Abstract
Interest in alkylamide, as a class of compound, has grown tremendously in recent years. This interest is due to the many presumed benefits in food, cosmetics, and medicine. This review focuses on the different alkylamides naturally occurring in many plant species. Several methods have been employed for their identification as well as isolation and several in vitro and in vivo studies have revealed their therapeutic effects in various diseases. In general, alkylamides have been reported to have several biological activities and pharmacological effects which include immunomodulatory, antithrombotic, antimicrobial, antiviral, antioxidant, anti-inflammatory, analgesic, anticancer, antidiabetic, and antiprotozoal activities. Moreover, many studies have reported on their mechanisms of action, structure-activity relationship, pharmacokinetics, and toxicity. We herein present an updated report on the chemistry and pharmacology of alkylamides of natural origin.
Graphical abstract
Electronic supplementary material
The online version of this article (10.1007/s43450-020-00095-5) contains supplementary material, which is available to authorized users.
Keywords: Immunomodulatory, Anti-inflammatory, Pharmacological effects, Natural origin, Alkylamides
Introduction
Alkylamides are a group of bioactive compounds that can be obtained from natural sources such as the plant families of Aristolochiaceae, Asteraceae, Brassicaceae, Convolvulaceae, Euphorbiaceae, Menispermaceae, Piperaceae, Poaceae, Rutaceae, and Solanaceae (Méndez-Bravo et al. 2011). In the Asteraceae family, alkylamides have been reported in the genera Achillea, Acmella, Echinacea, Heliopsis, and Spilanthes (Rios 2012). Plants containing alkylamides are used as spices for their pungent and tingling sensations and are incorporated into topical cosmetics for their wrinkle-smoothing and anti-aging properties (Veryser 2016). When they occur in plants, the abundance of alkylamides could vary in the various parts. These include but not limited to leaves and stems of Aristolochia gehrtii Hoehne, Aristolochiaceae, Phyllanthus fraternus G.L.Webster, Phyllanthaceae, Amaranthus hypochondriacus L., Amaranthaceae, Achyranthes ferruginea Roxb, Amaranthaceae; the fruits of Piper longum L., Piperaceae; the flowers of Spilanthes acmella (L.) L., Asteraceae; the seeds of the Piper species, the pericarpium of Zanthoxylum piperitum DC., Rutaceae, and Piper sp.; the placenta of Capsicum sp.; tubers of Lepidium meyenii Walp., Brassicaceae; and roots of Heliopsis longipes (A. Gray) S. F. Blake, Asteraceae, Echinacea purpurea (L.) Moench, Asteraceae, Achillea wilhelmsii K. Koch, Asteraceae, Acmella oppositifolia (Lam.) R.K. Jansen, Asteraceae, Asarum heterotropoides F. Schmidt, Aristolochiaceae, and Cissampelos glaberrima A.St.-Hil., Menispermaceae (Rios 2012).
Alkylamides are structurally diverse with a wide range of pharmacological effects (Boonen et al. 2012a). Thus, their application in the pharmaceutical, cosmetic, and food industry is anticipated. Those obtained from plants are usually lipophilic substances with some growth regulatory functions, like those of N-acylethanolamines (NAEs) (Hajdu et al. 2014). They have been reported to promote primary root growth and root hair elongation in a manner independent of auxin (Ramírez-Chávez et al. 2004) and dependent on the cytokinin-signaling pathway (López-Bucio et al. 2007). NAAs also induce plant immunity through jasmonic acid (JA)–dependent and MPK6-regulated signaling pathways (Méndez-Bravo et al. 2011).
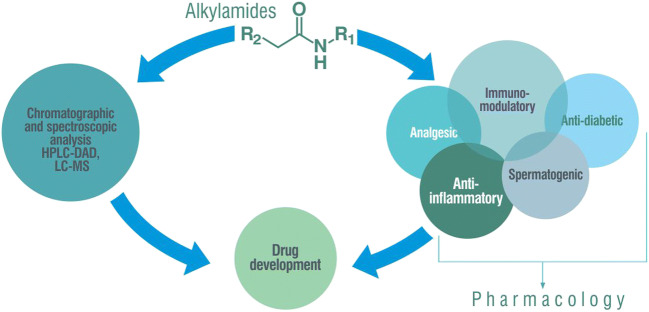
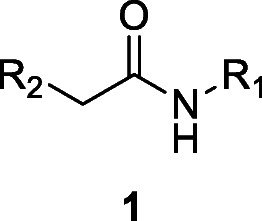
Despite their structural diversity, alkylamides have a common central feature of an amide bond (1). Plant NAAs also usually contain an aliphatic chain of poly-unsaturated fatty acids connected to a short-chain amine, which forms the basis for their structural classification. An online database with more information on the classification of alkylamide called Alkamid® is currently available for public use (Boonen et al. 2012a). This review is a comprehensive compilation of alkylamides from natural sources, with specific reference to their chemistry and pharmacological effects. It is aimed that the article would provide up-to-date scientific information on these compounds that can generate further interest in their development as drugs.
Chemistry
Structurally, alkylamides are fatty acid amides related to N-acyl-l-homoserine lactones (AHLs) from gram-negative bacteria and to N-acylethanolamines (NAEs) from plants and mammals (Méndez-Bravo et al. 2011). Despite such a relatively simple molecular constitution (1), however, alkylamides are diverse in their structural variability. They each consist of saturated or unsaturated (including double or triple bond) fatty acid chain with length ranging from C8 to C18 and an aliphatic, cyclic, or aromatic amine moiety. The characteristics of both the acids (level of saturation, number of carbon atoms, and stereochemistry) and the amide portion have found usefulness in chemotaxonomy. A book chapter describing the chemical distribution of alkylamides and their relevance to plant classification is available (Rios 2012).
Detection and Analytical Methods
Various analytical methods involving chromatographic and spectroscopic techniques are used in medicinal plant research to ensure the quality of herbal preparations. These analytical methods could be individual or hyphenated. Thin-layer chromatography (TLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), and electrophoretic methods (ECs) have been used for plant analysis individually as well in the variously hyphenated modes. In recent years, however, combining chromatographic methods with spectroscopic detector has been the method of choice for obtaining structural information on the constituents present in herbal samples. Thus, the combination of column liquid chromatography (LC) or gas chromatography (GC) with a UV-VIS or a mass spectrometer (HPLC-DAD, CE-DAD, GC-MS, and LC-MS, respectively) is a good approach for the analysis of herbal products (Kamboj 2012). Different hyphenated techniques including LC-IR, LC-MS, LC-NMR, GC-MS, HPLC–DAD, HPLC–MS, hyphenation of EC, and many others can however be applied depending on the goal of the research and accessibility to instruments. Overall, liquid chromatography coupled with mass spectrometry (LC-MS) is a more sensitive and selective analytical method to detect and identify compounds (Marston 2007). Despite the increased interest in alkylamides as pharmaceuticals, limited analytical methods have been reported in the literature for their quantification. A sensitive and specific method was developed for the identification and quantification of alkylamides in human plasma using HPLC-electrospray ionization (ESI) ion trap MS and used to analyze samples obtained after oral administration of Echinacea angustifolia DC., Asteraceae, root extracts (Woelkart et al. 2005a). Another study reported the use of HPLC coupled to a single quad MS with an atmospheric pressure chemical ionization (APCI) source operating in positive ion selected ion monitoring (SIM) mode to detect alkylamides in plasma samples of individuals given tablets manufactured from ethanolic liquid extracts of E. angustifolia and E. purpurea (Matthias et al. 2005). Alkylamides from the liquid and tablet preparations obtained from a mixture of E. purpurea root and E. angustifolia root were analyzed using the same method with no significant difference in results (Matthias et al. 2007). The pharmacokinetics and tissue distribution of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides in rats after a single oral dose administration were studied using liquid chromatography-tandem mass spectrometry (HPLC-ESI quadrupole MS) (Woelkart et al. 2009). Another HPLC-MS/MS method was developed and validated for the detection and quantification of undeca-2-ene-8,10-diynoic acid isobutylamide in human plasma (Goey et al. 2011) and used for the analysis of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides (Goey et al. 2012). Quantification of spilanthol and pellitorine across the skin, oral/gut mucosa, and the blood-brain barrier was carried out using HPLC-UV and bio-analytical UPLC-MS methods (Veryser et al. 2014). A rapid, sensitive, and accurate UHPLC–MS/MS method was developed for the determination of hydroxy-α-sanshool, hydroxy-β-sanshool, and hydroxy-γ-sanshool isolated from Zanthoxylum bungeanum Maxim., Rutaceae, in rat plasma (Rong et al. 2016). A comprehensive review of analytical methods used to study compounds, including alkylamides in Echinacea species, has been reported (Bruni et al. 2018). A recent method involving molecularly imprinted polymer solid-phase extraction was developed to extract Zanthoxylum alkylamides from prickly ash (Li and Kan 2020). In this method, surface molecularly imprinted polymers (SMIPs) were prepared using the molecular structural analogs of sanshool as a dummy template molecule and acylated silica as the backbone and structurally characterized by Fourier-transform infrared spectroscopy and scanning electron microscopy. SMIP was thereafter used to prepare a molecularly imprinted solid-phase extraction column (SPEC) for the extraction of Zanthoxylum alkylamides followed by HPLC determination. This combination produced alkylamides with improved purity levels from 27.26 to 93.58%. Also, good linearity for alkylamides’ standard solutions was obtained in the range of 1–800 μg/ml, as well as low detection and quantification limits indicating that the method can be useful in the rapid detection and enrichment of alkylamides (Li and Kan 2020).
Extraction, Isolation, and Identification
Extraction generally refers to the process of separating a substance from a matrix. Different methods are available and used for plant extraction. Plant extraction methods can either be cold or hot depending on whether heat is applied and several methods including maceration, infusion, decoction, Soxhlet, counter-current, sonication, supercritical fluid extraction, hydrodistillation, microwave-assisted extraction, and ultrasound-assisted extraction have been used in natural product research (Azwanida 2015, Ingle et al. 2017, Xi 2017). Some extraction processes could be targeted towards specific phytoconstituents such as phenolics (Oreopoulou et al. 2019; Wang et al. 2020). Several methods and solvents including hexane, chloroform, ethyl acetate, ethanol, and methanol have been used for the extraction of alkylamides with chloroform considered the best (Rios 2012).
Natural product extracts are usually multi-components, containing several variations of bioactive compounds of different polarities. This situation makes the separation of these components and, hence, their identification and structural characterization a big challenge in natural product research. However, several chromatographic- and non-chromatographic-based techniques have been successfully applied in the separation of multi-component natural products. Chromatographic methods include thin-layer chromatography (TLC), column chromatography (CC), flash chromatography (FC), size exclusion chromatography (SEC), and HPLC among others while non-chromatographic techniques such as crystallization, immunoassay, which use monoclonal antibodies (MAbs), electrophoresis, and membrane separation have also been used. Pure compounds obtained through appropriate separation methods are thereafter subjected to spectroscopic (NMR, MS) analysis for structural elucidation before additional biological testing.
Different methods have been used for the extraction and purification of alkylamides. Hexane extract of the root of E. purpurea separated on flash column chromatography using silica gel followed by medium pressure liquid chromatography (MPLC) and preparative HPLC on reversed-phase material (RP-8) led to the isolation of nine alkylamides, including a mixture of Z/E isomers (Bauer et al. 1988b). Structures of the alkylamides (2–11) were confirmed by spectroscopy: UV, IR, 1H, and COSY NMR and MS.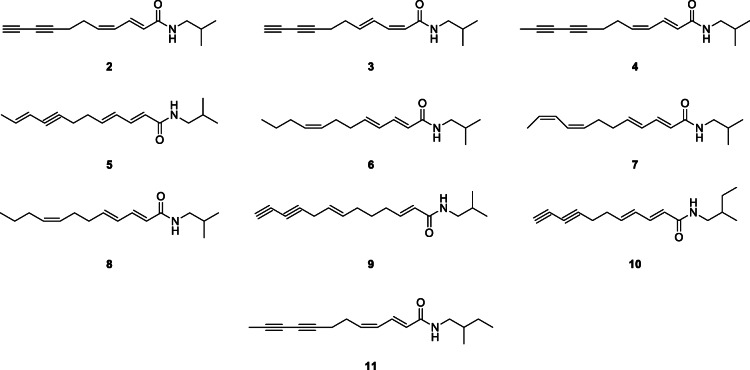
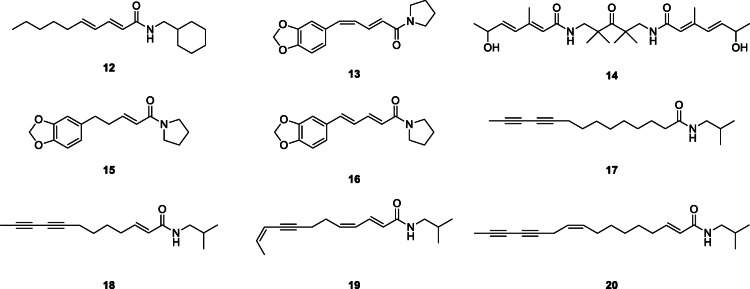
Silica gel chromatography of the petroleum ether extract of aerial parts of Artemisia dracunculus L., Asteraceae, yielded three alkylamides (Saadali et al. 2001). One known alkylamide, pellitorine, and two novel ones, neopellitorine A and neopellitorine B (12), were characterized using UV, IR, MS, 1H, and 13C NMR.
Using column chromatography on silica gel (Merck 230–400 mesh), two new alkylamides, N-[10-(13,14-methylenedioxyphenyl)-7(E),9(Z)-pentadienoyl]-pyrrolidine (13) and arboreumine (14) together with ten other known compounds: N-[10-(13,14-methylenedioxyphenyl)-7(E)-pentaenoyl]-pyrrolidine (15) and N-[10-(13,14-methylenedioxyphenyl)-7(E),9(E)-pentadienoyl]-pyrrolidine (16) including six amides and two cinnamoyl derivatives, were reported from the seeds and leaves of Piper tuberculatum Jacq., Piperaceae. Catalytic hydrogenation of 17 N-[10-(13,14-methylenedioxyphenyl)-7(E)-pentaenoyl]-pyrrolidine yielded the amide N-[10-(13,14-methylenedioxyphenyl)-pentanoyl]-pyrrolidine (da Silva et al. 2002).
In another study, the root of E. angustifolia extracted with supercritical CO2 and purified by semi-preparative HPLC led to the isolation of twelve alkylamides identified by their chromatographic behavior, mass data on LC-MS, and comparison with literature. These are tetradeca-2E-ene-10,12-diynoic acid isobutylamide (17), undeca-2E/Z,4Z/E-diene-8,10-diynoic acid isobutylamides (2), undeca-2E/Z-ene-8,10-diynoic acid isobutylamides, dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide, dodeca-2E-ene-8,10-diynoic acid isobutylamide (18), dodeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide (11), dodeca-2E,4Z,10Z-triene-8-ynoic acid isobutylamide (19), dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides (7), pentadeca-2E,9Z-diene-12,14-diynoic acid isobutylamide (20), dodeca-2E-ene-8,10-diynoic acid 2-methylbutylamide, dodeca-2E,4E,8Z-trienoic acid isobutylamide (6), and dodeca-2E,4E-dienoic acid isobutylamide (Woelkart et al. 2005a).
Thirteen N-alkylamides (five N-isobutylamides, three N-methyl isobutylamides, four tyramides, and one 2-phenylethylamide) were identified using MS1 and MS2 fragmentation data from the ethanolic extract of Anacyclus pyrethrum (L.) DC., Asteraceae (Boonen et al. 2012b). These included novel ones not reported before in the plant which were undeca-2E,4E-diene-8,10-diynoic acid isobutylamide, undeca-2E,4E-diene-8,10-diynoic acid N-methyl isobutylamide, tetradeca-2E,4E-diene-8,10-diynoic acid tyramide, deca-2E,4E-dienoic acid N-methyl isobutylamide, tetradeca-2E,4E,XE/Z-trienoic acid tyramide, and tetradeca-2E,4E,XE/Z,YE/Z-tetraenoic isobutylamide (Boonen et al. 2012b).
Purification of the dichloromethane extract of Otanthus maritimus (L.) Hoffmanns. & Link, Asteraceae, using silica gel vacuum-liquid chromatography (VLC), column chromatography (silica gel and Sephadex LH 20), and semi-preparative NP (normal-phase) or RP (reversed-phase) HPLC, yielded two new thienylheptatrienamides (21, 25) and eight known amides among other compounds (Ruiu et al. 2013). These were fully characterized based on the spectroscopic (1D, 2D, and MS) data as (2E,4E,6E)-N-isopentyl-7-(2-thienyl)-2,4,6-heptatrienamide (21), (2Z)-5-{[(2E,4E,6E)-7-(2-thienyl)-2,4,6-heptatrienoyl]-amino}-2-pentenyl-3-methylbutanoate (25), (2E,4E,6E)-N-isobutyl-7-(2-thienyl)-2,4,6-heptatrienamide (22), 1-[(2E,4E,6E)-7-(2-thienyl)-2,4,6-heptatrienoyl]piperidine (23), 1-[(2E,4E,6E)-7-(2-thienyl)-2,4,6-heptatrienoyl]2,3-dehydropiperidine (24), dodeca-2E,4E-dienoic acid isobutylamide, tetradeca-2E,4E-dienoic acid isobutylamide, tetradeca-2E,4E,8Z-trienoic acid isobutylamide, 1-[(2E,4E)-tetradecadienoyl]piperidine (26), and 1-[(2E,4E,8Z)-tetradecatrienoyl]piperidine (27). The 70% EtOH/H2O extract of dried pericarp of Z. bungeanum chromatographed on a 2.5 × 40 cm Sephadex LH-20 column, eluted with CH2Cl2/MeOH (1:1, v/v) and purified by HPLC led to the isolation of (2E,4E,8E,10E,12E)-N-isobutyl-2,4,8,10,12-tetradecapentaenamide, (γ-sanshool) (28), a promising lead antidiabetic alkylamide (Dossou et al. 2013).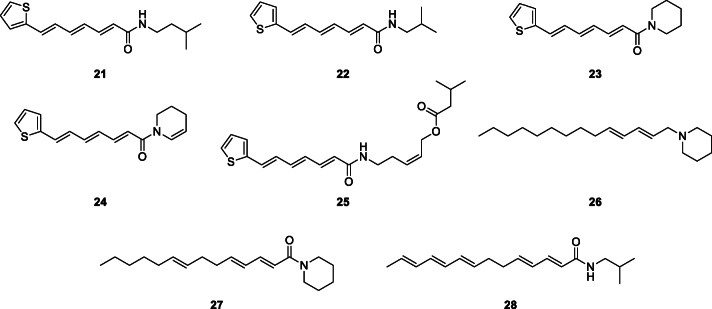
N-alkylamide profiling of the ethanol extract of the roots of A. pyrethrum was carried out using a gradient reversed-phase HPLC/UV/electrospray ionization–ion trap mass spectrometry (MS) method on an embedded polar column. The alkylamides were identified and quantified using MS1 and MS2 fragmentation data and UV detection, respectively. Thirteen alkylamides (five N-isobutylamides, three N-methyl isobutylamides, four tyramides, and one 2-phenylethylamide) were detected. These included undeca-2E,4E-diene-8,10-diynoic acid 2-PEA, deca-2E,4E-dienoic acid 4-OH PEA, tetradeca-2E,4E-diene-8,10-diynoic acid 4-OH PEA, deca-2E,4E-dienoic acid IBA (pellitorine (12), tetradeca-2E,4E-diene-8,10-diynoic acid IBA (anacycline) (29), dodeca-2E,4E-dienoic acid 4-OH PEA (30), deca-2E,4E-dienoic acid N-Me IBA (31), tetradeca-2E,4E-diene-8,10-diynoic acid N-Me IBA (32), tetradeca-2E,4E, XE/Z-trienoic acid 4-OH PEA (33), tetradeca-2E,4E, XE/Z, YE/Z-tetraenoic IBA, and dodeca-2E,4E-dienoic acid IBA (IBA, isobutylamide; PEA, phenylethylamide) (Sharma et al. 2013).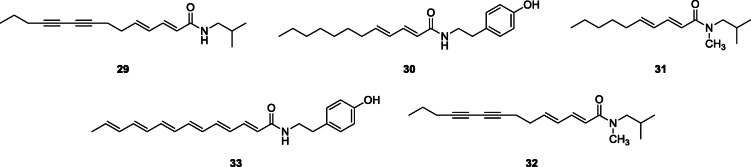
One new alkylamide, octadeca-2E,4E,8E,10Z,14Z-pentaene-12-ynoic acid isobutylamide, and three known ones: octadeca-2E,4E,8E,10Z,14Z-pentaene-12-ynoic acid 2-methylbutylamide, hexadeca-2E,4E,9Z-triene-12,14-diynoic acid isobutylamide, and hexadeca-2E,4E,9,12-tetraenoic acid 2-methylbutylamide, were identified from Heliopsis helianthoides var. scabra (Dunal) Fernald, Asteraceae. Also, previously described macamides, N-(3-methoxybenzyl)-(9Z,12Z,15Z)-octadecatrienamide (34), N-benzyl-9Z,12Z,15Z)-octadecatrienamide (35), and N-benzyl-(9Z,12Z)-octadecadienamide (36), were isolated from Lepidium meyenii Valp. (Maca) (Hajdu et al. 2014).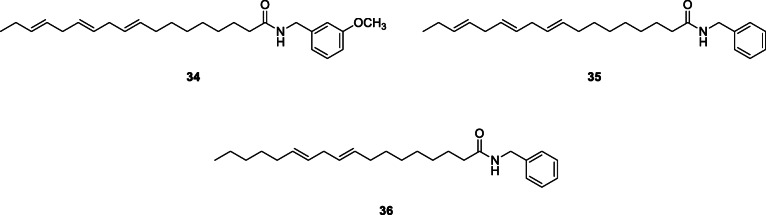
Three alkylamides, hydroxy-α-sanshool (37), hydroxy-β-sanshool (38), and hydroxy-γ-sanshool (39), were isolated from the powdered pericarp of Z. bungeanum through column chromatography and semi-preparative HPLC (Rong et al. 2016). Hydroxy-α-sanshool (37), hydroxy-β-sanshool (38), and hydroxy-γ-sanshool (39) were again purified from Z. bungeanum oleoresin by HPLC with yields of 26.48, 39.83, and 29.19%, respectively, in another study (Ren et al. 2017a).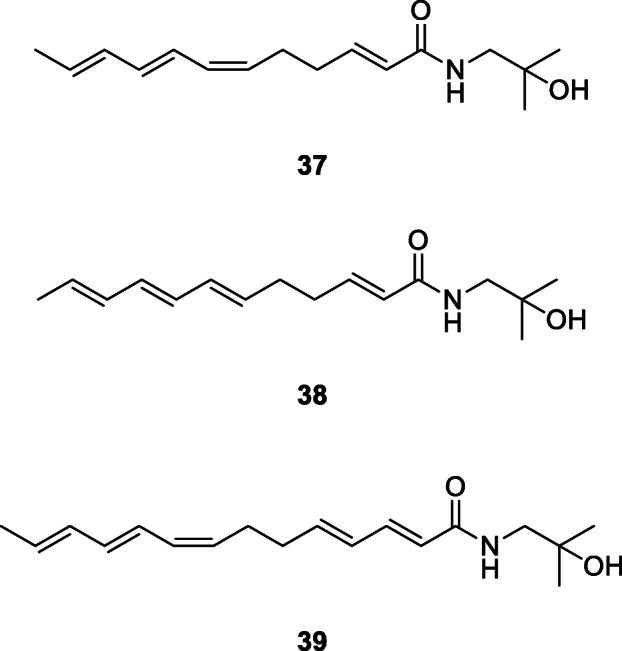
Two new alkylamides, zanthoamides E (40) and F (41), along with seven known ones (ZP-amide A (42), ZP-amide B (43), ZP-amide C (44), ZP-amide D (45), ZP-amide E (46), tetrahydrobungeanool (47), (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide (48)) were isolated from the pericarps of Z. bungeanum and structures determined by analysis of spectroscopic data (IR, UV, HRESIMS, 1D, and 2D NMR) (Wang et al. 2017).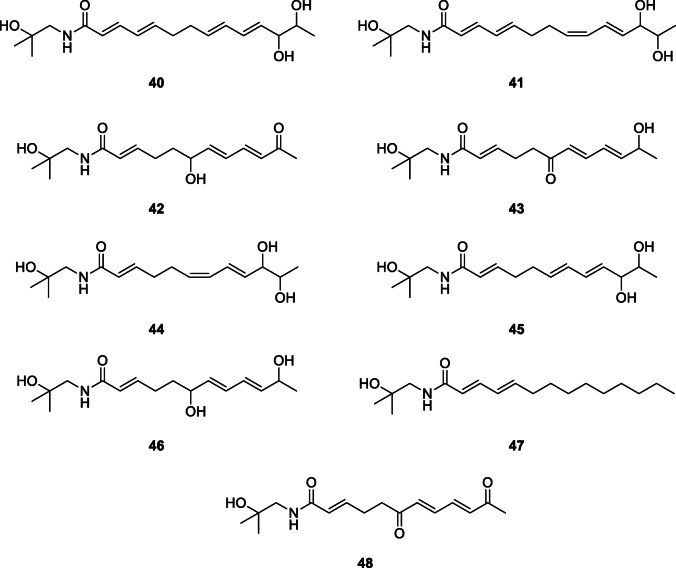
Abdubakiev et al. (2019) isolated sixteen known N-alkylamides from the dried fruits of P. longum. These included piperine (49), isopiperine, piperlonguminine (50), α,β-dihydropiperine, dihydropiperlonguminine, dehydropipernonaline (51), retrofractamide A (52), pipercide, guineensine, pipernonaline, retrofractamide C, trans-fagaramide (53), and cis-fagaramide (54). Their structures were established based on spectroscopic data and comparison to reported literature and five were being reported in the plant for the first time.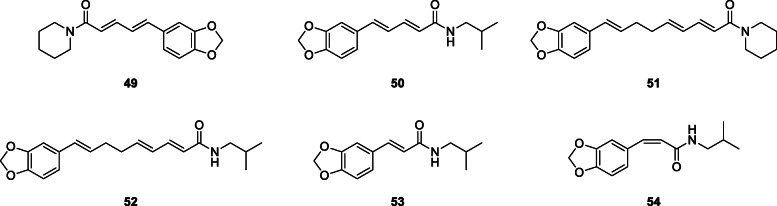
Other plants containing alkylamides include Capsicum species containing highly pungent capsacinoids that induce a hot or burning sensation with capsaicin and dihydrocapsaicin constituting approximately 90% (Rios 2012). m-Methoxybenzyl and N-benzyl amine and macamides have been reported in the roots of L. meyenii (Valerio and Gonzales 2005). Piperovatine, a buccal local anesthesic isobutyl amide along with piperlonguminine isopiperlonguminine, corcovadine, and isocorcovadine were isolated from Piper corcovadensis (Miq.) C. DC., Piperaceae (synonym of Ottonia corcovadensis Miq.) (Costa and Mors 1981), and N-isobutyl-6-(p-methoxyphenyl) 2E,4E-hexadieneamide from Ottonia propinqua Miq., Piperaceae (probably a synonymous of Piper baptisianum C.DC.) (Antunes et al. 2001).
Alkylamides from Insects
Another natural source of alkylamides is the tobacco hornworm, Manduca sexta (Linnaeus, 1763) (Lait et al. 2003). N-Linolenoyl-L-glutamine identified in oral secretions of the insect was shown to induce defensive responses in plants by producing volatile chemicals that can attract predators and parasites (Lait et al. 2003).
Ethnomedicinal Uses of Alkylamide-Containing Plants
Alkylamide-containing plants have been used in several traditional medical practices. Preparations from Echinacea species, an herb native to North America and Europe, are used in the prevention and management of common cold and as immunostimulant (Gertsch et al. 2004). It is also used to prevent infections, colds, respiratory infections, and influenza (Rios 2012). The root of O. maritimus, an aromatic herb belonging to Asteraceae, was shown to contain fatty acid amides (Bohlmann et al. 1974). In ethnomedicine, O. maritimus is used for the management of asthma, bronchitis, dysentery, and inflammation of the urinary bladder (Ruiu et al. 2013). H. helianthoides has been used by the North American Indians in pain remedy (Hajdu et al. 2014). H. longipes is a Mexican plant used as a flavoring in food preparation and in traditional medicine as a condiment, buccal anesthetic, analgesic in toothache, antiparasitic, anti-inflammatory, and anti-ulcerative agent, and to prepare homemade insecticides. Chewing of H. longipes stem produces intense salivation and a local analgesic effect (Molina-Torres et al. 1996). The roots of A. pyrethrum are used as sialagogue, insecticide, aphrodisiac, and local anesthesia and for treating paralysis, epilepsy, rheumatism, and toothache (Boonen et al. 2012b). P. longum is used as a spice and for the treatment of stomach disease and analgesia in traditional Chinese medicine (Abdubakiev et al. 2019). It is also used for treatment of gonorrhea, menstrual and chronic intestinal pain, tuberculosis, sleeping problems, respiratory infections such as coughs, bronchitis and asthma, malarial fever, diarrhea, jaundice, and arthritis (Rios 2012). Spilanthes sp. is used in African and Indian traditional medicine for the management of malaria (Spelman et al. 2011). S. acmella is used in traditional medicine to treat toothaches, stammering, and stomatitis (Rios 2012). Z. bungeanum, popularly called “toothache tree” and widely distributed in China and Southeast Asian countries, is used as a condiment and in traditional medicine as anesthetic and analgesic for toothache and sore throats (Rong et al. 2016). The pericarps from the fruits of Z. bungeanum are used in traditional medicine for the treatment of vomiting, toothache, stomachache, abdominal pain, and diarrhea (Wang et al. 2017). Acmella oleracea (L.) R.K. Jansen, Asteraceae, is used in ethnomedicine to relieve toothache (Dallazen et al. 2018). A. pyrethrum is used as an aphrodisiac in Indian ayurvedic medicine for the treatment of male sexual dysfunction, including infertility (Sharma et al. 2013). A. oleracea is used in the north of Brazil as a female aphrodisiac (de Souza et al. 2020). Phyllanthus fraternus is used for the treatment of malaria, jaundice, and various liver diseases by traditional healers and tribes in the northern region of India (Ghatapanadi et al. 2011; Sarkhel 2015). The fruits of Capsicum species are used in food preparation, to tone body muscles after workouts, for toothache and muscle pain (Rios 2012). The roots of L. meyenii are used to enhance fertility and sexual behavior in men and women and as a remedy for menopausal symptoms, regulation of hormone secretion, immunostimulation, memory enhancer, antidepressant, anticancer, and to prevent anemia (Valerio and Gonzales 2005). O. corcovadensis is used to relieve toothache (McFerren et al. 2002).
Reported Pharmacological Effects of Alkylamides
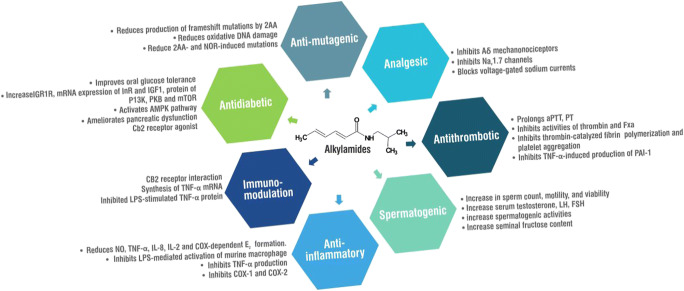
Several pharmacological effects (Table 1; supplementary material) of alkylamides from various plants have been reported in the literature with some mode of action as well as the molecular basis of the reported activity described (Fig. 1). The reported pharmacological actions justify the use of alkylamide-containing plants in ethnomedicine.
Fig. 1.
Some mechanisms of action of alkylamides and their corresponding pharmacological effects
Antiviral Activity of Alkylamides
Alkylamides in E. purpurea at 12 μg/kg body weight/day significantly increased the phagocytic activity and phagocytic index of the alveolar macrophages in an in vivo experiment using Sprague-Dawley rats (Goel et al. 2002).
Phagocytosis is important for the clearance of infections, killing of pathogens, and activation of host defenses (Labbe and Saleh 2008) while alveolar macrophages are the first line of defense in the lungs against pathogens (Espinosa and Rivera 2016). Phagocytosis by macrophages has been linked directly to viral infections including the influenza virus (Hashimoto et al. 2007). It also brings about antibody-mediated removal of pulmonary-infected SARS coronavirus through the activation of B cells by CD4+ T cells (Yasui et al. 2014). Thus, alkylamides may be considered useful in the management of coronavirus infections.
The effects of alkamides on T lymphocytes, which are key mediators of antiviral immunity, have also been reported. Two alkylamides obtained from Echinacea sp. were tested for their inhibitory effect on IL-2 production. Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide and dodeca-2E,4E-dienoic acid isobutylamide at a dose range of 0.6 to 25 μg/ml significantly reduced production of IL-2 by activated Jurkat T cells (Sasagawa et al. 2006).
In a separate study, four alkylamides were investigated for their inhibitory effect on the production of cytokines, chemokines, and PGE2 from RAW 264.7 macrophage-like cells infected with the H1N1 influenza A strain PR/8/34. The alkylamides, undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide, dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamide, dodeca-2E,4E-dienoic acid isobutylamide, and undeca-2E-ene-8,10-diynoic acid isobutylamide, had a suppressive effect on the production of TNF-α and PGE2 with dodeca-2E,4E-dienoic acid isobutylamide being most effective. It also strongly inhibited production of G-CSF, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES. This suggests the potential of alkylamides in alleviating symptoms of infections with influenza A (Cech et al. 2012). A comprehensive review of the antiviral potential of Echinacea sp. is available (Hudson and Vimalanathan 2011) and evidence supporting its antiviral effect through innate immunity-boosting by JAK1 responsive gene expression and epigenetic regulation of HERVs in monocytes has recently been reported (Declerck et al. 2020).
Antifungal and Antibacterial Activity of Alkylamides
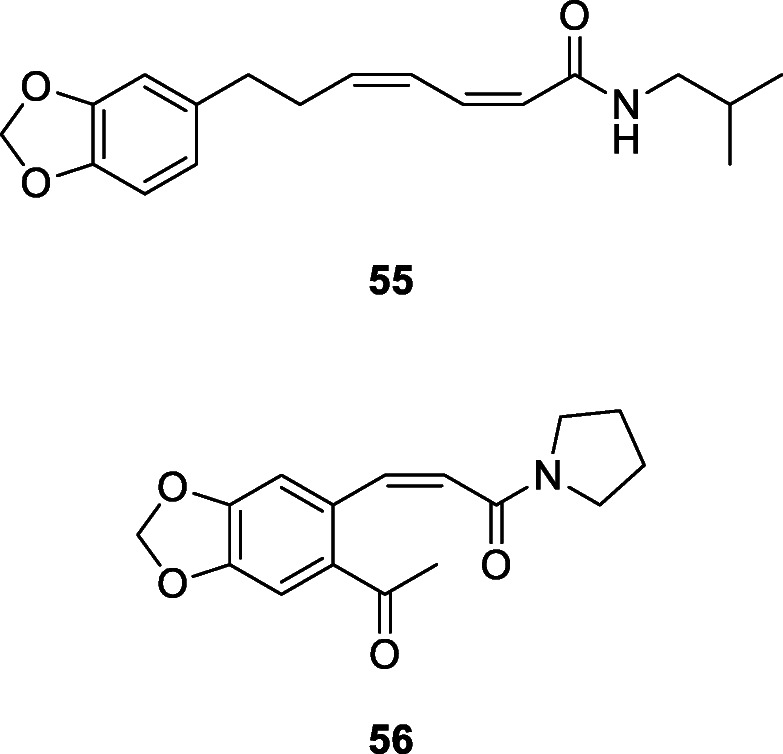
Affinin and capsaicin isolated from H. longipes roots and Capsicum sp. fruits respectively have been previously reported as an antimicrobial against Escherichia coli, Pseudomonas solanacearum, Bacillus subtilis, and Saccharomyces cerevisiae (Molina-Torres et al. 1999). The fungistatic and bacteriostatic activities of affinin, isolated from H. longipes roots, were also reported, along with two other alkamides (N-isobutyl-2E-decenamide and N-isobutyl-decanamide) obtained by catalytic reduction of affinin. The fungal test species used in the study were Rhizoctonia solani groups AG3 and AG5, Sclerotium rolfsii, S. cepivorum, Fusarium sp., Verticillium sp., phytopathogenic fungi; Phytophthora infestans, a phytopathogenic chromista; and Saccharomyces cerevisiae, a nonphytopathogenic ascomycete, while the bacteria were Escherichia coli, Erwinia carotovora, and Bacillus subtilis. Affinin completely inhibited (100%) S. rolfsii, S. cepivorum, P. infestans, and R. solani AG-3 and AG-5 and S. cerevisiae while none of the alkamides obtained by catalytic reduction of affinin showed activity against the fungi. Affinin also inhibited the growth of E. coli and B. subtilis, but not E. carotovora. N-Isobutyl-2E-decenamide inhibited E. coli and E. carotovora while N-isobutyl-decanamide inhibited the growth of B. subtilis (Molina-Torres et al. 2004). Twelve alkylamides including two new ones, N-[10-(13,14-methylenedioxyphenyl)-7(E),9(Z)-pentadienoyl]-pyrrolidine and arboreumine from the leaves of Piper auritum Kunth, Piperaceae, all showed antifungal activity against Cladosporium sphaerospermum and C. cladosporioides (da Silva et al. 2002). Other antifungal amides include N-[7-(3′,4′-methylenedioxyphenyl)-2(Z),4(Z)-heptadienoyl]pyrrolidine, (3Z,5Z)-N-isobutyl-8-(3′,4′-methylenedioxyphenyl)-heptadienamide (55) isolated from leaves of Piper hispidum Sw., Piperaceae (Alécio et al. 1998) and 8(Z)-N-(12,13,14-trimethoxycinnamoyl)-Δ3-pyridin-2-one, N-[3-(6′-methoxy-3′,4′-methylenedioxyphenyl)-2(Z)-propenoyl]pyrrolidine (56), piperamine, N-(12,13,14-trimethoxydihydrocinnamoyl)-Δ3-pyridin-2-one, piplartine, piperine (49), Δα,β-dihydropiperine, 5,6-dihydropiperlonguminine, and pellitorine from P. tuberculatum (Navickiene et al. 2000).
These reported antifungal and antibacterial activities exhibited by alkylamides corroborate the traditional uses of plants containing these compounds against bacterial infections such as dysentery, upper respiratory infections, eye infections pulmonary infection, wounds, and cough (Rios 2012; Ruiu et al. 2013).
N-Alkylamides as Immunomodulators
Alkylamides have been reported as potent immunomodulators. The analysis of the constituents of the standardized tincture of Echinacea plant, Echinaforce™, revealed that the main constituents were alkylamides (Gertsch et al. 2004). This tincture promoted syntheses of tumor necrosis factor-α (TNF-α) mRNA in primary human monocytes/macrophages and inhibited LPS-stimulated TNF-α protein in the early phase while prolonging it in the late phase. The increased production of TNF-α mRNA was found to be mediated through CB2 receptors, increased cAMP, p38/MAPK, and JNK signaling, as well as NFκB and ATF2/CREB1 activation, thus proposing alkylamides as potential ligands for CB2 receptors. The alkylamides identified in the tincture were dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides (7), trienoic, and dienoic acid (Gertsch et al. 2004). In a related study, Woelkart et al. (2006) reported that alkylamides exhibited selective affinity to CB2 receptors and can, therefore, be considered CB ligands. The study also suggested that CB2 interactions may be the molecular mode of action of Echinacea alkylamides as immunomodulators. Other researches corroborating the immunomodulatory effect of alkylamides are available in the scientific literature (Raduner et al. 2006; Ruiu et al. 2013; Dossou et al. 2013; Bauer et al. 1988a).
Preparations that contain alkylamides, such as those from Echinacea sp., have been used as immunostimulant (Gertsch et al. 2004). In particular, the promotion of TNF-α mRNA synthesis in primary human monocytes/macrophages and inhibition of LPS stimulated TNF-α protein demonstrated by alkylamides could make this class of compound promising as immunomodulators in COVID and similar infections. In COVID-19 patients, high levels of (TNF)-α has been shown especially in patients requiring intensive-care-unit hospitalization (Perricone et al. 2020). Similarly, activation of CB2 is also associated with intracellular pathways that tone down immune responses, thus making CB2 agonists like alkylamides good therapeutic agents in autoimmune diseases by suppression of antibody production through T cell mechanisms (Sexton 2020).
Alkylamides as Antidiabetic
Compounds with dual activities as CB1 receptor inhibitor and CB2 activator have been proposed to have some usefulness in type 1 diabetes (Dossou et al. 2013). (2E,4E,8E,10E,12E)-N-Isobutyl-2,4,8,10,12-tetradecapentaenamide (γ-sanshool) was identified from Z. bungeanum as antagonists of the CB1 receptor that displayed simultaneously agonistic activity against the CB2 receptor (Dossou et al. 2013). This alkylamide is thought to hold promise in the management of diabetes. The hypoglycemic effect of ten Zanthoxylum alkylamides and their mechanism of action in streptozotocin (STZ)-induced diabetic rats have also been reported (You et al. 2015). The alkylamides dose-dependently increased the relative weights of the liver and kidney as well as significantly decreased fasting blood glucose and fructosamine levels. Increased body weight and improved oral glucose tolerance were also observed in rats following treatment with alkylamides. Overall, alkylamides prevented STZ-induced hyperglycemia by altering the expression levels of the genes related to glucose metabolism and by ameliorating pancreatic dysfunction (You et al. 2015). Another report on alkylamides from Z. bungeanum evaluated their effects on protein metabolism and their potential mechanism in STZ-induced diabetic rats (Ren et al. 2017a). Diabetic rats were treated orally with 2, 4, and 8 mg/kg body weight of alkylamides (hydroxy-α-sanshool, hydroxy-β-sanshool, and hydroxy-γ-sanshool) daily for 28 days. The alkylamides decreased the relative weight of the liver and food intake and significantly decreased the blood urea nitrogen levels while significantly increasing the relative skeletal muscle weight. Insulin, insulin-like growth factor 1, total protein (TP), albumin (ALB), globular proteins, and ALB proteins/globulin protein levels in serum were also significantly increased. Real-time quantitative polymerase chain reaction results revealed that the alkylamides also significantly increased the mRNA expression levels of insulin receptor (InR), IGF1, and insulin-like growth factor 1 receptor (IGF1R) in the liver and skeletal muscle. There was also an increase in the expression levels of mRNAs and proteins of PI3K, PKB, and mTOR while those of atrogin-1, muscle ring finger 1, and FOXO in the skeletal muscle significantly decreased (Ren et al. 2017a). These studies also reported that the same alkylamides at the same doses could reduce blood glucose by activating the adenosine monophosphate-activated protein kinase (AMPK) pathway (Ren et al. 2017b). The combination of CB1 receptor antagonism and CB2 receptor agonism of alkylamides, together with their hypoglycemic activity in STZ-induced diabetic rats through amelioration of pancreatic dysfunction as well as stimulation of insulin, insulin-like growth factor 1, justifies the use of alkylamide containing plants like Zanthoxylum schinifolium Siebold & Zucc., Rutaceae, as antidiabetic.
Alkylamides as Anti-inflammatory Agent
Various studies have shown that alkylamides display anti-inflammatory activity. Echinacea alkylamides showed anti-inflammatory activity by reduction in nitric oxide, TNF-α, interleukin (IL)-8, IL-2, and cyclooxygenase (COX)-dependent prostaglandin E2 formation. In in vivo experiments in rats, these alkylamides were reported to significantly increase phagocytic activity and index of alveolar macrophages at a dose of 12 μg/kg body weight/day with the alveolar macrophages from the study also producing higher TNF-α and nitric oxide after in vitro stimulation with LPS (Goel et al. 2002). Dodeca-2Z,4E,10Z-trien-8-ynoic acid isobutylamide and dodeca-2Z,4E-diene-8,10-diynoic acid isobutylamide from Echinacea species inhibited LPS-mediated activation of the murine macrophage line RAW264.7 and reduction in NO production suggesting their anti-inflammatory activity (Chen et al. 2005). This finding was also supported by the work of Stevenson et al. (2005). The mixture of undeca-2E-ene-8,10-diynoic acid isobutylamide and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide inhibited TNF-α production under LPS-stimulated conditions in the mouse macrophage cell line RAW 264 as well as NO production at a concentration of 2.0 μg/ml (Stevenson et al. 2005). Alkylamides have also been reported to inhibit both COX-1 and of COX-2 to a different extent with COX-1 inhibition highest for undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide, dodeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide, and undeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide at concentrations of 100 μg/ml, while undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide had the highest COX-2 inhibition (Müller-Jakic et al. 1994; Clifford et al. 2002). The anti-inflammatory assessment of alkylamides from H. longipes evaluated in arachidonic acid (AA)– and phorbol myristate acetate (PMA)–induced mouse ear edema showed that affinin and isobutyl-decanamide had a good anti-inflammatory effect with ED50 = 0.8, 1.2, and 0.9 mg/ear, respectively, in the AA model as well as a dose-dependent anti-inflammatory effect with the extract, affinin, and isobutyl-decanamide having ED50 of 2.0, 1.3, and 1.1 mg/ear, respectively, in the PMA model (Hernández et al. 2009). Other anti-inflammatory-related reports include antiseptic effects of pellitorine in HMGB1-induced inflammatory responses (Ku et al. 2014) and its vascular barrier protective effects in LPS-induced inflammation (Lee et al. 2014). Inflammatory reactions through several mechanisms are implicated in several disease conditions such as infections (Van Dyke and van Winkelhoff 2013), cardiovascular disease (Schenkein and Loos 2013), obesity (Gregor and Hotamisligil 2011), depression (Raedler 2011), and infertility (Vannuccini et al. 2016). Thus anti-inflammatory agents such as alkylamides can be safely assumed to have wide therapeutic applications.
Analgesic Activity of Alkylamides
Affinin (spilanthol), showed analgesic activity through the release of gamma-aminobutyric acid (GABA) in mice brain slices 0.5 min after administration at 1 × 10−4 M concentration (Rios et al. 2007). The analgesic properties of hydroxyl-α-sanshool, from Zanthoxylum, were assessed in mice. It was observed that this alkylamide inhibited Aδ mechanonociceptors that mediate both sharp acute pain and inflammatory pain; inhibited action potential firing by blocking voltage-gated sodium currents in a subset of somatosensory neurons, which express a unique combination of voltage-gated sodium channels; and strongly inhibited heterologously expressed Nav1.7 compared with other sodium channels expressed in sensory neurons (Tsunozaki et al. 2013). GABA supplementation has been found useful in the treatment of central pain (Goldberg 2010). Plants containing alkylamides are used as pain remedies (Hajdu et al. 2014) in such conditions as rheumatism and toothache (Boonen et al. 2012b). Findings on analgesic effect generally support the traditional use of these plants in pain management.
Antinociceptive Effects of Alkylamides
The antinociceptive effect of affinin (spilanthol) was studied in mice using the writhing and capsaicin tests (Déciga-Campos et al. 2010). Intraperitoneal administration of affinin gave a dose-dependent antinociceptive response that was blocked by naltrexone (1 mg/kg, s. c.), p-chlorophenylalanine (80 mg/kg, i. p.), and flumazenil (5 mg/kg, s. c.), thus suggesting that the antinociceptive effect could be due to activation of opiodergic, serotoninergic, and GABAergic systems. Also, the NO-K+ channel pathway was thought to be involved in its mechanism of action since pretreatment with 1H-[1,2,4]oxadiazolo[1,2-a]quinoxalin-1-one (1 mg/kg, s. c.) and glibenclamide (10 mg/kg, s. c.) reduced the antinociceptive effects of affinin (Déciga-Campos et al. 2010). Other studies showing the mechanisms associated with the antinociceptive and pro-nociceptive effects of alkylamides as evaluated in chemical and sensorial tests revealed that they promoted analgesia in neurogenic and inflammatory phases of formalin test against glutamate-induced nociception (Dallazen et al. 2018).
Antioxidant Effect of Alkylamides
The antioxidant activity of alkylamide fraction of Echinacea species was investigated along with other fractions of the plant by measuring their inhibition of in vitro Cu(II)-catalyzed oxidation of human low-density lipoprotein (LDL). Synergistic antioxidant effects were observed when other components were combined with a natural mixture of alkylamides (Dalby-Brown et al. 2005). Alkylamides from black pepper (Piper nigrum L., Piperaceae, at 25 μg/ml) inhibited lipid peroxidation (LPO) by 45–85%, thus demonstrating the antioxidant potential (Liu et al. 2010). Given that hydroxyl radical and superoxide anion generation leading to lipid peroxidation and oxidative stress is implicated in many chronic diseases, the antioxidant potential of alkylamides could be seen as a general mode of action for their use in managing various ailments.
Antithrombotic Activities of Alkylamides
The anticoagulant activity of pellitorine was studied by monitoring activated partial thromboplastin time (aPTT), prothrombin time (PT), and the activities of cell-based thrombin and activated factor X (FXa). Its effects on the expressions of plasminogen activator inhibitor type 1 (PAI-1) and tissue-type plasminogen activator (t-PA) were also tested in TNF-α-activated human umbilical vein endothelial cells (HUVECs) (Ku et al. 2013). The results showed that pellitorine prolonged aPTT, PT, and inhibition of the activities of thrombin and FXa and inhibited the production of thrombin and FXa in HUVECs. It also inhibited thrombin-catalyzed fibrin polymerization and platelet aggregation, thus showing its anticoagulant effects in mice. Additionally, pellitorine inhibited TNF-α-induced production of PAI-1 and had a significant reduction effect on PAI-1 to t-PA ratio.
Antimutagenic and Anticancer Activity of Alkylamides
The antimutagenic activity of affinin was evaluated using Ames assay at doses 12.5, 25, and 50 mg/plate added to several mutagens with or without S9 metabolic activation in Salmonella typhimurium (TA98, TA100, and TA102 strains) (Arriaga-Alba et al. 2013). Affinin at 25 and 50 mg/plate significantly reduced the production of frameshift mutations by 2-aminoanthracene (2AA) as well as the oxidative DNA damage caused by norfloxacin (NOR). Affinin also showed an antioxidant effect that was able to reduce 2AA- and NOR-induced mutations in S. typhimurium TA98 and TA102 (Arriaga-Alba et al. 2013). Pellitorine isolated from the roots of P. nigrum has also been shown to possess cytotoxic activities against HL60 and MCT-7 cell lines (Ee et al. 2010). The human cancer cell proliferation inhibitory activities of piperine and alklyl amides in Capsicum sp. and P. nigrum against stomach, breast, lung, and colon cancer cells were reportedly dose-dependent with an IC50 range of 13–200 μg/ml (Liu et al. 2010). The anticancer activity of spilanthol on HeLa, K-562, MCF-7, and HaCaT cells was evaluated with the effects measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cell counting by flow cytometry, cell cycle analysis, Annexin V-FITC/PI activation, and nuclear DAPI staining. Spilanthol showed a pronounced inhibitory effect on cell lines with a good selectivity index (Willig et al. 2019). The reported cytotoxic effects on cancer cell lines are in line with the ethnomedical uses of plants that contain alkylamides as an anticancer agent (Valerio and Gonzales 2005).
Antimalarial Activity of Alkylamides
Two alkylamides E, E-2,4-octadienamide and E,Z-2,4-decadienamide isolated from P. fraternus, were reported to possess moderate antiplasmodial activity (Sittie et al. 1998). Alkylamides, spilanthol, and undeca-2E-ene-8,10-diynoic acid isobutylamide isolated from S. acmella were reported to have antimalarial activity against Plasmodium falciparum strain PFB and the chloroquine-resistant P. falciparum K1 strain (Spelman et al. 2011). The alkylamides had IC50 values of 16.5 μg/ml and 41.4 μg/ml on P. falciparum strain PFB and IC50 values of 5.8 μg/ml and 16.3 μg/ml against the chloroquine-resistant P. falciparum K1 strain, respectively. In the in vivo experiment, low concentrations of spilanthol reduced parasitemia level by 59% in mice infected with P. yoelii yoelii 17XNL at 5 mg/kg (Spelman et al. 2011). Althaus et al. (2014) also reported the antimalarial activity of pellitorine against P. falciparum with an IC50 value of 3.3 μg/ml. There are several reports of different plants with alkylamides as constituents for the management of malaria in various parts of the world. P. fraternus is used to treat malaria and jaundice in the northern region of India (Ghatapanadi et al. 2011; Sarkhel 2015), Spilanthes sp. is used in Africa (Spelman et al. 2011), and P. longum is used in traditional Chinese medicine (Abdubakiev et al. 2019). The effect of alkylamides against P. falciparum provides the rationale for this use.
Antitrypanosomal Activity of Alkylamides
The antiprotozoal activity of five alkylamides from the flowering aerial parts of Achillea ptarmica L., Asteraceae against Trypanosoma brucei rhodesiense was reported by Althaus et al. (2014). The alkylamide 8,9-Z-dehydropellitorine was the most active against T. b. rhodesiense with an IC50 of 2.0 μg/ml. This also corroborates the use of alkylamides in protozoal infections.
Insecticidal Activity of Alkylamides
A pungent isobutylamide of an unsaturated C18 acid isolated from the roots of Heliopsis scabra Dunal, Asteraceae (synonym of Heliopsis helianthoides subsp. scabra (Dunal) T.R. Fisher) and named “scabrin” was reported to be toxic to house flies (Jacobson 1951). Before this report, N-isobutyl-2,6,8-decatrienamide has been reported as insecticidal from a plant later identified as H. longipes (McGovran et al. 1947). Heliopsin, an unsaturated isobutylamide from the roots of H. helianthoides var. scabra and closely related to scabrin, was also reported to have toxicity against house flies (Jacobson 1957). Pellitorine and neopellitorine A and B isolated from A. dracunculus were reported to show insecticidal activity against Sitophilus oryzae and Rhyzopertha dominica at 200 μg/ml concentrations (Saadali et al. 2001). Clifford et al. (2002) reported the mortality of Aedes aegypti against 100 and 10 μg/ml of some alkylamides including undeca-2E,4Z-dien-8,10-diynoic acid isobutylamide, undeca-2Z,4E-dien-8,10-diynoic acid isobutylamide, dodeca-2E,4Z-dien-8,10-diynoic acid isobutylamide, undeca-2E,4Z-dien-8,10-diynoic acid 2-methylbutylamide, dodeca-2E,4Z-dien-8,10-diynoic acid 2-methylbutylamide, dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide, and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide. Three alkylamides: spilanthol, (E)-N-isobutylundeca-2-en-8,10-diynamide, and (R,E)-N-(2-methylbutyl)undeca-2-en-8,10-diynamide, from A. oleracea were investigated against Tuta absoluta the main tomato pest in Latin America as well as against the predator Solenopsis saevissima and the pollinator Tetragonisca angustula. All the alkylamides showed insecticidal activity, with spilanthol being the most active (LD50 0.13 μg/mg against T. absoluta) (Moreno et al. 2012). The documented insecticidal properties of alkylamides provide a scientific justification for the traditional use of plants like H. longipes as homemade insecticides (Molina-Torres et al. 1996).
Alkylamides as Anesthesia
Hydroxy-α-sanshool, hydroxy-β-sanshool, and hydroxy-γ-sanshool isolated from Z. bungeanum were shown to have anesthetic effect using the infiltrating anesthesia test (Rong et al. 2016). H. longipes is one plant that contains alkylamides which has been used as buccal anesthetic (Molina-Torres et al. 1996). The roots of A. pyrethrum are also used as local anesthesia (Boonen et al. 2012b).
Psychopharmacological Activity of Alkylamides
The psychopharmacological activity of affinin was evaluated in several models (Déciga-Campos et al. 2012). Affinin had antinociceptive effects, modified anxiety behavior, and prolonged the time of sodium pentobarbital-induced hypnosis. It also decreased the time of clonic and tonic seizures in the PTZ-induced seizure model suggesting its anxiolytic potential (Déciga-Campos et al. 2012). This study validates the use of such a plant as A. pyrethrum in the traditional management of epilepsy (Boonen et al. 2012b).
Alkylamides in Neurodegeneration
The nerve growth factor (NGF)–potentiating effect of nine alkylamides (zanthoamide E (40), zanthoamide F (41), ZP-amide A (42), ZP-amide B (43), ZP-amide C (44), ZP-amide D (45), ZP-amide E (46), tetrahydrobungeanool (47), and (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide (48)) from Z. bungeanum were evaluated using rat pheochromocytoma PC-12 cells as a model system of neuronal differentiation. All the nine compounds gave an increase in neurite-bearing cells with compound 47 (tetrahydrobungeanool) having the highest neurite outgrowth-promoting activity while compounds 45, 46, and 48 showed moderate activities. The neurotrophic mimicking activity of these alkylamides may be the basis for their use in the management of neurodegenerative diseases such as Alzheimer’s disease (Wang et al. 2017).
Alkylamides in Skin Disorders
Piperine (49), isopiperine, piperlonguminine (50), retrofractamide A (52), and retrofractamide C from the dried fruits of P. longum increased the melanin content and weakly stimulated tyrosinase activity in a concentration-dependent manner indicating their possible effectiveness in skin disorders (Abdubakiev et al. 2019). The amount of melanin pigment is directly related to the diagnosis and management of pigmentary skin diseases (Lee et al. 2001). The skin protects the body from the external environment and depends on melanocytes for its photoprotective and thermoregulating job by producing melanin. Melanocytes absorb ultraviolet rays and can endure genotoxic stress (Lin and Fisher 2007). Tyrosinase on the other hand is an enzyme involved in melanin biosynthesis whose activation is useful in managing hypopigmentation disorders (Hamid et al. 2012). Thus, the reported alkylamides may find usefulness in managing skin disorders.
Androgenic and Spermatogenic Activity of Alkylamides
The androgenic and spermatogenic potential of the alkylamide-rich ethanol extract of A. pyrethrum was evaluated in male Wistar strain rats. The ethanol extract of A. pyrethrum administered at doses of 50, 100, and 150 mg/kg for 28 days produced a significant increase in body weight, sperm count, motility, and viability along with serum testosterone, luteinizing hormone, and follicle-stimulating hormone concentrations at all tested doses. Histology of the testis also showed an increase in spermatogenic activities with a corresponding increase in seminal fructose content after 28 days of treatment (Sharma et al. 2013). Thirteen alkylamides including undeca-2E,4E-diene-8,10-diynoic acid 2-PEA, deca-2E,4E-dienoic acid 4-OH PEA, tetradeca-2E,4E-diene-8,10-diynoic acid 4-OH PEA, deca-2E,4E-dienoic acid IBA (pellitorine), tetradeca-2E,4E-diene-8,10-diynoic acid IBA (anacycline), dodeca-2E,4E-dienoic acid 4-OH PEA, deca-2E,4E-dienoic acid N-Me IBA, tetradeca-2E,4E-diene-8,10-diynoic acid N-Me IBA, tetradeca-2E,4E, XE/Z-trienoic acid 4-OH PEA, tetradeca-2E,4E, XE/Z, YE/Z-tetraenoic IBA, and dodeca-2E,4E-dienoic acid IBA (IBA, isobutylamide; PEA, phenylethylamide) were detected in the extract by using HPLC/UV/electrospray ionization mass spectrometry methods (Sharma et al. 2013). Plants containing alkylamides have been used traditionally for fertility and related purposes. These include L. meyenii used to enhance fertility and sexual behavior and as a remedy for menopausal symptoms (Valerio and Gonzales 2005), A. pyrethrum used as aphrodisiac in Indian ayurvedic medicine (Sharma et al. 2013), and A. oleracea used as a female aphrodisiac in Brazil (de Souza et al. 2020).
Structure-Activity Relationship of Alkylamides
Plant alkylamides have different carbon lengths, saturation patterns, and double-bond configurations and these reflect in the extent of their various pharmacological activities. Antifungal activity of affinin was suggested to require conjugation with the instauration in either positions 6Z, 8E, or both since conjugation of the carbonyl to the instauration 2E alone was found to be insufficient for its fungitoxic action (Molina-Torres et al. 2004). The α,β,γ,δ-unsaturated conjugation of E,E-2,4-octadienamide and E,Z-2,4-decadienamide isolated from P. fraternus is believed to enhance their antiplasmodial activity (Sittie et al. 1998). The structure-activity relationship (SAR) study of affinin-derived natural and synthetic fatty acid amides with similar chain lengths on root developmental responses of Arabidopsis sp. seedlings showed that N-isobutyl-decanamide, a C:10 saturated alkylamide, was the most active compound in inhibiting primary root growth and stimulating lateral root formation (Méndez-Bravo et al. 2010). Wang et al. (2017) reported that tetrahydrobungeanool, having a non-oxygenated unsaturated long fatty acid chain, had the highest neurite outgrowth-promoting activity, thus suggesting that the non-oxygenated unsaturated long carbon chain appears to play an important role in the neurite outgrowth-promoting activity of the tested alkylamides. Further studies on the SAR of alkylamides would provide more insight into their various observed pharmacological activities.
N-Alkylamides in Plant Immunity
Plants usually produce several intracellular and systemic responses to abiotic (non-pathogen-induced) and biotic (pathogen-induced) stress. Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are three main phytohormones triggered in plants in response to biotic stress (Méndez-Bravo et al. 2011). Pathways that depend on phytohormones lead to the expression of defense-related genes and the production of antimicrobial secondary metabolites (Glazebrook 2001). Alkylamides are one of such secondary metabolites produced by some plants in response to stress and as a chemical defense against plant competitors or microbial and herbivorous predators (Molina-Torres et al. 2004). Echinacea species accumulate unsaturated alkylamides in response to Jas (Romero et al. 2009). Affinin was also reported to have in vitro antimicrobial activity against some plant microbial pathogens, including bacteria and fungi (Molina-Torres et al. 2004).
Pharmacokinetics of Alkylamide-Containing Products
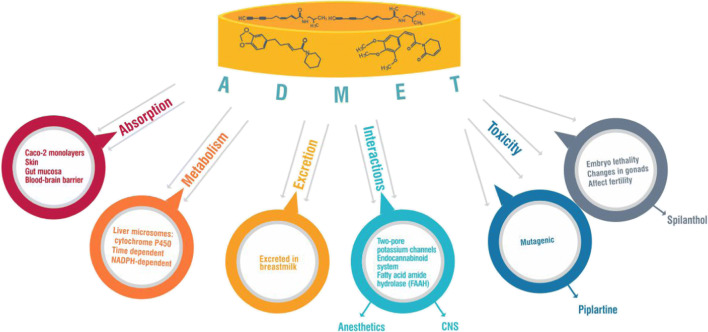
Different products containing alkylamides are commercially available as food supplements, medicinal products, cosmetics, and medical devices. E. angustifolia, E. pallida, and E. purpurea are Echinacea species with reported alkylamide profiles that are used in commercial preparations (Rios 2012). A compilation of widely used alkylamide-containing products and their regulatory status has been reported (Wynendaele et al. 2018). Also, a few studies involving pharmacokinetic characteristics (Fig. 2) of alkylamide-containing plants/products are available in the literature. In a study, both the 2,4-diene and the 2-ene alkylamides penetrated Caco-2 monolayers with permeability coefficient ranging from 3 × 10−6 to 3 × 10−4 cm s−1 and the saturated compounds as well as those with N-terminal methylation having lower permeability coefficients (Matthias et al. 2004). Dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides were also shown to be bioavailable in humans after oral administration of ethanolic extract of E. purpurea (Dietz et al. 2001) as well as transport across Caco-2 monolayers in an in vitro model for the intestinal epithelial barrier (Jager et al. 2002). Other studies on the bioavailability of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides are available (Woelkart et al. 2005b; Matthias et al. 2005). The trend observed was that the higher the unsaturation of the alkylamide, the higher the absorbability (Spelman 2012).
Fig. 2.
ADMET characteristics and interactions of alkylamides
The permeability kinetics of spilanthol and pellitorine across the skin, oral/gut mucosa, and blood-brain barrier showed that the alkylamides can penetrate the skin after topical administration and that they may likely pass the endothelial gut as they easily pass the Caco-2 cells in the monolayer model. The study also revealed spilanthol crosses the oral mucosa as well as the blood-brain barrier (Veryser et al. 2014). Transdermal and pharmacokinetic properties of spilanthol and pellitorine have been studied (Veryser 2016).
Drug interaction studies that investigated the metabolism of alkylamides by human liver microsomes in vitro reported a time- and NADPH-dependent degradation of alkylamides in microsomal fractions suggesting that alkylamides are metabolized by cytochrome P450 enzymes in human liver (Butterweck et al. 2004). In the study, 2-ene and 2,4-diene pure synthetic alkylamides susceptibility to microsomal degradation differ. Also, lower degradation was observed for dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides mixture in an ethanolic Echinacea species extract indicating the dependence of liver P450 metabolism on both alkylamide chemistry (Butterweck et al. 2004).
Alkylamides have been reportedly excreted in the breastmilk of a mother (Perri et al. 2006). In a woman given tablets of Echinacea each containing the equivalent of 675 mg of E. purpurea root and 600 mg of E. angustifolia root made from the dried ethanolic extracts, 13.1 mg of N-isobutyldodeca-2E,4E,8Z,10E/Z-tetraenamide was reported in the breast milk between 1 and 4 h after ingestion (Matthias et al. 2008).
Alkylamides are also known to produce pharmacological effects through various mechanisms of action. These observations include the tingling effect caused by the blockage of the two-pore potassium channels found widespread in the body which can also be activated by some anesthetic drugs (Mathie and Veale 2007). Thus, potential interaction is expected between an alkylamide-containing product and an anesthetic drug acting on the two-pore potassium channels if taken together. Also, alkylamides interact with the endocannabinoid system (Antonella and Federica 2019) as well as inhibit fatty acid amide hydrolase (FAAH) both of which are linked to CNS activities.
From the pharmacokinetic properties and various reported pharmacological activities of alkylamides, they appear to show beneficial health effects but require adequate studies to balance their activities with inherent risks.
Toxicity Studies of Alkylamides
Limited toxicity studies on alkylamides are currently available in the literature. Piplartine isolated from Piper species was shown to induce mutagenicity in eukaryotic models both in vivo and in vitro (Bezerra et al. 2009). Acute toxicity study of affinin was carried out in mice using oral administration of 0.2 ml/10 g body weight at doses 1, 10, and 100 mg/kg. The LD50 value was calculated with a geometrical median according to the Lorke method with affinin having LD50 of 113.13 mg/kg and considered safer than H. longipes extract where it was isolated (Déciga-Campos et al. 2012). Its toxicity was also assessed in zebrafish. Orally administered spilanthol (3 mg/kg) was evaluated for its effects on reproductive performance and embryonic development of zebrafish F1 generation (de Souza et al. 2020). Spilanthol interrupted spawning in the treated group and histopathological evaluation of the gonads revealed a reduction in the percentage of mature cells in the spermatozoa and in females. Also, spilanthol caused embryo lethality in parental groups treated with it as well as caused severe changes in the gonads and on fertility (de Souza et al. 2020). More studies evaluating the toxicity of the so many available alkylamides with good pharmacological effects are required.
Concluding Remarks
Alkylamides are secondary metabolites found in several medicinal plants belonging to different families. They have found usefulness in different traditional medical practices across the world. Several studies on the chemical compositions and pharmacological activities of these plants have established the contributions of alkylamides in pharmaceuticals, cosmetology, and the food industry. Though alkylamides have relatively simple structures, they have been the subject of several research interests related to their diversity, distribution, and chemical, and pharmacological effects. Various chromatographic and spectroscopic approaches have been employed in their detection, isolation, and characterization. Alkylamide-containing plants as well as pure alkylamides are known to have pungent and/or irritating taste, analgesic, and anesthetic effects and as such are useful in treating dental, muscular, and arthritic pains. They are also used to boost immune response, relieve colds, respiratory infections, and influenza. Associated pharmacological activities include immunomodulatory, anti-inflammatory, analgesic, anesthetic, antiviral, and antifungal. This class of compounds, therefore, holds good promise in different product developments and is worthy of thorough investigations.
Electronic Supplementary Material
(DOCX 22 kb).
Authors’ Contribution
TOE wrote the first draft of the manuscript, produced the final version, and was responsible for the submission; SH and AA did the critical revision of the manuscript. All authors read and approved the final manuscript submission.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Abdubakiev S, Li H, Lu X, Li J, Aisa HA. N-Alkylamides from Piper longum L. and their stimulative effects on the melanin content and tyrosinase activity in B16 melanoma cells. Nat Prod Res. 2019;3:1–4. doi: 10.1080/14786419.2018.1539982. [DOI] [PubMed] [Google Scholar]
- Alécio AC, Bolzani VD, Young MC, Kato MJ, Furlan M. Antifungal amide from leaves of Piper hispidum. J Nat Prod. 1998;61:637–639. doi: 10.1021/np9703656. [DOI] [PubMed] [Google Scholar]
- Althaus JB, Kaiser M, Brun R, Schmidt TJ. Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents. Molecules. 2014;19:6428–6438. doi: 10.3390/molecules19056428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonella B, Federica P. Old strategies and new perspectives in modulating the endocannabinoid system. Curr Bioact Compd. 2019;15:159–173. doi: 10.2174/1573407214666180627144214. [DOI] [Google Scholar]
- Antunes PA, Chierice GO, Constantino CJ, Aroca RF. Spectroscopic characterization of N-isobutyl-6-(p-methoxyphenyl)2E,4E-hexadieneamide extracted from Ottonia propinqua. Vib Spectrosc. 2001;27:175–181. doi: 10.1016/S0924-2031(01)00132-1. [DOI] [Google Scholar]
- Arriaga-Alba M, Rios MY, Déciga-Campos M. Antimutagenic properties of affinin isolated from Heliopsis longipes extract. Pharm Biol. 2013;51:1035–1039. doi: 10.3109/13880209.2013.775161. [DOI] [PubMed] [Google Scholar]
- Azwanida NN (2015) A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med Aromat Plants 4. 10.4172/2167-0412.1000196
- Bauer R, Jurcic K, Puhlmann J, Wagner H. Immunologische In-vivo-und in-vitro-untersuchungen mit Echinaceae-extrakten. Arzneimittel-Forschung. 1988;38:276–281. [PubMed] [Google Scholar]
- Bauer R, Remiger P, Wagner H. Alkamides from the roots of Echinacea purpurea. Phytochemistry. 1988;27:2339–2342. doi: 10.1016/0031-9422(88)80156-0. [DOI] [Google Scholar]
- Bezerra DP, Vasconcellos MC, Machado MS, Villela IV, Rosa RM, Moura DJ, Pessoa C, Moraes MO, Silveira ER, Lima MA, Aquino NC. Piplartine induces genotoxicity in eukaryotic but not in prokaryotic model systems. Mutat Res Genet Toxicol Environ Mutagen. 2009;677:8–13. doi: 10.1016/j.mrgentox.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Bohlmann F, Zdero C, Suwita A. Polyacetylenverbindungen, 225. Weitere amide aus der tribus Anthemideae. Chem Ber. 1974;107:1038–1043. doi: 10.1002/cber.19741070331. [DOI] [Google Scholar]
- Boonen J, Bronselaer A, Nielandt J, Veryser L, DeTre G, De Spiegeleer B. Alkamid database: chemistry, occurrence, and functionality of plant N-alkylamides. J Ethnopharmacol. 2012;142:563–590. doi: 10.1016/j.jep.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Boonen J, Sharma V, Dixit VK, Burvenich C, De Spiegeleer B. LC-MS N-alkylamide profiling of an ethanolic Anacyclus pyrethrum root extract. Planta Med. 2012;78:1787–1795. doi: 10.1055/s-0032-1315371. [DOI] [PubMed] [Google Scholar]
- Bruni R, Brighenti V, Caesar LK, Bertelli D, Cech NB, Pellati F. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J Pharm Biomed Anal. 2018;160:443–477. doi: 10.1016/j.jpba.2018.07.044. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Derendorf H, Gaus W, Nahrstedt A, Schulz V, Unger M. Pharmacokinetic herb-drug interactions: are preventive screenings necessary and appropriate? Planta Med. 2004;70:784–791. doi: 10.1055/s-2004-827223. [DOI] [PubMed] [Google Scholar]
- Cech NB, Kandhi V, Davis JM, Hamilton A, Eads D, Laster SM. Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE2 from RAW 264.7 macrophage-like cells. Int Immunopharmacol. 2012;10:1268–1278. doi: 10.1016/j.intimp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X. Macrophage activating effects of new alkamides from the roots of Echinacea species. J Nat Prod. 2005;68:773–776. doi: 10.1021/np040245f. [DOI] [PubMed] [Google Scholar]
- Clifford LJ, Nair MG, Rana J, Dewitt DL. Bioactivity of alkamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine. 2002;9:249–254. doi: 10.1078/0944-7113-00105. [DOI] [PubMed] [Google Scholar]
- Costa SS, Mors WB. Amides of Ottonia corcovadensis. Phytochemistry. 1981;20:1305–1307. doi: 10.1016/0031-9422(81)80027-1. [DOI] [Google Scholar]
- da Silva RV, Navickiene HM, Kato MJ, Bolzani VD, Méda CI, Young MC, Furlan M. Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry. 2002;59:521–527. doi: 10.1016/S0031-9422(01)00431-9. [DOI] [PubMed] [Google Scholar]
- de Souza GC, Viana MD, Goés LD, Sanchez-Ortiz BL, Silva GD, Pinheiro WD, Santos CR, Carvalho JT. Reproductive toxicity of the hydroethanolic extract of the flowers of Acmella oleracea and spilanthol in zebrafish: In vivo and in silico evaluation. Hum Exp Toxicol. 2020;39:127–146. doi: 10.1177/0960327119878257. [DOI] [PubMed] [Google Scholar]
- Dalby-Brown L, Barsett H, Landbo AK, Meyer AS, Mølgaard P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. J Agric Food Chem. 2005;53:9413–9423. doi: 10.1021/jf0502395. [DOI] [PubMed] [Google Scholar]
- Dallazen JL, Maria-Ferreira D, da Luz BB, Nascimento AM, Cipriani TR, de Souza LM, Glugoski LP, Silva BJ, Geppettim P, De Paula Werner MF. Distinct mechanisms underlying local antinociceptive and pronociceptive effects of natural alkylamides from Acmella oleracea compared to synthetic isobutylalkyl amide. Fitoterapia. 2018;131:225–235. doi: 10.1016/j.fitote.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Déciga-Campos M, Rios MY, Aguilar-Guadarrama AB. Antinociceptive effect of Heliopsis longipes extract and affinin in mice. Planta Med. 2010;76:665–670. doi: 10.1055/s-0029-1240658. [DOI] [PubMed] [Google Scholar]
- Déciga-Campos M, Arriaga-Alba M, Ventura-Martínez R, Aguilar-Guadarrama B, Rios MY. Pharmacological and toxicological profile of extract from Heliopsis longipes and affinin. Drug Dev Res. 2012;73:130–137. doi: 10.1002/ddr.21002. [DOI] [Google Scholar]
- Declerck K, Novo CP, Grielens L, Camp GV, Suter A, Berghe WV. (2020) Standardized Echinacea purpurea extract primes an IFN specific innate immune monocyte response via JAK1-STAT1 dependent gene expression and epigenetic control of endogenous retroviral sequences. Preprint: 10.21203/rs.3.rs-28886/v1
- Dietz B, Heilmann J, Bauer R. Absorption of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides after oral application of Echinacea purpurea tincture. Planta Med. 2001;67:863–864. doi: 10.1055/s-2001-18846. [DOI] [PubMed] [Google Scholar]
- Dossou KS, Devkota KP, Morton C, Egan JM, Lu G, Beutler JA, Moaddel R. Identification of CB1/CB2 ligands from Zanthoxylum bungeanum. J Nat Prod. 2013;76:2060–2064. doi: 10.1021/np400478c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ee GC, Lim CM, Rahmani M, Shaari K, Bong CF. Pellitorine, a potential anti-cancer lead compound against HL60 and MCT-7 cell lines and microbial transformation of piperine from Piper nigrum. Molecules. 2010;15:2398–2404. doi: 10.3390/molecules15042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa V, Rivera A. First line of defense: innate cell-mediated control of pulmonary aspergillosis. Front Microbiol. 2016;7:272. doi: 10.3389/fmicb.2016.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNFα gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004;577:563–569. doi: 10.1016/j.febslet.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Ghatapanadi SR, Johnson N, Rajasab AH. Documentation of folk knowledge on medicinal plants of Gulbarga district, Karnataka. Indian J Tradit Knowl. 2011;10:349–353. [Google Scholar]
- Glazebrook J. Genes controlling expression of defense responses in Arabidopsis—2001 status. Curr Opin Plant Biol. 2001;4:301–308. doi: 10.1016/S1369-5266(00)00177-1. [DOI] [PubMed] [Google Scholar]
- Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, Basu TK. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. Int Immunopharmacol. 2002;2:381–387. doi: 10.1016/S1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- Goey AK, Sparidans RW, Meijerman I, Rosing H, Schellens JH, Beijnen JH. A sensitive LC-MS/MS method for the quantitative analysis of the Echinacea purpurea constituent undeca-2-ene-8,10-diynoic acid isobutylamide in human plasma. J Chromatogr B. 2011;879:41–48. doi: 10.1016/j.jchromb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Goey AK, Rosing H, Meijerman I, Sparidans RW, Schellens JH, Beijnen JH. The bioanalysis of the major Echinacea purpurea constituents dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides in human plasma using LC-MS/MS. J Chromatogr B. 2012;902:151–156. doi: 10.1016/j.jchromb.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Goldberg JS. Selected gamma aminobutyric acid (GABA) esters may provide analgesia for some central pain conditions. Persp Med Chem. 2010;4:23–31. doi: 10.4137/PMC.S5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu Z, Nicolussi S, Rau M, Lorántfy L, Forgo P, Hohmann J, Csupor D, Gertsch J. Identification of endocannabinoid system-modulating N-alkylamides from Heliopsis helianthoides var. scabra and Lepidium meyenii. J Nat Prod. 2014;77:1663–1669. doi: 10.1021/np500292g. [DOI] [PubMed] [Google Scholar]
- Hamid MA, Sarmidi MR, Park CS. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int J Mol Med. 2012;29:209–217. doi: 10.3892/ijmm.2011.840. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- Hernández I, Márquez L, Martínez I, Dieguez R, Delporte C, Prieto S, Molina-Torres J, Garrido G. Anti-inflammatory effects of ethanolic extract and alkamides-derived from Heliopsis longipes roots. J Ethnopharmacol. 2009;124:649–652. doi: 10.1016/j.jep.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Hudson J, Vimalanathan S. Echinacea-a source of potent antivirals for respiratory virus infections. Pharmaceuticals. 2011;4:1019–1031. doi: 10.3390/ph4071019. [DOI] [Google Scholar]
- Ingle KP, Deshmukh AG, Padole DA, Dudhare MS, Moharil MP, Khelurkar VC. Phytochemicals: extraction methods, identification, and detection of bioactive compounds from plant extracts. J Pharmacog Phytochem. 2017;6:32–36. [Google Scholar]
- Jacobson M. Constituents of Heliopsis species. I. Scabrin, an insecticidal amide from the roots of H. scabra Dunal1. J Am Chem Soc. 1951;73:100–103. doi: 10.1021/ja01145a037. [DOI] [Google Scholar]
- Jacobson M. Constituents of Heliopsis species. V. Heliopsin, a second insecticidal amide from the roots of H. helianthoides var. scabra. J Am Chem Soc. 1957;79:356–358. doi: 10.1021/ja01559a031. [DOI] [Google Scholar]
- Jager H, Meinel L, Dietz B, Lapke C, Bauer R, Merkle HP, Heilmann J. Transport of alkamides from Echinacea species through Caco-2 monolayers. Planta Med. 2002;68:469–471. doi: 10.1055/s-2002-32076. [DOI] [PubMed] [Google Scholar]
- Kamboj A. Analytical evaluation of herbal drugs. Drug Dis Res Pharmacogn. 2012;3:23–55. [Google Scholar]
- Ku SK, Lee IC, Kim JA, Bae JS. Antithrombotic activities of pellitorine in vitro and in vivo. Fitoterapia. 2013;91:1–8. doi: 10.1016/j.fitote.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Ku SK, Lee IC, Kim JA, Bae JS. Anti-septic effects of pellitorine in HMGB1-induced inflammatory responses in vitro and in vivo. Inflammation. 2014;37:338–348. doi: 10.1007/s10753-013-9745-5. [DOI] [PubMed] [Google Scholar]
- Labbe K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- Lait CG, Alborn HT, Teal PE, Tumlinson JH. Rapid biosynthesis of N-linolenoyl-L-glutamine, an elicitor of plant volatiles, by membrane-associated enzyme (s) in Manduca sexta. Proc Natl Acad Sci. 2003;100:7027–7032. doi: 10.1073/pnas.1232474100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Kim JH, Im S, Lee KB, Sohn S, Kang WH. Application of computerized image analysis in pigmentary skin diseases. Int J Dermatol. 2001;40:45–49. doi: 10.1046/j.1365-4362.2001.00084.x. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Min BW, Lee S, Jee JG, Kim JA, Bae JS. Vascular barrier protective effects of pellitorine in LPS-induced inflammation in vitro and in vivo. Fitoterapia. 2014;92:177–187. doi: 10.1016/j.fitote.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Kan J. Separation and determination of alkylamides from prickly ash powder using molecularly imprinting technique. J Food Compos Anal. 2020;86:103387. doi: 10.1016/j.jfca.2019.103387. [DOI] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yadev VR, Aggarwal BB, Nair MG. Inhibitory effects of black pepper (Piper nigrum) extracts and compounds on human tumor cell proliferation, cyclooxygenase enzymes, lipid peroxidation, and nuclear transcription factor-kappa-B. Nat Prod Commun. 2010;5:1934578X1000500. doi: 10.1177/1934578X1000500822. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Millán-Godínez M, Méndez-Bravo A, Morquecho-Contreras A, Ramírez-Chávez E, Molina-Torres J, Pérez-Torres A, Higuchi M, Kakimoto T, Herrera-Estrella L. Cytokinin receptors are involved in alkamide regulation of root and shoot development in Arabidopsis. Plant Physiol. 2007;145:1703–1713. doi: 10.1104/pp.107.107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. Role of advances in chromatographic techniques in phytochemistry. Phytochemisry. 2007;68:2786–2798. doi: 10.1016/j.phytochem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8:555–562. [PubMed] [Google Scholar]
- Matthias A, Blanchfield JT, Penman KG, Toth I, Lang CS, De Voss JJ, Lehmann RP. Permeability studies of alkylamides and caffeic acid conjugates from Echinacea using a Caco-2 cell monolayer model. J Clin Pharm Ther. 2004;29:7–13. doi: 10.1046/j.1365-2710.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- Matthias A, Addison RS, Penman KG, Dickinson RG, Bone KM, Lehmann RP. Echinacea alkylamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sci. 2005;77:2018–2029. doi: 10.1016/j.lfs.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Matthias A, Addison RS, Agnew LL, Bone KM, Watson K, Lehmann RP. Comparison of Echinacea alkylamide pharmacokinetics between liquid and tablet preparations. Phytomedicine. 2007;14:587–590. doi: 10.1016/j.phymed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Matthias A, Merika H, Addison RS, Bone KM, Lehmann RP (2008) Bioavailability of Echinacea alkylamides in human breast milk. Planta Med 74. 10.1055/s-0028-1083939
- McFerren MA, Cordova D, Rodriguez E, Rauh JJ. In vitro neuropharmacological evaluation of piperovatine, an isobutylamide from Piper piscatorum (Piperaceae) J Ethnopharmacol. 2002;83:201–207. doi: 10.1016/S0378-8741(02)00224-6. [DOI] [PubMed] [Google Scholar]
- McGovran ER, Bottger GT, Gersdorff WA, Fales JH. Insecticidal action of Heliopsis longipes and Erigeron spp. U S Bur Ent and Plant Quar. 1947;E-736:5. [Google Scholar]
- Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J. Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol. 2010;51:1612–1626. doi: 10.1093/pcp/pcq117. [DOI] [PubMed] [Google Scholar]
- Méndez-Bravo A, Calderón-Vázquez C, Ibarra-Laclette E, Raya-González J, Ramírez-Chávez E, Molina-Torres J, Guevara-García AA, López-Bucio J, Herrera-Estrella L. Alkamides activate jasmonic acid biosynthesis and signaling pathways and confer resistance to Botrytis cinerea in Arabidopsis thaliana. PLoS One. 2011;6:e27251. doi: 10.1371/journal.pone.0027251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Torres J, Salgado-Garciglia R, Ramirez-Chavez E, Del Rio RE. Purely olefinic alkamnides in Heliopsis longipes and Acmella (Spilanthes) oppositifolia. Biochem Syst Ecol. 1996;24:43–47. doi: 10.1016/0305-1978(95)00099-2. [DOI] [Google Scholar]
- Molina-Torres J, García-Chávez A, Ramírez-Chávez E. Antimicrobial properties of alkylamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. J Ethnopharmacol. 1999;64:241–248. doi: 10.1016/S0378-8741(98)00134-2. [DOI] [PubMed] [Google Scholar]
- Molina-Torres J, Salazar-Cabrera CJ, Armenta-Salinas C, Ramírez-Chávez E. Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: affinin and reduced amides. J Agric Food Chem. 2004;52:4700–4704. doi: 10.1021/jf034374y. [DOI] [PubMed] [Google Scholar]
- Moreno SC, Carvalho GA, Picanço MC, Morais EG, Pereira RM. Bioactivity of compounds from Acmella oleracea against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and selectivity to two non-target species. Pest Manag Sci. 2012;68:386–393. doi: 10.1002/ps.2274. [DOI] [PubMed] [Google Scholar]
- Müller-Jakic B, Breu W, Pröbstle A, Redl K, Greger H, Bauer R. In vitro inhibition of cyclooxygenase and 5-lipoxygenase by alkamides from Echinacea and Achillea species. Planta Med. 1994;60:37–40. doi: 10.1055/s-2006-959404. [DOI] [PubMed] [Google Scholar]
- Navickiene HM, Alécio AC, Kato MJ, Bolzani VD, Young MC, Cavalheiro AJ, Furlan M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry. 2000;55:621–626. doi: 10.1016/S0031-9422(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Oreopoulou A, Tsimogiannis D, Oreopoulou V. Polyphenols in Plants. Cambridge: Academic Press; 2019. Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters; pp. 243–259. [Google Scholar]
- Perri D, Dugoua JJ, Koren G. Safety and efficacy of Echinacea (Echinacea angustifolia, E. purpurea and E. pallida) during pregnancy and lactation. J Popul Ther Clin Pharmacol. 2006;13:268–276. [PubMed] [Google Scholar]
- Perricone C, Triggianese P, Bartoloni E, Cafaro G, Bonifacio AF, Bursi R, Perricone R, Gerli R. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J Autoimmun. 2020;111:102468. doi: 10.1016/j.jaut.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J. Alkylamides from Echinacea are a new class of cannabinomimetics cannabinoid type 2 receptor-dependent and-independent immunomodulatory effects. J Biol Chem. 2006;281:14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24:519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Ramírez-Chávez E, López-Bucio J, Herrera-Estrella L, Molina-Torres J. Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiol. 2004;134:1058–1068. doi: 10.1104/pp.103.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Zhu Y, Xia X, Ding Y, Guo J, Kan J. Zanthoxylum alkylamides ameliorate protein metabolism disorder in STZ-induced diabetic rats. J Mol Endocrinol. 2017;58:113–125. doi: 10.1530/JME-16-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Zhu Y, Kan J. Zanthoxylum alkylamides activate phosphorylated AMPK and ameliorate glycolipid metabolism in the streptozotocin-induced diabetic rats. Clin Exp Hypertens. 2017;39:330–338. doi: 10.1080/10641963.2016.1259332. [DOI] [PubMed] [Google Scholar]
- Rios MY. Natural alkylamides: pharmacology, chemistry, and distribution. Drug Discov Res Pharmacogn. 2012;16:107–144. [Google Scholar]
- Rios MY, Aguilar-Guadarrama AB, del Carmen GM. Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae) J Ethnopharmacol. 2007;110:364–367. doi: 10.1016/j.jep.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Romero FR, Delate K, Kraus GA, Solco AK, Murphy PA, Hannapel DJ. Alkamide production from hairy root cultures of Echinacea. In vitro Cell Dev Biol Plant. 2009;45:599–609. doi: 10.1007/s11627-008-9187-1. [DOI] [Google Scholar]
- Rong R, Cui MY, Zhang QL, Zhang MY, Yu YM, Zhou XY, Yu ZG, Zhao YL. Anesthetic constituents of Zanthoxylum bungeanum maxim.: a pharmacokinetic study. J Sep Sci. 2016;39:2728–2735. doi: 10.1002/jssc.201600295. [DOI] [PubMed] [Google Scholar]
- Ruiu S, Anzani N, Orrù A, Floris C, Caboni P, Maccioni E, Distinto S, Alcaro S, Cottiglia F. N-alkyl dien-and trienamides from the roots of Otanthus maritimus with binding affinity for opioid and cannabinoid receptors. Bioorg Med Chem. 2013;21:7074–7082. doi: 10.1016/j.bmc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Saadali B, Boriky D, Blaghen M, Vanhaelen M, Talbi M. Alkamides from Artemisia dracunculus. Phytochemistry. 2001;58:1083–1086. doi: 10.1016/S0031-9422(01)00347-8. [DOI] [PubMed] [Google Scholar]
- Sarkhel S. Ethnomedicinal uses of some plants in treatment of jaundice by tribal communities of Paschim Medinipur district, West Bengal, India. Med Arom Plants. 2015;4:4. [Google Scholar]
- Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharmacol. 2006;6:1214–1221. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84:S51–S69. doi: 10.1902/jop.2013.134006. [DOI] [PubMed] [Google Scholar]
- Sexton M. Cannabis in the time of coronavirus disease 2019: the Yin and Yang of the Endocannabinoid system in Immunocompetence. J Altern Complement Med. 2020;26:444–448. doi: 10.1089/acm.2020.0144. [DOI] [PubMed] [Google Scholar]
- Sharma V, Boonen J, Spiegeleer BD, Dixit VK. Androgenic and spermatogenic activity of alkylamide-rich ethanol solution extract of Anacyclus pyrethrum DC. Phytother Res. 2013;27:99–106. doi: 10.1002/ptr.4697. [DOI] [PubMed] [Google Scholar]
- Sittie AA, Lemmich E, Olsen CE, Hviid L, Christensen SB. Alkamides from Phyllanthus fraternus. Planta Med. 1998;64:192–193. doi: 10.1055/s-2006-957405. [DOI] [PubMed] [Google Scholar]
- Spelman K (2012) The pharmacodynamics, pharmacokinetics, and clinical use of Echinaceae purpurea. Continuing Education. http://www.ncpa.co/issues/APOCT12-CE.pdf. Accessed April 17, 2020
- Spelman K, Depoix D, McCray M, Mouray E, Grellier P. The traditional medicine Spilanthes acmella, and the alkylamides spilanthol and undeca-2e-ene-8,10-diynoic acid isobutylamide, demonstrate in vitro and in vivo antimalarial activity. Phytother Res. 2011;25:1098–1101. doi: 10.1002/ptr.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson L, Matthias A, Banbury L, Penman K, Bone K, Leach D, Lehmann R. Modulation of macrophage immune responses by Echinacea. Molecules. 2005;10:1279–1285. doi: 10.3390/10101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunozaki M, Lennertz RC, Vilceanu D, Katta S, Stucky CL, Bautista DM. A ‘toothache tree’ alkylamide inhibits Aδ mechanonociceptors to alleviate mechanical pain. J Physiol. 2013;591:3325–3340. doi: 10.1113/jphysiol.2013.252106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio LG, Gonzales GF. Toxicological aspects of the south American herbs cat’s claw (Uncaria tomentosa) and maca (Lepidium meyenii) Toxicol Rev. 2005;24:11–35. doi: 10.2165/00139709-200524010-00002. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Clin Periodontol. 2013;40:S1–S7. doi: 10.1111/jcpe.12088. [DOI] [PubMed] [Google Scholar]
- Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22:104–115. doi: 10.1093/humupd/dmv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veryser L (2016) Analytical, pharmacokinetic and regulatory characterization of selected plant N-alkylamides (Doctoral dissertation, Ghent University)
- Veryser L, Wynendaele E, Taevernier L, Verbeke F, Joshib T, Tatke P, De Spiegeleer B. N-alkylamides: from plant to brain. Fun Foods Health Dis. 2014;4:264–275. doi: 10.31989/ffhd.v4i6.6. [DOI] [Google Scholar]
- Wang Y, Liao ZB, Cao R, Li H, Wei AZ, Gao JM. Isolation, structural characterization and neurotrophic activity of alkylamides from Zanthoxylum bungeanum. Nat Prod Commun. 2017;12:1934578X1701200. doi: 10.1177/1934578X1701200730. [DOI] [Google Scholar]
- Wang Z, Li S, Ge S, Lin S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J Agric Food Chem. 2020;68:3330–3343. doi: 10.1021/acs.jafc.9b06574. [DOI] [PubMed] [Google Scholar]
- Willig JB, Salomón J, Vianna DR, Moura S, Pilger DA, Buffon A, Konrath EL. Heliopsis longipes SF Blake (Asteraceae) extract causes cell cycle arrest and induces caspase-dependent apoptosis against cancer cell lines. S Afr J Bot. 2019;125:251–260. doi: 10.1016/j.sajb.2019.07.035. [DOI] [Google Scholar]
- Woelkart K, Koidl C, Grisold A, Gangemi JD, Turner RB, Marth E, Bauer R. Bioavailability and pharmacokinetics of alkylamides from the roots of Echinacea angustifolia in humans. J Clin Pharmacol. 2005;45:683–689. doi: 10.1177/0091270004273493. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Med. 2005;71:701–705. doi: 10.1055/s-2005-871290. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Bauer R, Hinz B (2006) Alkamides from Echinacea angustifolia roots inhibit cyclooxygenase-2-dependent prostaglandin synthesis in human neuroglioma cells. Planta Med 72. 10.1055/s-2006-950070
- Woelkart K, Frye RF, Derendorf H, Bauer R, Butterweck V. Pharmacokinetics and tissue distribution of dodeca-2E,4E,8E,10E/Z-tetraenoic acid isobutylamides after oral administration in rats. Planta Med. 2009;75:1306–1313. doi: 10.1055/s-0029-1185631. [DOI] [PubMed] [Google Scholar]
- Wynendaele E, De Spiegeleer B, Gevaert B, Janssens Y, Suleman S, Cattoor S, Saunders JH, Veryser L. Regulatory status of N-alkylamide containing health products. Regul Toxicol Pharmacol. 2018;98:215–223. doi: 10.1016/j.yrtph.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Xi J. Ultrahigh pressure extraction of bioactive compounds from plants—a review. Crit Rev Food Sci Nutr. 2017;57:1097–1106. doi: 10.1080/10408398.2013.874327. [DOI] [PubMed] [Google Scholar]
- Yasui F, Kohara M, Kitabatake M, Nishiwaki T, Fujii H, Tateno C, Yoneda M, Morita K, Matsushima K, Koyasu S, Kai C. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014;454:157–168. doi: 10.1016/j.virol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Ren T, Zhang S, Shirima GG, Cheng Y, Liu X. Hypoglycemic effects of Zanthoxylum alkylamides by enhancing glucose metabolism and ameliorating pancreatic dysfunction in streptozotocin-induced diabetic rats. Food Funct. 2015;6:3144–3154. doi: 10.1039/C5FO00432B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 22 kb).