Abstract
Introduction
Data regarding real-world impact on cancer clinical research during COVID-19 are scarce. We analysed the impact of the COVID-19 pandemic on the conduct of paediatric cancer phase I–II trials in Europe through the experience of the Innovative Therapies for Children with Cancer (ITCC).
Methods
A survey was sent to all ITCC-accredited early-phase clinical trial hospitals including questions about impact on staff activities, recruitment, patient care, supply of investigational products and legal aspects, between 1st March and 30th April 2020.
Results
Thirty-one of 53 hospitals from 12 countries participated. Challenges reported included staff constraints (30% drop), reduction in planned monitoring activity (67% drop of site initiation visits and 64% of monitoring visits) and patient recruitment (61% drop compared with that in 2019). The percentage of phase I, phase II trials and molecular platforms closing to recruitment in at least one site was 48.5%, 61.3% and 64.3%, respectively. In addition, 26% of sites had restrictions on performing trial assessments because of local contingency plans. Almost half of the units suffered impact upon pending contracts. Most hospitals (65%) are planning on improving organisational and structural changes.
Conclusion
The study reveals a profound disruption of paediatric cancer early-phase clinical research due to the COVID-19 pandemic across Europe. Reported difficulties affected both patient care and monitoring activity. Efforts should be made to reallocate resources to avoid lost opportunities for patients and to allow the continued advancement of oncology research. Identified adaptations to clinical trial procedures may be integrated to increase preparedness of clinical research to futures crises.
Keywords: COVID-19, Clinical trials, Drug development, Paediatric haematology and oncology, Healthcare policy
1. Introduction
The COVID-19 pandemic has led to a global healthcare crisis, affecting more than 28 million individuals and resulting in 917,417 deaths as of 14th September 2020 [1]. All European states, although not equally affected, have been forced to make unprecedented adjustments to prioritise resources against COVID-19 [2,3].
Although clinical research is key to advancing medical knowledge and improving patient care, priority has been given to COVID-19–related patient care during the pandemic. This has severely impacted essential clinical research in other fields, such as cancer research [4,5]. Coronavirus mitigation measures can affect the conduct of clinical trials in many ways, e.g. patient recruitment, completion of trial assessments, or provision of investigational medicinal products (IMPs) [6]. Furthermore, pharmaceutical companies have redirected their focus to developing diagnostics and treatments against COVID-19, thus neglecting other research areas [7].
To limit the downstream impact for patients and for the drug industry, the European Medicines Agency [8] and several national agencies [[9], [10], [11]] have issued guidelines on the management of clinical trial activities during the pandemic. They are all aligned in the intention to maintain trial activity, while assuring patient safety and trial activities traceability, but no definitive recommendations have been provided, making it difficult to create a unified action plan. Across Europe, all parties (i.e. healthcare authorities, regulators, sponsors) have implemented different measures depending on the impact of the pandemic in their countries and their capacity to adapt.
Particular attention should be given to patients in cancer clinical trials because of their remarkable vulnerability: cancer patients not only require timely evaluation and treatment that does not allow for delays [12], but also they may be vulnerable to COVID-19–related serious events [13,14]. The European Society of Medical Oncology [15] and International Society of Paediatric Oncology [16] have developed guidelines to mitigate the deleterious effects of the pandemic on the treatment of cancer patients. However, data regarding real-world impact on cancer clinical research during COVID-19 are scarce [17,18].
In this study, we evaluate the impact of the COVID-19 pandemic on the early-phase clinical trial activity in paediatric haematology–oncology in Europe. We analyse the experience of the Innovative Therapies for Children with Cancer (ITCC) consortium, an academic consortium dedicated to improve the access to novel treatments for children and adolescents with cancer in 13 European countries [19,20].
2. Methods
The local representatives from all 53 ITCC centres accredited to conduct early-phase clinical trials in paediatric cancer were contacted and sent a 93-item questionnaire. This questionnaire investigates the impact of the pandemic on phase I/II clinical trials in solid and haematological paediatric malignancies and molecular screening cancer platforms (Appendix 1) between 1st March and 30th April 2020. The online survey was closed on 21st June. Descriptive statistics are provided using SPSS Statistics® (V.23 TM, Chicago). Additional information about the status of the trials was obtained from EudraCT (https://eudract.ema.europa.eu/) and the National Library of Medicine clinical trial registry (https://clinicaltrials.gov/).
3. Results
Of the 53 ITCC sites, 31 (58.5%) responded, including 22 university hospitals, 6 cancer centres and 3 private hospitals. All responses were complete and could be included in the analysis. Participating sites accounted for 12 European countries, with a fair representation from Northern, Southern and Western Europe. Fourteen sites were exclusively paediatric, while 17 treated adults and children.
Sixty-four different phase I (n = 33) and phase II (n = 31) trials and 14 different molecular platforms were active and recruiting before 1st March. Phase I and phase II trials and molecular platforms were open in a median of four sites (interquartile range [IQR]: 1–7), three sites (IQR: 1–9) and one site (IQR: 1–3.25), respectively (Table 1 ).
Table 1.
Summary of responses to the survey provided by the 31 early-phase clinical trials units.
| Items | Total, n | Total, %b | |
|---|---|---|---|
| Baseline dataa | Phase I trials active, recruiting | 33 | |
| Phase II trials active, recruiting | 31 | ||
| Molecular platforms active, recruiting | 14 | ||
| Academic-sponsored trials and academic-sponsored molecular platforms actively recruiting | 38 | ||
| Industry-sponsored trials actively recruiting | 40 | ||
| SIV previously scheduled for March/April 2020 | 52 | ||
| MV previously scheduled for March/April 2020 | 309 | ||
| Unit's full-time workers | 570 | ||
| Total number of patients recruited in phase I/II studies in 2019 | 408 | ||
| Total number of patients recruited in molecular platforms in 2019 | 495 | ||
| Impact on personnel | Number of units suffering any kind of shortages of on-site staff | 21 | 68% (21/31) |
| Total reduction in number of on-site workers | 170 | 30% (170/570) | |
Cause of on-site personnel shortage:
|
59 98 13 |
35% (59/170) 58% (98/170) 7% (13/170) |
|
| Units performing remote data entry | 28 | 90% (28/31) | |
| Units with appropriate tools for homeworking (e.g remote access to hospital software [VPN]) | 24 | 77% (24/31) | |
| Impact on on-site activities performed by the sponsors | Units suffering SIV cancellation | 20 | 65% (20/31) |
| Number of SIV cancelled | 35 | 67% (35/52) | |
Postponing SIV decided by:
|
17 1 9 8 |
49% (17/35) 3% (1/35) 25% (9/35) 23% (8/35) |
|
| Number of SIV performed remotely | 15 | 29% (15/52) | |
| Number of SIV performed on-site | 2 | 4% (2/52) | |
| Units suffering MV cancellation | 27 | 87% (27/31) | |
| Number of MV cancelled | 196 | 64% (196/309) | |
Postponing MV decided by:
|
22 2 83 89 |
11% (22/196) 1% (2/196) 42% (83/196) 45% (89/196) |
|
| Number of MV performed remotely | 109 | 35% (109/309) | |
| Number of MV performed on-site | 4 | 1% (4/309) | |
| Impact on recruitment | Units that interrupted recruitment in all trials and molecular platforms | 5 | 16% (5/31) |
| Units that interrupted recruitment in at least one trial | 22 | 71% (22/31) | |
| Units that interrupted recruitment in at least one molecular platformc | 11 | 45% (11/24) | |
| Trials with interrupted recruitment in at least one unit | 35 | 55% (35/64) | |
| Trials with interrupted recruitment in at least 50% of the units | 17 | 26% (17/64) | |
| Trials with interrupted recruitment in 100% of the units | 9 | 14% (9/64) | |
| Molecular platforms with interrupted recruitment in at least one unit | 9 | 64% (9/14) | |
| Molecular platforms with interrupted recruitment in at least 50% of the units | 7 | 50% (7/14) | |
| Molecular platforms with interrupted recruitment in 100% of the units | 5 | 36% (5/14) | |
| New patients recruited to early-phase trials or to molecular platformsd | 59 | ||
| Impact on IMP and research devices | Trials with IMP shortages | 0 | 0% |
| Units shipping IMPs in at least one trial to the patient's home or to the local healthcare institution | 18 | 58% (18/31) | |
| Trials in which IMP were shipped to the patient's home or to the local healthcare centres | 26 | ||
| Units managing to increase IMP supply to patients | 8 | 26% (8/31) | |
| Units suffering shortages of research devices (e.g. sample kits) | 3 | 10% (3/31) | |
| Units that experienced difficulties to ship research samples | 13 | 42% (13/31) | |
| Impact on patient care organisation | Units suffering any kind of limitations in patients' care | 15 | 48% (15/31) |
| Units conducting remote patient visits in other healthcare institutions or by phone | 24 | 77% (24/31) | |
Total number of patient visits conducted:
|
809 646 59 104 |
80% (646/809) 7% (59/809) 13% (104/809) |
|
| Patient visits rescheduled | 48 | 6% (48/809) | |
| Patient visits cancelled | 8 | ||
| Patients suffering treatment delays | 10 | ||
Cause for patients suffering treatment delay:
|
0 8 1 1 |
0% 80% (8/10) 10% (1/10) 10% (1/10) |
|
| Patients suffering treatment discontinuation | 0 | ||
| Units suffering restrictions to perform trial assessments | 8 | 26% (8/31) | |
| Potential patients not being able to be recruited into trials | 23 | ||
Alternative provided to patients not being able to be recruited into trials:
|
4 19 |
17% (4/23) 83% (19/23) |
|
| Impact on legal aspects | Units suffering impact in pending contracts (e.g. contracts postponed, or revision of budget delayed) | 15 | 48% (15/31) |
| Pending contracts postponed | 43 | ||
| Units procured with sponsor contingency plans for the management of issues related to the COVID-19 | 29 | 94% (29/31) | |
| Units developing individual contingency plans for the management of issues related to the COVID-19 | 29 | 94% (29/31) | |
| ITCC countries whose National Regulatory Agency developed contingency plans for the management of issues related to COVID-19 | 10 | 83% (10/12) | |
| Future perspectives | Unit Leads recruitment expectations in 2020 compared to 2019:
|
11 20 0 |
35% (11/31) 65% (20/31) 0% |
| Unit Leads planning changes in organisation over the next months to promote homeworking and remote MVs or SIVs | 20 | 65% (20/31) | |
| Unit Leads that believe that this crisis will make their units better prepared for future crises | 31 | 100% (31/31) | |
IMP: investigational medical product; ITCC: Innovative Therapies for Children with Cancer consortium.
MV: monitoring visit; SIV: site initiation visit; VPN: virtual private network.
Baseline data refer to units' status before 1st March. These are aggregated data from all units.
Percentages may not always sum up to 100% due to rounding error.
Among the 31 sites, 77% (n = 24) had molecular platforms.
Defined by having started trial treatment or having had their tumour profiled.
3.1. Impact on clinical research staff
Of 31 units, 21 (68%) suffered shortages of on-site staff. The median decrease in personnel across all professional categories was 30% (IQR 0–48) per site. Main causes were institutional contingency policies (58%), COVID-19–infected workers (35%) and redeployment to other hospital areas (7%).
Almost all units (28/31) managed to perform remote data entry, although 23% (n = 7) were not adequately equipped for it and reported difficulties performing trial activities from home-based offices.
3.2. Impact on on-site activities performed by sponsors
Most units experienced cancellations of site initiation visits (SIVs) (65%) and monitoring visits (MVs) (87%).
Sixty-seven percent (n = 35) of planned SIVs were cancelled, while 29% (n = 15) were performed remotely and 4% (n = 2) on-site. The decision to postpone visits was taken by sponsors in half of the cases (49%), by institutions (25%) and by sponsors and institutions together (23%)
Sixty-four percent (n = 196) of planned MVs were cancelled, whereas 35% (n = 109) were performed remotely and 1% (n = 4) on-site. The decision to postpone visits was predominantly taken jointly by sponsors and institutions (45%) or by institutions alone (42%).
3.3. Impact on recruitment
The percentage of phase I and phase II trials and molecular platforms that closed to recruitment in at least one site was 48.5% (n = 16), 61.3% (n = 19) and 64.3% (n = 9), respectively.
Eighty-one percent (n = 25) of the units halted recruitment of at least one trial or molecular platform from their own portfolio. Five sites (16%) temporarily interrupted recruitment for all active trials and molecular platforms after institutional decision. Among the other 26 sites, the proportion of industry-sponsored trials and molecular platforms that halted recruitment was 18% against the 9% of academic trials/platforms.
Fifty-nine new patients were enrolled across all units in this period, 61% less than the bimonthly 2019 average (n = 150).
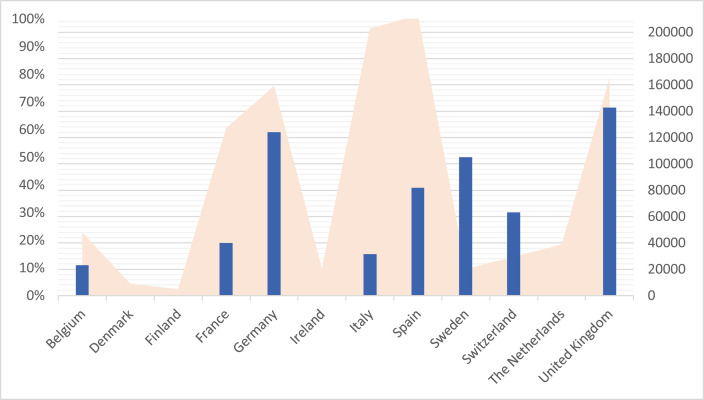
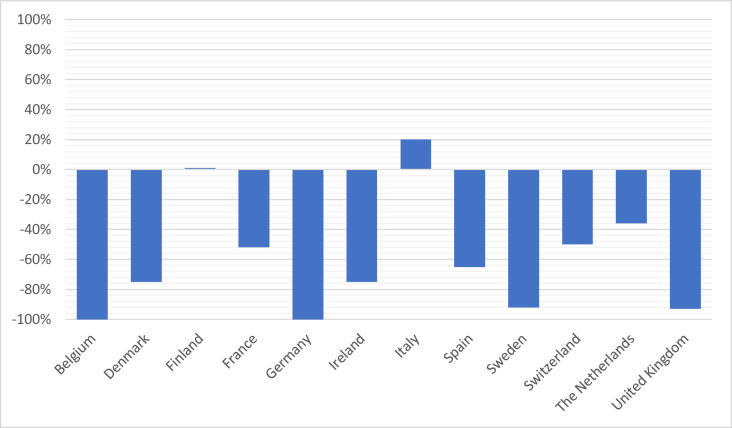
The impact on clinical trial activities, as measured by the rate of studies that were put on hold during that period (Fig. 1 ), or the relative change in the number of recruited patients compared with that in 2019 (Fig. 2 ), was heterogeneous across countries.
Fig. 1.
Overview of COVID-19 diffusion and studies interruption in ITCC countries during the time of the present survey.  The percentage of trials and molecular platforms whose recruitment was interrupted per country during the period between 1st of March and 30th April 2020.
The percentage of trials and molecular platforms whose recruitment was interrupted per country during the period between 1st of March and 30th April 2020.  COVID-19 confirmed infected cases per countries during the period between 1st of March and 30th April 2020. Based on data from the World Health Organization. Coronavirus Disease (COVID-19) Situation Report-137 Highlights Situation in Numbers (by WHO Region); 20201.
COVID-19 confirmed infected cases per countries during the period between 1st of March and 30th April 2020. Based on data from the World Health Organization. Coronavirus Disease (COVID-19) Situation Report-137 Highlights Situation in Numbers (by WHO Region); 20201.
Fig. 2.
Relative change in recruitment per country during the period between 1st of March and 30th April 2020 compared with that the 2019 bimonthly mean.
3.4. Impact on organisation of patient care
All units continued to perform on-site patient visits, but 48% (n = 15) reported limitations in providing best care for patients. Eight units (26%), seven of which were located in mixed adult–paediatric centres, had restrictions on performing trial assessments due to local strategies (e.g. procedures requiring anaesthesia because of shortage of specialists, fewer slots due to prolonged inter-patient intervals or cancellation of out-patient visits in other disciplines).
Of 809 patient visits performed, 80% were conducted on-site (n = 646), while 13% (n = 104) were conducted by phone and 7% (n = 59) in local hospitals closer to families’ home. Only eight patient visits were definitively cancelled.
Among patients being actively treated, 10 experienced treatment delays because of COVID-19–related concerns: physician's decision to guarantee patient's safety (n = 8), hospital organisational policy (n = 1) and parents' fear of COVID-19 (n = 1). No patient on the trial had study discontinuation due to COVID-19.
Twenty-three potentially eligible patients could not be screened for trials becuse of COVID-19–related constraints. Of those, 83% (n = 19) received other anticancer standard-of-care treatment, and 17% (n = 4) did not receive anticancer treatment at that time point.
3.5. Impact on supply of investigational medicinal products and research devices
Provision of IMPs was uniformly maintained at all times. Only three units (10%) suffered research devices shortage (e.g. sample kits). Forty-two percent (n = 13) of sites experienced difficulties shipping research samples.
Eighteen units could ship IMPs to patients’ homes or local hospitals. In all these cases, the sponsor supported the shipment of the IMPs. For 16 trials, eight units managed to provide larger amounts of trial medications to patients to minimise hospital visits.
3.6. Impact on administrative and regulatory aspects
Almost half of the units (48%, n = 15) suffered impact on administrative and regulatory aspects (e.g. delays in ethics committee submissions, review of trials budget), and the completion of 43 contracts was delayed.
In nearly all units (94%, n = 29), local protocols were created, and sponsors procured contingency plans for the management of COVID-19 issues. National regulatory authorities from 10 of 12 countries surveyed (83%) and developed specific guidance for the conduct of clinical trials during the COVID-19 emergency.
3.7. Future perspective
Sixty-five percent of sites foresee a similar recruitment in 2020 compared with 2019, whereas 35% lower. Most units (65%) are implementing changes to promote remote working and SIVs/MVs.
Fifty-eight percent (n = 18) of interviewees responded to the open question about COVID-19–related considerations. Homogenously, they pointed out the proactive attitude from the site staff to develop strategies to minimise potential risks to patient safety and to assure compliance with trial procedures. All investigators agreed that this episode will make units better prepared for future crises.
4. Discussion
This study reveals a profound disruption of paediatric cancer early clinical research due to the COVID-19 pandemic across Europe. Healthcare adjustments were made initially assuming that the pandemic would be brief, but we have come to realise that the pandemic and its consequences are unlikely to dissipate soon. Because children with cancer have an unmet need to access innovative therapies [21], now is the right time to think how to fulfil their needs in this ‘new normal’ setting.
With hospitals diverting resources to care for COVID-19 patients, personnel shortages were a major concern in most units. Physicians and nurses’ activities are patient-centred and therefore more susceptible to be compromised by COVID-19 mitigation measures. Nevertheless, the efforts of healthcare workers to address the issues resulting from this crisis enabled continued care provision for most patients. Other professionals, such as study coordinators or data managers, may continue to perform home-based work more easily. In this regard, most units managed remote data entry, although 23% of them reported to not having the appropriate means to do so. Efforts should be made to implement homeworking solutions under the perspective of COVID-related restrictions expected throughout upcoming months. Although reallocation of staff to other areas to fight against COVID-19 is logical in this context, it has an undeniable impact on areas with highly specialised and multidisciplinary activities, such as clinical research.
Our study shows drastic suspension of SIVs and MVs. The decisions leading to delaying SIVs are understandable because of the inherent complexity of early-phase studies. However, SIVs should be rescheduled as soon as possible to avoid lost opportunities for patients with no other therapeutic options. In our study, 15 SIVs were performed remotely, demonstrating the feasibility of this procedure. Regarding MVs, only 36% were performed. MVs ensure trial safety and data quality; their suspension may lead to unknown consequences upon the integrity of trials. Hence, it is necessary that sponsors implement alternative mechanisms to resume MVs in collaboration with research teams via reinforced remote communication. Potential impacts of weakening monitoring could be magnified by protocol deviations or delayed visits. Although sponsors provided guidelines to record deviations related to COVID-19 [22,23], regulators should take a sensible approach when reviewing these deviations, especially as most would not compromise patient safety.
More than 50% of the trials and 60% of the molecular platforms interrupted recruitment in at least one unit, and 80% of the sites halted recruitment of at least one trial or molecular platform. The proportion of industry trials stopped is twice the proportion of academic trials, highlighting the key role that academic researchers plays in times of crises and their commitment to cancer patient health over other considerations. Furthermore, patient recruitment during these two months was severely impacted with a 61% drop compared with that in 2019. The reasons for this drop cannot be explained by the halt in trial recruitment alone, but also by individual national lockdown strategies and institutional contingency policies, among others. All efforts should be made to resume recruitment as soon as possible, particularly for phase II trials or molecularly driven studies that have higher chances of success [24].
In an attempt to minimise patient risk, 77% of sites performed remote patient visits in other healthcare institutions or by phone. Although the general population was advised not to visit hospitals because of infection risk, this could constitute a major problem for patients receiving investigational treatments if adverse events do not receive the necessary medical attention or if they suffer treatments delays. Cortiula et al. [12] highlighted the negative implications of excessive focus on COVID-19 and of overshadowing other aspects of clinical practice, especially in cancer care.
Fortunately, according to our data, no patient discontinued treatment because of COVID-19. Nevertheless, 23 potentially eligible patients could not be recruited because of COVID-19–related issues. It should be pointed out that whereas COVID-19 has a low mortality rate in children [25], more than 90% of children with relapsed cancers will continue to die. Therefore, considering the safety profile and oral nature of the treatment for most novel anticancer agents, a risk–benefit assessment should be conducted, and cancer treatment continuation should be promoted. Moreover, the stalling process on experimental medicines may extend the already lengthy marketing process, meaning that some new medicines will take longer to reach the patients across the globe [26].
Several stakeholders are advocating seizing the opportunity to keep the positive clinical trial adaptations implemented during this pandemic permanently [[27], [28], [29]]. Doherty et al. [27] recently suggested changes in trial design and trial implementation so that research units can better cope with future crisis. They include generalisation of virtual visits leaving face-to-face visits for patients and trial monitors only if necessary, administration of medication at home, and efficacy assessments performed closer to patient's home. These suggestions are already in line with some of those raised by the ITCC investigators, and we agree that this is the right time to invest in structural and regulatory changes, so that clinical research is not compromised again to the extent we have seen in this crisis.
Although there are multiple publications about the impact of generic emergency situations in health settings [30,31], there is minimal information that focuses specifically on repercussions on paediatric clinical trials sites [17,18]. The American Society of Clinical Oncology recently published an analysis of the impact of COVID-19 on the conduct of oncology clinical trials [17]. Although focused on the United States research programs in adults, it reports numerous challenges with conducting clinical trials that we have also observed, including enrolment and protocol adherence difficulties or staff constraints.
We acknowledge some limitations of this study. First, its retrospective nature may have limited the accuracy of the data reported, magnified by the fact that during that period site staff were working under unprecedented pressure. Second, some countries were only represented by a fraction of their ITCC sites, therefore potentially limiting the interpretation of individual countries impact. On the other hand, the site coverage, measured by the response rate from the sites, was high (58.5%); 12 of the 14 countries that are part of ITCC were represented, and all sites provided substantially complete responses valid for the analysis; this strengthens the reproducibility and generalisation of the results.
With this study we have identified an extensive disruption in paediatric cancer research during COVID-19 crisis across Europe. We also provided insight into the efforts of sponsors and research teams to maintain access to experimental therapies despite the difficulties. Disruption of ongoing trials wastes time and resources, may reduce patient access to active treatment and carry long-term knock-on effects on drug development. To mitigate the impact, knowledge provided in this study may help reach a consensus strategy for enhancing the adaptability of paediatric clinical trials sites to future crises.
Authors' contributions
Alba Rubio-San-Simón: Study concepts, study design, data analysis, stadistical analysis, manuscript preparation, data acquisition, manuscript editing, manuscript review.
Nicolas André: Data acquisition, manuscript editing, manuscript review.
Maria Giuseppina Cefalo: Data acquisition, manuscript editing, manuscript review.
Isabelle Aerts: Data acquisition, manuscript editing, manuscript review.
Alicia Castañeda: Data acquisition, manuscript editing, manuscript review.
Sarah Benezech: Data acquisition, manuscript editing, manuscript review.
Guy Makin: Data acquisition, manuscript editing, manuscript review.
Natasha van Eijkelenburg: Data acquisition, manuscript editing, manuscript review.
Karsten Nysom: Data acquisition, manuscript editing, manuscript review.
Lynley Marshall: Data acquisition, manuscript editing, manuscript review.
Marion Gambart: Data acquisition, manuscript editing, manuscript review.
Raquel Hladun: Data acquisition, manuscript editing, manuscript review.
Claudia Rossig: Data acquisition, manuscript editing, manuscript review.
Luca Bergamaschi: Data acquisition, manuscript editing, manuscript review.
Franca Fagioli: Data acquisition, manuscript editing, manuscript review.
Ben Carpenter: Data acquisition, manuscript editing, manuscript review.
Stephane Ducassou: Data acquisition, manuscript editing, manuscript review.
Cormac Owens: Data acquisition, manuscript editing, manuscript review.
Ingrid Øra: Data acquisition, manuscript editing, manuscript review.
Antonio Juan Ribelles: Data acquisition, manuscript editing, manuscript review.
Bram De Wilde: Data acquisition, manuscript editing, manuscript review.
Pilar Guerra-García: Data acquisition, manuscript editing, manuscript review.
Marion Strullu: Data acquisition, manuscript editing, manuscript review.
Carmelo Rizzari: Data acquisition, manuscript editing, manuscript review.
Torben Ek: Data acquisition, manuscript editing, manuscript review.
Simone Hettmer: Data acquisition, manuscript editing, manuscript review.
Nicolas U. Gerber: Data acquisition, manuscript editing, manuscript review.
Christine Rawlings: Data acquisition, manuscript editing, manuscript review.
Manuel Diezi: Data acquisition, manuscript editing, manuscript review.
Sauli Palmu: Data acquisition, manuscript editing, manuscript review.
Antonio Ruggiero: Data acquisition, manuscript editing, manuscript review.
Jaime Verdú: Study concepts, study design, data acquisition, manuscript editing, manuscript review.
Teresa de Rojas: Study concepts, study design, data acquisition, manuscript editing, manuscript review.
Gilles Vassal: Data acquisition, manuscript editing, manuscript review.
Birgit Geoerger: Data acquisition, manuscript editing, manuscript review.
Lucas Moreno: Data acquisition, manuscript editing, manuscript review.
Francisco Bautista: Study concepts, study design, data analysis, stadistical analysis, manuscript preparation, data acquisition, manuscript editing, manuscript review.
Funding
JV was supported by the 2019 ITCC-Fellowship grant. There was no other specific support for this study.
Conflict of interest statement
N. André reports a consulting or advisory role for Bayer, BMS and Akina. He received funding for trial from BMS and molecules for trials from BMS and Pierre Fabre. He received travel support from BMS.
K. Nysom reports a consulting or advisory role for YmAbs and Bayer and receiving honoraria from YmAbs and Bayer.
L. Marshall reports a consulting or advisory role for Bayer, BMS, Eisai and Tesaro and receiving honoraria from Bayer for speaking in symposia/educational events.
M. Gambart reports a consulting or advisory role for Bayer and receiving grants for travel expenses from Jazz pharmaceutical and Eusapharma.
C. Rossig reports receiving honoraria for educational presentations and advice by Amgen, Pfizer, Celgene, Roche, BMS, Novartis and Genentech.
F. Fagioli reports a consultant or advisory role for Amgen, Jazz Pharmaceuticals, Pfizer, Sanofi Genzyme and Takeda.
I. Øra reports a consulting or advisory role for and receiving honoraria from Bayer.
B. De Wilde reports a consultant or advisory role for Bayer, Roche and Novartis.
C. Rizzari reports receiving personal and/or institutional grants, travel support and consultation fees from Amgen, Jazz Pharmaceuticals, SOBI and Servier.
S. Palmu reports a consulting or advisory role for Boehringer Ingelheim and receiving honoraria for educational events and travel expenses from Octapharma and Sobi.
L. Moreno reports being a member of a data monitoring committees for clinical trials sponsored by Novartis, Actuate Therapeutics, Shionogi, Incyte, the University of Southampton and the Royal Marsden NHS Foundation Trust; a consulting role for Novartis and Shionogi. He also reports being a member of the Executive Committee of SIOPEN (European neuroblastoma research cooperative group), organisation which receives royalties for the sales of dinutuximab beta. He reports that his institution receives funding from sponsors for DMC participation, advisory role or conducting industry-sponsored clinical trials.
F. Bautista reports a consultant or advisory role for Bayer, Amgen, Sanofi and EusaPharma and receiving honoraria for speaking at symposia from Amgen and Jazz Pharmaceuticals and support for attending symposia from Takeda, EusaPharma, Shire and Jazz Pharmaceuticals.
The rest of the authors declare that they have no conflict of interest.
Acknowledgements
The authors are grateful to Carole Lecinse, ITCC project manager in Gustave Roussy, for her endless support and help to communicate with each ITCC site.
They are also thankful to all the staff from the ITCC Clinical Trial Units at each site for their invaluable contribution to the advancement of research in paediatric cancer despite this very difficult situation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2020.09.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . 2020. Coronavirus disease (COVID-19) situation report-137 highlights situation in numbers (by WHO region)https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200914-weekly-epi-update-5.pdf?sfvrsn=cf929d04_2 [Google Scholar]
- 2.The common EU response to COVID-19 | European Union. https://europa.eu/european-union/coronavirus-response_en
- 3.European Centre for Disease Prevention and Control Infection prevention and control and preparedness for COVID-19 in healthcare settings - third update. https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings
- 4.Safeguard research in the time of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0852-1. [DOI] [PubMed] [Google Scholar]
- 5.Service R. ‘The disruption is enormous.’ Coronavirus epidemic snarls science worldwide. Science. 2020 doi: 10.1126/science.abb3556. [DOI] [PubMed] [Google Scholar]
- 6.McDermott M.M., Newman A.B. Preserving clinical trial integrity during the coronavirus pandemic. JAMA, J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 7.Ledford H. Coronavirus shuts down trials of drugs for multiple other diseases. Nature. 2020 doi: 10.1038/d41586-020-00889-6. [DOI] [PubMed] [Google Scholar]
- 8.EUROPEAN MEDICINES AGENCY Guidance for medicine developers and companies on COVID-19 | European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-medicine-developers-companies-covid-19
- 9.Medidas excepcionales aplicables a los ensayos clínicos para gestionar los problemas derivados de la emergencia por COVID-19 - Agencia Española de Medicamentos y Productos Sanitarios. https://www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/2020-medicamentosusohumano-3/medidas-excepcionales-aplicables-a-los-ensayos-clinicos-para-gestionar-los-problemas-derivados-de-la-emergencia-por-covid-19/
- 10.NHS Making changes to a research study to manage the impact of COVID-19 - health Research Authority. https://www.hra.nhs.uk/covid-19-research/covid-19-guidance-sponsors-sites-and-researchers/
- 11.Agenzia Italiana del Farmaco Gestione degli studi clinici in Italia in corso di emergenza COVID-19. https://www.aifa.gov.it/documents/20142/871583/Comunicato_gestione_studi_clinici_in_emergenza_COVID-19_07.04.2020.pdf/34d8c749-a329-990b-9ce3-2ea044cecc80
- 12.Cortiula F., Pettke A., Bartoletti M., Puglisi F., Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouffet E., Challinor J., Sullivan M., Biondi A., Rodriguez-Galindo C., Pritchard-Jones K. Early advice on managing children with cancer during the COVID-19 pandemic and a call for sharing experiences. Pediatr Blood Canc. 2020 doi: 10.1002/pbc.28327. [DOI] [PubMed] [Google Scholar]
- 14.Liang Wenhua, Guan Weijie, Chen Ruchong, Wang Wei, Li Jianfu, Xu Ke, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ESMO Cancer patient management during the COVID-19 pandemic | ESMO. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
- 16.Sullivan Michael, Bouffet Eric, Rodriguez-Galindo Carlos, Luna-Fineman Sandra, Saghir Khan Muhammad, Kearns Pam, et al. The COVID-19 pandemic: a rapid global response for children with cancer from SIOP, COG, SIOP-E, SIOP-PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr Blood Canc. 2020 doi: 10.1002/pbc.28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterhouse David M., Harvey R. Donald, Hurley Patricia, Levit Laura A., Kim Edward S., Klepin Heidi D., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of clinical oncology survey. JCO Oncol Pract. 2020 doi: 10.1200/op.20.00275. [DOI] [PubMed] [Google Scholar]
- 18.Rubio-San-Simón A., Verdú-Amorós J., Hladun R., Juan-Ribelles A., Molero M., Guerra-García P., et al. Challenges in early phase clinical trials for childhood cancer during the COVID-19 pandemic: a report from the new agents group of the Spanish Society of Paediatric Haematology and Oncology (SEHOP) Clin Transl Oncol. May 2020 doi: 10.1007/s12094-020-02399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno Lucas, Pearson Andrew D.J., Paoletti Xavier, Jimenez Irene, Geoerger Birgit, Kearns Pamela R., et al. Early phase clinical trials of anticancer agents in children and adolescents-an ITCC perspective. Nat Rev Clin Oncol. 2017 doi: 10.1038/nrclinonc.2017.59. [DOI] [PubMed] [Google Scholar]
- 20.Zwaan C.M., Kearns P., Caron H., Verschuur A., Riccardi R., Boos J., et al. The role of the “innovative therapies for children with cancer” (ITCC) European consortium. Canc Treat Rev. 2010 doi: 10.1016/j.ctrv.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Vassal G., Geoerger B., Morland B. Is the European Pediatric Medicine Regulation working for children and adolescents with cancer? Clin Canc Res. 2013 doi: 10.1158/1078-0432.CCR-12-2551. [DOI] [PubMed] [Google Scholar]
- 22.Myers Squibb Bristol. Investor fact sheet related to COVID-19. https://www.bms.com/assets/bms/us/en-us/pdf/investor-info/BMY-Investor-Factsheet-COVID-19.pdf
- 23.COVANCE Monitoring drug safety during COVID-19 - five key questions and answers - insights from our labs to yours. https://blog.covance.com/2020/07/monitoring-drug-safety-during-covid-19-five-key-questions-and-answers/
- 24.Bautista Francisco, Di Giannatale Angela, Dias-Gastellier Nathalie, Fahd Mony, Valteau-Couanet Dominique, Couanet Dominique, et al. Patients in pediatric phase I and early phase II clinical oncology trials at gustave roussy: a 13-year center experience. J Pediatr Hematol Oncol. 2015 doi: 10.1097/MPH.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020 doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul Steven M., Mytelka Daniel S., Dunwiddie Christopher T., Persinger Charles C., Munos Bernard H., Lindborg Stacy R., et al. How to improve RD productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010 doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 27.Doherty G.J., Goksu M., de Paula B.H.R. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Can (Que) 2020 doi: 10.1038/s43018-020-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inf Assoc. 2020 doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan A.C., Ashley D.M., Khasraw M. Adapting to a pandemic - conducting oncology trials during the SARS-CoV-2 pandemic. Clin Canc Res. 2020 doi: 10.1158/1078-0432.CCR-20-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDuffie Roberta, Summerson John, Reilly Patricia, Blackwell Caroline, Goff David, Kimel Angela R., et al. The action to control cardiovascular risk in diabetes (ACCORD) trial and Hurricane Katrina: Lessons for managing clinical trials during and after a natural disaster. Contemp Clin Trials. 2008 doi: 10.1016/j.cct.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunt H., Heenan H. Mitigating the impact of disasters and emergencies on clinical trials site conduct: a site perspective following major and minor unforeseen events. Contemp Clin Trials Commun. 2019 doi: 10.1016/j.conctc.2019.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.