Summary
The BCR-ABL1 fusion gene is caused by a translocation between chromosomes 9 and 22, resulting in an abnormal chromosome 22 (Philadelphia chromosome; Ph). Prior to the introduction of tyrosine kinase inhibitors (TKI), the presence of BCR-ABL1 conferred a poor prognosis in patients with acute lymphoblastic leukaemia (ALL). We compared the survival of Ph+ and Ph−ALL during the period when TKIs were universally available in the US for Ph+ALL, using a Surveillance, Epidemiology, and End Results (SEER) Database analysis. A total of 2694 patients with pre-B ALL (206 Ph+ALL; 2488 Ph−ALL) aged ≥18 years, who were diagnosed between 2010 and 2014, were identified in SEER registries. The median overall survival (OS) was 32 months in Ph+ALL (95% confidence interval [CI] 18 months-not reached) and 27 months (95% CI 24–30 months) in Ph−ALL (Log-rank test P-value 0·34). Older age was associated with worse prognosis in both Ph+ALL and Ph−ALL. Age-adjusted OS was inferior in Hispanics and African-Americans compared to non-Hispanic whites. Survival of pre-B ALL shows continued improvement with time. Philadelphia chromosome status does not confer poor prognosis in pre-B ALL in the TKI era: prognostic factors in pre-B ALL should be re-evaluated in the light of this finding.
Keywords: philadelphia chromosome, acute lymphoblastic leukaemia, survival, tyrosine kinase inhibitors, SEER
Philadelphia chromosome positive acute lymphoblastic leukaemia (Ph+ALL) is distinguished by the presence of a novel fusion gene, BCR-ABL1, which arises from a reciprocal translocation between chromosomes 9 and 22, resulting in a constitutively activated ABL tyrosine kinase. Ph+ALL constitutes about 25% of patients with pre-B ALL (Pui et al, 2004). Its incidence increases with age; nearly 50% of pre-B ALL patients aged over 50 years are Ph+ (Ravandi & Kebriaei, 2009). The presence of Philadelphia chromosome has been identified as a bad prognostic factor in pre-B ALL: in the pre-tyrosine kinase inhibitor (TKI) era, fewer than 25% of patients achieved long-term remission with conventional chemotherapy (Thomas et al, 2004; Rowe et al, 2005).
Imatinib (Gleevec®), a TKI with activity against the ABL kinase, has revolutionized the care of patients with chronic myeloid leukaemia, which is also driven by the BCR-ABL1 fusion gene. Imatinib was approved by the US Food and Drug Administration (FDA) for the treatment of Ph+ALL in 2004, followed by other, more potent, TKIs (Ravandi et al, 2010, 2015; Foa et al, 2011; Jabbour et al, 2015; Kim et al, 2015; Rousselot et al, 2016; Sasaki et al, 2016; Short et al, 2016).
The use of BCR-ABL1 TKIs appears to have significantly improved the survival in Ph+ALL, based on post-hoc comparisons between prospective clinical trials conducted before and after the introduction of this class of drugs (Thomas et al, 2004; Yanada et al, 2006; de Labarthe et al, 2007; Mizuta et al, 2011; Fielding et al, 2014). However, to date there has been no large-scale population based study comparing the survival of Ph+ALL with Philadelphia negative (Ph−) ALL in the TKI era. This is important because survival of patients on clinical trials often exceeds results seen in “real world” population-based studies, due to study exclusion criteria, disparities in the number of elderly patients who receive TKI therapy, omission of patients with central nervous system involvement, etc. Thus the apparent improvement in outcomes has awaited confirmation at the population level. The analysis of the effect of Ph+ status on outcome of B-ALL has not been feasible up until now because translocation status (by fluorescent in situ hybridization, FISH) has only been captured as a separate code in the US Surveillance, Epidemiology, and End Results (SEER) cancer registries since 2010. Thus, other studies have assessed trends in survival of ALL or acute leukaemia but have not been able to assess the effect of distinct mutations (Pulte et al, 2013). We hypothesized that the widespread adoption of TKIs had indeed abrogated the adverse risk of Ph+ALL, and that this would be reflected in an improved survival of Ph+ALL.
In this study, we used data from the population-based US SEER cancer registries to analyse the survival of Ph+ALL and Ph−ALL in patients diagnosed between 2010 and 2014 (a period during which TKI were widely used in Ph+ALL in the United States). We quantified the proportions of patients with Ph+ subtype, by racial and ethnic groups, age and sex. We also analysed the survival differences by age, sex, racial/ethnic classification, year of diagnosis, marital status and insurance status.
Methods
Data for patients diagnosed with ALL aged ≥18 years between 1973 and 2014 (N = 10 064) were extracted from the most recent version of SEER database (Version April 2017) (SEER 2017). The SEER program of the National Cancer Institute documents data on newly diagnosed cancer patients and uses the International Classification of Disease for Oncology (ICD-O) to categorize ALL. The ICD-O classification codes from 1973 to 2009 were 9835–9836 whilst those for 2010–2014 were 9811/3 for B lymphoblastic leukaemia/lymphoma, not otherwise specified (NOS), 9813/3 for B lymphoblastic leukaemia/lymphoma with t(v;11q23); MLL (KMT2A) rearranged, 9812/3 for B lymphoblastic leukaemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1, 9817/3 for B lymphoblastic leukaemia/lymphoma with t(5;14)(q31;q32); IL3-IGH, 9818/3 for B lymphoblastic leukaemia/lymphoma with t(1;19)(q23;p13.3); E2A-PBX1 (TCF3-PBX1), 9814/3 for B lymphoblastic leukaemia/lymphoma with t(12;21)(p13; q22); TEL-AML1 (ETV6-RUNX1), 9816/3 for B lymphoblastic leukaemia/lymphoma with hypodiploidy (Hypodiploid ALL) and 9815/3 for B lymphoblastic leukaemia/lymphoma with hyperdiploidy. 9812/3 was classified as Ph+ALL while others were classified as Ph−ALL. Patients were categorized according to their age, sex, race/ethnicity, marital status at diagnosis, insurance and year of diagnosis. Detailed patient selection is shown in Fig 1.
Fig 1.
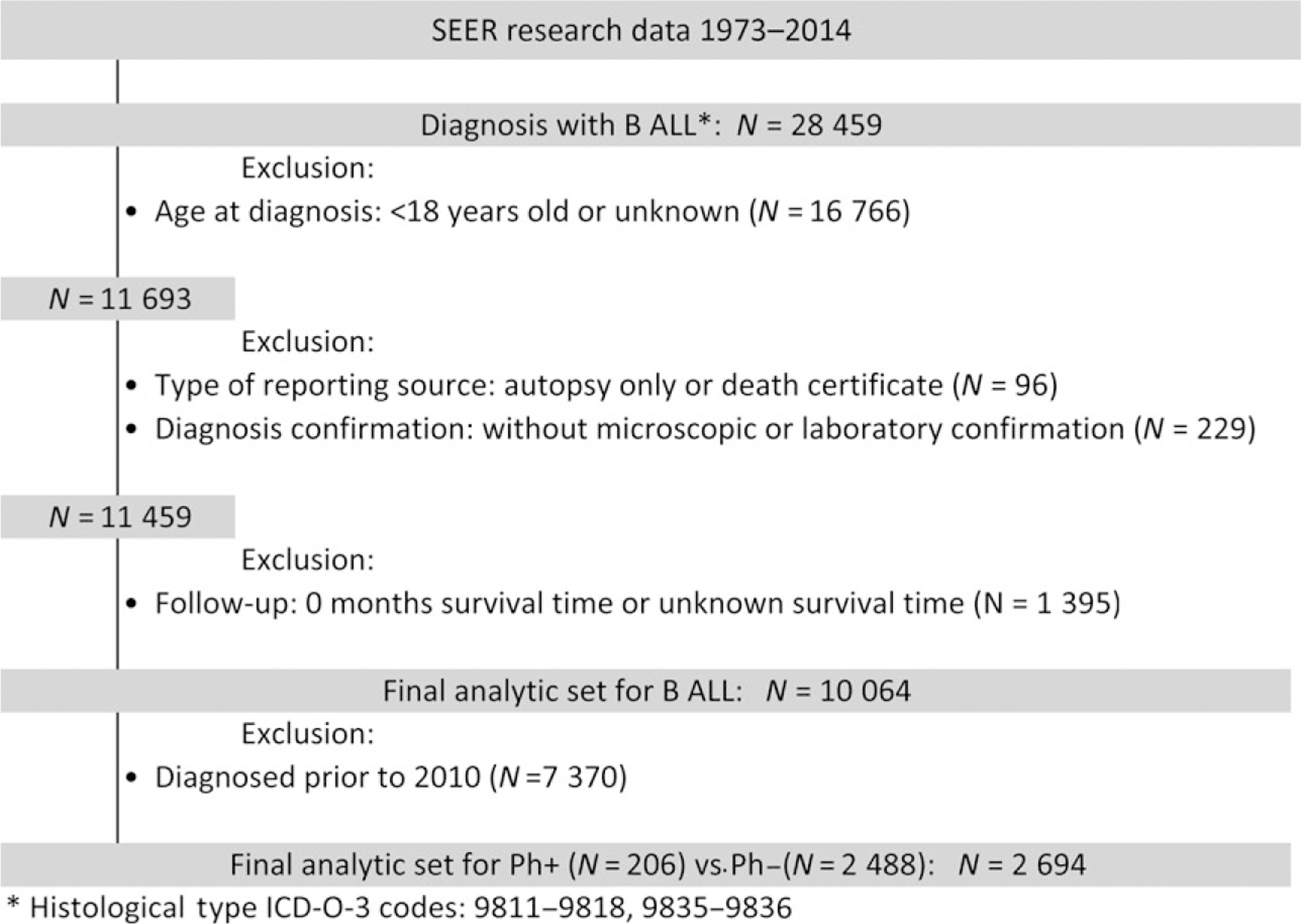
Patient selection diagram for pre-B ALL patients from SEER 1973 to 2014. ALL, acute lymphoblastic leukaemia; SEER, surveillance, epidemiology, and end results database.
Statistical analysis
The primary endpoints of the study were overall survival (OS), defined as the period from date of diagnosis to the date of death or censored at date of last contact, 31 December 2014 or 10 years after diagnosis, whichever came first. Both univariate survival analysis [Kaplan Meier (KM) curves, medians, hazard ratios and log-rank tests] and multivariate Cox regression model (adjusted hazard ratios and Wald tests) were used to evaluate the difference in OS by Ph status and other patient characteristics. Adjusted median OS estimates were derived from the direct adjusted survival probabilities from the multivariate Cox regression model (Zhang et al, 2007). Interactions between Ph status and age (18–39 vs. 40+ years) and year of diagnosis (2010–2012 vs. 2013–2014) on OS were tested by including the interaction term in the multivariate Cox regression model. The analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC) at a significance level of 0·05. All tests are 2-sided.
Results
A total of 10 064 patients were diagnosed with pre-B ALL from 1973 to 2014 in SEER. Ph status was not initially recorded in the SEER database, however, from 2010 onwards, the SEER database began categorizing BCR-ABL1 (Philadelphia chromosome) positive ALL (Ph+ALL) using a separate code. A total of 2694 patients (median age 50 years, range 18–102 years) were diagnosed between 2010 and 2014, of which 2488 patients were diagnosed with Ph−ALL and 206 patients with Ph+ALL (Fig 1).
Characteristics of patients in 2010–2014 cohort
Ph+ALL patients were significantly older than those with Ph−ALL (median age 53 vs. 49 years respective, P = 0·01) (Tables I and II); 54·9% were male with no sex differences between Ph+ALL and Ph−ALL. Analysis of the racial/ethnic composition between Ph+ALL and Ph−ALL showed a lower percentage of Hispanics in the Ph+ALL group compared to Ph−ALL, with 18·9% of Ph+ALL categorized as Hispanic compared to 35·7% of Ph−ALL. There was no significant difference in the insurance type between Ph+ALL and Ph−ALL. Only 35·4% of Ph+ALL patients were reported to be married at the time of diagnosis compared to 50·2% of Ph−ALL (P < 0·0001).
Table I.
Characteristics of Ph+ and Ph−ALL patients in SEER 2010–2014
| Ph+ (N = 206) | Ph− (N = 2488) | Total (N = 2694) | P value | |
|---|---|---|---|---|
| Age at diagnosis, years | ||||
| Median | 53·0 | 49·0 | 50·0 | 0·010 |
| Interquartile range | 400,630 | 320,625 | 330,630 | |
| Range | (18·0–88·0) | (18·0–102·0) | (18·0–102·0) | 0·015 |
| 18–39 | 51 (24·8%) | 866 (34·8%) | 917 (34%) | |
| 40–49 | 36 (17·5%) | 384 (15·4%) | 420 (15·6%) | |
| 50–59 | 48 (23·3%) | 455 (18·3%) | 503 (18·7%) | |
| 60–69 | 46 (22·3%) | 425 (17·1%) | 471 (17·5%) | |
| 70+ | 25 (12·1%) | 358 (14·4%) | 383 (14·2%) | |
| Sex | ||||
| Male | 114 (55·3%) | 1365 (54·9%) | 1479 (54·9%) | 0·89 |
| Female | 92 (44·7%) | 1123 (45·1%) | 1215 (45·1%) | |
| Race/ethnicity | ||||
| Non-Hispanic White | 120 (58·3%) | 1247 (50·1%) | 1367 (50·7%) | <0·0001 |
| African American | 21 (10·2%) | 152 (6·1%) | 173 (6·4%) | |
| Asian | 21 (10·2%) | 173 (7%) | 194 (7·2%) | |
| Hispanic | 39 (18·9%) | 889 (35·7%) | 928 (34·4%) | |
| Native American | 5 (2·4%) | 23 (0·9%) | 28 (1%) | |
| Unknown | 0 (0%) | 4 (0·2%) | 4 (0·1%) | |
| Insurance | ||||
| Insured | 154 (74·8%) | 1658 (66·6%) | 1812 (67·3%) | 0·0503 |
| Any Medicaid | 38 (18·4%) | 637 (25·6%) | 675 (25·1%) | |
| Uninsured/unknown | 14 (6·8%) | 193 (7·8%) | 207 (7·7%) | |
| Married | ||||
| Yes | 73 (35·4%) | 1250 (50·2%) | 1323 (49·1%) | <0·0001 |
| Not | 133 (64·6%) | 1238 (49·8%) | 1371 (50·9%) | |
| Year of diagnosis | ||||
| 2010 | 18 (8·7%) | 511 (20·5%) | 529 (19·6%) | <0·0001 |
| 2011 | 30 (14·6%) | 523 (21%) | 553 (20·5%) | |
| 2012 | 4 (21·4%) | 482 (19·4%) | 526 (19·5%) | |
| 2013 | 45 (21·8%) | 497 (20%) | 542 (20·1%) | |
| 2014 | 69 (33·5%) | 475 (19·1%) | 544 (20·2%) |
Table II.
Effect of sex, age, race/ethnicity, marital status, insurance status and year of diagnosis on survival in pre-B ALL from 2010 to 2014
| N | Median OS (95% CI), months | HR (95% CI) | Log-rank test P value | Adjusted HR (95% CI)* | Wald test P value* | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 1479 | 28 (24, 33) | Reference | 0·47 | Reference | 0·39 |
| Female | 1215 | 27 (22, 32) | 1·04 (0·93, 1·17) | 0·95 (0·84, 1·07) | ||
| Age at diagnosis, years | ||||||
| 18–39 | 917 | NE (58, NE) | Reference | <0·001 | Reference | <0·001 |
| 40–49 | 420 | 33 (25, NE) | 1·50 (1·24, 1·82) | 1·58 (1·30, 1·93) | ||
| 50–59 | 503 | 23 (18, 33) | 1·78 (1·49, 2·12) | 1·92 (1·59, 2·32) | ||
| 60–69 | 471 | 20 (16, 23) | 2·18 (1·84, 2·59) | 2·48 (2·06, 2·99) | ||
| 70+ | 383 | 8 (6, 10) | 3·84 (3·24, 4·55) | 4·74 (3·93,5·72) | ||
| Race/ethnicity | ||||||
| Non-Hispanic White | 1367 | 28 (24, 33) | Reference | 0·090 | Reference | 0·009 |
| African American | 173 | 21 (16, 27) | 1·14 (0·91, 1·43) | 1·43 (1·13, 1·83) | ||
| Asian | 194 | NE (24, NE) | 0·78 (0·61, 1·00) | 0·93 (0·70, 1·23) | ||
| Hispanic | 928 | 26 (22, 33) | 0·94 (0·82, 1·06) | 1·22 (1·04, 1·43) | ||
| Native American | 28 | 17 (10, NE) | 1·21 (0·73, 2·03) | 1·56 (0·91, 2·68) | ||
| Unknown | 4 | NE | NE | NE | ||
| Married | ||||||
| Yes | 1323 | 34 (28, 42) | Reference | 0·003 | Reference | 0·19 |
| Not | 1371 | 23 (21, 27) | 1·19 (1·06, 1·33) | 1·09 (0·96, 1·23) | ||
| Insurance | <0·001 | |||||
| Insured | 1812 | 28 (24, 33) | Reference | 0·60 | Reference | |
| Any medicaid | 675 | 25 (20, 31) | 1·06 (0·92, 1·21) | 1·40 (1·20, 1·64) | ||
| Uninsured/unknown | 207 | 30 (21, NE) | 0·95 (0·76, 1·19) | 1·25 (0·99, 1·58) | ||
| Year of diagnosis | ||||||
| 2010 | 529 | 23 (18, 30) | Reference | 0·18 | Reference | 0·33 |
| 2011 | 553 | 28 (23, 33) | 0·90 (0·77, 1·05) | 0·94 (0·80, 1·10) | ||
| 2012 | 526 | 28 (23, NE) | 0·90 (0·76, 1·06) | 0·92 (0·78, 1·09) | ||
| 2013 | 542 | NE | 0·89 (0·75, 1·07) | 0·88 (0·74, 1·06) | ||
| 2014 | 544 | NE | 0·76 (0·59, 0·98) | 0·77 (0·60,0·99) | ||
| Ph status | ||||||
| Ph+ | 206 | 32 (18, NE) | Reference | 0·34 | Reference | 0·63 |
| Ph− | 2488 | 27 (24, 30) | 1·12 (0·88, 1·42) | 1·06 (0·83, 1·37) |
CI, confidence interval; HR, hazard ratio; NE, not reached or not evaluable.
Based on the Cox multivariate regression model adjusted variables included in the table and stratified by SEER registry site.
Overall survival of patients with ALL
The OS of all patients with pre-B ALL estimated from 1973 to 2014 showed significant increase with each decade (adjusted P < 0·001) (Fig 2): 1973–1979 [adjusted median OS = 11 months, 95% confidence interval (CI) = 10·0–13·0, 1980–1989] (adjusted median OS = 13 months, 95% CI = 12·0–14·0), 1990–1999 (adjusted median OS = 17 months, 95% CI = 15·0–18·0), 2000–2009 (adjusted median OS = 20 months, 95% CI = 19·0–22·0), 2010–2014 (adjusted median OS = 26 months, 95% CI = 23·0–30·0). The highest increase in median OS (6 months) was seen in the period comparing 2000–2009 to 2010–2014. The adjusted OS estimates by period were calculated from the multivariate Cox regression model, which included age, sex, race/ethnicity, marital status at diagnosis and insurance type.
Fig 2.
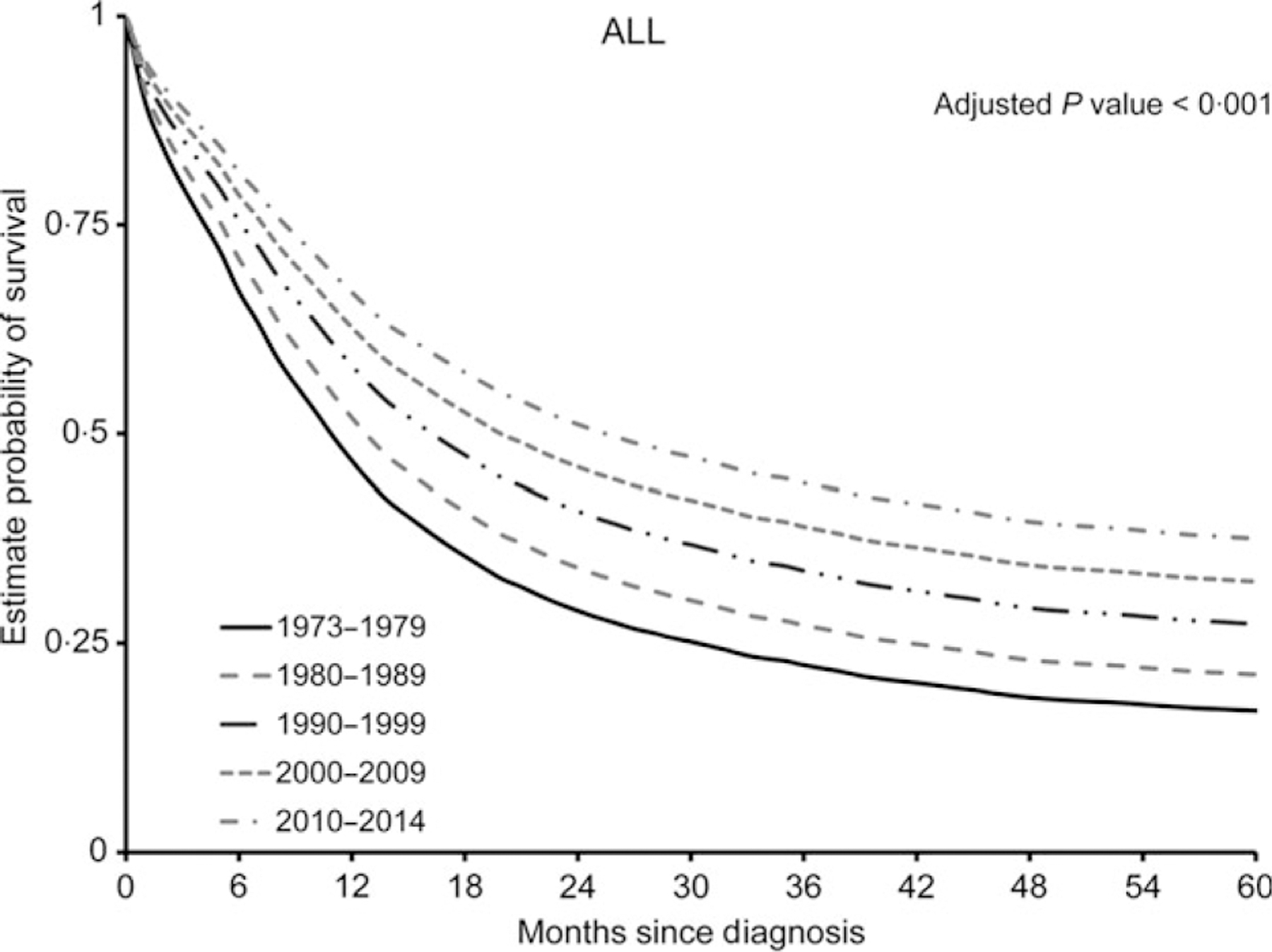
Improvement in 5-year overall survival from 1973 to 2014. Adjusted overall curves of pre-B acute lymphoblastic leukaemia (ALL) from 1973 to 2014. Estimated rates of overall survival for 5 years are illustrated according to the decade of diagnosis.
We next evaluated the survival of Ph+ALL verses Ph−ALL in the 2010–2014 cohort. The median follow-up among patients who were alive at the time of analysis (N = 1516) was 19·0 months (range: 1–59 months). Based on KM curves, the median OS between Ph+ALL [32 months, 95% CI = 18-not evaluated (NE)] and Ph−ALL (27 months, 95% CI = 24–30) was not statistically significant [unadjusted hazard ratio (HR) = 1·12, 95% CI: 0·88–1·42, comparing Ph−ALL to Ph+ALL, Log-rank test P-value = 0·34] (Fig 3A). The 36-month survival rate for Ph+ALL was 46·9% (95% CI = 36·3–56·9%) compared to 44·6% (95% CI = 42·2–47·0%) for Ph−ALL.
Fig 3.
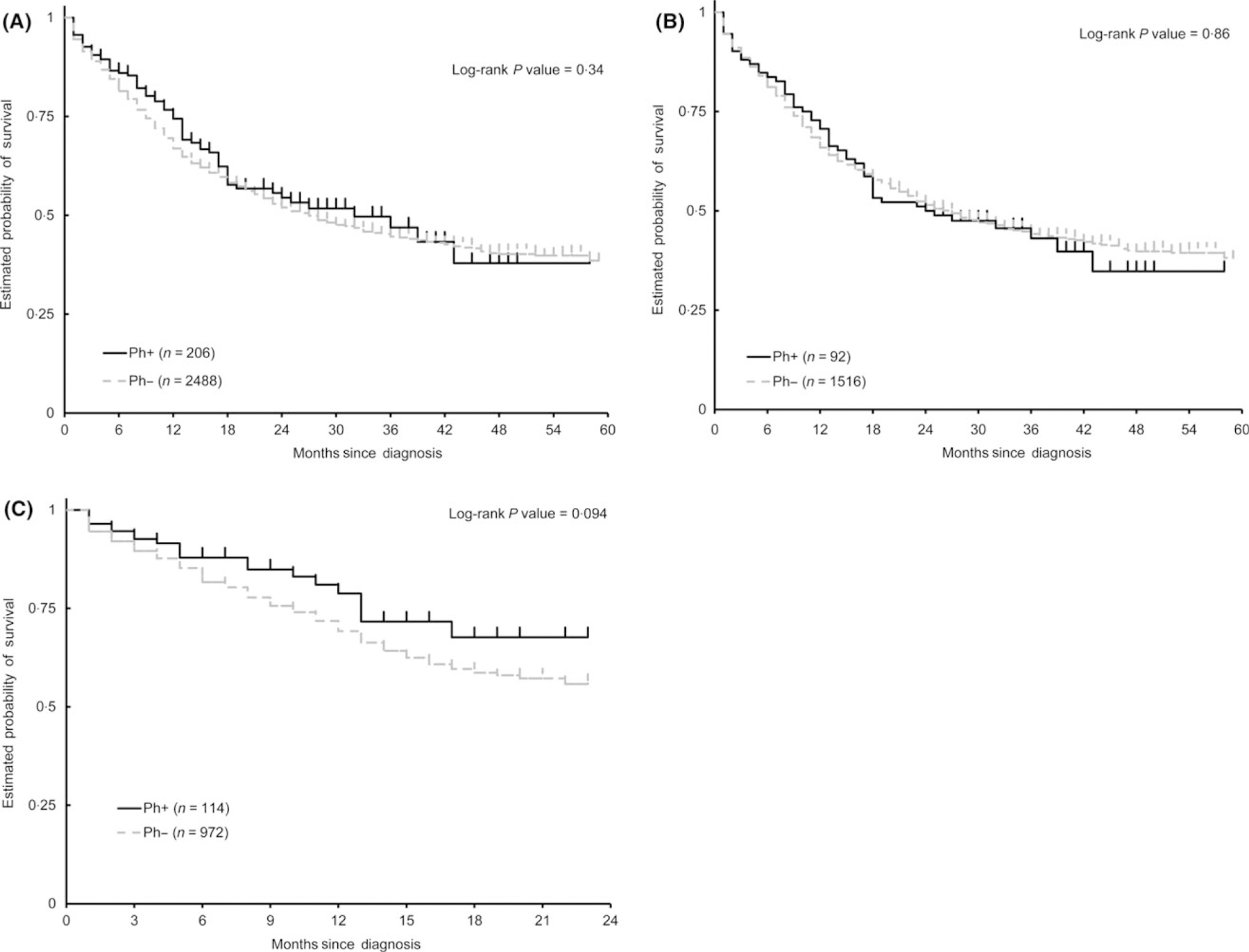
No significant difference in survival between Ph+ALL and Ph−ALL. (A) Kaplan–Meier curves of overall survival (OS) for Ph+ALL compared to Ph−ALL from 2010 to 2014. Estimated 5-year OS rates are illustrated comparing Ph+ALL and Ph−ALL. (B) Estimated 5-year OS of Ph+ALL compared to Ph−ALL diagnosed in 2010–2012. (C) Estimated 2-year survival of Ph+ALL compared to Ph−ALL diagnosed in 2013–2014.
Trend towards better survival in later Ph+ cohorts
To evaluate the progress in the survival of Ph+ALL compared to Ph−ALL with time, the OS was estimated separately for patients diagnosed between 2010–2012 and 2013–2014 (Fig 3B, C and Table SI, SII). For 2010–2012, there was no significant difference (unadjusted HR = 0·97, 95% CI: 0·74–1·29, comparing Ph−ALL to Ph+ALL, Log-rank P-value = 0·86) in the OS between Ph+ALL (median OS = 24·5 months, 95% CI = 17·0–43·0) and Ph−ALL (median OS = 27, 95% CI = 23·0–30·0). For patients diagnosed between 2013 and 2014 period, there was a trend towards better survival for patients diagnosed with Ph+ALL (1-year survival rate: 78·8%, 95% CI = 67·1–86·7%) compared to Ph− (1-year survival rate: 69·2%, 95% CI = 65·5–72·6%), although the difference was not statistically significant (unadjusted HR = 1·45, 95% CI: 0·93–2·26, comparing Ph−ALL to Ph+ALL, Log-rank P-value = 0·094). Interaction test for Ph status and the period of diagnosis on OS was not significant in the multivariate model including the interaction term and other variables in Table I (P = 0·11).
Standard front line induction therapy for younger adult ALL typically involves high-dose multi-agent chemotherapy. Older patients are often unable to tolerate regimens given at standard doses and this may contribute to the very poor outcomes seen in these patients. Since TKIs are comparatively less toxic and easier to tolerate, we analysed the survival of Ph+ and Ph−ALL by young adult ALL (18–39 years) and older ALL (≥40 years) patient groups (Fig 4A, B and Table SIII). There was no significant difference in the OS between Ph+ALL and Ph−ALL for young adults (unadjusted HR = 0·83, 95% CI: 0·50–1·37, comparing Ph−ALL to Ph+ALL, Log-rank P-value = 0·46). Older adults with Ph+ALL (median OS = 32 months, 95% CI = 18·0–43·0) had a significantly better OS (unadjusted HR = 1·32, 95% CI: 1·01–1·73, comparing Ph−ALL to Ph+ALL, Log rank test P-value = 0·037) compared to older adults with Ph−ALL (median OS = 18 months, 95% CI = 17·0–21·0), using a univariate survival analysis. However, after adjusting for confounding variables using a multivariate Cox regression model, the difference was not statistically significant (adjusted HR = 1·25, 95% CI: 0·95–1·64, comparing Ph−ALL to Ph+ALL, Wald Chi-Square P-value = 0·11). Interaction test for Ph status and age at diagnosis on OS was not significant in the multivariate model including the interaction term and other variables in Table I (P = 0·21).
Fig 4.
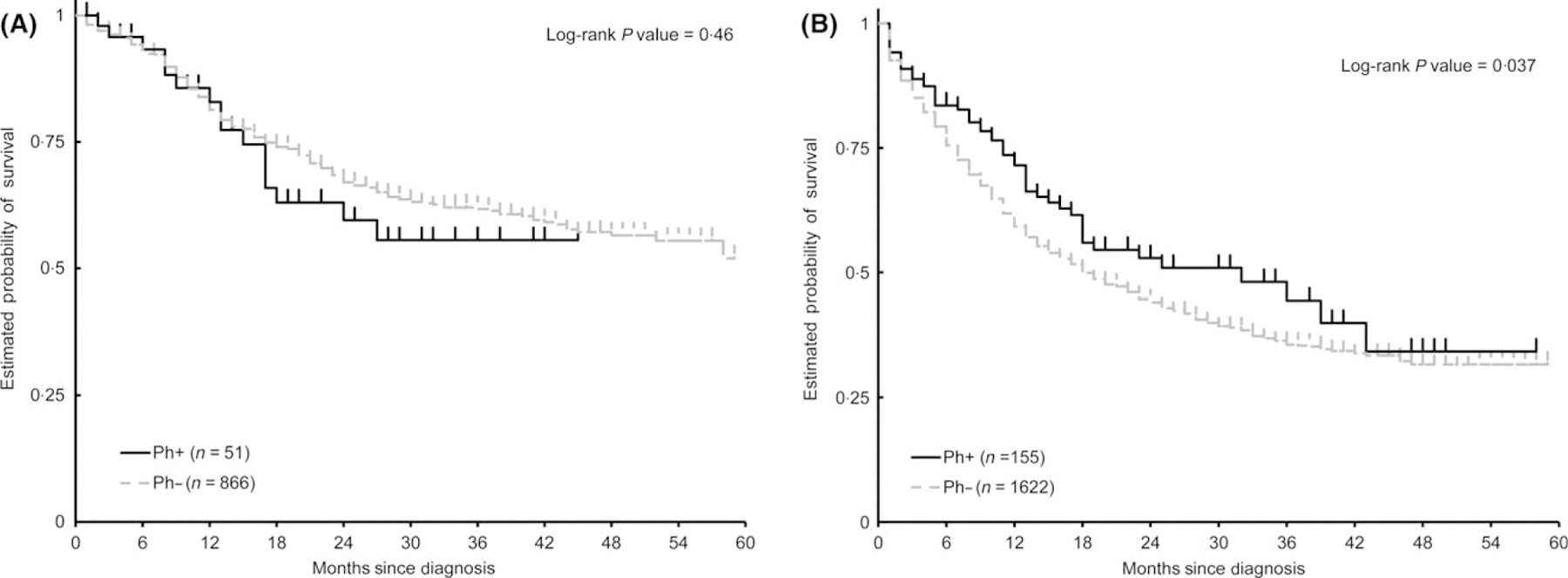
Kaplan–Meier curves of overall survival of Ph+ALL compared to Ph−ALL in age group 18–39 years (A), and in patients aged over 40 years (B).
Analysis was also performed to evaluate other variables that might influence the survival for patients diagnosed with pre-B ALL. After adjusting for the covariates (age, sex, race/ethnicity, marital status, type of insurance and year of diagnosis), only age (Wald chi-square P < 0·001), race/ethnicity (Wald chi-square P-value = 0·009) and insurance type (Wald chi-square P < 0·001) significantly influenced the outcome. Increase in age, patients with Medicaid insurance and those of African-American and Hispanic ethnicity/race were associated with worse OS.
Discussion
Our study is the first to compare the survival of Ph+ALL and Ph−ALL in the TKI era using a large population-based database. The use of TKIs in Ph+ALL were reported as early as 2001, however use of TKIs as a standard part of front-line induction therapy for Ph+ALL is not part of the FDA label for any of the currently approved TKIs (Druker et al, 2001; Ottmann et al, 2002; Champagne et al, 2004). Treatment information, including TKI use, is not captured in the SEER database; however, by 2010, the use of TKIs was widespread and recommended by experts in the field and consensus guidelines (Fielding, 2010).
Our results indicate that in the TKI era, the survival outcome of Ph+ALL is not statistically different from that of Ph−ALL. Current recommendations continue to use Ph status to risk-stratify ALL patients. Our study shows that since the introduction and adoption of BCR-ABL1 TKIs for the treatment of Ph+ALL, Ph status no longer serves as a prognostic marker, but rather a predictive marker for response to TKI.
In the patients diagnosed between 2013 and 2014, there was a statistically non-significant trend towards better survival for Ph+ALL compared to Ph−ALL. The follow-up in this cohort was limited, but it is possible that with longer follow-up, the survival difference would be significant. Recent years have seen an increase in the use of second-generation TKIs for treatment of Ph+ALL (dasatinib, nilotinib, ponatinib). Newer-generation TKIs offer higher response rates and earlier complete molecular response, which may confer better survival in Ph+ALL when used in place of imatinib (Ravandi et al, 2010, 2015; Foa et al, 2011; Jabbour et al, 2015; Kim et al, 2015; Rousselot et al, 2016; Sasaki et al, 2016; Short et al, 2016). Thus the apparent improvement in survival in 2013–2014 could be a reflection of increased use of second-generation TKIs in Ph+ALL.
Currently, Ph+ adults less than 65 years of age with no substantial comorbidities who achieve complete remission with induction chemotherapy are offered allogeneic stem cell transplantation (AlloSCT), if available. In the pre-imatinib era, AlloSCT resulted in improvement in survival compared to chemotherapy alone (Cornelissen et al, 2001; Dombret et al, 2002; Avivi & Goldstone, 2003; Esperou et al, 2003; Goldstone et al, 2008). Studies suggest that the incorporation of TKI (imatinib) into the treatment regimen for Ph+ALL patients has led to improvements in outcomes over chemotherapy alone and, by increasing remission rates, may have lead to an increase in the percentage of patients preceding to AlloSCT (Thomas et al, 2004; Yanada et al, 2006; Fielding et al, 2014). A recent study showed excellent outcome for Ph+ALL patients who achieved complete molecular remission at 3 months with a combination of TKI and chemotherapy but without AlloSCT (Short et al, 2016). AlloSCT could potentially be restricted to patients who do not achieve a complete molecular remission or at the time of relapse. This study shows that we have made tremendous progress in treating Ph+ALL. But, since our data does not capture the specific treatment the patients received, the relative contribution of TKIs versus AlloSCT cannot be determined. It is possible that TKI therapy would have resulted in increased complete remission enabling a higher proportion of Ph+ALL patients to undergo AlloSCT. TKIs in combination with novel agents could also have contributed to rescue of many relapsed Ph+ALL patients, enabling them to undergo AlloSCT. Also, the ability to monitor minimal residual disease in Ph+ALL using the BCR-ABL1 transcript could have contributed to early detection of relapse and hence, early intervention. Prospective randomized studies to evaluate the role of AlloSCT in Ph+ALL are warranted.
Older patients are less likely to tolerate intense chemotherapy and to undergo AlloSCT. Univariate analysis showed that the outcome of Ph+ALL in the elderly age group showed better outcome compared to Ph−ALL, however the difference was not statistically significant after adjusting for co-variables. Increasingly, TKIs are being recognized as the backbone therapy for Ph+ALL, and some have called into question the need for chemotherapy, given that this disease is inherently chemoresistant, particularly in older patients. TKI-based induction regimens have shown complete response rates comparable to, or better than, cytotoxic chemotherapy-based regimens, often with lower toxicity and outpatient treatment (Vignetti et al, 2007; Foa et al, 2011; Chalandon et al, 2015; Rousselot et al, 2016). Ultimately, dual targeting of ABL kinase with catalytic site and allosteric inhibitors, such as ABL001, may make chemotherapy unnecessary in this disease (Wylie et al, 2017). TKI-only therapies are invariably associated with the development of resistance mutations in in ABL kinase (Foa et al, 2011) and therefore newer combinations such as TKI + blinatumomab are currently being tested [Southwestern Oncology Group (SWOG) 1318, NCT02143414].
The 2010–2014 cohort showed a worse prognosis for African-American and Hispanic patients. African Americans and Native Americans have worse prognosis compared to other ethnic groups, as shown for haematological malignancies and several other cancers (Maskarinec et al, 2011; Kahn et al, 2016; Jemal et al, 2017). Our study shows that a lower proportion of Ph+ALL were Hispanics compared to Ph−ALL. Several subtypes of ALL occur more frequently in certain races and ethnic groups. For example, a higher frequency of TCF3-PBX1 ALL is seen in African Americans (Hunger & Mullighan, 2015). It also has been reported that African American children with ALL were significantly more likely to have higher-risk prognostic features (Pui et al, 2004; Pui & Evans, 2006). Hispanic patients are known to have a higher proportion of Ph−like ALL, which confer worse prognosis (Harvey et al, 2010; Hunger & Mullighan, 2015; Jain et al, 2017). Both biological and socio-economic factors could be playing a role in the differences in outcomes seen with ethnicity/race. Patients with Medicaid insurance had a worse survival compared to patients with other insurance. This again, could be a reflection of socio-economic factors.
Age has historically been considered an important prognostic factor in pre-B ALL, including Ph+ALL (Rowe et al, 2005; Gokbuget & Hoelzer, 2006; Bassan & Hoelzer, 2011). The increasing incidence of Ph with age in ALL was attributed to contributing a worse prognosis in elderly ALL (Pui et al, 2004; Burmeister et al, 2008; Moorman et al, 2010). However, even with the introduction of TKIs, the prognosis of elderly Ph+ALL continues to be worse than younger Ph+ALL, suggesting the contribution of other biological factors, both cancer intrinsic and extrinsic (Gleissner et al, 2002; Rowe et al, 2005; Moorman et al, 2010). Moreover, younger children (1–9 years) with Ph+ALL have a better prognosis than adolescents with this subtype (Arico et al, 2000).
The proportion of Ph+ALL in the 2010–2014 SEER database was lower than expected (Ravandi & Kebriaei, 2009). It is possible the database under-reports the Ph status of the patients (for example, some of the patients in the B-ALL NOS category may not have been tested and may have been Ph+), suggesting a potential weakness of this study.
In summary, the introduction of TKIs has resulted in significant improvement in the survival of patients with Ph+ALL. Our study highlights the need for randomized studies of AlloSCT in the TKI era and a re-evaluation of prognostic and treatment algorithms for patients with pre-B ALL.
Supplementary Material
Acknowledgement
II performed the research and wrote the paper, DY performed the research and wrote the paper, AM wrote the paper, NM wrote the paper, GY wrote the paper, KK designed the research and wrote the paper, GR designed the study, performed the research and wrote the paper.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table SI. Survival estimates for Ph+ALL and Ph−ALL diagnosed 2010–2014
Table SII. Survival estimates for Ph+ALL and Ph−ALL diagnosed 2010–2012 and 2013–2014
Table SIII. Survival estimates for Ph+ALL and Ph−ALL for age groups 18–39 and 40 years and above
References
- Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G, Kamps W, Pui CH & Masera G (2000) Outcome of treatment in children with philadelphia chromosome-positive acute lymphoblastic leukemia. The New England Journal of Medicine, 342, 998–1006. [DOI] [PubMed] [Google Scholar]
- Avivi I & Goldstone AH (2003) Bone marrow transplant in ph+ ALL patients. Bone Marrow Transplantation, 31, 623–632. [DOI] [PubMed] [Google Scholar]
- Bassan R & Hoelzer D (2011) Modern therapy of acute lymphoblastic leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29, 532–543. [DOI] [PubMed] [Google Scholar]
- Burmeister T, Schwartz S, Bartram CR, Gokbuget N, Hoelzer D, Thiel E; GMALL study group. (2008) Patients’ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood, 112, 918–919. [DOI] [PubMed] [Google Scholar]
- Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F, Raffoux E, Leguay T, Rousselot P, Lepretre S, Escoffre-Barbe M, Maury S, Berthon C, Tavernier E, Lambert JF, Lafage-Pochitaloff M, Lheritier V, Chevret S, Ifrah N & Dombret H; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). (2015) Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with ph-positive acute lymphoblastic leukemia. Blood, 125, 3711–3719. [DOI] [PubMed] [Google Scholar]
- Champagne MA, Capdeville R, Krailo M, Qu W, Peng B, Rosamilia M, Therrien M, Zoellner U, Blaney SM & Bernstein M; Children’s Oncology Group phase 1 study. (2004) Imatinib mesylate (STI571) for treatment of children with philadelphia chromosome-positive leukemia: results from a children’s oncology group phase 1 study. Blood, 104, 2655–2660. [DOI] [PubMed] [Google Scholar]
- Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B & Anasetti C (2001) Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood, 97, 1572–1577. [DOI] [PubMed] [Google Scholar]
- Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, Delannoy A, Buzyn A, Bilhou-Nabera C, Cayuela JM, Fenaux P, Bourhis JH, Fegueux N, Charrin C, Boucheix C, Lheritier V, Esperou H, MacIntyre E, Vernant JP & Fiere D; Groupe d’Etude et de Traitement de la Leucemie Aigue Lymphoblastique de l’Adulte (GET-LALA Group). (2002) Outcome of treatment in adults with philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood, 100, 2357–2366. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R & Talpaz M (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the philadelphia chromosome. The New England Journal of Medicine, 344, 1038–1042. [DOI] [PubMed] [Google Scholar]
- Esperou H, Boiron JM, Cayuela JM, Blanchet O, Kuentz M, Jouet JP, Milpied N, Cahn JY, Faucher C, Bourhis JH, Michallet M, Tanguy ML, Vernant JP, Gabert J, Bordigoni P, Ifrah N, Baruchel A, Dombret H; French Bone Marrow Transplantation. (2003) A potential graft-versus-leukemia effect after allogeneic hematopoietic stem cell transplantation for patients with philadelphia chromosome-positive acute lymphoblastic leukemia: results from the french bone marrow transplantation society. Bone Marrow Transplantation, 31, 909–918. [DOI] [PubMed] [Google Scholar]
- Fielding AK (2010) How I treat philadelphia chromosome-positive acute lymphoblastic leukemia. Blood, 116, 3409–3417. [DOI] [PubMed] [Google Scholar]
- Fielding AK, Rowe JM, Buck G, Foroni L, Gerrard G, Litzow MR, Lazarus H, Luger SM, Marks DI, McMillan AK, Moorman AV, Patel B, Paietta E, Tallman MS & Goldstone AH (2014) UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in philadelphia positive acute lymphoblastic leukemia. Blood, 123, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, Paoloni F, Fazi P, Cimino G, Nobile F, Ferrara F, Castagnola C, Sica S, Leoni P, Zuffa E, Fozza C, Luppi M, Candoni A, Iacobucci I, Soverini S, Mandelli F, Martinelli G, Baccarani M; GIMEMA Acute Leukemia Working Party. (2011) Dasatinib as first-line treatment for adult patients with philadelphia chromosome-positive acute lymphoblastic leukemia. Blood, 118, 6521–6528. [DOI] [PubMed] [Google Scholar]
- Gleissner B, Gokbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW, Fonatsch C, Heyll A, Voliotis D, Beck J, Lipp T, Munzert G, Maurer J, Hoelzer D & Thiel E; German Multicenter Trials of Adult Acute Lymphoblastic Leukemia Study Group. (2002) Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the german multicenter trial group and confirmed polymerase chain reaction analysis. Blood, 99, 1536–1543. [DOI] [PubMed] [Google Scholar]
- Gokbuget N & Hoelzer D (2006) Treatment of adult acute lymphoblastic leukemia. Hematology American Society of Hematology. Education Program, 2006, 133–141. [DOI] [PubMed] [Google Scholar]
- Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, Chopra R, Wiernik PH, Foroni L, Paietta E, Litzow MR, Marks DI, Durrant J, McMillan A, Franklin IM, Luger S, Ciobanu N & Rowe JM (2008) In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood, 111, 1827–1833. [DOI] [PubMed] [Google Scholar]
- Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, Kang H, Liu W, Dobbin KK, Smith MA, Carroll WL, Devidas M, Bowman WP, Camitta BM, Reaman GH, Hunger SP, Downing JR & Willman CL (2010) Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, hispanic/latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood, 115, 5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger SP & Mullighan CG (2015) Acute lymphoblastic leukemia in children. The New England Journal of Medicine, 373, 1541–1552. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian H, Ravandi F, Thomas D, Huang X, Faderl S, Pemmaraju N, Daver N, Garcia-Manero G, Sasaki K, Cortes J, Garris R, Yin CC, Khoury JD, Jorgensen J, Estrov Z, Bohannan Z, Konopleva M, Kadia T, Jain N, DiNardo C, Wierda W, Jeanis V & O’Brien S (2015) Combination of hyper-CVAD with ponatinib as first-line therapy for patients with philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. The Lancet. Oncology, 16, 1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, Zweidler-McKay P, Lu X, Fawcett G, Wang SA, Konoplev S, Harvey RC, Chen IM, Payne-Turner D, Valentine M, Thomas D, Garcia-Manero G, Ravandi F, Cortes J, Kornblau S, O’Brien S, Pierce S, Jorgensen J, Shaw KR, Willman CL, Mullighan CG, Kantarjian H & Konopleva M (2017) Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood, 129, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ & Weir HK (2017) Annual report to the nation on the status of cancer, 1975–2014, featuring survival. Journal of the National Cancer Institute, 109, 10.1093/jnci/djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JM, Keegan TH, Tao L, Abrahao R, Bleyer A & Viny AD (2016) Racial disparities in the survival of american children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and hodgkin lymphoma. Cancer, 122, 2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Joo YD, Lim SN, Kim SD, Lee JH, Lee JH, Kim DH, Kim K, Jung CW, Kim I, Yoon SS, Park S, Ahn JS, Yang DH, Lee JJ, Lee HS, Kim YS, Mun YC, Kim H, Park JH, Moon JH, Sohn SK, Lee SM, Lee WS, Kim KH, Won JH, Hyun MS, Park J, Lee JH, Shin HJ, Chung JS, Lee H, Eom HS, Lee GW, Cho YU, Jang S, Park CJ, Chi HS & Lee KH; Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology. (2015) Nilotinib combined with multiagent chemotherapy for newly diagnosed philadelphia-positive acute lymphoblastic leukemia. Blood, 126, 746–756. [DOI] [PubMed] [Google Scholar]
- de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, Rea D, Cayuela JM, Vekemans MC, Reman O, Buzyn A, Pigneux A, Escoffre M, Chalandon Y, MacIntyre E, Lheritier V, Vernant JP, Thomas X, Ifrah N & Dombret H; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). (2007) Imatinib combined with induction or consolidation chemotherapy in patients with de novo philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood, 109, 1408–1413. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Sen C, Koga K & Conroy SM (2011) Ethnic differences in breast cancer survival: status and determinants. Women’s Health (London, England), 7, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, Ueda Y, Kanamori H, Usui N, Akiyama H, Miyazaki Y, Ohtake S, Atsuta Y, Sakamaki H, Kawa K, Morishima Y, Ohnishi K, Naoe T & Ohno R (2011) Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia, 25, 41–47. [DOI] [PubMed] [Google Scholar]
- Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N & Proctor SJ (2010) A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood, 115, 206–214. [DOI] [PubMed] [Google Scholar]
- Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT, Tura S, Fischer T, Deininger MW, Schiffer CA, Baccarani M, Gratwohl A, Hochhaus A, Hoelzer D, Fernandes-Reese S, Gathmann I, Capdeville R & O’Brien SG (2002) A phase 2 study of imatinib in patients with relapsed or refractory philadelphia chromosome-positive acute lymphoid leukemias. Blood, 100, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Pui CH & Evans WE (2006) Treatment of acute lymphoblastic leukemia. The New England Journal of Medicine, 354, 166–178. [DOI] [PubMed] [Google Scholar]
- Pui CH, Relling MV & Downing JR (2004) Acute lymphoblastic leukemia. The New England Journal of Medicine, 350, 1535–1548. [DOI] [PubMed] [Google Scholar]
- Pulte D, Redaniel MT, Jansen L, Brenner H & Jeffreys M (2013) Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica, 98, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F & Kebriaei P (2009) Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology/Oncology Clinics of North America, 23, 1043–1063, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, Dara S, Jorgensen J, Kebriaei P, Champlin R, Borthakur G, Burger J, Ferrajoli A, Garcia-Manero G, Wierda W, Cortes J & Kantarjian H (2010) First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with philadelphia chromosome-positive (ph+) acute lymphoblastic leukemia. Blood, 116, 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F, O’Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S, Burger JA, Rytting ME, Ferrajoli A, Wierda WG, Verstovsek S, Champlin R, Kebriaei P, McCue DA, Huang X, Jabbour E, Garcia-Manero G, Estrov Z & Kantarjian HM (2015) Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer, 121, 4158–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselot P, Coude MM, Gokbuget N, Gambacorti Passerini C, Hayette S, Cayuela JM, Huguet F, Leguay T, Chevallier P, Salanoubat C, Bonmati C, Alexis M, Hunault M, Glaisner S, Agape P, Berthou C, Jourdan E, Fernandes J, Sutton L, Banos A, Reman O, Lioure B, Thomas X, Ifrah N, Lafage-Pochitaloff M, Bornand A, Morisset L, Robin V, Pfeifer H, Delannoy A, Ribera J, Bassan R, Delord M, Hoelzer D, Dombret H & Ottmann OG; European Working Group on Adult ALL (EWALL) group. (2016) Dasatinib and low-intensity chemotherapy in elderly patients with philadelphia chromosome-positive ALL. Blood, 128, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N, Prentice HG, Durrant J, Tallman MS, Goldstone AH; ECOG & MRC/NCRI Adult Leukemia Working Party. (2005) Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood, 106, 3760–3767. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, Daver NG, Kadia TM, Konopleva MY, Jain N, Issa GC, Jeanis V, Moore HG, Garris RS, Pemmaraju N, Cortes JE, O’Brien SM & Kantarjian HM (2016) Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer, 122, 3650–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEER. (2017) SEER Research Data 1973–2014 – ASCII Text Data: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2014), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. Available at: https://seer.cancer.gov/data/options.html
- Short NJ, Jabbour E, Sasaki K, Patel K, O’Brien SM, Cortes JE, Garris R, Issa GC, Garcia-Manero G, Luthra R, Thomas D, Kantarjian H & Ravandi F (2016) Impact of complete molecular response on survival in patients with philadelphia chromosome-positive acute lymphoblastic leukemia. Blood, 128, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Faderl S, Cortes J, O’Brien S, Giles FJ, Kornblau SM, Garcia-Manero G, Keating MJ, Andreeff M, Jeha S, Beran M, Verstovsek S, Pierce S, Letvak L, Salvado A, Champlin R, Talpaz M & Kantarjian H (2004) Treatment of philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood, 103, 4396–4407. [DOI] [PubMed] [Google Scholar]
- Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, Meloni G, Ambrosetti A, Quarta G, Pagano L, Rege-Cambrin G, Elia L, Bertieri R, Annino L, Foa R, Baccarani M & Mandelli F (2007) Imatinib plus steroids induces complete remissions and prolonged survival in elderly philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the gruppo italiano malattie ematologiche dell’adulto (GIMEMA) LAL0201-B protocol. Blood, 109, 3676–3678. [DOI] [PubMed] [Google Scholar]
- Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J, Zhu W, Buonamici S, Hassan AQ, Lombardo F, Iyer V, Palmer M, Berellini G, Dodd S, Thohan S, Bitter H, Branford S, Ross DM, Hughes TP, Petruzzelli L, Vanasse KG, Warmuth M, Hofmann F, Keen NJ & Sellers WR (2017) The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature, 543, 733–737. [DOI] [PubMed] [Google Scholar]
- Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, Kobayashi T, Ueda Y, Takeuchi M, Miyawaki S, Maruta A, Emi N, Miyazaki Y, Ohtake S, Jinnai I, Matsuo K, Naoe T & Ohno R; Japan Adult Leukemia Study Group. (2006) High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the japan adult leukemia study group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 24, 460–466. [DOI] [PubMed] [Google Scholar]
- Zhang X, Loberiza FR, Klein JP & Zhang MJ (2007) A SAS macro for estimation of direct adjusted survival curves based on a stratified cox regression model. Computer Methods and Programs in Biomedicine, 88, 95–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


