Abstract
Objective
This study explored the association of patients’ demographics, health status, symptom severity, previous osteoarthritis (OA) care, and psychological status with the change in pain severity following a first-line intervention including education and exercise for OA provided nationwide in Swedish primary care.
Methods
This register-based cohort study included 23,309 people with knee or hip OA from the Better Management of Patients with OA register. Linear regression models were used to assess the association of independent variables with the change in pain from baseline to 3 and 12 months. All the analyses were stratified based on the affected joint (hip vs knee).
Results
In people with hip and people with knee OA, high levels of baseline pain were associated with decreased pain at both follow-ups (3 months: knee B = −.67; hip B = −.64; 12 months: knee B = −.70; hip B = −.66), whereas being older, overweight, or female had a weak or no association. Finally, at both follow-ups, bilateral OA was associated with increased pain only in people with knee OA, whereas comorbidities and the willingness to undergo surgery were associated with increased pain regardless of the affected joint.
Conclusions
Baseline pain showed the strongest association among the analyzed variables, whereas sex, age, and body mass index appear to be weakly associated with the pain change after a first-line intervention. Comorbidities and willingness to undergo surgery showed a potentially important association and may have a negative impact on the pain change following a first-line intervention.
Impact
In people with hip or knee OA, age, sex, body mass index, and previous surgery are only weakly associated with the change in pain after a first-line intervention supporting the evidence recommending exercise and education as a foundation for all OA therapy. Having comorbidities and being willing to undergo surgery is associated with a worse outcome from a first-line intervention, including exercise and education. Individualized treatments addressing the disease perception and the specific comorbidity profile may improve the outcomes.
Osteoarthritis (OA) is one of the main causes of activity limitations in older adults, which places it among the world’s 10 most disabling conditions.1 Roughly 40% of the world population aged older than 65 years is estimated to have OA, with an annual health care cost estimated to be as high as 7 billion euros in Europe only.2,3 Without a cure for OA, the management of the disease focuses primarily on pain reduction and symptomatic relief. International guidelines recommend education and exercise as the first-line intervention for OA to reduce pain and improve patients’ quality of life.4,5
Yet, results from education- and exercise-based interventions vary largely between individuals and joint affected (hip vs knee).6,7 Treatments for OA tend to be one-size-fits-all, largely due to the lack of information regarding the effect that patients' specific characteristics have on treatment response. Indeed, OA is caused by a multitude of factors (eg, biomechanical, metabolic, genetic), which influence both the disease process and the individual’s perception of pain.8–11 In this context, OA is hypothesized to be a syndrome comprised of multiple phenotypes.8,12 Identifying a relationship between patient- and disease-specific factors and treatment response represents a key step toward personalized OA care.
Information such as patients’ demographics (age, sex, education), health status (physical activity, body mass index [BMI]), symptom severity (pain frequency, bilateral OA, walking difficulties), previous OA care (previous surgery, drug intake, previous rehabilitation), and psychological status (fear of movement, self-efficacy) is often collected in clinical practice and used by clinicians to design interventions. Previous evidence has shown that these factors have the potential to impact disease status and progression and may be important when planning interventions for people with OA.10,13–16
However, little is known about the association of these factors with the outcome of first-line interventions. Previous studies often examined single factors in isolation using samples originally recruited for randomized controlled trials, potentially missing key interactions and generating results that may not be transferrable to the general population seeking OA care.17,18 Finally, people with knee and/or hip OA appear to respond differently to first-line interventions, with different factors potentially responsible for this difference.
This study aims to provide a comprehensive exploration of the joint-specific association of patients’ demographics; health, disease, and psychological status; and previous OA care with the change in pain severity following a first-line intervention provided nation-wide in Swedish primary care.
Methods
Study Design
This is a register-based cohort study with sociodemographic, health status, disease severity, previous OA care, and psychological factors assessed at baseline and after 3 and 12 months following a first-line intervention. The study was approved by the Regional Ethical Review Board in Gothenburg (1059–16).
Intervention: Better Management of Patients With Osteoarthritis (BOA)
BOA is a national quality register including data from a first-line intervention provided nationwide in Sweden to people with OA of the knee, hip, hand, and shoulder. All the patients taking part in BOA receive a minimum of 2 theoretical group sessions led by a physical therapist focusing on the disease pathophysiology and on the benefit of exercise, including self-management advice and strategy to incorporate exercise into daily life.19 Participants can then take part in a 1-on-1 session with a physical therapist who designs a rehabilitation program in accordance with OA clinical guidelines and based on the patient’s specific needs and goals.20 During this session, the participants are instructed on how to perform the program independently and how to manage the pain during the exercise using a tolerable pain model.21 Finally, participants can decide to perform their exercise program at home or under the supervision of a physical therapist in 12 group sessions of the duration of 1 hour provided twice per week for a total of 6 weeks. Further details on the BOA program can be found elsewhere.19
Study Sample
The study sample consisted of people with knee and/or hip OA who participated in BOA between 2008 and December 2016. The presence of a clinical diagnosis of OA from primary or secondary care is the only criteria necessary to be eligible for BOA. The exclusion criteria are joint pain caused by another disease (eg, hip fractures, inflammatory joint disease, cancer), total joint replacement within the past 12 months; other surgery to the knee or hip within the past 3 months (eg, meniscectomy); or does not understand Swedish. The index joint was selected by the physical therapist based on the participant’s medical history, symptoms, and results of the clinical examination. In the case of OA affecting multiple joints, the most symptomatic joint was considered as the index joint for the treatment. The patient-reported outcomes were assessed at the baseline and the 3-month follow-up during a visit with a physical therapist. During these visits, the physical therapist performed a clinical examination, delivered the questionnaires for the self-reported outcomes, and entered the data into the register. At the 12-month follow-up, the questionnaires for the self-reported outcomes were sent to the participants by mail with a prepaid envelope to return the completed questionnaires. Patients who attended the two education sessions provided in BOA and either home or supervised exercise with data available at both follow-ups were included in this study. Participants followed-up later than the indicated period or with data missing at one of the follow-ups were excluded from the study.
Included Variables
Pain intensity
Mean pain intensity during the last week in the index joint was evaluated at baseline and follow-ups on a numeric rating scale (NRS) ranging from 0 (no pain) to 10 (maximum pain). The NRS is a valid, reliable, and responsive measure of pain widely used in people with OA.22 The change in pain was used as the dependent variable and was calculated as the difference between follow-up pain and baseline pain.
Sociodemographics
Participants reported their age, sex, level of education (<14 years; ≥14 years), and living situation (living alone, living with someone).
Health-related factors
Participants rated their general health status on a visual analogue scale with a score ranging from 0 (poor health) to 100 (excellent health). Body weight and height were self-reported at the first visit from which the BMI was calculated as kg/m2.
Pain frequency
Pain frequency was assessed by a question: “How often do you have pain in your knee/hip,” with 5 possible answers: never, every month, every week, every day, or all the time. For the purpose of the study, we have dichotomized this question to frequent pain (every day or all the time) and infrequent pain (every week, every month, never).
Intake of drugs for OA
Intake of drugs was evaluated by the physiotherapist asking the patients whether they had taken any drugs for OA during the last 3 months because of their knee/hip pain. The question was dichotomized into “yes” or “no.” Any kind of drug prescribed for OA or taken to subside the OA-related joint symptoms was considered for this variable.
Self-efficacy
Self-efficacy was assessed by the Arthritis Self-Efficacy Scale, which is designed to assess participants’ confidence in their ability to manage symptoms of arthritis. The final score ranges from 10 to 100, with higher values representing higher self-efficacy. In BOA, only the scale assessing pain and other symptoms self-efficacy have been included. Arthritis Self-Efficacy Scale has previously been used to evaluate patient education programs for patients with arthritis and is validated in Swedish.23,24
Willingness to undergo surgery
The willingness to undergo surgery was assessed by the question “Are your knee/hip symptoms so severe that you wish to undergo surgery? (yes/no).”
Fear of movement
Fear of movement was assessed by the question “Are you afraid your joints will be injured by physical training/activity? (yes/no).”
Physical activity
Physical activity was assessed by the question “How active are you during a regular, typical week,” with 7 possible answers: inactive, less than 30 minutes, 30 to 60 minutes, 60 to 90 minutes, 90 to 150 minutes, 150 to 300 minutes, and more than 300 minutes. The question was dichotomized into <150 min/wk and ≥150 min/wk based on the international recommendation for physical activity.25
Charnley classification
The Charnley classification categorizes patients into 3 classes: A, unilateral OA (knee or hip); B, bilateral OA (both knees or both hips); C, OA in multiple joint sites (eg, hip and knee) and the presence of any other disease that affects walking ability.26,27
Treatment modality
BOA participants received a personalized exercise program and could decide to carry it out (1) unsupervised at home or (2) supervised by a physical therapist in 12 group exercise sessions.
Statistical Analysis
All the analyses were stratified based on the index joint. Separate linear regression models were used to assess the association of the independent variables with the change in pain from baseline to 3 months, and from baseline to 12 months. Categorical variables were coded as dummy variables before being included in the model. Negative values indicate a reduction of pain. Assumptions for multiple linear regressions were checked and examination of multicollinearity between variables conducted. Initially, all the independent variables were entered simultaneously in the model. An augmented backward elimination process was adopted.28 Variables with α ≥ .2 were excluded one-by-one from the model unless their exclusion led to a change in the estimates of the other factors >10%, in which case the variable was retained as a confounder together with variables with α > .05 and <.2.28,29 Alpha-level was set at .05, all the variables reaching statistical significance were retained in the models. All statistical analyses were conducted using SPSS software (v 25.0, SPSS, Chicago, IL). Results are presented as unstandardised regression coefficients (B) and are accompanied by 95% CI. A change of 1 point on the NRS scale at the follow-ups was considered clinically relevant. This cut-off was previously validated in a sample of people with OA and other chronic rheumatic conditions and indicates subjects feeling slightly better.30
Results
Among the people who took part in BOA, 44,228 had knee or hip OA and took part in the one-to-one session with a physical therapist receiving a personalized exercise program. Of these, 20,919 did not report data at one or both follow-ups, were followed-up outside the established time frame, or were not yet followed-up at the time of the data extraction (Figure). Baseline characteristics of the subjects who were not included in the study are reported in Table 1. Excluded patients showed similar characteristics to the people included in the study despite a higher prevalence of people willing to undergo joint replacement.
Figure.
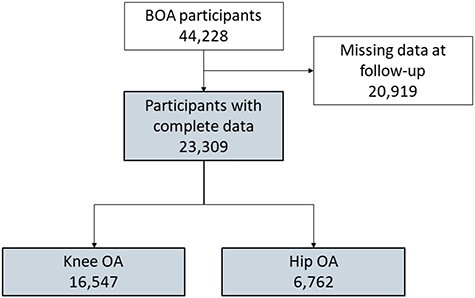
Study flowchart
Table 1.
Baseline Characteristics for Excluded and Included Patientsa
| Cohort and No. of Patients | Excluded (n = 20,919) | Knee OA (n = 16,547) | Hip OA (n = 6762) | ||
|---|---|---|---|---|---|
| Baseline Characteristics | Mean (SD) or % | Patients Reporting Outcome | Mean (SD) or % | Patients Reporting Outcome | Mean (SD) or % |
| Baseline pain (0–10) | 5.5 (1.95) | 16,507 | 5.1 (1.95) | 6745 | 5.1 (1.93) |
| Age (y) | 65.7 (9.55) | 16,547 | 66.3 (9.02) | 6762 | 67.1 (9.14) |
| BMI | 28.1 (4.85) | 16,268 | 28.2 (4.93) | 6657 | 26.7 (4.3) |
| Sex | 16,547 | 6762 | |||
| Men | 31.3 | 4929 | 29.8 | 21,985 | 29.4 |
| Women | 68.7 | 11,618 | 70.2 | 4777 | 70.6 |
| Quality of life (0–100) | 65.0 (19.63) | 13,278 | 68.6 (18.46) | 5560 | 67.1 (18.71) |
| Physical activity (min/wk) | 13,331 | 5585 | |||
| <150 | 60.6 | 7420 | 55.7 | 3024 | 54.1 |
| ≥150 | 39.4 | 6064 | 44.3 | 2561 | 45.9 |
| Living situation | 16,495 | 6747 | |||
| With someone | 71.6 | 12,122 | 73.5 | 4941 | 73.2 |
| Alone | 28.4 | 4373 | 26.5 | 1806 | 26.8 |
| Education (y) | 16,501 | 6741 | |||
| Low (0–13) | 72.6 | 11,560 | 70.1 | 4666 | 69.2 |
| High (≥14) | 27.4 | 4941 | 29.9 | 2075 | 30.8 |
| Treatment modality | 16,441 | 6730 | |||
| Home exercise | 41.2 | 6722 | 40.9 | 2813 | 41.8 |
| Supervised exercise | 58.8 | 9719 | 59.1 | 3917 | 58.2 |
| Pain frequency | 16,490 | 6730 | |||
| Every week or less often | 15.5 | 3191 | 19.4 | 1237 | 18.4 |
| Every day or all the time | 84.5 | 13,299 | 80.6 | 5493 | 81.6 |
| Charnley class | 16,547 | 6733 | |||
| A (1 joint with OA) | 38.2 | 6465 | 39.0 | 2540 | 38.0 |
| B (bilateral OA) | 17.8 | 3970 | 24.3 | 775 | 11.5 |
| C (bilateral OA + other comorbidities) | 44.0 | 6112 | 37.7 | 3418 | 50.5 |
| Walking difficulties due to OA | 16,466 | 6725 | |||
| No | 16.4 | 3530 | 21.4 | 1467 | 21.8 |
| Yes | 82.7 | 12,936 | 78.6 | 65,258 | 78.2 |
| Drugs for OA in last 3 mo | 16,496 | 6,733 | |||
| No | 22.7 | 4238 | 25.7 | 1740 | 25.8 |
| Yes | 76.8 | 12,258 | 74.1 | 4993 | 74.2 |
| Previous surgery index joint | 16,520 | 6748 | |||
| No | 87.0 | 13,704 | 83.0 | 6627 | 98.2 |
| Yes | 13.0 | 2816 | 17.0 | 121 | 1.8 |
| Previous surgery contralateral | 16,472 | 6737 | |||
| No | 87.2 | 14,642 | 88.9 | 6284 | 93.3 |
| Yes | 11.3 | 1830 | 11.1 | 453 | 6.7 |
| Previous physical therapy | 16,498 | 6740 | |||
| No | 54.4 | 8829 | 53.5 | 3660 | 54.3 |
| Yes | 45.6 | 7669 | 46.5 | 3080 | 45.7 |
| Willingness to undergo surgery | 16,495 | 6705 | |||
| No | 69.5 | 13,158 | 80.3 | 5334 | 79.6 |
| Yes | 30.5 | 3237 | 19.7 | 1371 | 20.4 |
| Fear of movement | 16,459 | 6728 | |||
| No | 82.6 | 13,738 | 83.5 | 5823 | 86.5 |
| Yes | 17.4 | 2271 | 16.5 | 905 | 13.5 |
| ASES pain (0–100) | 61.2 (19.24) | 16,167 | 65.2 (18.26) | 6602 | 62.5 (18.34) |
| ASES symptoms (0–100) | 66.1 (17.41) | 16,064 | 69.0 (16.44) | 6556 | 67.7 (16.4) |
a ASES = Arthritis Self-Efficacy Scale; BMI = body mass index; OA = osteoarthritis.
A total of 23,309 people were included in this study. The mean change in pain was −1.27 (SD = 2.14) and −0.98 (SD = 2.34) at 3 months and −0.93 (SD = 2.10) and −0.47 (SD = 2.32) at 12 months for people with knee and/or hip OA, respectively.
Knee OA
Baseline pain, BMI, health-related quality of life, education, treatment group, pain frequency, Charnley class, previous surgery to the index knee, previous physical therapy, willingness to undergo surgery, fear of movement, and pain self-efficacy were associated with the change in pain at 3 months (P < .001, adjusted R2 = .28) (Tab. 2). Age, previous contralateral surgery, and other symptoms self-efficacy did not reach statistical significance but were retained in the model as confounders.
Table 2.
Regression Coefficients of Factors Associated With Pain Reduction at 3 and 12 Months After Interventiona
| Knee OA b | Hip OA b | |||
|---|---|---|---|---|
| Baseline Characteristics | 3 Months B (95% CI) | 12 Months B (95% CI) | 3 Months B (95% CI) | 12 Months B (95% CI) |
| Baseline pain (0–10) | −0.67 (−0.69 to − 0.65) | −0.70 (−0.72 to − 0.67) | −0.64 (−0.67 to 0.66) | −0.66 (−0.70 to − 0.63) |
| Age (y) | 0.00 (0.00 to 0.01) | 0.01 (0.01 to 0.02) | n/a | n/a |
| BMI | 0.02 (0.02 to 0.03) | 0.03 (0.02 to 0.04) | 0.02 (0.01 to 0.03) | 0.04 (0.03 to 0.06) |
| Sex | ||||
| Men | Reference | Reference | Reference | Reference |
| Women | n/a | n/a | 0.078 (−0.23 to 0.18) | n/a |
| Quality of life (0–100) | −0.01 (−0.01 to − 0.01) | −0.01 (−0.01 to − 0.01) | −0.01 (−0.01 to − 0.00) | −0.01 (−0.01 to − 0.00) |
| Physical activity (min/wk) | ||||
| <150 | Reference | Reference | Reference | Reference |
| ≥150 | n/a | n/a | n/a | −0.15 (−0.26 to − 0.04) |
| Living situation | ||||
| Live with someone | Reference | Reference | Reference | Reference |
| Live alone | n/a | n/a | n/a | 0.12 (−0.00 to 0.24) |
| Education (y) | ||||
| Low (0–14) | Reference | Reference | Reference | Reference |
| High (>14) | −0.12 (−0.19 to − 0.05) | −0.24 (−0.32 to − 0.16) | −0.13 (−0.23 to − 0.02) | −0.16 (−0.28 to −0.38) |
| Pain frequency | ||||
| Every week or less often | Reference | Reference | Reference | Reference |
| Every day or all the time | 0.36 (0.27 to 0.45) | 0.35 (0.26 to 0.44) | 0.28 (0.14 to 0.41) | 0.34 (0.19 to 0.50) |
| Charnley class | ||||
| A (1 joint with OA) | Reference | Reference | Reference | Reference |
| B (bilateral OA) | 0.38 (0.30 to 0.46) | 0.50 (0.34 to 0.67) | 0.13 (−0.03 to 0.29) | 0.10 (−0.84 to 0.28) |
| C (bilateral OA + other comorbidities) | 0.49 (0.41 to 0.56) | 0.71 (0.56 to 0.86) | 0.22 (0.11 to 0.33) | 0.32 (0.20 to 0.44) |
| Walking difficulties due to OA | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | n/a | 0.20 (0.11 to 0.29) | 0.16 (0.03 to 0.29) | 0.38 (0.24 to 0.52) |
| Drugs for OA in last 3 mo | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | n/a | n/a | n/a | 0.16 (0.04 to 0.28) |
| Previous surgery | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.12 (0.03 to 0.20) | 0.33 (0.23 to 0.42) | n/a | n/a |
| Previous surgery contralateral | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.07 (−0.29 to 0.18) | 0.07 (−0.02 to 0.19) | n/a | n/a |
| Previous physical therapy | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.15 (0.08 to 0.21) | 0.16 (0.09 to 0.23) | 0.09 (−0.011 to 0.186) | 0.16 (0.05 to 0.27) |
| Willingness to undergo surgery | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.36 (0.28 to 0.45) | 0.51 (0.42 to 0.61) | 0.50 (0.363 to 0.632) | 0.50 (0.35 to 0.65) |
| Fear of movement | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | −0.10 (−0.01 to − 0.03) | −0.23 (−0.33 to − 0.13) | n/a | −0.15 (−0.31 to 0.01) |
| ASES pain (0–100) | −0.01 (−0.01 to − 0.01) | −0.01 (−0.01 to − 0.01) | −0.01 (−0.013 to − 0.007) | −0.01 (−0.01 to − 0.01) |
| ASES symptoms (0–100) | −0.00 (−0.01 to 0.00) | −0.00 (−0.01 to 0.00) | n/a | n/a |
a ASES = Arthritis Self-Efficacy Scale; B = unstandardized regression coefficient; BMI = body mass index; n/a = not available due to being dropped during the backward elimination process; OA = osteoarthritis.
Statistically significant results are reported in bold.
Baseline pain, BMI, age, quality of life, education, pain frequency, Charnley class, walking difficulties, previous surgery to the index knee, previous physical therapy, willingness to undergo surgery, fear of movement, and pain self-efficacy were associated with the change in pain at 12 months (P < .001, adjusted R2 = .26). Previous contralateral surgery and other symptoms self-efficacy were the only non-significant variables retained in this model as confounders.
Hip OA
Baseline pain, BMI, health-related quality of life, education, pain frequency, Charnley class C, willingness to undergo surgery, and pain self-efficacy pain were associated with the change in pain at 3 months (P < .001, adjusted R2 = .25) (Tab. 2). Sex, Charnley class B, walking difficulties, and previous physical therapy were not statistically significant but were retained in the model as confounders.
Baseline pain, BMI, quality of life, physical activity, pain frequency, Charnley class C, walking difficulties, drugs for OA in the last 3 months, previous physical therapy, willingness to undergo surgery, and pain self-efficacy were associated with the change in pain at 12 months (P < .001, adjusted R2 = .23) (Tab. 2). Sex, living situation, education, Charnley class A, and fear of movement were not significant but were retained in the model as confounders.
Discussions
To the best of our knowledge, this is the first study exploring the association between person- and disease-specific factors and the change in pain after 3 and 12 months in more than 20,000 people with knee and/or hip OA who underwent a first-line intervention.
Pain is the most disabling symptom for people with OA and one of the major drivers of clinical decision-making. In this study, higher levels of baseline pain were associated with a larger absolute reduction in pain intensity at both follow-ups, confirming previous evidence suggesting that exercise for knee and/or hip OA is effective regardless of the intensity of pain.4 This can be partially explained through a regression to the mean effect. However, previous evidence from BOA data showed the superiority of exercise compared with a minimal intervention including only education, suggesting that the treatment plays a role in explaining the observed changes.31 As opposed to pain intensity, frequent pain at baseline was associated with increased pain at both follow-ups, especially in people with knee OA. This can be explained by the fact that frequent pain is linked to worse symptomatic and structural disease severity, which may negatively impact the outcome of the intervention.32
Older age, female sex, and high BMI have been shown to be associated with more severe symptoms and faster disease progression.33,34 In our study, only BMI was associated with an increase in pain after the treatment across all the follow-ups and regardless of the joint involved (hip or knee), but the association was of questionable clinical importance. The current results suggest that first-line interventions should be advised regardless of the person’s age, sex, and BMI. However, addressing weight reduction in overweight or obese people with OA is key to improve health and maximize exercise benefits.35
Baseline physical activity had no effect on pain change after treatment with the exception of people with hip OA at 12 months, where the effect is of scarce clinical importance. This result confirms previous findings from similar first-line interventions suggesting that people with OA may benefit from education and exercise regardless of their initial level of physical activity.14
In this study, higher levels of pain self-efficacy and quality of life were associated with lower levels of pain at both follow-ups, confirming results from previous studies. People with high self-efficacy tend to report less pain and higher quality of life and are more willing to pursue challenges, which may explain these results.15,36 Despite this, the association of baseline pain self-efficacy on the change in pain was somewhat weak. Similarly, quality of life had a small association with the change of pain, which could hardly be clinically significant when considered in isolation.
Having OA in multiple joints and comorbidities is associated with worse symptoms and physical function.16,37 In the current study, bilateral OA (Charnley class B) was associated with an increase in pain in people with knee OA at both follow-ups. The addition of other comorbidities influencing the gait (Charnley class C) led to increased pain in both joint subgroups, with a potentially clinically important association in people with knee OA, especially at 12 months. These results underline the potential importance of adapting first-line interventions to the patient’s comorbidity profile, which may maximize treatment benefits.38 More information regarding the severity of the symptoms in the contralateral joint and the number and severity of comorbidities may help clarify the difference in the association between people with knee and/or hip OA.
Previous care for OA had a somewhat contrasting association with the outcome under examination. Taking OA-related drugs in the 3 months preceding the intervention was not associated with the change in pain following the intervention (3 months). However, in people with hip OA, taking drugs for OA was associated with a small pain increase at 12 months (B: 95% CI = 0.05 to 0.27). This result is hard to interpret due to the lack of information in the BOA register regarding the type of drug, dose, and treatment plan. However, taking drugs before the intervention does not seem to be associated with additional benefit, reinforcing the idea that exercise and education should be proposed before attempting other treatments.
Having received previous physical therapy was associated with increased pain in both joint sub-groups at both follow-ups. Due to the lack of information regarding the number and content of rehabilitation sessions undertaken, interpretation of these results requires care. It is possible that having sought care from a physical therapist may be a proxy for disease severity, explaining the increase in pain. It can also be hypothesized that people who received rehabilitation may have already implemented changes in their daily life that are suggested in BOA, thus reducing the benefit of the intervention.
The presence of walking difficulties was the only available measure to capture disability in the cohort and was associated in both the joint subgroups with increased pain at 12 months only. More objective measures of disability are needed to clarify the association between physical impairments and pain reduction after first-line interventions.
Having received previous surgery (eg, anterior cruciate ligament reconstruction, meniscectomy) had a potentially important association with increased pain only in people with knee OA, particularly at 12 months. These results suggest that for those with knee OA who underwent surgery, first-line intervention may be effective in the short term, while longer interventions (>12 sessions) may be necessary to prolong the benefits. The absence of an association between previous surgery and pain reduction in people with hip OA may be due to the small number of people with hip OA who received surgery before enrolment in BOA (hip OA = 1.8%; knee OA = 17%).
Willingness to undergo surgery at baseline was associated with a potentially important increase in pain at both follow-ups, regardless of the affected joint. More severe symptoms and disability, as well as previous experiences and expectations, are linked to the desire for surgery and have the potential to influence the outcome of treatments.39,40 This suggests that willingness to undergo surgery may be a proxy for disease severity and other factors potentially explaining some of the variation not fully captured by the included variables. Previous evidence has shown that exercise and education are effective in patients listed for surgery and can delay surgery for at least 2 years.41-43 However, willingness to undergo surgery appears to be linked to a worse outcome, and it should be taken into consideration when designing and delivering first-line interventions.
Preliminary evidence suggests that people with knee OA experience greater improvements after undergoing first-line interventions compared with people with hip OA.6,7,31 Despite several hypotheses based on differences in joint biomechanics and disease mechanisms, the reason for the different response to first-line interventions is still not clear. This study showed a similar pattern in the association between the examined variables and the change in pain after the intervention in people with knee and hip OA. Among the variables that show a different association, most are of scarce clinical importance due to the small magnitude of the association (ie, physical activity, previous drugs for OA, fear of movement), and they could hardly explain the difference in treatment outcomes. Of potential clinical importance, bilateral OA and additional comorbidity showed an association with increased pain only in people with knee OA. Similarly, fear of movement in people with knee OA, but not in those with hip OA, seem to be associated with greater pain reduction following the intervention. Considering that the knee joint is more biomechanically unstable than the hip, it can be hypothesized that the improvement of neuromuscular control that follows exercise may lead to greater benefits in people with knee OA and fear of movement. However, further research is needed to clarify the reason for these differences.
Overall, among the variables analyzed, only having Charnley class C, walking difficulties, and having received previous surgery showed different levels of association (without overlapping CI) between 3 and 12 months. People presenting these characteristics may be thought to have a more severe disease, which, in turn, can explain the larger pain increase showed at 12 months after the treatment was terminated. The different associations between follow-ups may suggest that longer intervention may be needed to mitigate the increase in pain in the year following the intervention, possibly mitigating the pain increase observed in the long term. The other analyzed variables showed, instead, similar associations between the follow-ups, suggesting that these variables have a similar influence on the pain change regardless of the follow-up time.
Finally, baseline pain appeared to be the factor more strongly associated with the change in pain after a first-line intervention followed by the presence of comorbidity (Charnley class), willingness to undergo surgery, and baseline pain frequency. These results seem to suggest that factors linked to disease severity are more strongly associated with absolute pain reduction than other factors such as age and BMI. However, while higher pain is associated with greater pain reduction, the presence of walking disability or comorbidities appear to be associated with worse pain following a first-line intervention. However, little can be said regarding the role of the analyzed variable in the mechanisms leading to the pain reduction. Further research building on these results and exploring the causal pathways is warranted to disentangle the relationship between these variables and the pain reduction.
This study has some limitations that need to be discussed. First of all, the observational nature of the study does not allow us to establish causality and to draw any conclusion on the effect of the treatment on the pain change. A large number of variables were not reported at baseline, most likely due to a missed upload to BOA by the physical therapist responsible for uploading the data at the local unit. For this reason, we assumed that missing data at baseline may be considered to be missing at random and should introduce no or minimal bias in the analysis. However, it was not possible to verify the real reason for the data missing data. Thus, we recommend interpreting the results cautiously, taking into account possible biases due to missing data. Pain was the only outcome tested for association with baseline variables. This implies that examined variables may have important associations with other key therapeutically outcomes like physical function, which is not available in the register. Including additional variables may increase the low variance explained by the models. Despite being hard to capture, contextual factors including patient-physical therapist relation, expectations, and personal preferences have an important role in determining treatment outcomes and may account for part of the unexplained variance.44 Finally, none of the variables analyzed affected the change in pain that approached a 1-unit change, which is often considered to be the minimal clinically important difference for pain when measured on an NRS scale30; however, certain characteristics may tend to cluster together, potentially leading to clinically significant effects. For this reason, future research carefully exploring characteristics of responders to first-line management interventions is warranted. Despite these limitations, this study used data from more than 20,000 patients who received treatment in clinical practice nationwide, strengthening the clinical relevance and external validity of the results.
Conclusions
Providing the right treatment to the right patient is a key step in reducing the burden of OA for society and the patients. In this study, we showed that higher baseline pain was associated with greater (absolute) pain reduction and that participants’ age, sex, BMI, and previous surgery are only weakly associated with the change in pain after a first-line intervention, somewhat supporting the evidence recommending exercise and education as a foundation for all OA therapy. Comorbidities and willingness to undergo surgery instead appear to be associated with a worse outcome from a first-line intervention and may require individualized treatments.
Contributor Information
Andrea Dell’Isola, Department of Clinical Sciences Orthopaedic, Faculty of Medicine, Lund University, Entrégatan 8 Lund 22100, Sweden, and Department of Clinical Sciences Orthopaedics, Clinical Epidemiology Unit, Lund University.
Therese Jönsson, Department of Clinical Sciences Orthopaedic, Faculty of Medicine, Lund University.
Håkan Nero, Department of Clinical Sciences Orthopaedic, Faculty of Medicine, Lund University.
Frida Eek, Department of Health Sciences, Division of Physiotherapy, Lund University.
Leif Dahlberg, Department of Clinical Sciences Orthopaedic, Faculty of Medicine, Lund University.
Author Contributions and Acknowledgments
Concept/idea/research design: A. Dell’Isola, T. Jönsson, H. Nero, F. Eek, L. Dahlberg
Writing: A. Dell’Isola, T. Jönsson, H. Nero, F. Eek, L. Dahlberg
Data collection: A. Dell’Isola, T. Jönsson
Data analysis: A. Dell’Isola, T. Jönsson, H. Nero, F. Eek, L. Dahlberg
Project management: A. Dell’Isola, L. Dahlberg
Fund procurement: L. Dahlberg
Consultation (including review of manuscript before submitting): H. Nero
The authors thank all participating patients, the physical therapists reporting data to the BOA register, and others involved in BOA.
Ethics Approval
This study was approved by the Regional Ethical Review Board in Gothenburg (1059–16).
Funding
There is no funding to report for this study.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
T. Jönsson is a member of the steering committee of the BOA register, for which she does not receive any financial compensation. L. Dahlberg is cofounder and chief medical officer of Joint Academy, a company that provides digital nonsurgical treatment for patients with hip and/or knee OA. L. Dahlberg owns stocks in, is a board member of, and is a paid part-time consultant for Joint Academy since May 1, 2019. H. Nero is a part-time employee at Joint Academy. Of note, Joint Academy has not supported this work financially and has thus played no role concerning study conception, study design, analysis of data, and manuscript compilation. All other authors report no competing interests.
References
- 1. World Health Organization . Chronic Rheumatic Conditions. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2. Salmon JH, Rat AC, Sellam J, et al. Economic impact of lower-limb osteoarthritis worldwide: a systematic review of cost-of-illness studies. Osteoarthritis Cartilage. 2016;24: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 3. European Medical Agency (EMA) Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. https://www.ema.europa.eu. Accessed September 24, 2019.
- 4. Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–636. [DOI] [PubMed] [Google Scholar]
- 5. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019; 27:1578–1589. [DOI] [PubMed] [Google Scholar]
- 6. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015; 2015;1:CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4:CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dell'Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dell'Isola A, Smith SL, Andersen MS, Steultjens M. Knee internal contact force in a varus malaligned phenotype in knee osteoarthritis (KOA). Osteoarthritis Cartilage. 2017; 25:2007–2013. [DOI] [PubMed] [Google Scholar]
- 10. Kittelson AJ, George SZ, Maluf KS, Stevens-Lapsley JE. Future directions in painful knee osteoarthritis: harnessing complexity in a heterogeneous population. Phys Ther. 2014; 94:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dell'isola A, Wirth W, Steultjens M, Eckstein F, Culvenor AG. Knee extensor muscle weakness and radiographic knee osteoarthritis progression. Acta Orthop. 2018;89:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dell'Isola A, Steultjens M. Classification of patients with knee osteoarthritis in clinical phenotypes: data from the osteoarthritis initiative. PLoS One. 2018;13:e0191045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. The Lancet. 2019;393:1745–1759. [DOI] [PubMed] [Google Scholar]
- 14. Skou ST, Bricca A, Roos EM. The impact of physical activity level on the short- and long-term pain relief from supervised exercise therapy and education: a study of 12,796 Danish patients with knee osteoarthritis. Osteoarthritis Cartilage. 2018;26:1474–1478. [DOI] [PubMed] [Google Scholar]
- 15. Jackson T, Wang Y, Wang Y, Fan H. Self-efficacy and chronic pain outcomes: a meta-analytic review. J Pain. 2014;15: 800–814. [DOI] [PubMed] [Google Scholar]
- 16. van Dijk GM, Veenhof C, Schellevis F, et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eyles JP, Lucas BR, Patterson JA, et al. Does clinical presentation predict response to a nonsurgical chronic disease management program for endstage hip and knee osteoarthritis? J Rheumatol. 2014;41:2223–2231. [DOI] [PubMed] [Google Scholar]
- 18. Veenhof C, Dekker J, Bijlsma JW, van den Ende CH. Influence of various recruitment strategies on the study population and outcome of a randomized controlled trial involving patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2005;53:375–382. [DOI] [PubMed] [Google Scholar]
- 19. Thorstensson CA, Garellick G, Rystedt H, Dahlberg LE. Better management of patients with osteoarthritis: development and nationwide implementation of an evidence-based supported osteoarthritis self-management programme. Musculoskeletal Care. 2015;13:67–75. [DOI] [PubMed] [Google Scholar]
- 20. Ageberg E, Roos EM. Neuromuscular exercise as treatment of degenerative knee disease. Exerc Sport Sci Rev. 2015;43:14–22. [DOI] [PubMed] [Google Scholar]
- 21. Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for pain (VAS pain), Numeric Rating Scale for pain (NRS pain), McGill Pain Questionnaire (MPQ), Short-Form Mcgill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240–S252. [DOI] [PubMed] [Google Scholar]
- 23. Brand E, Nyland J, Henzman C, McGinnis M. Arthritis self-efficacy scale scores in knee osteoarthritis: a systematic review and meta-analysis comparing arthritis self-management education with or without exercise. J Orthop Sports Phys Ther. 2013;43:895–910. [DOI] [PubMed] [Google Scholar]
- 24. Lomi C, Nordholm LA. Validation of a Swedish version of the arthritis self-efficacy scale. Scand J Rheumatol. 1992;21: 231–237. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organisation (WHO). Global Recomendation on Physical Activity for Health. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 26. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charnley J, Halley DK. Rate of wear in total hip replacement. Clin Orthop Relat Res. 1975;170–179. [PubMed] [Google Scholar]
- 28. Dunkler D, Plischke M, Leffondre K, Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PLoS One. 2014;9:e113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heinze G, Dunkler D. Five myths about variable selection. Transpl Int. 2017;30:6–10. [DOI] [PubMed] [Google Scholar]
- 30. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–291. [DOI] [PubMed] [Google Scholar]
- 31. Dell'Isola A, Jonsson T, Ranstam J, Dahlberg LE, Ekvall Hansson E. Education, home exercise, and supervised exercise for people with hip and knee osteoarthritis as part of a Nationwide implementation program: data from the better management of patients with osteoarthritis registry. Arthritis Care Res (Hoboken). 2020;72:201–207. [DOI] [PubMed] [Google Scholar]
- 32. Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63:1115–1125. [DOI] [PubMed] [Google Scholar]
- 34. Janssen I, Mark AE. Separate and combined influence of body mass index and waist circumference on arthritis and knee osteoarthritis. Int J Obes (Lond). 2006;30:1223–1228. [DOI] [PubMed] [Google Scholar]
- 35. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32: 37–44. [DOI] [PubMed] [Google Scholar]
- 37. Riddle DL, Stratford PW. Unilateral vs bilateral symptomatic knee osteoarthritis: associations between pain intensity and function. Rheumatology (Oxford). 2013;52:2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reeuwijk KG, de Rooij M, van Dijk GM, Veenhof C, Steultjens MP, Dekker J. Osteoarthritis of the hip or knee: which coexisting disorders are disabling? Clin Rheumatol. 2010;29: 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barlow T, Griffin D, Barlow D, Realpe A. Patients' decision making in total knee arthroplasty: a systematic review of qualitative research. Bone Joint Res. 2015;4:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toye FM, Barlow J, Wright C, Lamb SE. Personal meanings in the construction of need for total knee replacement surgery. Soc Sci Med. 2006;63:43–53. [DOI] [PubMed] [Google Scholar]
- 41. Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597–1606. [DOI] [PubMed] [Google Scholar]
- 42. Cronstrom A, Nero H, Dahlberg LE. Factors associated with patients' willingness to consider joint surgery after completion of a digital osteoarthritis treatment program: a prospective cohort study. Arthritis Care Res (Hoboken). 2019;71:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skou ST, Roos EM, Laursen MB, et al. Total knee replacement and non-surgical treatment of knee osteoarthritis: 2-year outcome from two parallel randomized controlled trials. Osteoarthritis Cartilage. 2018;26:1170–1180. [DOI] [PubMed] [Google Scholar]
- 44. Rossettini G, Carlino E, Testa M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskelet Disord. 2018;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]


