Abstract
We describe the case of a COVID-19 patient with severely impaired consciousness after sedation hold, showing magnetic resonance imaging (MRI) findings of (i) acute bilateral supratentorial ischemic lesions involving the fronto-parietal white matter and the corpus callosum and (ii) multiple diffuse susceptibility weighted imaging (SWI) hypointense foci, infra and supratentorial, predominantly bithalamic, suggestive of microhemorrhage or alternatively microthrombi. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) RNA was detected in the cerebrospinal fluid. Our findings suggest the occurrence of vascular damage, predominantly involving microvessels. The underlying mechanisms, which include direct and indirect penetration of the virus to the central nervous system and systemic cardiorespiratory complications, are yet to be elucidated, and a direct correlation with SARS-CoV-2 infection remains uncertain.
Keywords: Covid-19, Neurological complications, Coagulopathy, Microthrombi, Inflammation, Ischemia
Introduction
Neurological manifestations have been observed in a relevant proportion of patients with COVID-19 [1] with different magnetic resonance imaging (MRI) patterns [2]. We report the case of a patient with severe neurological complications and brain MRI evidence of multiple supratentorial areas of restricted diffusion at diffusion-weighted imaging (DWI), consistent with acute ischemic lesions, and several susceptibility-weighted imaging (SWI) punctate hypointensities, most referable to microbleeds or alternatively microthrombi.
Collection and scientific use of the patient’s data were approved by the regional ethics committee (CER Liguria: 173/2020-DB id 10512). Consent to data publication was verbally obtained from the next of kin.
Case report
On March 25, a 68-year-old man with previous history of hypertension and diabetes mellitus presented to the emergency department with dyspnea, fever, fatigue, and productive cough. Diagnosis of coronavirus 2 (SARS-CoV-2) pneumonia was confirmed by nasopharyngeal swab specimen using the transcriptase-polymerase chain reaction assay. Following the development of severe acute respiratory syndrome and acute kidney failure, the patient was admitted to our intensive care unit (ICU) and intubated; mechanical ventilation and continuous veno-venous hemodialysis (CVVHD) were started. Table 1 reports the main mechanical ventilation settings, data on respiratory function, and laboratory results. He was started on steroids, hydroxychloroquine, and ceftaroline. A prophylactic dose of enoxaparin (6000 u once/day) was subsequently switched to 6000 u/bd, due to increase of D-dimer values. Respiratory function improved over days, and he was switched to assisted mechanical ventilation, while CVVHD was continued for persistent anuria. On day 9, on sedation hold, his Glasgow Coma Score was 3 (Eyes 1,Verbal 1, Motor 1). Photomotor reflex was present, and brainstem reflexes (including cough) were preserved. After 2 days from sedation hold, an electroencephalography (EEG) revealed a severe voltage attenuation (signal diffusely < 20 μV), with background activity substituted by irregular theta and delta activities widely distributed, without antero-posterior gradient. No focal or generalized interictal/ictal epileptiform discharges nor periodic discharges were observed. Two days later, a second EEG showed no significant changes. At brain MRI (Fig. 1), DWI shows multiple small supratentorial areas of restricted diffusion, consistent with acute ischemic foci, involving the bilateral fronto-parietal deep and subcortical white matter and the genu and body of the corpus callosum. SWI revealed several hypointense foci throughout the brain, both infratentorial and supratentorial, mostly bithalamic. On T1-weighted images obtained after intravenous gadolinium injection, there was no evidence of parenchymal or meningeal enhancement. Cerebrospinal fluid (CSF) analysis showed 0.492 g/L [0.130–0.520 g/L] proteins, 88 mg/dL [44–74 mg/L] glucose, no leukocytes and red blood cells, CSF/serum albumin ratio 7.74 [1.92–7.30], and CSF/serum immunoglobulin G (IgG) ratio 4.48 [0.82–3.26]. SARS-CoV-2 RNA was detected in the CSF. Over the following days, the patient developed a systemic candida and gram-negative bacteria infection. His respiratory function significantly worsened, and he developed a septic shock with multiorgan failure. He died on day 21.
Table 1.
Laboratory data, respiratory function, and mechanical ventilation†
| Variable | Reference range, adults | On admission | ICU day 3 | ICU day 7 | ICU day 14 |
|---|---|---|---|---|---|
| White blood cell (109/L) | 4.50–9.80 | 9.58 | 13.99 | 24.14 | 23.58 |
| C-reactive protein (mg/L) | 0.0–5.0 | 137 | 77.7 | 138 | 121 |
| Procalcitonin (mcg/L) | 0.00–0.25 | 1.37 | 2.09 | 1.67 | 2.07 |
| IL (interleukin)-6 (ng/L) | < 3.4 | 491 | 32.7 | NA | NA |
| Ferritin (mcg/L) | 30–400 | 605 | 378 | NA | NA |
| D-Dimer (mcg/L) | 0.00–500.0 | 1605 | 810 | 3497 | 6941 |
| Fibrinogen (g/L) | 2.00–4.00 | 6.10 | 3.84 | 6.06 | 8.47 |
| Activated partial-thromboplastin time (sec) | 28.0–40.0 | 39 | 27.3 | 33.6 | 32.2 |
| Creatinine (mg/dL) | 0.51–0.95 | 2.4 | 5 | 2.9 | 2.6 |
| Urea nitrogen (mg/dL) | 21–54 | 90 | 172 | 135 | 167 |
| Lactate dehydrogenase (U/L) | 135–214 | 425 | 282 | 357 | 265 |
| Arterial pH | 7.35–7.45 | 7.36 | 7.35 | 7.23 | 7.34 |
| Arterial partial pressure of oxygen (mmHg) | 83–108 | 71.8 | 73.3 | 80.8 | 56.1 |
| Arterial partial pressure of carbon dioxide (mmHg) | 32–45 | 42.4 | 48.1 | 54.1 | 55.4 |
| Lactate (mmol/L) | 0.5–1.6 | 1.1 | 1.1 | 1.6 | 0.8 |
| Nasopharyngeal swab | Positive-quite positive-negative | Positive | Positive | Positive | Quite positive |
| Ventilation mode | Controlled | Controlled | Controlled | Assisted | |
| Fraction of inspired oxygen | 0.8 | 0.6 | 0.6 | 0.6 | |
| Positive end-expiratory pressure (cmH2O) | 15 | 15 | 13 | 12 | |
| Continuous renal replacement therapy | NO | YES | YES | YES |
†To convert the values for urea nitrogen to millimoles per liter, multiply by 0.357. To convert values for creatinine to micromoles per liter, multiply by 88.4. NA denotes not available
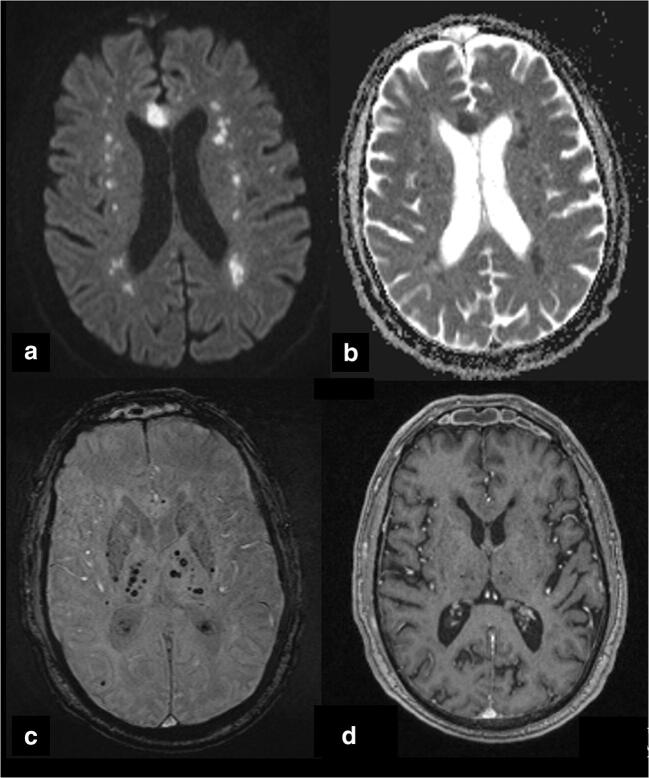
Fig. 1.
Diffusion-weighted imaging (DWI) b1000 (a) and apparent diffusion coefficient map (b) show multiple areas of restricted diffusion, involving the bilateral fronto-parietal white matter and the genu of the corpus callosum, compatible with acute ischemic foci. Susceptibility-weighted imaging (SWI) (c) shows several millimetric hypointense areas, predominantly distributed in the thalami bilaterally, but also in the genu of the corpus callosum and in the parietal iuxtacortical white matter, consistent with microhemorrhages or alternatively microthrombi. T1-weighted post-contrast image (d) demonstrates the lack of parenchymal or meningeal enhancement at the level of these lesions (lack of enhancement was also observed in the rest of the brain, not shown)
Discussion
We present a patient with pulmonary COVID-19 infection who developed neurological symptoms characterized by severely impaired consciousness, with multiple DWI areas of restricted diffusion and scattered SWI hypointensities at brain MRI and CSF positivity for SARS-CoV-2 RNA.
Neurological complications following COVID-19 infection are not uncommon [2], and various putative mechanisms for brain involvement have been suggested [3]. Our patient presented MRI signs of brain vascular injury, characterized by the coexistence of acute ischemic areas and scattered microbleeds or alternatively microthrombi. The underlying mechanisms, which include direct and indirect penetration of the virus to the central nervous system and systemic cardiorespiratory complications [4], are yet to be elucidated, and a direct correlation with SARS-CoV-2 infection remains uncertain, but we tried to make some observations.
First, the multiple supratentorial ischemic lesions seen on DWI, with corpus callosum involvement, do not depict a territorial distribution, inconsistent with COVID-19-associated large vessel occlusion [5]. Instead, they might reflect diffuse local thrombosis or, less likely, embolism. In this setting, high D-dimer values may reflect thrombus formation, but they can also act as acute-phase enhancer of the inflammatory cascade and stimulates monocyte synthesis and release of proinflammatory cytokines, which can contribute to the occurrence of stroke [6]. We cannot ascertain a specific correlation of the DWI findings with SARS-CoV2 infection in our patient. Nevertheless, similar MRI findings of bilateral supratentorial and corpus callosum DWI lesions have been described in COVID-19 patients with similar clinical manifestations [2].
The SWI hypointense foci in our patient are also of difficult interpretation. In fact, SWI hypointensities in the corpus callosum and in the cortical/iuxtacortical regions of COVID-19 patients with neurological impairment have been reported [7], but their pathological correlate is not univocal, as they could represent microbleeds or microthrombi. In one pathology study, microhemorrages were detected on brain tissue at the location of SWI hypointensities [8]. Though a coagulopathic disorder has been reported in COVID-19 patients, diffuse, mostly peripherally distributed brain microbleeds can also be the expression of critical illness in ICU patients [9].
Acute necrotizing encephalopathy (ANE) [2], with similar bithalamic SWI hypointensities, has been reported in COVID-19 patients, but the MRI picture we describe is different, because no signs of edema and necrosis were evident.
Our patient did not develop any EEG abnormalities typically related to meningoencephalitis, hypoxic encephalopathy, or renal failure, such as epileptiform focal abnormalities and lateralized or generalized periodic discharges. Accordingly, post-contrast MRI showed a lack of parenchymal and meningeal enhancement, and no MRI signs of encephalitis were present.
The meaning of SARS-CoV-2 RNA positivity in the CSF of our patient is unclear. A direct effect of the virus, with normal proteins and no pleocytosis in the CSF, is questionable. Viral neurotropism has been largely theorized, and virus particles have been observed to cross the capillary endothelium of the brain blood barrier (BBB) [10], but direct implication of SARS-CoV-2 in neurological manifestations is controversial, and viral RNA in the CSF of patients with neurological complications is only rarely detected [11]. In a recent study including 31 patients with neurological manifestations, none had CSF viral positivity, but many had signs of disturbance of the blood brain barrier (BBB) integrity [12]. Our patient showed signs of mild damage of the BBB as well, which might also be a possible consequence of systemic inflammation, in a patient with multiple organ failure. Therefore, an indirect mechanism of neurovascular unit breakdown, resulting in a passage of the virus in the CSF through the damaged BBB, cannot be excluded. Contamination or artifact could also be a possibility [11].
In COVID-19 pneumonia, the extensive microvascular damage seems to be related to a macrophage activation syndrome (MAS)-like mechanism [13], which induces a coagulopathic cascade with subsequent local microthrombosis and microbleeding in the lungs. A similar mechanism could be responsible for central nervous system manifestations, but supporting data are scarce.
We consider this case an example of neurological manifestations possibly related to COVID-19, characterized by vascular damage predominantly involving microvessels. The absence of pathological confirmation represents a major limitation, but similar MRI patterns [2] and recent pathological correlations seem to support a microangiopathic substrate [8]. Different mechanisms including a systemic procoagulative status and systemic and local inflammatory processes involving the endothelium, most likely via the ACE2 receptor, and eventual immune-mediated vascular injury could be considered [11]. Clinicians should be aware of the possible severe neurological complications in COVID-19 patients and of the role of MRI in their characterization.
Authors’ contribution
Laura Saitta, Alexandre Molin, Luca Roccatagliata, Chiara Robba, and Nicolò Patroniti contributed to conception and design of the study. Material preparation, data collection, and analysis were performed by Laura Saitta, Alexandre Molin, Flavio Villani, Angelo Insorsi, Luca Roccatagliata, Chiara Robba, and Nicolò Patroniti. The first draft of the manuscript was written by Chiara Robba and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
Dr. Bassetti reports personal fees from Achaogen, personal fees and other from Angelini, personal fees and other from Astellas, personal fees from Bayer, personal fees and other from Basilea, personal fees from Biomerieux, personal fees and other from Cidara, personal fees and other from Gilead, personal fees from Menarini, personal fees and other from MSD, personal fees from Nabiva, personal fees from Paratek, personal fees and other from Pfizer, personal fees from Roche, personal fees and other from Melinta, personal fees and other from Shionogi, personal fees from Tetraphase, personal fees from VenatoRx and Vifor, outside the submitted work; nothing to disclose. All the other authors have nothing to disclose.
Ethical approval
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. 77:683. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed]
- 2.Kremer S, Lersy F, De Seze J et al (2020) Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology:202222. 10.1148/radiol.2020202222
- 3.Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglini D, Brunetti I, Anania P, Fiaschi P, Zona G, Ball L, Giacobbe DR, Vena A, Bassetti M, Patroniti N, Schenone A, Pelosi P, Rocco PRM, Robba C (2020) Neurological manifestations of severe SARS-CoV-2 infection: potential mechanisms and implications of individualized mechanical ventilation settings. Front Neurol 11. 10.3389/fneur.2020.00845 [DOI] [PMC free article] [PubMed]
- 5.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, de Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao T, Tian BL, Li G, Cui Q, Wang CF, Zhang Q, Peng B, Gao Y, Zhan YQ, Hu D, Xu L, Wang GH. Elevated plasma D-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: a prospective, observational study. BMC Neurol. 2019;19:175. doi: 10.1186/s12883-019-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, Borja MJ, Zan E, Fatterpekar GM. COVID-19 –associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297:E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaunmuktane Z, Mahadeva U, Green A, Sekhawat V, Barrett NA, Childs L, Shankar-Hari M, Thom M, Jäger HR, Brandner S. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020;140:397–400. doi: 10.1007/s00401-020-02190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanou EM, Coutinho JM, Shannon P, Kiehl TR, Levi MM, Wilcox ME, Aviv RI, Mandell DM. Critical illness-associated cerebral microbleeds. Stroke. 2017;48:1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 10.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellon M, Schweblin C, Lambeng N, Cherpillod P, Vazquez J, Lalive PH, Schibler M, Deffert C (2020) Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin Infect Dis. 10.1093/cid/ciaa1165 [DOI] [PMC free article] [PubMed]
- 13.McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]