Abstract
Introduction
In light of the current COVID-19 pandemic, during which the world is confronted with a new, highly contagious virus that suppresses innate immunity as one of its initial virulence mechanisms, thus escaping from first-line human defense mechanisms, enhancing innate immunity seems a good preventive strategy.
Methods
Without the intention to write an official systematic review, but more to give an overview of possible strategies, in this review article we discuss several interventions that might stimulate innate immunity and thus our defense against (viral) respiratory tract infections. Some of these interventions can also stimulate the adaptive T- and B-cell responses, but our main focus is on the innate part of immunity. We divide the reviewed interventions into: 1) lifestyle related (exercise, >7 h sleep, forest walking, meditation/mindfulness, vitamin supplementation); 2) Non-specific immune stimulants (letting fever advance, bacterial vaccines, probiotics, dialyzable leukocyte extract, pidotimod), and 3) specific vaccines with heterologous effect (BCG vaccine, mumps-measles-rubeola vaccine, etc).
Results
For each of these interventions we briefly comment on their definition, possible mechanisms and evidence of clinical efficacy or lack of it, especially focusing on respiratory tract infections, viral infections, and eventually a reduced mortality in severe respiratory infections in the intensive care unit. At the end, a summary table demonstrates the best trials supporting (or not) clinical evidence.
Conclusion
Several interventions have some degree of evidence for enhancing the innate immune response and thus conveying possible benefit, but specific trials in COVID-19 should be conducted to support solid recommendations.
Keywords: COVID-19, Immune response, Innate, Exercise, Mindfulness, Sleep, Bacterial vaccine, Trained immunity, Bacillus calmette-guérin, MMR, NK-Cell
Abbreviations: ACE2, Angiotensin converting enzime-2; APC, Antigen-presenting cell; BCG, Bacillus Calmette-Guérin; BV, Bacterial vaccine; CCL-5, Chemokine (C–C motif) ligand 5; CI, Confidence interval; CNS, Central nervous system; COVID-19, Coronavirus disease-2019; CXCR3A, CXC chemokine receptor 3A; DAMPs, Damage-associated molecular patterns; DC, Dendritic cell; DLE, Dialyzable leukocyte extract; Gαs: G protein coupled receptor alfa-subunits, HSP; Heat shock proteins, HLA-DR; Major histocompatibility complex class II cell surface receptor, ICAM-1; Intercellular adhesion molecule type 1, IFN; Interferon, IG; Immunoglobulin, IGFBP6; Insulin-like growth-factor-binding-protein 6, IL; Interleukin, MBSR; Mindfulness-based stress reduction, mCa++: Intramitochondrial calcium; MCP-1, Monocyte chemoattractant protein-1; MODS, Multi-organ dysfunction syndrome; MyD88, Myeloid differentiation primary response 88; NF-κB, Nuclear factor kappaB; NK, Natural killer; NOD2, Nucleotide-binding oligomerization domain-containing protein 2; OR, Odds ratio; OxPhos: Oxidative phosphorylation, PAMPs; Pathogen-associated molecular patterns, PBMC; Peripheral blood mononuclear cell, PI3K/Akt: Phosphatidylinositol 3-kinase pathway; PKC, Protein kinase C; PPD, Purified protein derivative (tuberculin); PUFA, Polyunsaturated fatty acid; R0: Basic reproduction number, REM; Rapid eye movement, RIPK2; Receptor iteracting serine/threonine kinase 2, RNA; Ribonucleic acid, RNS; Reactive nitrogen species, ROS; Reactive oxygen species, SARS-CoV-2; Severe acute respiratory syndrome coronavirus 2, SIRS; Systemic inflammatory response syndrome, TCR:T-cell receptor; Th, T helper-cell; TLR, Toll-like receptor; TNF-α, Tumor necrosis factor alpha; TRPV, Thermolabile calcium channels; URTI, Upper-respiratory tract infection
Introduction
Taking a closer look at the evolution of the coronavirus disease 2019 (COVID-19) pandemic and learning from previous historical events with similar conditions, such as the Spanish flu (an H1N1 influenza virus) pandemic in 1918–1919, it is very probable that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shall still be around for a while.1 Due to its high basic reproduction number (R0), close to 3.5 under natural conditions without social distancing,2,3 the virus shall probably keep on infecting human beings until an effective vaccine has been developed, herd immunity (possibly both, cellular and humoral) has been built, or the virus has undergone loss-of-potency mutations.
At first contact with the respiratory mucosa, coronaviruses including SARS-Cov-2 enter the cells via the angiotensin converting enzime-2 (ACE2) receptor and, after transcription of its ribonucleic acid (RNA), produces structural and non-structural proteins. Among those proteins, the N-protein is capable of blocking the interferon (IFN) production and its effects on adjacent cells, see Fig. 1.4 Thus, the first days after infection the virus subverts the host's initial innate immune response; this enables it to replicate without being noticed by the body. Once symptoms start, they are often aggressive with high fever and generalized malaise, as already many cells have been infected. Approximately 7 days after the onset of the initial symptoms is the decisive moment: the immune system eliminates the virus, giving rise to the patient's recovery, or the virus advances into the lungs, attacking specifically type 2 pneumocytes, and into the systemic circulation, causing destruction of the endothelium and oxidative stress. Further into the second week, in about 3-5% of all patients, this leads to an overactivation of the inflammatory response, specifically caused by interleukin (IL)-6 that activates macrophages, monocytes, and T-cells, causing what some investigators describe as a “cytokine storm”.5 In this context it should be borne in mind that the cytokine levels in COVID-19 are 100 to 1000-fold lower than in the true cytokine storm syndrome described years back; and at this point it is not fully understood at what level there is an actual immune overactivation in COVID-19 and if so, what the prime site is.6
Fig. 1.
SARS-CoV-2 infects type 2 pneumocytes by entering the cells via the ACE2 receptor on their surfaces. Unlike the normal anti-viral response with increased IFN type I and III and with it the activation of genes stimulated by IFN in adjacent cells and thereby increasing its anti-viral defense, the coronavirus has mechanisms that lower this anti-viral innate defense mechanism by interfering with IFN production and its effects. In addition, chemotactic molecules are released in a viral infection that attract macrophages (M∅), natural killer (NK) cells, and neutrophils. This reaction is not fully achieved during early infection with coronavirus, so the initial innate immune response appears incomplete and slow. After this first innate response, adaptive immunity is triggered via activation of dendritic cells (DC) that stimulate specific Th1 lymphocytes, which in turn activate cytotoxic T cells (Tccell) to eliminate infected cells, in the more advanced stages of the infection, along with plasma cell development and the production of antiviral IgM and IgG antibodies (not shown). However, in some patients with COVID-19 at this stage a dysregulated activation of macrophages (ie, by IL6) is seen, causing the feared cytokine storm.
Summarizing, together with the total viral load and the adaptive immune response the role of the innate immune system in the outcome of COVID-19 seems of the utmost importance. When innate immune cells, natural killer (NK)-cells, macrophages, and neutrophils, would recognize virus infected mucosal cells right from the start, they could reduce viral replication and eliminate virus infected cells and SARS-Cov-2 would not be able to advance in the body — even less so, if the adaptive immune response would develop satisfactorily and take care of the remaining virus. And, at a later phase, when immune regulation would be able to balance immune activation better the immune over-activation could be avoided. Finally, a higher level of antioxidants in the patient's body could counterbalance the mechanisms by which the coronavirus causes endothelial damage. In each of these steps there might be an opportunity for intervention.
With this in mind, the question arises as to how the innate immune system could be reinforced, to enhance its preparedness to fight SARS-CoV-2 from the start and as a consequence to stimulate the cellular adaptive immune response further downstream. Although it has become clear that T-cells are key-players in the adaptive stage, with epitope pools of SARS-CoV-2 detecting CD4+ and CD8+ T cells in 100% and 70% of convalescent COVID patients7 and also a special subpopulation of B lymphocytes, the B-1a cells that produce natural IgM in an autocrine manner, might have a special therapeutic potential,8 we would like to focus our review on interventions that primarily stimulate and regulate the innate immune system.
Alongside, one could wonder if there are ways to enhance the redox balance and reduce oxidative stress. We present in several short chapters, see Box 1, different interventions that might help in these directions, discuss the evidence, and conclude on their practical usefulness.
Box 1. Review content: Interventions that could enhance the innate immune response or improve the redox balance.
-
1.Life style factors
-
a.Exercise
-
b.Forest bathing
-
c.Sleep
-
d.Meditation/mindfulness
-
e.Supplementation
-
i.Vitamin D
-
ii.Vitamin C
-
iii.Zinc
-
iv.Others
-
i.
-
a.
-
2.Non-specific immune stimulants
-
a.Fever and hyperthermia
-
b.Bacterial vaccines
-
c.Probiotics
-
d.Dialyzable leukocyte extract (DLE)
-
e.Synthetic molecules (Pidotimod)
-
a.
-
3.Specific vaccines with heterologous effect
-
a.Bacillus Calmette-Guérin (BCG) vaccine
-
b.Other heterologous vaccines
-
a.
Alt-text: Box 1
Lifestyle factors
First, we review some of the life style factors that might improve the innate immunity or the redox balance.
Exercise
Even though there are still no published clinical or experimental data about the role of exercise in the context of the COVID-19 pandemic, extrapolated evidence from previous studies shows that regular, moderate-to-vigorous exercise improves immune competency across the lifespan and may help to reduce the frequency of upper-respiratory tract infections (URTIs).9,10 Importantly, this has been observed also in older individuals (>65 year-olds) who regularly exercise, whose URTIs incidence per year has been inversely associated with the energy expenditure utilized during physical activity.11,12
There is growing scientific evidence showing that regular exercise may induce modifications of cell populations with antiviral properties in the bloodstream. Zamani et al observed that moderate exercise had an effect on cytokine responses: post-exercise peripheral blood mononuclear cell (PBMC) cultures showing a significant increase of IFN-γ and IL-12 compared to their paired pre-exercise samples, with a significant reduction of the level of IFN-γ after 2 months of post-exercise inactivity. Interestingly, IL-6 levels were not significantly affected by exercise.13
During a 40–60 min period of intense exercise, transient lymphocytosis occurs, typified by a sudden influx of both NK and CD8+ T-cells (rising up to 10-fold and 2.5-fold, respectively), which favors an effector-memory immune phenotype.14 This effect is governed by stimulation of beta-2-adrenergic receptors on the surface of lymphocytes (arising from adrenaline released during exercise), causing lymphocyte endothelial detachment and recirculation,15 which also induces the expression of CD4+ B-cells and regulatory T-cells.16 Also, exercise helps to maintain immune homeostasis through bone marrow homing and increasing apoptosis of exhausted/senescent T-cells, thus stimulating the production and release of new progenitor cells (ie, IFN-producing CD8+ T-cells).14 Lastly, exercise has been associated with enhanced vaccination responses.17
Another relevant anti-inflammatory effect of constant exercise is the potential capacity to modulate the activation of TLRs signaling, as TLRs have been shown to be important in the innate immune response during a SARS-CoV infection.18 It has been demonstrated that physical inactivity correlates with augmented TLRs activation and, conversely, that chronic exercise has an effect of decreasing cell-surface expression of TLRs on immune cells.19 In addition, there is evidence showing that moderate, enduring exercise has the potential to reduce the activation of the NLRP3 inflammasome as well as IL-1β, a cytokine processed by the inflammasome.20
Conversely, some studies report that during prolonged periods of strenuous exercise (such as marathons) and after approximately 2 weeks following intense competitions, there is an immunodepression span (the “open window” period), related to a higher rate of URTI-like symptoms (mainly self-reported) vs those who undergo lower physical activity.21,22 This theory, attributed to the consequences of psychological stress and excessive training,23,24 is however highly debated today in light of a better understanding of the variability in the intensity-related response to physical activity, which depends largely on the functional capacity of the immune system. As a result, up-to-date perceptions on this topic tend to discard the concept of exercise-induced immunosuppression. In fact, it is widely accepted that during and after exercise, when performed regularly, the immune system is in a heightened state of immune surveillance and regulation.25
During the COVID-19 quarantine it is important to stay active and to exercise regularly (gradually increasing intensity), not only as a way to keep a healthy lifestyle, but also as an immunoprotective and immunoregulatory activity. It is possible that older adults (who are at higher risk of a severe SARS-CoV-2 infection) may obtain the greatest exercise-induced benefits to their immunological health.
Forest bathing (Shinrin Yoku)
In a series of several, subsequent small studies, Japanese investigators in cooperation with experts from the Stanford University have shown that walking in a forest seems to enhance NK cell activity, partly stimulated by volatile substances produced by trees (particularly phytoncides: antimicrobial essential oils from trees). The first experiment showed in vitro that phytoncides activate NK cell activity and augment NK cell granule content.26 Then they confirmed this in peripheral blood lymphocytes of subjects submitted to a prospective trial of a 3-day, 2-night trip to the forest including three 2-h walks.27 In a third case-control trial they demonstrated that the effect was not related to the walking tour, as they compared the rise in NK-cell activity after a 3-day tour to a small city with that after a 3-day tour to the forest. Both tours included three 2-h walks, but NK-cell activity was only enhanced after the forest trip. Moreover, NK-cell activity stayed elevated one week and still somewhat 30 days after the tour.28 In the last experiment investigators sought to confirm it is the inhalation of phytoncides, that cause the NK cell activation, by having study-subjects sleep in rooms where phytoncide was sprinkled in the air.29 Even though all studies are small, confounders have been neatly corrected for.
Sleep
To date, no studies have been published that demonstrate a preventive effect of sleep against COVID-19. However, it has been proposed that a healthy lifestyle, with good sleep quality, could increase and modulate the functions of the immune system.30
A first mechanism is the optimization of the cytotoxic T cell response by functional downregulation of the G protein coupled receptor alfa-subunits (Gαs) receptor during sleep. This receptor has an immunosuppressive effect by inhibiting intercellular adhesion molecule type 1 (ICAM-1) and with it the function of the β2-integrins, essential for the ability of T-cells to adhere to their target cells (including those infected by virus) through the T-cell receptor (TCR). A paired in vitro study with cell cultures confirmed that, compared to wakefulness, sleep (8 h) significantly increased fluorescence uptake of ICAM-1 multimers in T cells, infected with cytomegalovirus or Epstein-Barr virus [F (1, 9) = 4.1; P = 0.05 and F (1.8) = 6.0; P = 0.05, respectively]. Interestingly, a 2-h sleep deprivation appeared to be sufficient to affect the adhesion capacity of T cells.31
Other studies have shown that sleep deprivation, both complete or only selective to the rapid eye movement (REM) stage, modifies various components of the innate immune system, such as dendritic cells (DCs) and the percentage of some subpopulations of T cells (CD4+, CD8+ and NK) and cytokine levels (IFN-γ, TNF-α and IL-1).32, 33, 34 Specifically, in a clinical study in women 77 h of sleep deprivation (total and REM phase) resulted in a proinflammatory state and affected the cellular immune response, presenting changes in the production of IFNs and in the phagocytic activity. Sleep deprivation also decreased lymphocytic blastogenesis, NK cell activity, and regulation of IL-1 and IL-2.35
Moreover, in the elderly, a sleep pattern with poor quality (self-reported) was related to increased levels of IL-6 and this association was not explained by other factors, such as depressive symptoms, stress or loneliness, but exclusively by sleep disturbance as an independent factor.36
As for the clinical evidence, an adequate sleep pattern also appears to be beneficial in modulating the adaptive immune response. Spiegel et al evaluated the effect of sleep deprivation on the antibody response to influenza vaccination on 25 volunteers. The control group (who slept 8 h per night) developed a rise in IgG antibody titer against influenza virus 10 days after vaccination twice as high compared to the sleep deprived group (who slept 4 h for 6 nights, but then were allowed to recover with several days of 12-h catch-up sleep). Antibody levels were determined once again after 21–30 days, this time showing no difference in antibody levels between the groups. This might be explained by the effect of normalization of the sleep pattern in the group initially subjected to wakefulness.37
Table 1 presents 2 studies evaluating the effect of sleep in volunteers with an experimentally induced rhinovirus common-cold.38,39 Shorter sleep duration was associated with an increased risk of developing a common cold in a dose-dependent manner. These results support the concept that people with a normal sleep pattern (7–9 h a night in adults) could increase the effectiveness of the immune system in the context of responses to viral URTIs. Up to now there are no specific studies on the effect of sleep and the immune response to SARS-COV-2.
Table 1.
Effect of sleep duration and common cold symptoms after experimental inoculation with rhinovirus.
| Cohen et al. | Prather et al. | |
|---|---|---|
| Design | Prospective | Prospective |
| Number of volunteers | 153 | 164 |
| Age | 21–55 years old | 18–55 years old |
| Duration of the study | 5 days | 7 days |
| Monitoring | Duration and effectiveness of sleep | Duration and continuity of sleep |
| Objective sleep measurement | Actiographya | Actiographya |
| Results | OR = 2.94 (95% CI 1.18–7.30) of having flu symptoms in those who had <7 h of sleep | OR = 4.50 (95% CI 1.08–18.69) of having flu symptoms in those who slept < 5 hOR = 4.24 (95% CI, 1.08–16.71) those who slept 5–6 h vs. >7 h of sleep per night |
| Virus | Rhinovirus | Rhinovirus |
| Covariate Adjustment Resultsb | No effect | No effect |
OR: Odds ratio.
Actigraphy: validated method for measuring objective l sleep parameters.
Covariates: age, antibody levels before surgery, sex, body mass index, race, season of the year during the study, socio-economic level, educational level, income, smoking, physical activity, alcohol consumption, stress, kindness and lifestyle
Mindfulness
Albeit limited, there is some evidence suggesting that enhancing general physical and mental health may reduce URTI prevalence and severity.40 Specifically, mindfulness meditation has been proposed as a practice progressively capable to reduce experienced stress and negative emotion through increased awareness of physical, emotional, and cognitive aspects and heightened sensitivity to bodily sensation, which may lead to a healthier mind-body response to stress, albeit with little quantitative expression.41
One interesting clinical randomized trial on 94 adults aged 50 years or older who underwent either a professionally guided standardized 8-week meditation-based program of mindfulness-based stress reduction (MBSR) with weekly 2-h group sessions and 45 min of daily at-home practice (N = 51) or placebo (n = 51), showed that incidence, duration, and global severity of URTIs were 33%, 43%, and 60% lower in the meditation group compared with controls (whereas not all comparisons reached statistical significance). Also, URTI-related work absenteeism was significantly lower for the meditation group (P < 0.001).42
Based on the above, a constant program of professionally guided meditation strategy could be beneficial to achieve an effective stress reduction and probably to reduce the burden of URTIs, mainly in older adults.
Supplementation
-
i.
Vitamin D
The hypothesis that vitamin D use could reduce the risk of respiratory infections began from observational studies showing that the most vulnerable populations (older adults, individuals with comorbidities, malnutrition, etc), shared an increased risk of vitamin D deficiency and that in addition, the incidence of respiratory infections increased in winter season where vitamin D levels drop, secondary to a shorter time of sun exposure.43
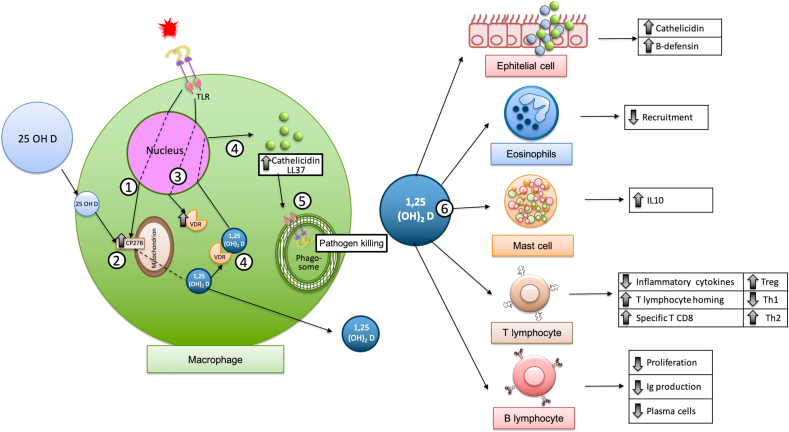
The effects of vitamin D on the immune system have been well studied, including the induction of catelicidine and β-defensins, modulation of immune cells migration to the site of infection, direct antiviral effects such as disruption of the viral membrane interfering with glycoproteins necessary for viral replication downregulating viral receptors, modulation of TLRs, clearance of respiratory pathogens by inducing infected epithelial cell apoptosis, and selective suppression of pro-inflammatory cytokine production (TNF-α, IFN-β, IL-8, IL-6, chemokine (C–C motif) ligand 5 [CCL-5]), among many others.44,45 See Fig. 2.45, 46, 47
Fig. 2.
The conversion of 25 OHD2 to 1,25-(OH)2 D3 can be done directly in some cells of the immune system such as macrophages. The macrophage, like kidney cells, contains the enzyme 1-alpha hydroxylase that is capable of transforming Vitamin D into its active form, calcitriol. Exposure of the macrophage to some pathogens induces the production of CP27B (step 1) (which allows 25 OHD to enter the mitochondria for transformation into its active form 1,25-(OH)2 D3), (step 2) as well as vitamin D receptor (VDR) (step 3), which by binding to 1,25-(OH)2 D3 increases the production of cathelicidin. (step 4). The antimicrobial activity of vitamin D appears to be primarily dependent on the induction of cathelicidins, which perform numerous functions that enhance both innate and adaptive immunity; help improve the digestion process within the phagolysosome through a non-oxygen dependent mechanism (step 5),46 that can promote pathogen clearance by inducing apoptosis of infected epithelial cells, and induce paracrine responses in monocytes and T and B lymphocytes, among others.45 Vitamin D also has direct effects on other cells, for example, epithelial cells, eosinophils, mast cells as well as T and B lymphocytes. (step 6)47
Although the biological basis of vitamin D intervention is clear, and multiple studies have described the benefits of vitamin D supplementation to reduce the risk of respiratory infections48 and even to reduce intrahospital stays in mechanically ventilated patients,49 the published study results so far have not been conclusive.
Of the 6 meta-analysis reviewed, only 2 agree on the benefit of vitamin D supplementation for decreased respiratory infections.50,51 Subgroup analysis shows that the most consistent benefits are with daily or weekly supplementation50, 51, 52 vs monthly supplementation, in patients with basal serum vitamin D < 25 nmol/L51 and in asthma patients to reduce the risk of URTI-induced asthma exacerbations;53 however, the high heterogeneity of the studies, the bias of having not measured the basal serum levels in most studies and the difficulty of establishing the diagnosis of outcome in a reliable way makes it difficult the attribution of real benefits and therefore the general application of their results.
An important point to be highlighted is the low level of adverse effects reported despite the administration of high doses of vitamin D, so the intervention could be considered relatively safe.54
Finally, the most important fact in considering supplementation in the context of COVID-19 would be to identify subjects with vitamin deficiency who would likely benefit most from supplementation.55,56 At the moment, there is no universal consensus on ideal vitamin D levels,57 and even less, the values that will permit to achieve a benefit on viral infections. Nonetheless, 40–50 ng/mL could be considered an appropriate value in most scenarios.48
-
ii.
Vitamin C
The antioxidant properties of Vitamin C might influence the regulatory function of the immune system.58 These effects have been observed in in vivo murine models, showing an increase in the production of IFN-α and -β that up-regulate immune regulatory responses.55,59 Also, an in vitro study provided information that the addition of vitamin C to iron and/or cupper was related to an increase in the production of hydrogen peroxide and other free radicals, thus conferring antiviral properties to vitamin C.58 Because of the previously mentioned effects, it has been proposed that vitamin C could have a protective effect against viruses in humans. A systematic review with Cochrane collaboration failed to demonstrate that vitamin C supplementation with doses over 200 mg reduced the incidence of colds in the general population, but the disease duration was reduced by 8% in adults and 14% in children.60 Another study did not find a conclusive effect on prevention of COVID-19 with high doses of vitamin C.55 Despite the inconclusive results with vitamin C prophylaxis for COVID-19, some authors recommend its use with the premise of the low risk of given intravenous vitamin C or through the oral route, added to the theoretical benefit in case of a serious infectious disease.58,61, 62, 63, 64
-
ii.
Omega 3 and adiponectin
Animal studies have shown that polyunsaturated fatty acids (PUFAs) with omega-3 could improve adiponectin plasma levels,64 increasing the possibility of a regulatory immunologic response improvement, through blockage of pro-inflammatory cytokines like TNF-α, IL-2 and IL-6. This might be of particular interest in COVID-19 as one of the pathophysiologic mechanisms clearly demonstrated is inflammation of the pulmonary vascular endothelium, accompanied by increased angiogenesis.65 An appropriate ingestion of PUFAs and omega-3 at doses of 250–500 mg/day could have a positive effect on the response to viral infections through messenger RNA export blockage of intracellular pathogens.64,66 To date, there is no evidence of PUFA supplementation in prophylaxis of URTIs.
-
iv.
Flavonoids
Among polyphenols epigallo-catechin-3 gallate is a flavonoid present in green tea, that blocked TNF-β translocation, in mice models of COVID-19 attenuating lung injury.64,67 Another option is the use of traditional Chinese herbs, many of which contain cinanserin, quercetin, and herbacetin, among other substances. These have been shown to inhibit the coronavirus main proteinase (3CLpro) and thus inhibit viral replication in SARS-CoV.68, 69, 70 Glycyrrhizin acid derived from licorice root with a binding capability to ACE2 in combination with vitamin C has been used for SARS-CoV-2 pneumonia.61,71
The previously mentioned interventions are potential options that could improve the immune system against SARS- CoV-2, but more evidence is required.70 See Table 2.
-
v.
Zinc
Table 2.
Possibly potentiating defense against COVID-19 of some vitamins and micronutrients and their main natural sources.
| Dietary Supplementation | Potential Effects in COVID-19 infection | Main Natural Sources |
|---|---|---|
| Vitamin C |
|
Oranges, lemons, mangoes. |
| Flavonoids |
|
Red wine, oranges, red fruits and vegetables. |
| Omega 3 and Fatty acids |
|
Seafood (salmon, tuna), Nuts and seeds, plant oils. |
| Polyphenol |
|
Green tea, broccoli, apples. |
Modified from: Husson MO et al. Modulation of host defense against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect 2016; 73; 523–35.66
Zinc is an essential trace element for the proper functioning of multiple biological processes within the human body, and its contribution to immunity is not an exception.72
The complete mechanism by which zinc could decrease the number or severity of viral infectious processes in general and of COVID-19 in particular is not exactly understood yet; however, effects have been observed on the binding of the viral agent to the mucosa and on its replication, as well as on the regulation of the inflammatory process;73 enhanced benefits have been hypothesized when co-administered with other medications such as (hydroxy)chloroquine that could function as an ionophore, facilitating the entrance of zinc into the cells.74 The human body's ability to store zinc is known to be low; its deficiency compromises the immune system, as has been evidenced occasionally by thymic atrophy, lymphopenia, and altered lymphocyte responses.75 Its supplementation may reduce the risk of infectious processes, especially URTI, pneumonia, and diarrhea in both children and older adults.76,77
In relation to the clinical evidence, all of the 4 reviewed meta-analyses reported beneficial effects of zinc supplementation in children for the prevention of pneumonia76,78, 79, 80 (especially for objectively diagnosed disease),79 continuous administration vs short courses,78 and with doses above 75 mg/day.80
Adverse effects are considered mild and include nausea and headache. However, it is important to avoid over-administration due to the risk of copper deficiency.80
Non-specific immune stimulants
Fever and hyperthermia
Fever is a complex autonomic response of vertebrates generated in response to endogenous damage secondary to an infection, which has evolved over 600 million years and has been associated with increased survival and resolution of an infection.81,82 It is a defensive response capable of improving innate and probably also the adaptive immune response, due to its effect on the DCs, whose functioning depends on body temperature, Fig. 3.83,84
Fig. 3.
In DCs, derived from human monocytes, subjected to temperatures >38 °C, a decrease in mitochondrial metabolism has been seen inducing a decrease in oxidative phosphorylation (OxPhos) and an increase in reactive oxygen and nitrogen species (ROS/RNS), intramitochondrial calcium and glycolysis. All this produces the release of proinflammatory cytokines that activate T lymphocytes. Fever favors the production of heat shock proteins (HSP) by all eukaryotic cells. DCs release insulin-growth-factor-binding-protein (IGFBP6) that has stimulating, but also self-regulating effects
Temperature rise from 39 to 40° Celsius (°C) increases the production of IL-6, IL-12, TNFα, IL-1β, IFNs, and prostaglandins (endogenous pyrogens) by various cells, while macrophage production of IL-10 and TNFα is reduced, driving the T helper (Th)-cell response towards Th1. Temperature restitution does not completely reverse these effects.85 Endogenous pyrogens act on the thermoregulatory center in the hypothalamus, resulting in physiological responses that increase temperature.83 They stimulate the expression of L-selectin, which favors leukocyte migration.83 However, autonomic central nervous system (CNS)-derived norepinephrine has an opposite effect.86 On the other hand, unbeneficial heat stress can cause the muscle to produce IL-6 and other myokines.87
Volunteers have been subjected to 39.5 °C baths, to experimentally resemble fever, while monocyte activation molecules were measured. CD14, CD11b (complement receptor) and of CD62L are upregulated, the latter enhancing leukocyte adherence to the vascular endothelium. If exposed to endotoxin, they release a large amount of TNF-α.82,88 Feverish states also activate T lymphocytes with an increase in protein kinase C (PKC) and opening of thermolabile calcium channels (TRPV), increasing their cytotoxic activity.89
Thermal stress favors the release of acute phase reactants (C reactive protein, serum amyloid A, procalcitonin, complement C3, haptoglobin) and components of the coagulation cascade (fibrinogen and fibronectin A). These endogenous factors are related to the interaction with toll-like receptors (TLRs).90
Some investigators are evaluating whether the controlled induction of hyperthermia could have any therapeutic use in the control of infectious processes.82 In the 5th century B.C., Hippocrates described the calming effect of malaria fever in epileptics. In the 19th century heat-therapy was used in the treatment of psychiatric disorders, and an Austrian psychiatrist received the 1927 Nobel Prize for his work on fever therapy in the treatment of neurosyphilis.91
However, hyperthermia also has anti-inflammatory properties. Brunt et al showed that serum, collected from volunteers submitted to 8-week heat-therapy (4–5 times/week sufficient to maintain rectal body temperature 38.5 °C for 60 min) upregulates HSP and anti-inflammatory and anti-oxidative proteins, thus augmenting the resistance of endothelial cells against inflammatory and oxidative stress. This suggests heat therapy might be a promising intervention to reduce cellular damage following ischemic events.92
The temperature rise during an infection improves the conditions for the systemic inflammatory response syndrome (SIRS) to develop more effectively,88 Fig. 4. However, temperatures above 42 °C for more than an hour are no longer beneficial, as they produce a decreased recruitment and activity of NK cells, generate CNS damage and protein denaturation.84
Fig. 4.
The response to early damage has been clinically defined as systemic inflammatory response syndrome (SIRS) in which innate immune system cells and proinflammatory cytokines are involved; secondarily, the adaptive immune response also gets activated, allowing the host to heal, as long as a negative feedback is produced by regulatory cytokines to counteract the inflammation and limit the immune damage, generating a “temporary immunosuppression”. However, if at this moment an opportunistic infection occurs or the inflammatory stimulus persists, a second SIRS can be generated that favors the massive release of inflammatory cytokines (cytokine storm) that can cause death due to the development of the multi-organ dysfunction syndrome (MODS)
As for clinical data, in the elderly with community-acquired pneumonia, mortality was significantly lower in patients who developed a febrile response (4 versus 29%).93 Survival of infected patients admitted to the ICU was enhanced in those presenting 37.5–40 °C.94 Also, pathogens replicate better at 37 °C than at higher temperatures.95 Finally, during the 1918 Flu pandemic hydrotherapy with hot wrappings on chest and abdomen, soaked in boiling water thrice daily with the patient wrapped in a blanket resulted in 300% increased survival rate.96
Even so, the discussion whether fever is beneficial is still ongoing, as in most clinical trials withholding antipyretics increased survival, but not in all.97,98 Definitely temperatures above 40 °C are no longer favorable. At population level, hypothetic models of seasonal influenza also showed diminishing fever with antipyretics seemed to increased mortality by 5%.99 Surely enough, milder infections can have an afebrile course and even so a good outcome. Metanalyses have been inconclusive so far.97,98
Bacterial vaccines
Trained immunity refers to immunological memory of the innate immune system, thus being non-specific by definition. It enables a stronger immune response to a second stimulus.100 A bacterial vaccine (BV) contains bacterial lysates or whole, live attenuated or dead cells of one or several pathogens. BV can be administered sublingually, orally or subcutaneously. In order to induce trained immunity the following characteristics are needed:
-
I.
Contain trained immunity inducers, like pathogen and damage-associated molecular patterns (PAMPs and DAMPs) in addition to pathogen specific antigens.
-
II.
Demonstrate effectiveness against heterologous pathogens, apart from the specific pathogens, targeted by the BV
Interestingly, by activating the innate cells to release IL-1β, IL-12 and TNFα BV also simulates the adaptive immune response.
The best-known BVs are Bacillus Calmette-Guérin (BCG), Candida and mixes of respiratory bacteria. Anecdotal evidence exists from the 19th century onward, but since the past century its scientific bases were settled. BVs have been used as adjuvants not only for reducing RTI, but also in allergy, asthma, and cancer treatment. The observation that monocytes from patients who were vaccinated with BCG responded vigorously to stimulation with unrelated germs (Candida albicans, Schistosoma mansoni, or influenza virus)101 and epidemiologic reports that showed nonspecific protection in infants who were previously vaccinated with BCG, gave rise to the creation of the concept of “trained immunity”,102,103 (see section on BCG).
Nowadays, investigators have shown that trained immunity is based on epigenetic reprogramming of myeloid cells (dendritic cells, macrophages, NK-cells) that result in changes in their phenotypic and metabolic behavior. Consequently, they produce a stronger response to subsequent stimuli, since deoxyribonucleic acid sequences coding for proinflammatory cytokines and chemokines are partly kept open by histone methylation,100 (see Fig. 5). Signaling is dependent on dectin-1, TLRs, and nucleotide-binding oligomerization domain-containing protein 2 (NOD2), depending on the trained immunity inducer being BCG, bacteria, or candida, respectively. In murine and ex vivo human experiments monocyte-derived DCs, upon stimulation with MV130 (a sublingual poly-bacterial vaccine), produced IL-12-p70 and TNF-α enhancing Th1 proliferation, and IL-6, IL-1β, IL-8 stimulating the development of Th17 cells through the receptor interacting serine/threonine kinase 2 (RIPK2) and myeloid differentiation primary response 88 (MyD88) coding gene pathways; conversely, a rise in IL-10 simultaneously balanced the immune-regulation.104,105 Long-lasting effects, up to 12 months, have been documented as trained immunity also influences myeloid progenitor cells in the bone marrow.106
Fig. 5.
In murine and ex vivo human experiments monocyte-derived DCs, upon stimulation with MV130 (Bactek), produced IL-12p70 and TNF-a enhancing Th1 proliferation, and IL-6, IL-1β, IL-8 ie, stimulating the development of Th17 cells. MV130 effects were shown to be generated after TLR activation and the serine/threonine-protein kinase-2 receptor interaction (RIPK2) and myeloid-88 (MyD88) pathways under control of IL-10.104 Trained immunity effects have been shown to persist for months, as epigenetic changes also occur in the progenitor cells in the bone marrow100
As for clinical evidence, a metanalysis of randomized trials demonstrated its efficacy as a preventive agent during the 1918 Spanish flu pandemic, reducing case fatality up to 70% in those subjects who received the bacterial vaccine compared to controls.107 More recently, a prospective open pilot study of 17 subjects, six months’ daily sublingual administration of a heat-inactivated, respiratory BV, MV130 (including Streptococcus pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, Moraxella catarrhalis and Hemophilus influenzae, respectively 60-15-15-4-3-3%, respectively), significantly reduced the rate of recurrent RTIs.108 Similarly, OM85, a lyophilized alkaline extract of mixed respiratory pathogens reduced lower respiratory tract infections in high-risk infants from 65 to 42% the first year.109 Recently, investigators demonstrated that virus can also enhance trained immunity in cord-blood immune cells of newborn infants of HBV-infected mothers, enabling them to respond vigorously to a bacterial challenge.110
In conclusion, education of the innate immune response by the administration of multipurpose BV produces an intense pro-inflammatory response during subsequent heterogeneous infections (viral or bacterial) while maintaining immune system homeostasis.
Probiotics
Probiotics are “live microorganisms that, when administered in adequate amounts, confer a healthy benefit to the host".111 Administered orally, they activate TLR-2 and TLR-3 in intestinal epithelial cells, with subsequent increased production of IL-6 and other cytokines, chemoattractant for type I macrophages. This thus increases the number of macrophages and dendritic cells in the intestinal lamina propria, with greater production of TNF-α and IFN-γ that favor a state of tolerance with increased immunoglobulin (Ig)A+ cells.111, 112, 113 Cytokines produced locally in the intestine can influence the activity of immune cells at distant sites, including the bronchi, inducing the activation of regulatory T cells that release IL-10. Additionally, probiotics strengthen the intestinal barrier by increasing mucin and tight-junction proteins, as well as goblet and Paneth cells that produce bactericidal substances.111, 112, 113
Modulation of the gut microbiota is a mechanism by which probiotics maintain the balance and suppression of harmful pathogens. The viability of probiotics is crucial for the interaction with epithelial cells and macrophages, mainly favoring the innate immune response, with increased microbicidal activity of peritoneal and splenic macrophages.112 Probiotics protect against intestinal colonization of pathogens by direct induction of their death, by competition for nutrients and by activation of the mucosal-associated immune system. They also protect against some remote infections. In a study in human immunodeficiency virus (HIV)-positive patients, oral administration of probiotics was associated with an increase in type I and II interferons (particularly IFN-α) in intestinal cells and peripheral mononuclear cells, with an antiviral effect related to decreased viral spread.114 In a clinical trial in China, oral administration of probiotics was associated with increased serum and secretory IgA and IFN-γ after 12 weeks of treatment, concluding that probiotics are safe and effective in reducing episodes of the common cold. Likewise, the potentiating effect on the production of antibodies or increased activity of NK cells of specific strains has been documented in elderly people115,116
An intercommunication between the intestine and the lung has been proposed in the pathogenesis of some respiratory conditions. This opens the possibility of the potential benefit of probiotics under such conditions. In 2 meta-analyses, a modest efficacy of some probiotics (Lactobacillus and Bifidobacterium strains) was found in reducing the incidence and duration of viral respiratory infections55, 117 Jayawardena 2020.118,119 The existence of intestinal dysbiosis has been reported in critically ill patients.120 Two clinical trials found that mechanically ventilated patients receiving probiotics (Lactobacillius rhamnosus, Bacillius subtilis, and Enterococcus faecalis) developed ventilator-associated pneumonia less frequently compared to the control group (placebo).117
A small case series from China showed that some patients with COVID-19 had dysbiosis with decreased Lactobacillus and Bifidobacterium, and a link has been proposed between COVID-19 case fatality and diet, associating a diet rich in fermented vegetables —probably Lactobacillius-rich— reduced mortality.121 Although it will be interesting to carry out studies to evaluate the efficacy of probiotics in the treatment of COVID-19, it is essential to know more about the pathophysiology of this disease before conducting clinical trials with probiotics in these patients.117
Dialyzable leukocyte extract (“transfer factor”)
Dialyzable leukocyte extract (DLE) is a mixture of low-molecular-weight peptides (<10 kDa) that are released on disruption of peripheral blood leukocytes from healthy human subjects. It is used as a non-specific immune modulator. It originated from the historically controversial therapeutic resource defined as “Lawrence Transfer Factor”, a blood product with the ability to passively transfer antigen-specific cell mediated immunity. In 1949, Lawrence published the successful transfer of late skin sensitivity to tuberculin (PPD) to PPD negative patients,122 and in 1952 he successfully transferred sensitivity to partially purified preparations of the streptococcal substance M by the administration of suspensions of leukocyte components released by cell lysis by repeated freezing and thawing or distilled water lysis.
The further purified DLE, composed of only small peptides, caught the interest of a number of researchers in demonstrating mechanisms, mediators and reproducing clinical results in different diseases. Among others, Myles et al aiming to identify the source of DLE's effects, performed experimental studies on murine and human individuals, and they found that the cell extract derived from TCD8+ antigen-specific memory lymphocytes induces the release of IL-6 by antigen-presenting cells (APCs) (DCs and myeloid-origin monocytes).123 These cells also induce the release of IL-17 by naive CD8+ T cells, without the need for contact with the APCs.124
Ramirez-Ramirez et al found in an ex-vivo study of cells from umbilical cord of new born infants and bone marrow of adults, that DLE increases the differentiation of CD34+ toward a CD56+CD16+CD11c+ NK-like cell population endowed with properties such as IFN-y production, tumor cell cytotoxicity, and the capability of inducing γ-δ T lymphocyte proliferation, all together with an intense stimulation of the innate immune response.125 In addition, DLE appears to have the ability to regulate cytokine production by PBMCs. In in vitro studies, Rodríguez-Flores found that T-cells from healthy individuals could produce TNF-α, IL-12 and IL-10 after DLE stimulation and thus defining monocytes as the main cellular population that produced TNF-α after stimulation with DLE and showing the direct activation of monocytes via TLR2.126,127
Based on the above cited and results of several other researchers, the exact content of DLE is being elucidated: it is assumed to possess oligo-ribonucleopeptids and fragments of the T-cell receptor;123,128 these immunomodulating peptides stimulate the innate immune system by activating monocytes to produce IL-6 and IL-8, but also the adaptive immune system by enhancing the release of IL-2, IFN-γ and TNF-α from Th1 cells. Even so, it also enhances immune regulation as a rise in IL-10 has been documented.126
Clinically, it has shown benefit in some trials against infections,126,129,130 cancer —both in murine and in human studies—126,128,131) and autoimmune diseases. Even so, searching PubMed indexed journals randomized trials and DBPC studies with DLE are still very scarce and thus its use remains poorly sustained at the moment, but it might be a promising option for the future.
Synthetic molecules (pidotimod)
Pidotimod (3-L-pyroglutamyl-l-thiaziolidine-4-carboxylic acid, CAS 121808-62-6) is a synthetic dipeptide with immunomodulatory properties, which is employed in the prevention and treatment of respiratory tract infections in children and adults.132
Early studies in humans demonstrated that pidotimod induces the bactericidal activity of alveolar macrophages,133 while also activating innate immunity at NK cell level activity.134 Recently, research has shown that monocyte adhesion and chemotaxis, as well as the migration of IL-2 activated T cells, induced by pidotimod requires the activation of the phosphatidylinositol 3-kinase pathway (PI3K/Akt) signaling pathway through the CXC chemokine receptor 3A (CXCR3A) isoform.135
Pidotimod is also capable of inducing the maturation of DCs and increasing the expression of major histocompatibility complex class II cell surface receptor (HLADR) and co-stimulatory molecules CD83 and CD86. Pidotimod also induces DC to produce pro-inflammatory molecules monocyte chemoattractant protein-1 (MCP-1) and TNF-α, which drive T cell proliferation and differentiation towards a Th1 phenotype.136
Furthermore, the use of pidotimod for community-acquired pneumonia treatment in adults has demonstrated that it upregulates the expression genes involved in inflammation and chemotaxis.137
As for the clinical evidence, a meta-analysis assessing the effectiveness and safety of pidotimod reviewed 29 randomized controlled trials including 4344 pediatric patients were included. While the meta-analysis questioned the quality of some of the trials under review, it concluded that pidotimod treatment resulted in a significant decrease in instances of recurrent RTIs (RR 1.59; 95% CI 1.45–1.74, p < 0.00001) in comparison to conventional treatment.138
Specific vaccines with heterologous activity
Bacillus Calmette-Guérin vaccine
The Bacillus Calmette–Guérin (BCG) vaccine has been used in humans since 1921 to prevent tuberculosis and other infections due to Mycobacterium tuberculosis (MT). Its administration is a public policy in many developing and some developed countries.
More than 130 million children per year are vaccinated with BCG. Its safety has been demonstrated even in preterm and/or low-birth-weight infants.139 Infrequently, non-suppurating complications have been documented, be it of spontaneous resolution without treatment in 4 to 6 months. Less frequently seen suppurating complications, such as lymphadenitis abscess, might benefit from needle aspiration.140 It is a live vaccine and thus contraindicated in immune deficient subjects.
Interestingly, the effects of BCG vaccination are not limited to the prevention of MT infections. It has several non-specific (also known as heterologous) effects. These include the prevention of other infections, its use for the treatment of cancer (eg, bladder cancer) and others. A retrospective study using data from the Official Spanish Registry of Hospitalizations (CMBD-HA) to identify differences in hospitalization rates (HR) in BCG-vaccinated children showed a risk reduction in hospitalizations for sepsis not attributable to tuberculosis in children under 1 year of age of 52.8% and for respiratory infections in <15 years of 41.4%.141 A randomized controlled trial in Guinea-Bissau in low-birth-weight infants (<2500 grs), vaccinated with BCG at birth or at 6 weeks of life, as their routine practice, showed a reduction of neonatal mortality of 48%, of infant mortality of 21% and 41.4% for URTIs in <15 year-olds.142 A randomized controlled trial in elderly (60–75 years old) who received BCG vaccinations as 3 monthly doses, showed a reduction of the incidence of acute URTIs.143
Furthermore, BCG has shown to induce a better response to other vaccines. In a double blind placebo controlled DBPC study, subjects who received BCG vaccination 14 days before the trivalent influenza vaccine by intramuscular route had a more pronounced increase and accelerated induction of functional antibodies against the influenza strains contained in the vaccine and a larger production of IFN-γ and IL-6 in response to unrelated pathogens.144 In another study, subjects vaccinated with BCG who 28 days later received the yellow-fever live attenuated vaccine had a reduction of viremia that highly correlated with the upregulation of IL-1β, a heterologous cytokine associated to the induction of trained immunity or innate memory in monocytes. This has been validated through genetic, epigenetic and immunological studies, showing epigenetic reprogramming in monocytes resulting in a protection against non-related viral infection.145
During the COVID-19 pandemic, there seems to be a strong correlation between BCG index (the degree in which universal BCG vaccination is diploid in a country) and COVID-19 mortality: after correcting for many socioeconomic and pandemic-related confounders investigators found for every 10% increase in the BCG index a 10.4% reduction in COVID-19 mortality.146 This association does not allow us to conclude anything about causality, however. Likewise, countries that have maintained the universal BCG vaccination policy have less morbidity and mortality per capita in some studies, but not in others.147
Nevertheless, the development of a specific vaccine for COVID-19 is urgently needed. Its inclusion as a worldwide public policy could take more than a year and be expensive. In the meantime, it is desirable to consider the BCG as an accessible, cheap, and safe alternative to possibly reduce the COVID-19 burden.148 However, its efficacy to prevent SARS-CoV-2 infection or its severity has yet to be demonstrated in prospective randomized trials: up to now, there are 5 such clinical studies registered in Europe in high risk adult subjects. Four in healthcare professional who take care of infected subjects and 1 in subjects >60 years.(clinicaltrials.gov).
Other heterologous vaccines
Looking for other options to improve the response of our immune system against SARS-COV-2, one of the fields studied, is the immune response to vaccination. A clinical question is whether there is any secondary, non-specific immune response to immunizations that could favor the response to other viruses, including SARS-COV-2. There is a concept called heterologous immunity, which consists of a response of the immune system to one or more pathogens, after exposure to a different infectious agent. This type of response can occur after previous exposure to a pathogen or through immunization.149
During the first years of life, the immune system is in an active state of learning and development. Therefore, the child's innate and cellular adaptive immune responses are in an increased state of alertness, influenced by frequent exposure to different pathogens, but also to many immunizations with viral and bacterial vaccines. This scientific basis could may be explain that certain vaccines might help to generate an immune response to a pathogen, different from the one applied.150
Apart from BCG, another of the vaccines studied in this pandemic is measles, which could have a positive effect when exposed to other pathogens, reducing mortality from infections not related to vaccination, including SARS-CoV-2. This beneficial effect could be mediated by increased Th1-type responses and activation of CD4 + T-cells, producers of IFN-γ, as well as CD8 memory cells.150 The measles vaccine may have protective effects against unrelated infections, mainly in the airway and gastrointestinal tract.149,150
It has been proposed that influenza vaccination could also induce a heterologous response to other viral pathogens, such as respiratory syncytial virus, through the innate immune response, mediated by TLR3 and TLR7.151, 158
Despite the aforementioned, in the face of this pandemic it shall be important to continue the investigation and monitor whether heterologous vaccination might offer any additional protection. Hypothetically, it could be one of the factors explaining why children have been affected in a much lesser degree than adults, with a mortality rate 1:1000 compared to adults. However, studies are needed to know what role vaccines with a heterologous immune response could play at the moment a subject is exposed to SARS-CoV-2.
Final remarks
Without claiming to be complete, in this review article we presented a dozen of interventions that could possibly enhance the innate immune response, human's first-line defense when facing exposure to SARS-CoV-2. The interventions were divided into: 1) related to lifestyle, 2) actions with a non-specific booster effect on immunity, and 3) specific antigens causing a heterologous activation of the immune response. As for now, none of the interventions has proven effective for preventing COVID-19, though several are under investigation in clinical trials. However, there is evidence for almost all in the context of RTI and even directly in viral respiratory infections or severe RTI in the ICU; we tabulated what seemed to be studies with the best level of evidence for each intervention in Table 3. In the light of the threat for all of us of an imminent infection some interventions, even though evidence for their efficacy is not rock-solid yet, could be considered options that might help enhance the innate immune system and thus worthwhile, especially, as most lack the risk for serious adverse events. Together with the techniques focusing on reduction of the viral load, such as social distancing, equipment for personal protection and adequate hand hygiene, improving our first-line defense seems vital. Finally, we add in Table 4 bullet-points as a concluding summary, making it clear more direct evidence is needed in this important area.
Table 3.
Overall highest level of evidence for each intervention.152, 153, 154, 155, 156, 157 BCG: Bacillus Calmette-Guérin; BMI = body mass index; DLE: Dialyzable leukocyte extract; EPA: Eicosapentaenoic acid (an omega-3 fatty acid); GLA: gamma linoleic acid; I2: Heterogeneity; IgG: Immunoglobulin G; ICU: intensive care unit; IU: International units; IRR: Incidence rate ratio; LPS: Lipopolysaccharide; MCV: measles containing vaccines; MRR: Mortality Rate Ratio; MV: mechanical ventilation; OR: Odds ratio; RR = relative risk; RSV = respiratory syncytial virus; (U)RTI = (Upper) respiratory tract infection; TNF-: Tumor necrosis factor alpha
Table 4.
Concluding remarks per intervention in bullet-point format.
| Intervention | Conclusion |
|---|---|
|
Regular exercise has a probable immunoprotective and immunoregulatory effect.Older adults may obtain the greatest exercise-induced benefits for their immunological health. |
|
Forest walks might be beneficial to boost innate immunity, particularly in times of a pandemic; evidence is limited, but there are no direct health-related safety issues. |
|
The potential benefit of adequate sleep patterns overweighs the low level of evidence available up to date |
|
A constant program of a professionally guided meditation strategy could be beneficial to achieve an effective stress reduction and probably to reduce the burden of URTIs, mainly in older adults |
|
The published study results so far have not been conclusive.Subjects with vitamin deficiency would likely benefit most from supplementation.The intervention could be considered relatively safe |
|
A Cochrane collaboration systematic review showed daily doses of 0.2 mg reduced the duration of common cold. In ARDS patients very high IV doses might be beneficial. More data are needed. |
|
All 4 meta-analyzes have reported beneficial effects of zinc supplementation in children for the prevention of pneumonia. Important to restore the redox balance.Adverse effects are considered mild |
|
Several have anti-inflammatory potency, but there is no evidence of PUFA supplementation in prophylaxis of URTIs. Polyphenols display zinc ionophore capacity. |
|
Fever during infection enhances survival of living beings. Solid evidence in human beings still lacking. Positive effects unless temperature exceeds 40°C for prolonged periods of time. |
|
Reduced mortality according to metanalysis of trials conducted during the 1918–19 influenza pandemic. Mechanisms are progressively better understood. |
|
A modest efficacy of some probiotics (Lactobacillus and Bifidobacterium strains) was found in reducing the incidence and duration of viral respiratory infections |
|
Though it might seem of benefit, evidence of the reduction of infections is very scarce and quality trials are lacking. |
|
With the use of Pidotimod there is a lower recurrence of respiratory tract infections as compared to conventional treatment and less use of antibiotics |
|
We encourage to wait for the results of the ongoing RCT before using BCG vaccination for the prevention of COVID-19 |
|
The immune stimulation produced by heterologous vaccines enhances the response to other pathogens, different from the one in the vaccine and was associated with reduced all-cause infant mortality (e.g. measles vaccine, BCG). |
BCG = Bacillus Calmette-Guérin; DLE = Dialyzable leukocyte extract; PUFA = Polyunsaturated fatty acid; RCT = randomized controlled trials; URTIs = Upper-respiratory tract infection
Author contributions
Désirée Larenas-Linnemann,1: Concept, wrote original draft, translation intro-final and 7 chapters of the article into English and correction of all, led discussions on content, organized and led online meetings, correction of the draft and creation of the final version, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Noel Rodríguez-Pérez,2: Concept, corrected original draft, translation 2 chapters of the article into English and correction of all, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Alfredo Arias-Cruz,5: Reviewed and corrected original draft, translation 1 chapter of the article into English, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. María Virginia Blandón-Vijil,1: Reviewed and corrected original draft, translation of 1 chapter of the article into English, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Blanca E. Del-Río-Navarro,6: Reviewed and corrected original draft, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Alan Estrada-Cardona,7: Reviewed and corrected original draft, translation 1 chapter of the article into English, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. José E. Gereda: Reviewed and corrected original draft, translation 1 chapter of the article into English, attended online sessions, corrections of the draft. Jorge A. Luna-Pech,3: Reviewed and corrected original draft, translation 2 chapters of the article into English and correction of final doc, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Elsy Maureen Navarrete-Rodríguez,6: Reviewed and corrected original draft, translation 1 chapter of the article into English, attended 2 online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. Ernesto Onuma-Takane,1: Reviewed and corrected original draft, translation 2 chapters of the article into English and correction of final doc, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. César Fireth Pozo-Beltrán,9: Reviewed and corrected original draft, translation 2 chapters of the article into English and correction of final doc, attended online sessions, corrections of the draft, Participated in discussions on content, Did the research of literature, Wrote content of their intervention and reviewed the final complete draft, Are member of the group that suggested adjustments to the text, Gave their consent to the final version. María Isabel Rojo-Gutiérrez: Reviewed and corrected original draft, translation 2 chapters of the article into English and correction of final doc, attended online sessions, corrections of the draft.
Funding
Not applicable.
Consent for publication
All co-authors gave consent for publication.
Availability of data and materials
Not applicable.
Ethical approval and IRB board submission
As there are no patients involved: does not apply.
Declaration of competing interest
All authors declared or they have no potential conflicts of interest. The following authors, indicated relations with the pharmaceutical industry: Dr. Larenas Linnemann reports personal fees from Allakos, Amstrong, Astrazeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Grunenthal, GSK, MEDA, Menarini, MSD, Novartis, Pfizer, Novartis, Sanofi, Siegfried, UCB, Alakos, Gossamer, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Boehringer Ingelheim, Chiesi, Purina institute.
Acknowledgements
None.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Taubenberger J.K., Morens D.M. Influenza: the once and future pandemic. Publ Health Rep. 2010;125(Suppl 3):16–26. [PMC free article] [PubMed] [Google Scholar]
- 2.He W., Yi G.Y., Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. J Med Virol. 2020 doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou T., Liu Q., Yang Z. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Base Med. 2020;13(1):3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha P., Matthay M.A., Calfee C.S. Is a "cytokine storm" relevant to COVID-19? [published online ahead of print, 2020 jun 30] JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 7.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501 e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz M., Brenner M., Wang P. Therapeutic Potential of B-1a Cells in COVID-19. Shock. 2020 doi: 10.1097/SHK.0000000000001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews C.E., Ockene I.S., Freedson P.S., Rosal M.C., Merriam P.A., Hebert J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34(8):1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Fondell E., Lagerros Y.T., Sundberg C.J. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc. 2011;43(2):272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 11.Kostka T., Berthouze S.E., Lacour J., Bonnefoy M. The symptomatology of upper respiratory tract infections and exercise in elderly people. Med Sci Sports Exerc. 2000;32(1):46–51. doi: 10.1097/00005768-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Simpson R.J., Lowder T.W., Spielmann G., Bigley A.B., LaVoy E.C., Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11(3):404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Zamani A., Salehi I., Alahgholi-Hajibehzad M. Moderate exercise enhances the production of interferon-gamma and interleukin-12 in peripheral blood mononuclear cells. Immune Netw. 2017;17(3):186–191. doi: 10.4110/in.2017.17.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell J.P., Riddell N.E., Burns V.E. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23(6):767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Kruger K., Lechtermann A., Fobker M., Volker K., Mooren F.C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. 2008;22(3):324–338. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Clifford T., Wood M.J., Stocks P., Howatson G., Stevenson E.J., Hilkens C.M.U. T-regulatory cells exhibit a biphasic response to prolonged endurance exercise in humans. Eur J Appl Physiol. 2017;117(8):1727–1737. doi: 10.1007/s00421-017-3667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohut M.L., Cooper M.M., Nickolaus M.S., Russell D.R., Cunnick J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57(9):M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 18.Totura A.L., Whitmore A., Agnihothram S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6(3) doi: 10.1128/mBio.00638-15. e00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart L.K., Flynn M.G., Campbell W.W. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19(5):389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Xu Y., Sheng H., Ni X., Lu J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav Brain Res. 2016;307:18–24. doi: 10.1016/j.bbr.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Kakanis M.W., Peake J., Brenu E.W. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. 2010;16:119–137. [PubMed] [Google Scholar]
- 22.Moreira A., Delgado L., Moreira P., Haahtela T. Does exercise increase the risk of upper respiratory tract infections? Br Med Bull. 2009;90:111–131. doi: 10.1093/bmb/ldp010. [DOI] [PubMed] [Google Scholar]
- 23.Nieman D.C. Exercise, infection, and immunity. Int J Sports Med. 1994;15(Suppl 3):S131–S141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- 24.Couto M., Silva D., Delgado L., Moreira A. Exercise and airway injury in athletes. Acta Med Port. 2013;26(1):56–60. [PubMed] [Google Scholar]
- 25.Campbell J.P., Turner J.E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Nakadai A., Matsushima H. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol Immunotoxicol. 2006;28(2):319–333. doi: 10.1080/08923970600809439. [DOI] [PubMed] [Google Scholar]
- 27.Li Q., Morimoto K., Nakadai A. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2007;20(2):3–8. doi: 10.1177/03946320070200S202. [DOI] [PubMed] [Google Scholar]
- 28.Li Q., Morimoto K., Kobayashi M. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmacol. 2008;21(1):117–127. doi: 10.1177/039463200802100113. [DOI] [PubMed] [Google Scholar]
- 29.Li Q., Kobayashi M., Wakayama Y. Effect of phytoncide from trees on human natural killer cell function. Int J Immunopathol Pharmacol. 2009;22(4):951–959. doi: 10.1177/039463200902200410. [DOI] [PubMed] [Google Scholar]
- 30.Kim S.W., Su K.P. Using psychoneuroimmunity against COVID-19. Brain Behav Immun. 2020 Jul;87:4–5. doi: 10.1016/j.bbi.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrov S., Lange T., Gouttefangeas C. Galphas-coupled receptor signaling and sleep regulate integrin activation of human antigen-specific T cells. J Exp Med. 2019;216(3):517–526. doi: 10.1084/jem.20181169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dimitrov S., Lange T., Nohroudi K., Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30(4):401–411. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda S., Sredni B., Carasso R.L., Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29(7):393–398. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 34.Zager A., Andersen M.L., Ruiz F.S., Antunes I.B., Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- 35.Ibarra-Coronado E.G., Pantaleon-Martinez A.M., Velazquez-Moctezuma J. The bidirectional relationship between sleep and immunity against infections. J Immunol Res. 2015;2015:678164. doi: 10.1155/2015/678164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heffner K.L., Ng H.M., Suhr J.A. Sleep disturbance and older adults' inflammatory responses to acute stress. Am J Geriatr Psychiatr. 2012;20(9):744–752. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel K., Sheridan J.F., Van Cauter E. Effect of sleep deprivation on response to immunization. J Am Med Assoc. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen S., Tyrrell D.A., Smith A.P. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 41.Carmody J., Baer R.A. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 42.Barrett B., Hayney M.S., Muller D. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann Fam Med. 2012;10(4):337–346. doi: 10.1370/afm.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannell J.J., Vieth R., Umhau J.C. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321(2):103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2) doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 46.Muehleisen B., Gallo R.L. Vitamin D in allergic disease: shedding light on a complex problem. J Allergy Clin Immunol. 2013;131(2):324–329. doi: 10.1016/j.jaci.2012.12.1562. [DOI] [PubMed] [Google Scholar]
- 47.Adams J.S., Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metabol. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant W.B., Lahore H., McDonnell S.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han J.E., Jones J.L., Tangpricha V. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergman P., Lindh A.U., Bjorkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martineau A.R., Jolliffe D.A., Hooper R.L. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vuichard Gysin D., Dao D., Gysin C.M., Lytvyn L., Loeb M. Effect of vitamin D3 supplementation on respiratory tract infections in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0162996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao L., Xing C., Yang Z. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr. 2015;114(7):1026–1034. doi: 10.1017/S000711451500207X. [DOI] [PubMed] [Google Scholar]
- 54.Ross A.C., Manson J.E., Abrams S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alshahrani F., Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. 2013;5(9):3605–3616. doi: 10.3390/nu5093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020:100190. doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y., Kim H., Bae S. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-alpha/beta at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemila H. Vitamin C and common cold-induced asthma: a systematic review and statistical analysis. Allergy Asthma Clin Immunol. 2013;9(1):46. doi: 10.1186/1710-1492-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L., Hu C., Hood M. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12(4) doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simonson W. Vitamin C and coronavirus. Geriatr Nurs. May-Jun 2020;41(3):331–332. doi: 10.1016/j.gerinurse.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Messina G., Polito R., Monda V. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Husson M.O., Ley D., Portal C. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. 2016;73(6):523–535. doi: 10.1016/j.jinf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Ling L.J., Lu Y., Zhang Y.Y. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67:153150. doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- 68.Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjorklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215:108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang D., Zhang B., Lv J.T., Sa R.N., Zhang X.M., Lin Z.J. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res. 2020;157:104882. doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo P., Liu D., Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int J Antimicrob Agents. 2020;55(6):105995. doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skalny A.V., Rink L., Ajsuvakova O.P. Zinc and respiratory tract infections: perspectives for COVID19 (Review) Int J Mol Med. 2020 Jul;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shittu M.O., Afolami O.I. Improving the efficacy of Chloroquine and Hydroxychloroquine against SARS-CoV-2 may require Zinc additives - a better synergy for future COVID-19 clinical trials. Inf Med. 2020;28(2):192–197. [PubMed] [Google Scholar]
- 75.Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5-6):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aggarwal R., Sentz J., Miller M.A. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119(6):1120–1130. doi: 10.1542/peds.2006-3481. [DOI] [PubMed] [Google Scholar]
- 77.Barnett J.B., Hamer D.H., Meydani S.N. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev. 2010;68(1):30–37. doi: 10.1111/j.1753-4887.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhutta Z.A., Black R.E., Brown K.H. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J Pediatr. 1999;135(6):689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 79.Roth D.E., Richard S.A., Black R.E. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 2010;39(3):795–808. doi: 10.1093/ije/dyp391. [DOI] [PubMed] [Google Scholar]
- 80.Hemila H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J. 2011;5:51–58. doi: 10.2174/1874306401105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tournier J.N., Hellmann A.Q., Lesca G., Jouan A., Drouet E., Mathieu J. Fever-like thermal conditions regulate the activation of maturing dendritic cells. J Leukoc Biol. 2003;73(4):493–501. doi: 10.1189/jlb.1002506. [DOI] [PubMed] [Google Scholar]
- 83.Appenheimer M.M., Girard R.A., Chen Q. Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur J Immunol. 2007;37(10):2856–2867. doi: 10.1002/eji.200636421. [DOI] [PubMed] [Google Scholar]
- 84.Liso A., Capitanio N., Gerli R., Conese M. From fever to immunity: a new role for IGFBP-6? J Cell Mol Med. 2018;22(10):4588–4596. doi: 10.1111/jcmm.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Netea M.G., Kullberg B.J., Van der Meer J.W. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 86.Nicholls A.J., Wen S.W., Hall P., Hickey M.J., Wong C.H.Y. Activation of the sympathetic nervous system modulates neutrophil function. J Leukoc Biol. 2018;103(2):295–309. doi: 10.1002/JLB.3MA0517-194RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welc S.S., Clanton T.L. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013;98(2):359–371. doi: 10.1113/expphysiol.2012.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zellner M., Hergovics N., Roth E., Jilma B., Spittler A., Oehler R. Human monocyte stimulation by experimental whole body hyperthermia. Wien Klin Wochenschr. 2002;114(3):102–107. [PubMed] [Google Scholar]
- 89.Majhi R.K., Sahoo S.S., Yadav M., Pratheek B.M., Chattopadhyay S., Goswami C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. 2015;282(14):2661–2681. doi: 10.1111/febs.13306. [DOI] [PubMed] [Google Scholar]
- 90.Huntoon K.M., Wang Y., Eppolito C.A. The acute phase protein haptoglobin regulates host immunity. J Leukoc Biol. 2008;84(1):170–181. doi: 10.1189/jlb.0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papaioannou T.G., Karamanou M., Protogerou A.D., Tousoulis D. Heat therapy: an ancient concept re-examined in the era of advanced biomedical technologies. J Physiol. 2016;594(23):7141–7142. doi: 10.1113/JP273136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brunt V.E., Wiedenfeld-Needham K., Comrada L.N., Minson C.T. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol. 2018;596(20):4831–4845. doi: 10.1113/JP276559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahkee S., Srinath L., Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. 1997;90(3):296–298. doi: 10.1097/00007611-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Schulman C.I., Namias N., Doherty J. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect. 2005;6(4):369–375. doi: 10.1089/sur.2005.6.369. [DOI] [PubMed] [Google Scholar]
- 95.Small P.M., Tauber M.G., Hackbarth C.J., Sande M.A. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun. 1986;52(2):484–487. doi: 10.1128/iai.52.2.484-487.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elliott L. Review and Herald Publishing ASSN; Washington, D.C.: 1919, May. The Value of Sanitarium Treatment in Respiratory Diseases.http://documents.adventistarchives.org/Periodicals/LH/LH19190501-V34-05.pdf Available from: [Google Scholar]
- 97.Ludwig J., McWhinnie H. Antipyretic drugs in patients with fever and infection: literature review. Br J Nurs. 2019;28(10):610–618. doi: 10.12968/bjon.2019.28.10.610. [DOI] [PubMed] [Google Scholar]
- 98.Jefferies S., Weatherall M., Young P., Eyers S., Perrin K.G., Beasley C.R. The effect of antipyretic medications on mortality in critically ill patients with infection: a systematic review and meta-analysis. Crit Care Resusc. 2011;13(2):125–131. [PubMed] [Google Scholar]
- 99.Earn D.J., Andrews P.W., Bolker B.M. Population-level effects of suppressing fever. Proc Biol Sci. 2014;281(1778):20132570. doi: 10.1098/rspb.2013.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mulder W.J.M., Ochando J., Joosten L.A.B., Fayad Z.A., Netea M.G. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18(7):553–566. doi: 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kleinnijenhuis J., Quintin J., Preijers F. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Netea M.G., Latz E., Mills K.H., O'Neill L.A. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol. 2015;16(7):675–679. doi: 10.1038/ni.3178. [DOI] [PubMed] [Google Scholar]
- 103.Netea M.G., Joosten L.A., Latz E. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cirauqui C., Benito-Villalvilla C., Sanchez-Ramon S. Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways. Eur J Immunol. 2018;48(1):180–193. doi: 10.1002/eji.201747024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benito-Villalvilla C., Cirauqui C., Diez-Rivero C.M., Casanovas M., Subiza J.L., Palomares O. MV140, a sublingual polyvalent bacterial preparation to treat recurrent urinary tract infections, licenses human dendritic cells for generating Th1, Th17, and IL-10 responses via Syk and MyD88. Mucosal Immunol. 2017;10(4):924–935. doi: 10.1038/mi.2016.112. [DOI] [PubMed] [Google Scholar]
- 106.Mitroulis I., Ruppova K., Wang B. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172(1-2):147–161 e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chien Y.W., Klugman K.P., Morens D.M. Efficacy of whole-cell killed bacterial vaccines in preventing pneumonia and death during the 1918 influenza pandemic. J Infect Dis. 2010;202(11):1639–1648. doi: 10.1086/657144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alecsandru D., Valor L., Sanchez-Ramon S. Sublingual therapeutic immunization with a polyvalent bacterial preparation in patients with recurrent respiratory infections: immunomodulatory effect on antigen-specific memory CD4+ T cells and impact on clinical outcome. Clin Exp Immunol. 2011;164(1):100–107. doi: 10.1111/j.1365-2249.2011.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sly P.D., Galbraith S., Islam Z., Holt B., Troy N., Holt P.G. Primary prevention of severe lower respiratory illnesses in at-risk infants using the immunomodulator OM-85. J Allergy Clin Immunol. 2019;144(3):870–872 e11. doi: 10.1016/j.jaci.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 110.Hong M., Sandalova E., Low D. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins. 2018;10(1):11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maldonado Galdeano C., Cazorla S.I., Lemme Dumit J.M., Velez E., Perdigon G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 113.Kanmani P., Kim H. Immunobiotic strains modulate toll-like receptor 3 agonist induced innate antiviral immune response in human intestinal epithelial cells by modulating IFN regulatory factor 3 and NF-kappaB signaling. Front Immunol. 2019;10:1536. doi: 10.3389/fimmu.2019.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinacchio C., Scheri G.C., Statzu M. Type I/II interferon in HIV-1-Infected patients: expression in gut mucosa and in peripheral blood mononuclear cells and its modification upon probiotic supplementation. J Immunol Res. 2018;2018:1738676. doi: 10.1155/2018/1738676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H., Yeh C., Jin Z. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3(2):113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr Pharmaceut Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mak J.W.Y., Chan F.K.L., Ng S.C. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020 Jul;5(7):644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.King S., Glanville J., Sanders M.E., Fitzgerald A., Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hao Q., Dong B.R., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895. doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 120.Chernevskaya E., Beloborodova N., Klimenko N. Serum and fecal profiles of aromatic microbial metabolites reflect gut microbiota disruption in critically ill patients: a prospective observational pilot study. Crit Care. 2020;24(1):312. doi: 10.1186/s13054-020-03031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bousquet J., Anto J.M., Iaccarino G. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin Transl Allergy. 2020;10:16. doi: 10.1186/s13601-020-00323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawrence H.S. The cellular transfer of cutaneous hypersensitivity to tuberculin in man. Proc Soc Exp Biol Med. 1949;71(4):516–522. doi: 10.3181/00379727-71-17242. [DOI] [PubMed] [Google Scholar]
- 123.Myles I.A., Zhao M., Nardone G. CD8+ T cells produce a dialyzable antigen-specific activator of dendritic cells. J Leukoc Biol. 2017;101(1):307–320. doi: 10.1189/jlb.3A0216-082R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J.F., Park A.J., Rendini T., Levis W.R. Lawrence transfer factor: transference of specific immune memory by dialyzable leukocyte extract from a CD8+ T cell line. J Drugs Dermatol JDD. 2017;16(12):1198–1206. [PubMed] [Google Scholar]
- 125.Ramirez-Ramirez D., Vadillo E., Arriaga-Pizano L.A. Early differentiation of human CD11c(+)NK cells with gammadelta T cell activation properties is promoted by dialyzable leukocyte extracts. J Immunol Res. 2016;2016:4097642. doi: 10.1155/2016/4097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodriguez-Flores A., Nunez-Fernandez G., Estrada-Garcia I., Aguilar-Santelises M., Rojas-Espinosa O., Estrada-Parra S. Adjuvant treatment with a dialyzable leukocytes extract contributes to maintain HPV-infected women free of low-grade cervical lesions. Eur J Gynaecol Oncol. 2015;36(6):655–661. [PubMed] [Google Scholar]
- 127.Garcia-Hernandez U., Robledo-Avila F.H., Alvarez-Jimenez V.D. Dialyzable leukocyte extracts activate TLR-2 on monocytes. Nat Prod Commun. 2014;9(6):853–856. [PubMed] [Google Scholar]
- 128.Hernandez-Esquivel M.A., Perez-Torres A., Romero-Romero L. The dialyzable leukocyte extract Transferon(TM) inhibits tumor growth and brain metastasis in a murine model of prostate cancer. Biomed Pharmacother. 2018;101:938–944. doi: 10.1016/j.biopha.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 129.Castrejon Vazquez M.I., Resendiz-Albor A.A., Ynga-Durand M.A. Dialyzable leukocyte extract (transferon) administration in sepsis: experience from a single referral pediatric intensive care unit. BioMed Res Int. 2019;2019:8980506. doi: 10.1155/2019/8980506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Willeford B.V., Shapiro-Dunlap T., Willeford K.O. Serum derived transfer factor stimulates the innate immune system to improve survival traits in high risk pathogen scenarios. Drug Dev Res. 2017;78(5):189–195. doi: 10.1002/ddr.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whyte R.I., Schork M.A., Sloan H., Orringer M.B., Kirsh M.M. Adjuvant treatment using transfer factor for bronchogenic carcinoma: long-term follow-up. Ann Thorac Surg. 1992;53(3):391–396. doi: 10.1016/0003-4975(92)90256-4. [DOI] [PubMed] [Google Scholar]
- 132.Puggioni F., Alves-Correia M., Mohamed M.F. Immunostimulants in respiratory diseases: focus on Pidotimod. Multidiscip Respir Med. 2019;14:31. doi: 10.1186/s40248-019-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oddera S., Silvestri M., Sacco O., Eftimiadi C., Rossi G.A. [Effect of pidotimod on phagocytosis and intracellular killing of Staphylococcus aureus by human circulating polymorphonuclear neutrophils and alveolar macrophages] Drugs Exp Clin Res. 1993;19(Suppl):27–35. [PubMed] [Google Scholar]
- 134.Migliorati G., D'Adamio L., Coppi G., Nicoletti I., Riccardi C. Pidotimod stimulates natural killer cell activity and inhibits thymocyte cell death. Immunopharmacol Immunotoxicol. 1992;14(4):737–748. doi: 10.3109/08923979209009231. [DOI] [PubMed] [Google Scholar]
- 135.Caccuri F., Bugatti A., Corbellini S. The synthetic dipeptide pidotimod shows a chemokine-like activity through CXC chemokine receptor 3 (CXCR3) Int J Mol Sci. 2019;20(21) doi: 10.3390/ijms20215287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giagulli C., Noerder M., Avolio M. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int Immunopharm. 2009;9(12):1366–1373. doi: 10.1016/j.intimp.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 137.Trabattoni D., Clerici M., Centanni S., Mantero M., Garziano M., Blasi F. Immunomodulatory effects of pidotimod in adults with community-acquired pneumonia undergoing standard antibiotic therapy. Pulm Pharmacol Therapeut. 2017;44:24–29. doi: 10.1016/j.pupt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Niu H., Wang R., Jia Y.T., Cai Y. Pidotimod, an immunostimulant in pediatric recurrent respiratory tract infections: a meta-analysis of randomized controlled trials. Int Immunopharm. 2019;67:35–45. doi: 10.1016/j.intimp.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 139.Badurdeen S., Marshall A., Daish H., Hatherill M., Berkley J.A. Safety and immunogenicity of early Bacillus calmette-guerin vaccination in infants who are preterm and/or have low birth weights: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(1):75–85. doi: 10.1001/jamapediatrics.2018.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cuello-Garcia C.A., Perez-Gaxiola G., Jimenez Gutierrez C. Treating BCG-induced disease in children. Cochrane Database Syst Rev. 2013;1:CD008300. doi: 10.1002/14651858.CD008300.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Castro M.J., Pardo-Seco J., Martinon-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60(11):1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 142.Biering-Sorensen S., Aaby P., Lund N. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: A randomized controlled trial. Clin Infect Dis. 2017;65(7):1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–190. [PubMed] [Google Scholar]
- 144.Leentjens J., Kox M., Stokman R. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212(12):1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 145.Arts R.J.W., Moorlag S., Novakovic B. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100 e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 146.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. J Am Med Assoc. 2020 Jun 9;323(22):2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O'Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Agrawal B. Heterologous immunity: role in natural and vaccine-induced resistance to infections. Front Immunol. 2019;10:2631. doi: 10.3389/fimmu.2019.02631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gil A., Kenney L.L., Mishra R., Watkin L.B., Aslan N., Selin L.K. Vaccination and heterologous immunity: educating the immune system. Trans R Soc Trop Med Hyg. 2015;109(1):62–69. doi: 10.1093/trstmh/tru198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee Y.J., Lee J.Y., Jang Y.H., Seo S.U., Chang J., Seong B.L. Non-specific effect of vaccines: immediate protection against respiratory syncytial virus infection by a live attenuated influenza vaccine. Front Microbiol. 2018;9:83. doi: 10.3389/fmicb.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mao S., Huang S. Vitamin D supplementation and risk of respiratory tract infections: a meta-analysis of randomized controlled trials. Scand J Infect Dis. 2013;45(9):696–702. doi: 10.3109/00365548.2013.803293. [DOI] [PubMed] [Google Scholar]
- 153.Yakoob M.Y., Salam R.A., Khan F.R., Bhutta Z.A. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst Rev. 2016;11:CD008824. doi: 10.1002/14651858.CD008824.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fowler A.A., 3rd, Truwit J.D., Hite R.D. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J Am Med Assoc. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bousquet J., Schunemann H.J., Togias A. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80 e3. doi: 10.1016/j.jaci.2019.06.049. [DOI] [PubMed] [Google Scholar]
- 156.Rice T.W., Wheeler A.P., Thompson B.T. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. J Am Med Assoc. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Higgins J.P., Soares-Weiser K., Lopez-Lopez J.A. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Larenas-Linnemann Désirée, Rodríguez-Monroy Fernanda. Thirty-six COVID-19 cases preventively vaccinated with mumps-measles-rubella vaccine: All mild course. Allergy. 2020 doi: 10.1111/all.14584. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.