations for the dose needed.
This study has several strengths. We were able to successfully recruit, follow and demonstrate weight impacts in a disadvantaged group. The failure of several large trials to impact weight in high-risk preschoolers highlights the urgent need for earlier prevention.55 StEP provides an infrastructure for adding support for high-risk families into frequent health care visits, facilitating scalability, and allowing for seamless intervention from pregnancy through early childhood. Future researchers will explore whether changes in feeding styles and practices, or child sleep, activity, and screen time, serve as mediators of StEP weight impacts.
One limitation was using medical record reviews to obtain anthropometrics. Although researchers have demonstrated that clinical- and research-measured weights correlate well, inaccuracies are common in clinically measured length and height41; therefore, WFAz was used. Another limitation is that we did not track adherence to well-child visits or whether control families learned about the StEP curriculum from other families or providers. Given variability in dose received, future studies need to reduce participation barriers. Lack of sustained effects at age 3 years highlight the need to target the changing multifactorial causes of obesity when children have greater behavioral independence and exposure to obesogenic environments.
Conclusions
A health care–based child obesity prevention program from pregnancy through early childhood led to healthier weight trajectory and lower standardized weight in the first 2 years of life, although the prevalence and degree of obesity was not significantly different at age 3 years. Dose-dependent reduction in obesity prevalence was found. Further study is needed to determine if enhanced program engagement can sustain early effects beyond age 2 years.
Acknowledgments
We thank StEP staff who contributed to this project.
Glossary
- ATE
average treatment effect
- GEE
generalized estimating equation
- IV
instrumental variable
- SNAP
Supplemental Nutrition Assistance Program
- StEP
Starting Early Program
- WFA
weight for age
- WFAz
weight-for-age z-score
- WIC
Special Supplemental Nutrition Program for Women, Infants, and Children
Footnotes
Dr Messito conceptualized and designed the study, supervised acquisition of data, analyzed data, reviewed the analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Mendelsohn substantially contributed to the conception and design of the study and to analysis and interpretation of data and revised the manuscript; Dr Katzow substantially contributed to analysis, interpretation and review of data analyses, and manuscript content and revised the manuscript for important intellectual content; Dr Scott substantially contributed to analysis and interpretation of data, conducted and reviewed data analyses, and revised the manuscript for important intellectual content; Dr Vandyousefi substantially contributed to analysis and interpretation of data and revised the manuscript for important intellectual content; Dr Gross conceptualized and designed the study, supervised acquisition of data, analyzed data, reviewed the analyses, and reviewed and revised the manuscript; and all authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This study has been registered at www.clinicaltrials.gov (identifier NCT01541761).
Deidentified individual participant data will not be made available.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institute of Food and Agriculture, US Department of Agriculture, under award 2011-68001-30207. Funding was also provided by the National Institutes of Health/National Institute of Child Health and Human Development through a K23 Mentored Patient-Oriented Research Career Development Award (K23HD081077; principal investigator: Rachel S. Gross). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of obesity among youths by household income and education level of head of household — United States 2011–2014. MMWR Morb Mortal Wkly Rep. 2018;67(6):186–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377(22):2145–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth JN, Tomporowski PD, Boyle JME, et al. Obesity impairs academic attainment in adolescence: findings from ALSPAC, a UK cohort. Int J Obes. 2014;38(10):1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puhl RM, King KM. Weight discrimination and bullying. Best Pract Res Clin Endocrinol Metab. 2013;27(2):117–127 [DOI] [PubMed] [Google Scholar]
- 7.Johnson F, Pratt M, Wardle J. Socio-Economic Status and Obesity in Childhood In: Moreno LA, Pigeot I, eds.. Epidemiology of Obesity in Children and Adolescents. New York, NY: Springer; 2011:377–390 [Google Scholar]
- 8.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822–w831 [DOI] [PubMed] [Google Scholar]
- 9.Nader PR, Huang TT-K, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention: altering early life systems to support healthy parents, infants, and toddlers. Child Obes. 2012;8(3):195–204 [DOI] [PubMed] [Google Scholar]
- 10.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169(5):484–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell KJ, Lioret S, McNaughton SA, et al. A parent-focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. 2013;131(4):652–660 [DOI] [PubMed] [Google Scholar]
- 12.Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e109 [DOI] [PubMed] [Google Scholar]
- 13.Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children’s BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul IM, Savage JS, Anzman-Frasca S, et al. Effect of a responsive parenting educational intervention on childhood weight outcomes at 3 years of age. JAMA. 2018;320(5):461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at Age 1 year: a randomized clinical trial. JAMA Pediatr. 2016;170(8):742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ordway MR, Sadler LS, Holland ML, Slade A, Close N, Mayes LC. A home visiting parenting program and child obesity: a randomized trial. Pediatrics. 2018;141(2):e20171076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Home Visiting Resource Center 2017 Home Visiting Yearbook. Arlington, VA: James Bell Associates and the Urban Institute; 2017 [Google Scholar]
- 18.Cates CB, Weisleder A, Mendelsohn AL. Mitigating the effects of family poverty on early child development through parenting interventions in primary care. Acad Pediatr. 2016;16(3 suppl):S112–S120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moaddab A, Dildy GA, Brown HL, et al. Health care disparity and pregnancy-related mortality in the United States, 2005-2014. Obstet Gynecol. 2018;131(4):707–712 [DOI] [PubMed] [Google Scholar]
- 20.Partridge S, Balayla J, Holcroft CA, Abenhaim HA. Inadequate prenatal care utilization and risks of infant mortality and poor birth outcome: a retrospective analysis of 28,729,765 U.S. deliveries over 8 years. Am J Perinatol. 2012;29(10):787–793 [DOI] [PubMed] [Google Scholar]
- 21.Coker TR, Thomas T, Chung PJ. Does well-child care have a future in pediatrics? Pediatrics. 2013;131(suppl 2):S149–S159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross RS, Mendelsohn AL, Gross MB, Scheinmann R, Messito MJ. Randomized controlled trial of a primary care-based child obesity prevention intervention on infant feeding practices. J Pediatr. 2016;174:171–177.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross RS, Mendelsohn AL, Yin HS, et al. Randomized controlled trial of an early child obesity prevention intervention: impacts on infant tummy time. Obesity (Silver Spring). 2017;25(5):920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messito MJ, Katzow MW, Mendelsohn AL, Gross RS. AFRI_Starting Early Program impacts on feeding at infant 10 months age: A randomized controlled trial [published online ahead of print January 14, 2020]. Child Obes. doi:10.1089/chi.2019.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1986 [Google Scholar]
- 26.Birch LL, Parker L, Burns A. Early Childhood Obesity Prevention Policies. Washington, DC: National Academies Press, Institute of Medicine; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kathleen M, Rasmussen K, Yaktine A. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press. National Academy of Science (Institute of Medicine); 2009 [PubMed] [Google Scholar]
- 28.Barlow SE; Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberg SJ, Georgieff MK; Committee on Nutrition . Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics. 2018;141(2):e20173716. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services and US Department of Agriculture Dietary guidelines for Americans 2015-2020: 8th edition. 2015. Available at: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/. Accessed October 10, 2019
- 31.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics. 2010;125(4):686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167(8):731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheinmann R, Chiasson MA, Hartel D, Rosenberg TJ. Evaluating a bilingual video to improve infant feeding knowledge and behavior among immigrant Latina mothers. J Community Health. 2010;35(5):464–470 [DOI] [PubMed] [Google Scholar]
- 34.United States Department of Agriculture ChooseMyPlate.gov. Available at: https://www.choosemyplate.gov/resources/myplate-tip-sheets. Accessed September 29, 2018
- 35.Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124(suppl 3):S289–S298 [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine Health Literacy: A Prescription to End Confusion. Washington, DC: The National Academies Press; 2004 [PubMed] [Google Scholar]
- 37.Davis TC, Gazmararian J, Kennen EM. Approaches to improving health literacy: lessons from the field. J Health Commun. 2006;11(6):551–554 [DOI] [PubMed] [Google Scholar]
- 38.Sanders LM, Perrin EM, Yin HS, Bronaugh A, Rothman RL; Greenlight Study Team. “Greenlight study”: a controlled trial of low-literacy, early childhood obesity prevention. Pediatrics. 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richerson JE, Abularrage JJ, Almendarez Y, et al. ; Committee on Practice and Ambulatory Medicine; Bright Futures Periodicity Schedule Workgroup . 2019 recommendations for preventive pediatric health care. Pediatrics. 2019;143(3):e2018397130804073 [Google Scholar]
- 40.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7(4):56. [PMC free article] [PubMed] [Google Scholar]
- 42.WHO WHO Anthro for personal computers, version 322 : Software for assessing growth and development of the world’s children. 2011 [Google Scholar]
- 43.Bryant M, Santorelli G, Fairley L, et al. ; Born in Bradford Childhood Obesity Scientific Group . Agreement between routine and research measurement of infant height and weight. Arch Dis Child. 2015;100(1):24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention A SAS program for the 2000 CDC growth charts (ages 0 to <20 years). 2015. Available at: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed August 24, 2019
- 45.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman-Jensen A, Nord M, Singh A. Household Food Security in the United States in 2012. ERR-155. Washington, DC: U.S. Department of Agriculture: Economic Research Service; 2013 [Google Scholar]
- 47.Pascoe JM, Ialongo NS, Horn WF, Reinhart MA, Perradatto D. The reliability and validity of the maternal social support index. Fam Med. 1988;20(4):271–276 [PubMed] [Google Scholar]
- 48.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081 [DOI] [PubMed] [Google Scholar]
- 49.Mendelsohn AL, Huberman HS, Berkule SB, Brockmeyer CA, Morrow LM, Dreyer BP. Primary care strategies for promoting parent-child interactions and school readiness in at-risk families: the Bellevue Project for Early Language, Literacy, and Education Success. Arch Pediatr Adolesc Med. 2011;165(1):33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan J, Gijbels I. Local Polynomial Modelling and Its Applications: Monographs on Statistics and Applied Probability, vol. 66 Boca Raton, FL: CRC Press; 1996:263–306 [Google Scholar]
- 51.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16(22):2529–2542 [DOI] [PubMed] [Google Scholar]
- 52.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91(434):444–455 [Google Scholar]
- 53.Balestra P, Varadharajan-Krishnakumar J. Full information estimations of a system of simultaneous equations with error component structure. Econom Theory. 1987;3(2):223–246 [Google Scholar]
- 54.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. ; US Preventive Services Task Force . Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(23):2417–2426 [DOI] [PubMed] [Google Scholar]
- 55.Dietz WH. We need a new approach to prevent obesity in low-income minority populations. Pediatrics. 2019;143(6):e20190839. [DOI] [PubMed] [Google Scholar]
- 56.Moore SM, Borawski EA, Love TE, et al. Two family interventions to reduce BMI in low-income urban youth: a randomized trial. Pediatrics. 2019;143(6):e20182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkin SL, Heerman WJ, Sommer EC, et al. Effect of a behavioral intervention for underserved preschool-age children on change in body mass index. JAMA. 2018;320(5):450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486 [DOI] [PubMed] [Google Scholar]
- 59.Taylor BJ, Heath ALM, Galland BC, et al. Prevention of Overweight in Infancy (POI.nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health. 2011;11:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen LM, Baur LA, Simpson JM, et al. Sustainability of effects of an early childhood obesity prevention trial over time: a further 3-year follow-up of the healthy beginnings trial. JAMA Pediatr. 2015;169(6):543–551 [DOI] [PubMed] [Google Scholar]
- 61.French GM, Nicholson L, Skybo T, et al. An evaluation of mother-centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics. 2012;130(3). Available at: www.pediatrics.org/cgi/content/full/130/3/e507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machuca H, Arevalo S, Hackley B, et al. Well baby group care: evaluation of a promising intervention for primary obesity prevention in toddlers. Child Obes. 2016;12(3):171–178 [DOI] [PubMed] [Google Scholar]
- 63.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427–2444 [DOI] [PubMed] [Google Scholar]
- 64.Heerman WJ, JaKa MM, Berge JM, et al. The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression. Int J Behav Nutr Phys Act. 2017;14(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes. 2012;36(10):1292–1298 [DOI] [PubMed] [Google Scholar]