Abstract
Introduction
We analysed the neurological complications of patients with severe SARS-CoV-2 infection who required intensive care unit (ICU) admission.
Patients and methods
We conducted a retrospective, observational, descriptive study of consecutive patients admitted to the ICU due to severe respiratory symptoms secondary to SARS-CoV-2 infection between 1 April and 1 June 2020.
Results
We included 30 patients with neurological symptoms; 21 were men (72.40%), and mean age (standard deviation [SD]) was 57.41 years (11.61). The mean duration of ICU stay was 18.83 days (14.33). The neurological conditions recorded were acute confusional syndrome in 28 patients (93.33%), neuromuscular disease in 15 (50%), headache in 5 (16.66%), cerebrovascular disease in 4 (13.33%), and encephalopathies/encephalitis in 4 (13.33%). CSF analysis results were normal in 6 patients (20%). Brain MRI or head CT showed alterations in 20 patients (66.6%). EEG was performed in all patients (100%), with 8 (26.66%) showing abnormal findings. In 5 of the 15 patients with clinical myopathy, diagnosis was confirmed with electroneuromyography. We found a correlation between older age and duration of ICU stay (P = 0.002; 95% CI, 4.032–6.022; OR).
Conclusions
Severe COVID-19 mainly affects men, as observed in other series. Half of our patients presented acute myopathy, and almost all patients left the ICU with acute confusional syndrome, which fully resolved; no correlation was found with EEG or neuroimaging findings. Older age is associated with longer ICU stay.
Keywords: COVID-19, Critical patient, Acute myopathy, Encephalopathy, SARS-CoV-2
Abstract
Introducción
Nos proponemos analizar las complicaciones neurológicas de los pacientes con infección grave por SARS-CoV2, que han requerido ingreso en Unidad de Cuidados Intensivos (UCI).
Pacientes y métodos
Estudio descriptivo retrospectivo, observacional, de pacientes consecutivos ingresado en UCI por infección respiratoria grave por SARS-CoV-2, desde el 1 de Abril hasta el 1 de Junio de 2020.
Resultados
Registramos 30 pacientes con síntomas neurológicos, 21 hombres (72,40%), edad media: 57,41 años ± 11,61 desviación estándar (DE). Estancia media en UCI: 18,83 ± 14,33 DE. A nivel sindrómico: 28 pacientes (93,33%) con síndrome confusional agudo, 15 pacientes (50%) con patología neuromuscular, 5 (16,66%) cefalea, 4 (13,33%) con patología cerebrovascular, y 4 (13,33%) con encefalopatías/encefalitis. Punción lumbar normal en 6 pacientes (20%). La RMN craneal o TAC craneal mostró alteraciones en 20 casos (66,6%). Se realizó EEG en todos los pacientes (100%), alterado en 8 pacientes (26,66%). En 5 de los 15 pacientes con miopatía clínica se ha podido confirmar con ENMG. Hemos encontrado relación entre la mayor edad y los días de ingreso en UCI (P = 0,002, IC 95% 4,032-6,022; OR:).
Conclusiones
La infección grave por COVID-19 afecta mayoritariamente a hombres, similar a lo descrito en otras series. La mitad de nuestros pacientes presentan una miopatía aguda, y casi la totalidad de los pacientes salen de la UCI con sindromes confusionales agudos, que evolucionan a la resolución completa, sin correlacionarse con los resultados del EEG o de pruebas de neuroimagen. La mayor edad sí se asocia con un mayor número de días de estancia en UCI.
Palabras clave: COVID-19, Paciente crítico, Miopatía aguda, Encefalopatías, SARS-CoV2
Introduction
SARS-CoV-2 infection in humans was first reported in China in December 2019. In Spain, the first cases were reported in March 2020.
Although this novel virus mainly causes respiratory signs and symptoms (cough, fever, dyspnoea, interstitial infiltrate in chest radiography or lung CT) whose seriousness greatly determines disease severity and the risk of mortality, cases have been reported since the beginning of the pandemic of such neurological symptoms (or symptoms suggestive of neurological involvement) as headache, anosmia, muscle pain, insomnia, confusion, and bradyphrenia, both in patients with mild COVID-19 and in critical patients requiring orotracheal intubation and admission to an intensive care unit (ICU).
SARS-CoV-2 is currently believed to present tropism for angiotensin-converting enzyme 2 (ACE2) receptors, which the virus uses to enter the cell, where it begins replicating. ACE2 receptors are expressed in different organs and particularly the lungs, heart, kidneys, intestines, and brain.1, 2, 3, 4
In physiological conditions, ACE2 receptors can be found in glial cells and neurons in the brain; the hypothesis that the virus may cause neurological symptoms therefore seems plausible. This could be confirmed with routine neurological tests (brain MRI, CT, lumbar puncture, electroneuromyography [ENMG], or electroencephalography [EEG]), which may help us to understand the pathophysiology of the disease and the associated neurological complications.
Based on this assumption, our centre has developed a protocol aiming to provide specific neurological care to patients with severe COVID-19 admitted to the ICU and requiring orotracheal intubation; this protocol is intended to detect neurological complications as early as possible in order to reduce morbidity and mortality and minimise the risk of neurological sequelae.
The literature includes several series of patients with neurological involvement during acute SARS-CoV-2 infection.5, 6, 7, 8, 9, 10, 11, 12, 13
We analysed the neurological complications of severe SARS-CoV-2 infection within 48 hours after the critical period of the disease.
Patients and methods
We conducted a descriptive, observational, retrospective study of consecutive patients admitted to the ICU due to severe respiratory infection with SARS-CoV-2 between 1 April and 1 June 2020. Our patients underwent a neurological evaluation within 48 hours of discharge from the ICU and transfer to a conventional inpatient ward.
We analysed the following variables: age, sex, general comorbidities, neurological comorbidities, previous pharmacological treatments, source of infection, time since the first general symptom and the first neurological symptom, method used to establish diagnosis of SARS-CoV-2 infection, description of neurological symptoms, findings from the neurological examination, neurological syndrome, length of ICU stay (days), duration of hospitalisation (days), creatine kinase (CK) levels, lumbar puncture findings, CSF analysis results, head CT, brain or lumbar spine MRI, EEG, ENMG, causal relationship between COVID-19 and neurological syndrome in the opinion of the neurologist, treatment received for COVID-19 (antiviral drugs, antibiotics, sedatives), neurological treatment, clinical progression, and duration of neurological manifestations.
Data were anonymised and grouped. Statistical analysis was performed using SPSS, version 25 (IBM).
The study was approved by our hospital’s research committee (April 2020).
Results
A total of 54 patients were admitted to the ICU, 30 of whom presented neurological symptoms: 21 men (72.40%) and 9 women (27.60%). Mean age (SD) in our series was 57.41 (11.61) years (range, 25-74). None of the patients were healthcare professionals.
Mean duration of hospitalisation was 34.72 (11.44) days (range, 16-59). Mean stay at the ICU was 18.83 (14.33) days (range, 6-55). The source of infection was unknown in most cases (20 patients, 66.66%); the remaining patients were infected with SARS-CoV-2 in the family setting or by their partners (8 patients, 26.66%) and during social gatherings or travel (2 patients, 6.66%).
All patients presented bilateral pneumonia associated with severe respiratory insufficiency, and therefore required admission to the ICU and orotracheal intubation. All patients were sedated with remifentanil, propofol, midazolam, or morphine hydrochloride, which were suspended after discharge from the ICU.
Regarding medical history, 8 patients (26.66%) had arterial hypertension, 8 (26.66%) were obese, and 6 (20%) had type 2 diabetes mellitus. None had history of neurological disease. Only 8 patients (26.66%) were smokers, and 2 (6.66%) had history of respiratory disease (severe sleep apnoea-hypopnoea syndrome, treated with continuous positive airway pressure).
Mean time between onset of the respiratory infection and onset of neurological symptoms was 9.63 (4.1) days (range, 3-36). Twenty-one patients (70%) required anticoagulation therapy with tinzaparin due to segmental pulmonary thromboembolism observed in follow-up lung CT scans performed during hospitalisation.
Twenty-eight patients (93.33%) presented acute confusional syndrome, 15 (50%) neuromuscular disorders, 5 (16.66%) headache, 4 (13.33%) cerebrovascular disease, and 4 (13.33%) encephalopathy/encephalitis.
Six patients underwent lumbar puncture; CSF analysis revealed no alterations in cell counts, glucose levels, or protein levels. In all cases, PCR yielded negative results for SARS-CoV-2 in the CSF.
CK levels were elevated in 28 patients (93.33%); mean CK level was 379.69 (309.341) μg/L (range, 61-1206).
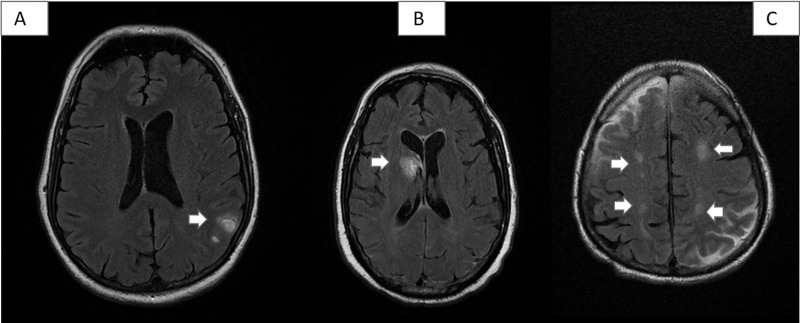
All patients underwent neuroimaging studies (brain MRI or CT scan, depending on the patient’s clinical status), which revealed no alterations in 10 cases (33.33%). The remaining patients presented intraparenchymal haemorrhage (2), venous thrombosis (2), encephalopathy (4), cavernoma (1), and chronic lacunar lesions (11) (Fig. 1 ).
Figure 1.
Some vascular lesions detected in our series. A) Patient 8: 2 small left parietal cortico-subcortical haematomas in the convexity. B) Patient 1: acute haematoma in the right caudate nucleus, with opening to the ventricular system. C) Patient 26: hyperintense subacute ischaemic lesions in both parietal lobes, in the border zone of the middle cerebral and anterior cerebral artery territories.
All patients underwent EEG, with 8 (26.66%) presenting alterations (mild diffuse slowing in all cases), with no epileptiform activity.
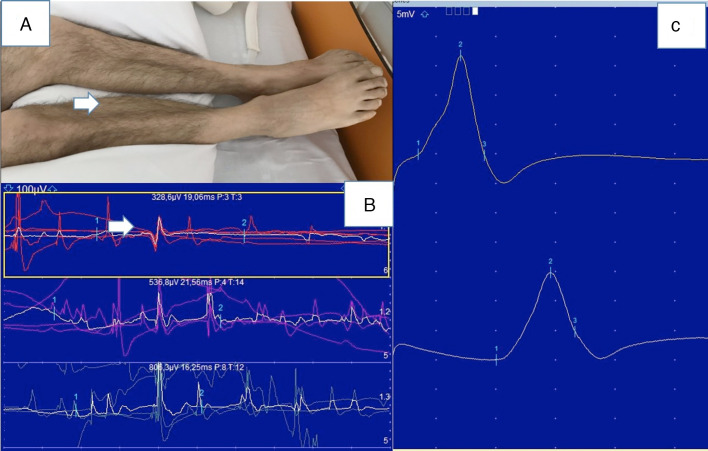
Six patients (20%) underwent ENMG studies, which revealed normal motor and sensory nerve conduction velocities and a myopathic pattern in 5 cases (Fig. 2 ). Only one patient presented polyneuropathy.
Figure 2.
Myopathy (patient 17). A) Atrophy of the gastrocnemius and tibialis anterior muscles. B) Electroneuromyography study: normal motor conduction velocity and motor potential amplitude in the right posterior tibial nerve. C) Needle electromyography of the right lateral head of the gastrocnemius muscle showing low amplitude and short duration of motor unit action potentials.
Patients were administered the following specific treatments for SARS-CoV-2 infection: chloroquine/hydroxychloroquine (30 patients; 100%), lopinavir/ritonavir (13; 43.33%), tocilizumab (17; 56.66%), boluses of methylprednisolone (15; 50%), remdesivir (2; 6.66%), and ceftriaxone (28; 93.33%). All patients received active treatment with antiviral drugs or empirical antibiotic treatment during their stay at the ICU.
Twenty patients (66.66%) progressed favourably or remained stable, 7 patients (23.33%) presented complications or progressive worsening, and 3 patients (10%) died.
According to the informing neurologist’s judgement, the relationship between COVID-19 and the neurological syndrome was probable causation in 66.66% of cases, definite causation in 20%, and coincidental in 13.34%.
No statistically significant association was observed between male sex and total hospitalisation time (P = .358), length of ICU stay (P = .292), or CK levels; between presence of confusional symptoms and EEG alterations (P = .486); or between age and presence of myopathy (P = 0.06). However, we did find a statistically significant association between older age (> 50 years) and longer ICU stay (P = .002; OR = 3.594; 95% CI, 1.032–6.022) (Table 1 ).
Table 1.
Descriptive and analytic study of our sample.
| N = 30 | P | OR | 95% CI |
||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Mean age (SD) | 57.41 years (11.61) | ||||
| Sex (men) | 21 (72.40%) | ||||
| Length of ICU stay (patients > 50 years) | .002 | 3.594 | 1.032 | 6.022 | |
| CK level | .226 | –1.240 | ↓380.301 | 115.529 | |
| Duration of hospitalisation | .0358 | 2.453 | 1.579 | 4.399 | |
| EEG alterations | .293 | 1.073 | –4.275 | 4.367 | |
CI: confidence interval; CK: creatine kinase; EEG: electroencephalography; ICU: intensive care unit; OR: odds ratio; SD: standard deviation.
Discussion
When the pandemic started, many neurologists had to become pulmonologists or internists to assist in the care of patients with SARS-CoV-2 infection; in this context, we observed the presentation of neurological symptoms in patients with COVID-19, and especially in severe cases. In the light of this, our centre decided to design a protocol for systematic, specific neurological evaluation of patients with COVID-19 who were admitted to the ICU and required orotracheal intubation.
From an epidemiological viewpoint, our series mainly included middle-aged men with no history of neurological disease, with few individuals presenting the most frequent vascular risk factors (hypertension, diabetes); this is consistent with the results from previous studies.6, 11, 12, 13, 14
Within 48−72 h of discharge from the ICU, most patients presented acute confusional syndrome presenting with disorientation to time and place, difficulty retrieving words, and fluctuating short-term memory problems. Some patients also presented irritability and aggressiveness within the first few hours, as has been described in other series.15 These patients presented similar symptoms, regardless of their age or the length of their ICU stay.
Their symptoms were not correlated with EEG or brain MRI alterations, and resolved completely. The only EEG alteration observed was mild generalised background slowing, which was not associated with epileptiform activity in any case. None of the patients presented seizures during hospitalisation.
Imaging studies revealed the most common vascular alterations (small intraparenchymal haemorrhages, chronic lacunar lesions, cerebral venous sinus thrombosis in patients with coagulation disorders in the context of COVID-19). These findings have been reported by different authors since the beginning of the pandemic.16, 17, 18, 19, 20, 21 However, cerebrovascular diseases were less frequent in our series than in other studies, probably due to the lower mean age of our sample and the lower prevalence of vascular risk factors.
Furthermore, some patients in our series presented radiological signs of encephalopathy, which were found to be poorly correlated with their symptoms.22, 23, 24, 25 Likewise, all CSF findings from patients undergoing lumbar puncture were normal (the procedure was contraindicated in most patients, since they were receiving anticoagulation therapy for pulmonary thrombosis). These results, together with negative PCR results in CSF, are increasingly being reported in the literature.26
Despite an increasing number of reports of Guillain-Barré syndrome and seizures or status epilepticus among patients with COVID-19,27, 28, 29, 30 these manifestations were not observed in any of our patients. However, we did find clinical evidence of myopathy in half of the patients evaluated (with several cases confirmed by ENMG); these findings have not previously been described in other series.11, 12, 13, 14 Marked amyotrophy of the lower limbs initially suggested Guillain-Barré-like polyneuropathy or critical illness polyneuropathy, but most patients showed normal motor and sensory nerve conduction velocities and a clearly myopathic pattern in the muscles studied (usually the abductor pollicis brevis, abductor digiti minimi, tibialis anterior, quadriceps, and gastrocnemius muscles), and normal tendon reflexes in the neurological examination. Only the patient with the longest ICU stay (55 days) showed critical illness polyneuropathy in an ENMG study. All patients showed slightly elevated CK levels. Lumbar puncture yielded normal results in patients undergoing this procedure.
Hypothesis testing revealed a statistically significant association between older age (> 50 years) and longer ICU stays, as similar studies have also reported.
Our series is smaller than other published series, such as that analysed by our colleagues from the ALBACOVID registry14 or the sample included in the multicentre study conducted in Madrid at the beginning of the pandemic and analysing smell and taste disorders.31 This may be due to the characteristics of our hospital, a small secondary healthcare centre with 270 beds serving a population of approximately 325 000 inhabitants (larger series include patients from tertiary hospitals).14, 31, 32, 33, 34 However, our series has the advantage that it is homogeneous: our patients were young, had no history of neurological disease or any other relevant medical history except for some cases of hypertension and diabetes mellitus, and they all presented severe respiratory involvement requiring admission to the ICU. All patients were attended by the same anaesthetists/intensivists, pulmonologists, and neurologists. As a result, the neurological examination, complementary tests, and pharmacological treatment were similar in all cases, minimising the risk of bias.
Although our patients presented similar disorders to those reported in previous studies (headache, intracranial haemorrhage, stroke, encephalopathy, etc),5, 35, 36, 37, 38 we found a significant number of patients with confusional syndrome and acute myopathy, which was most probably inflammatory (systemic reaction) or associated with critical illness polyneuropathy, rather than secondary to direct invasion by the virus. We believe that both complications are associated with the severity of COVID-19 in these patients.
Conclusions
In our series, severe COVID-19 was more frequent among men than women; this is consistent with previous series. Half of our patients presented acute myopathy, and nearly all presented acute confusional syndrome after discharge from the ICU; these symptoms resolved completely and were not correlated with EEG or neuroimaging findings. Older age was found to be associated with longer ICU stays. Studies with larger samples are needed to confirm our results.
Funding
After this study was completed, the lead author was awarded the COVID-19 research prize from the foundation for biomedical research and innovation of Hospital Universitario Infanta Sofía and Hospital Universitario del Henares, in Madrid.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Please cite this article as: Abenza-Abildúa MJ, Ramírez-Prieto MT, Moreno-Zabaleta R, Arenas-Valls N, Salvador-Maya MA. Algarra-Lucas C, et al. Complicaciones neurológicas en pacientes críticos por SARS-CoV-2. Neurología. 2020;35:621–627.
References
- 1.Mannan Baig A., Khaleeq A., Usman A. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 2.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020:1–4. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70:311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 4.Berger J.R. COVID-19 and the nervous system. J Neurovirol. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenk M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. New Engl Journal Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41:1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. Neurol Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed M.U., Hanif M., Ali M.J., Haider M.A., Kherani D., Memon G.M., et al. Neurological manifestations of COVID-19 (SARS-CoV2): a review. Front Neurol. 2020;11:518. doi: 10.3389/fneur.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Sánchez Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen A., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;10 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koftis K., Williams R., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV2 pandemic. Version 2. Crit Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular disease in COVID-19. AJNR Am J Neuroradiol. 2020;41:1170–1172. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodigiani C., Lapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Rudd A., Liu R. Challenges and potential solutions of Stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 2020;51:1356–1357. doi: 10.1161/STROKEAHA.120.029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leira E.C., Russman A.N., Biller J., Brown D.L., Bushnell C.D., Caso V., et al. Preserving stroke care during the COVID-19 pandemic: potential issues and solutions. Neurology. 2020;95:124–133. doi: 10.1212/WNL.0000000000009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A firs case of meningitus/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abenza-Abildúa M.J., Novo-Aparicio S., Moreno Zabaleta R., Algarra Lucas M.C., Rojo Moreno-Arcones B., Salvador Maya M.A., et al. Encephalopathy in severe SARS-COV2 infection: inflammatory or infectious? Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19 associated acute hemorrhagic necrotizing encephalopaty: imaging features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg R.K., Paliwal V.K., Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2020 doi: 10.1002/jmv.26207. Jun 19. [DOI] [PubMed] [Google Scholar]
- 26.De Melo Espíndola O., Siqueira M., Nascimento Soares C., Sales Dantas de Lima M.A., Celestino Bezerra Leite A.C., Queiroz Campos Araujo A., et al. Patients with COVID-19 and neurological manifestations show indetectable SARS-CoV2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. 2020;96:567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain-Barré syndrome associated with SARS-CoV2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guijarro-Castro C., Rosón-González M., Abreu A., García-Arratibel A., Ochoa-Mulas M. Síndrome de Guillain-Barré tras infección por SARS-CoV2. Comentarios tras la publicación de 16 nuevos casos. Neurología. 2020;35:412–415. doi: 10.1016/j.nrl.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Imbroda J., Reyes-Garrido V., Ciano-Petersen N.L., Serrano-Castro P.J. Implantación emergente de un servicio de Teleneurología en la Unidad de Neuromuscular del Hospital Regional de Málaga durante la pandemia por SARS-CoV2. Neurología. 2020;35:415–417. doi: 10.1016/j.nrl.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beltrán-Corbellini A., Chico-García J.L., Martínez-Poles J., Rodriguez-Jorge F., Natera-Villalba E., Gómez-Corral J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020 doi: 10.1111/ene.14273DOI./ene.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matías-Guiu J., Porta-Etessam J., López-Valdés E., García-Morales I., Guerrero-Solá A., Matías-Guiu J.A. Management of neurological care during the COVID-19 pandemic. Neurología. 2020;35:233–237. doi: 10.1016/j.nrl.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandas F., García Domínguez J.M., Díaz Otero F. Un servicio de Neurología de un hospital terciario en la pandemia COVID-19. Neurología. 2020;35:267–268. doi: 10.1016/j.nrl.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro E., Priori A., Beghi E., Helbok R., Campiglio L., Bassetti C.L., Bianchi E., et al. The international EAN survey on neurological symptoms in patients with COVID-19 infection. Eur J Neurol. 2020 doi: 10.1111/ene.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardi M., Padovani A., McArthur J.C. Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol. 2020;267:1573–1576. doi: 10.1007/s00415-020-09896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maassen Van Den Brink A., De Vries T., Danse A.H.J. Headache medication and the COVID-19 pandemic. Version 2. J Headache Pain. 2020;21:38. doi: 10.1186/s10194-020-01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pérez-Álvarez A.I., Suárez Cuervo C., Fernández Menéndez S. Infección por SARS-CoV2 asociada a diplopia y anticuerpos antirreceptor de acetilcolina. Neurología. 2020;35:264–265. doi: 10.1016/j.nrl.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escalada Pellitero S., Garriga Ferrer-Bergua L. Paciente con clínica neurológica como única manifestación de infección por SARS-CoV2. Neurología. 2020;35:271–272. doi: 10.1016/j.nrl.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]