Abstract
Corona virus disease 2019 (COVID-19) is triggered by the Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV2) and has rapidly developed into a worldwide pandemic. Unlike other SARS viruses, SARS-CoV2 does not solely impact the respiratory system, but additionally leads to inflammation of endothelial cells, microvascular injuries and coagulopathies, thereby affecting multiple organs. Recent reports of patients who were infected with SARS-CoV2 suggest persistent health problems even months after the initial infection. The French maritime pine bark extract PycnogenolⓇ has demonstrated anti-inflammatory, vascular and endothelium-protective effects in over 90 human clinical studies. It is proposed that PycnogenolⓇ may be beneficial in supporting recovery and mitigating symptoms and long-term consequences resulting from a SARS-CoV2 infection in COVID-19 patients.
Keywords: PycnogenolⓇ, COVID-19, SARS-CoV2, Pine bark extract, Endothelial dysfunction, Endotheliitis
1. COVID-19
1.1. COVID-19 symptoms and risk factors for a severe course of disease
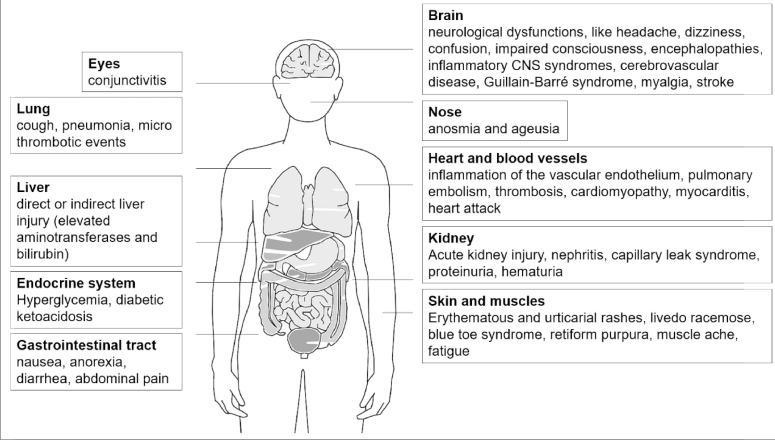
A new and highly infective corona virus, named SARS-CoV2 (Severe Acute Respiratory Syndrome Corona Virus 2), emerged in December 2019, which triggered Corona Virus Disease 2019 (COVID-19) and has led to an unprecedented worldwide pandemic [1], [2], [3], [4], [5]. Initially, the disease was thought to mainly affect the respiratory tract (hence the name `SARS´), with typical symptoms such as dry cough, sore throat, chest tightness and dyspnoea being accompanied by signs of infection (fever, throat congestion, tonsil swelling, enlarged lymph nodes, rash, fatigue, muscle aches, etc.) [6], [7], [8], [9], [10]. Atypically for SARS viruses, gastrointestinal symptoms (nausea, anorexia, diarrhoea and abdominal pain) and neurological dysfunction like olfactory and taste disorders (hypogeusia, hyposmia), headache, confusion, impaired consciousness or acute cerebrovascular disease have also been described [11], [12], [13], [14], [15], [16], [17], [18], [19]. In addition, recent studies have depicted an association with kidney diseases, like nephritis, resulting in capillary leak syndrome in severe cases of COVID-19 [20,21]. Furthermore, the dermatological symptoms that come with a SARS-CoV2 infection can be very diverse and severe, with erythematous and urticarial rashes, blue toe syndrome, vesicles lesions, retiform purpura, livedo racemose and other conditions being described in patients [22].The progression of the disease, regarding the onset and severity of the different symptoms and outcome, varies considerably between individuals [23]. Advanced age, obesity and comorbidities such as chronic heart disease, diabetes, chronic obstructive pulmonary disease and asthma increase the susceptibility of patients to the corona virus [23,24]. Interestingly, the association between diabetes and COVID-19 seems to be bidirectional, as there have been several reports of patients with new-onset type I, type II or a novel form of diabetes following a COVID-19 infection [25], [26], [27]. Furthermore, it has been suggested that glutathione deficiency has a negative influence on the progression of COVID-19, enhancing the oxidative damage of tissues [28]. Recent studies have suggested several long-term consequences and that patients who have recovered from the first COVID-19 symptoms are not necessarily completely healthy, as tracking apps for self-reported symptoms reveal [29,30]. For weeks or even months after the virus infection, affected patients have reported a persistent loss of smell and taste, fatigue, respiratory problems, rashes or different neurological problems, like memory or concentration issues [12,[29], [30], [31]]. Figure 1 and Table 1 summarise the affected organs during a SARS-CoV2 infection and provide examples of symptoms that are described in COVID-19 patients.
Figure 1.
Affected organs during COVID-19 and exemplary symptoms that are related to a SARS-CoV2 infection. In addition to pulmonary manifestations, several other organs can be impacted. The most frequent and dominant symptoms in the respective organs are summarised here.
Table 1.
Summary of the organs, route of infection, symptoms and a rough percentage of patients with the respective manifestation in COVID-19.
| Organ | Mode of virus entry (ACE2 receptor-harbouring cells) | Acute symptoms | Delayed symptoms and complications | Affected patients | References |
|---|---|---|---|---|---|
| Eyes | conjunctiva | conjunctivitis | - | 0.8–4.6% | Guan et al. [8], Zhou et al. [60] |
| Brain | haematogenous or retrograde route | headache, dizziness, confusion, impaired consciousness, encephalopathies, inflammatory CNS syndromes, cerebrovascular disease, Guillain-Barré syndrome, myalgia, stroke | delirium | 6.5–13.6% (headache) | Guan et al. [8], Wang et al. [61], Menni et al. [30] |
| Lung | epithelial cells in the respiratory tract | dry cough, sore throat, dyspnoea, pneumonia, micro thrombotic events | persistent cough, dyspnoea, chest pain | 59.4–82% (cough), 13.5–55% (dyspnoea) | Chen et al. [62], Wang et al. [61], Menni et al. [30], Huang et al. [9] |
| Nose | nasal epithelial cells | anosmia and ageusia | persistent anosmia and ageusia | 64.8–67.5% | Menni et al. [30] |
| Liver | cholangiocytes | direct or indirect liver injury | - | 10.5–18% (elevated bilirubin values) | Guan et al. [8], Chen et al. [62] |
| Heart and blood vessels | cardiovascular cells and vascular endothelium | inflammation of the vascular endothelium, pulmonary embolism, thrombosis, cardiomyopathy, myocarditis, heart attack | thrombosis | ca. 31% (thrombosis), ca. 0% (pulmonary embolism) | Klok et al. [44], Leisman et al. [35] |
| Endocrine glands | Islets of Langerhans (endocrine pancreatic beta cells) | hyperglycaemia, diabetic ketoacidosis | diabetes | ca. 6.4% (ketosis) | Li et al. [27] |
| Kidney | proximal tubular cells | acute kidney injury, nephritis, capillary leak syndrome, proteinuria, haematuria | - | 1.6–10% (elevated serum creatinine levels), ca. 98% (hypoalbuminaemia, risk for capillary leak syndrome) | Guan et al. [8], Huang et al. [9], Gross et al. [21], Chen et al. [62] |
| Gastrointestinal tract | gut enterocytes | nausea, vomiting, anorexia, diarrhoea, abdominal pain | diarrhoea, abdominal pain | 2–10.1% (diarrhoea), 2.2–21.3% (abdominal pain), 5–17.3% (nausea/vomiting) | Menni et al. [30], Chen et al. [62], Wang et al. [61], Guan et al. [8], Zhang et al. [10] |
| Skin and muscles | keratinocytes and skeletal muscle cells | erythematous and urticarial rashes, livedo racemose, blue toe syndrome, retiform purpura, muscle aches | - | ca. 0.2% (rash) | Guan et al. [8] |
| General health | Starting in the nasal epithelial cells | fatigue, fever, dysphonia | fatigue, fever dysphonia, loss of appetite | 82–98% (fever), 23.4–69.6% (fatigue) | Chen et al. [62], Wang et al. [61], Menni et al. [30], Guan et al. [8] |
Long-term and delayed complications that have already been described are listed. A detailed explanation of the virus entry mode into the respective organs and the acute and delayed symptoms can be found in the text.
1.2. Current knowledge of COVID-19 pathophysiology
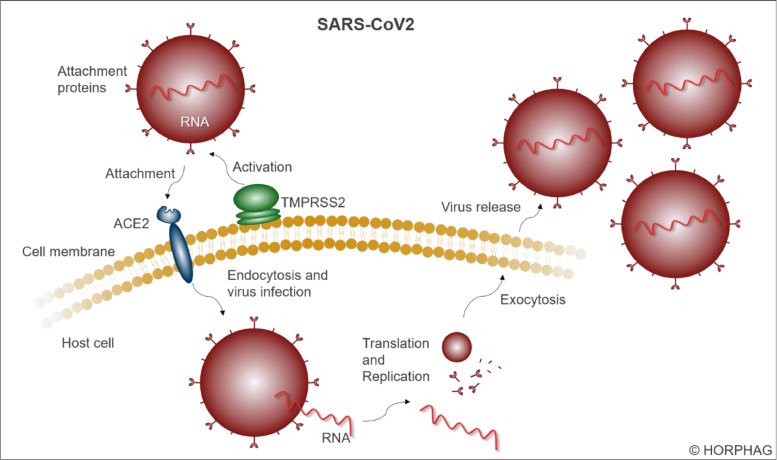
The disease apparently triggers a generalised vascular or endothelial pathology [32,33]. The virus enters cells expressing the angiotensin-converting enzyme 2 (ACE2) receptor and other receptors/facilitators on their surface that mediate the entry of SARS-CoV2, including transmembrane serine protease 2 (TMPRSS2), starting in nasal epithelial cells, which abundantly express these proteins [34], [35], [36], [37] (Figure 2 ). ACE2 receptors are densely present on the epithelial cells of the respiratory tract, but also on cell membranes of other organ tissues, like heart, kidney, stomach or colon tissue, as well as on glia cells, neurons and endothelial cells [38,39]. Being an essential part of the renin-angiotensin-system [40], ACE2 receptors are involved in the regulation of blood pressure and water balance [41]. The enzyme ACE2 metabolises angiotensin-II (AngII) to the vasodilatory and anti-inflammatory heptapeptide angiotensin (1-7) [40,41]. Upon virus admission in the early phases of infection, ACE2 is down-regulated, with loss of catalytic effect of these receptors [35]. Local AngII concentrations are consequently elevated, which results in vasoconstriction, endothelial activation and pro-inflammatory cytokine release [35]. In addition, cytokine paracrine signalling is dysregulated with the release of pro-inflammatory and anti-inflammatory molecules, as well as pro-apoptotic mediators [35,42]. Subsequently, lymphocytes are recruited through chemokines, resulting in depletion of natural killer, B-cell and T-cell decrease and possibly to lymphocytopenia, which is associated with severity of the disease [43]. This entails microvascular inflammation, triggering endothelial activation and eventual pro-thrombotic conditions [35]. Several cases of microvascular thrombosis or other thrombotic complications have been described in patients with COVID-19 [44], [45], [46], [47]. In most cases, the lungs become infected by the SARS-CoV2, in which the pneumocytes (the alveolar epithelial cells) express small amounts of ACE2 receptor proteins and TMPRSS2 protease, but enough to potentially bring this organ to its limits and to trigger a severe pneumonia [48]. The primary entrance of the virus via the respiratory epithelium can directly affect olfactory and taste abilities mediated by the olfactory epithelium [13,14]. The central nervous system can be infected when the virus uses the haematogenous or retrograde neuronal route [16,17]. To estimate the severity of neurological manifestations in COVID-19 patients, the NeuroCovid stages I–III classification scheme was proposed [49]. In stage I, the virus remains in the epithelial cells of the nose and mouth; stage II includes blood clotting; and in stage III, patients suffer from a cytokine storm that can damage the blood-brain barrier [49]. The virus spreads into the gastrointestinal tract via swallowed secretion from the nasopharynx space, where it might invade gut enterocytes, harbouring ACE2 receptors [50], [51], [52]. Additionally, cardiovascular cells expressing ACE2 receptors can be infected by SARS-CoV2, posing a risk of a severe cardiac injury [53,54]. Likewise, the liver can be potentially affected by infection of cholangiocytes [55,56]. Furthermore, the kidney mainly presents ACE2 receptors in the brush border of proximal tubular cells and podocytes, through which the coronavirus can infect this organ as well [20,57]. New-onset diabetes cases in COVID-19 patients could be explained by the expression of ACE2 receptors in endocrine pancreatic beta cells (islets of Langerhans) [25,39]. It has further been described that eyes can be infected by the virus, triggering conjunctivitis [58]. The various skin problems that occur after a SARS-CoV2 infection are possibly due to a high expression of the ACE2 receptor on keratinocytes [59]. Endothelial cells are quite strongly affected by SARS-CoV2 infection through the ACE2 receptors, in some cases leading to a severe endotheliitis, which often leads to microcirculation disorders and problems with blood clotting and coagulopathies [32,33]. Since endothelial cells line the inner membrane of blood vessels in the heart and other organs, an infection of those cells affects the whole body.
Figure 2.
Infection mechanism of a SARS-coronavirus 2. The virus can enter a cell using the attachment proteins (spikes), which are activated by the serin protease TMPRSS2 and can then attach to the transmembrane receptor ACE2. Via endocytosis, the virus infects the cell, releases the viral RNA genome, which is translated and replicated within the host. Subsequently, several copies of the virus leave the cell via exocytosis.
1.3. Kawasaki syndrome-like disease
Recent reports of increased incidences of a systemic vascular disorder in children and the SARS-CoV2 epidemic are possibly connected [63,64]. The symptoms of this disease strongly resemble Kawasaki syndrome, a mucocutaneous lymph node syndrome [63,65]. Kawasaki syndrome is a paediatric acute febrile systemic vasculitis, which can be potentially fatal [66]. Children with this disease develop high fever for at least 5 days, enlarged lymph nodes, rash in the genital area, red eyes, lips, palms and soles, a ‘strawberry tongue’, sore throat, diarrhoea, peeling skin, and coronary artery aneurysms in ca. 25% of untreated cases, which makes it the leading cause of acquired heart disease in children [66]. The disease predominantly affects children aged < 5 years and occurs relatively rarely, with ca. 25/100 000 children in the USA and ca. 30/100 000 children in Asia [66]. The cause is still unknown, but it is probably triggered by a classic antigen, possibly transferred via a viral infection that triggers the immune system [67]. Observational studies in Italy and England have shown an elevated rate of Kawasaki-like diseases [63,64]. In Bergamo, the incidence of this disease has increased by a factor of 30 since the beginning of the pandemic in February 2020. The affected children were older (7.5 vs. 3 years) and showed a higher rate of cardiac involvement compared with cases seen before the SARS-CoV2 epidemic [64]. Two London-based hospitals also found increasing numbers of patients with Kawasaki-like symptoms in communities with high rates of COVID-19, which was provisionally called paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [68], [69], [70].
2. Pycnogenol®
2.1. Description
PycnogenolⓇ is extracted from the bark of maritime pine trees (Pinus pinaster) originating from France. The result is a very fine, red/brown-coloured, water-soluble powder. PycnogenolⓇ mainly contains procyanidins and their monomers (catechin and epicatechin) as well as phenolic acids. The total amount of procyanidins is standardised to 70 ± 5%. PycnogenolⓇ meets the specifications for maritime pine extract, detailed in the United States Pharmacopeia (USP). The procyanidins are biopolymers consisting of units of catechin and epicatechin, with most chain lengths of 2–12 monomeric units [71].
2.2. Safety and toxicity
PycnogenolⓇ is self-affirmed GRAS (Generally Recognized As Safe) for use in conventional foods, based on the evaluation of clinical safety and preclinical toxicology data by an independent panel of toxicology experts. Non-mutagenicity, lethal dose (LD50) > 5.0 g/kg body weight, NOAEL > 1000 mg/kg/day have been determined [71]. Based on current European Food Safety Agency (EFSA) recommendations, this translates in a safe dosage of > 700 mg/day. Typically, oral dosages tested in the literature are in the 30–200 mg/day range, with some studies exploring higher dosages of 200–450 mg/day. Since it was introduced into the market in Europe around 1970, there have been no reports of serious adverse effects in any clinical study or from commercial use of PycnogenolⓇ. Mild side-effects of gastric discomfort have rarely been reported and linked to stomach-sensitive patients. No interactions of PycnogenolⓇ with other drugs, alcohol or food intake have been reported [71]. PycnogenolⓇ does not affect INR (a measure of bleeding tendency) or platelet function in patients taking aspirin [72]. One University study evaluated patients (n = 28; 49–73 years) with stable coronary artery disease treated with both optimal standard therapy and 200 mg/day PycnogenolⓇ for 8 weeks. Standard therapy included aspirin (100% of patients), statins (87%), ACE inhibitors/angiotensin receptor blockers (78%), β-blockers (74%), diuretics (35%), calcium antagonists (17%), clopidogrel (17%), ezetimibe (17%), oral antidiabetics (17%), phenprocoumon (4%), and α-antagonists (4%). There were no adverse drug-herb interactions [72].
2.3. Clinical studies on Pycnogenol® relating to conditions of a SARS-CoV2 infection
The symptoms of COVID-19 comprise endothelial dysfunction, coagulopathy, cytokine storm, microcirculation problems, and capillary leak syndrome. Data from clinical studies with PycnogenolⓇ exist for many of those conditions, showing beneficial effects in terms of normalising and stabilising signs and symptoms related to COVID-19 (Figure 3 ).
Figure 3.
Symptoms of a SARS-CoV2 infection and beneficial effects of PycnogenolⓇ in this context. The left box (red) summarises the most important conditions that occur in patients during COVID-19. The right box (green) shows related effects of PycnogenolⓇ showing a potential benefit through supplementation.
2.3.1. Improvement of endothelial health
Several clinical studies with patients having no or particular comorbidities showed that PycnogenolⓇ can improve endothelial function [72], [73], [74], [75], [76]. The suggested mechanism of action is activation of the endothelial nitric oxide synthase (eNOS), thus amplifying the NO generation from L-arginine, eventually leading to an increase in vessel lumen and adequate tissue perfusion. In patients with coronary artery disease, endothelial function was assessed by measuring the flow-mediated dilatation (FMD) of the brachial artery; 200 mg PycnogenolⓇ per day was supplemented in a randomised, double-blind, placebo-controlled cross-over study for 8 weeks [72]. FMD during supplementation was improved by 33%, whereas FMD slightly decreased during placebo [72]. In a double-blind placebo-controlled randomised study with hypertensive patients, endothelin-1 – which acts as a vasoconstrictor – was significantly lowered by 20% in the supplement group, whereas vasodilatory 6-keto prostaglandin F1a, the physiologically active and stable metabolite of prostacyclin, increased compared with the placebo group [76]. This indicates improved endothelial function. The patients had been taking 100 mg PycnogenolⓇ per day for 12 weeks [76]. Another double-blind, placebo-controlled study reported similar effects when supplementing type II diabetes and hypertensive patients, taking an ACE inhibitor together with 125 mg PycnogenolⓇ daily for 3 months. Here, the serum endothelin-1 levels were lowered by 17.8% compared with scarcely any change in placebo patients [74]. Nishioka et al. investigated the pharmacological effects of PycnogenolⓇ on the endothelium-dependent vasodilation via NO production by measuring the forearm blood flow in response to acetylcholine (an endothelium-dependent vasodilator) [75]. Following 200 mg PycnogenolⓇ intake per day for 14 days, forearm blood flow in response to acetylcholine of healthy volunteers significantly increased up to 41% [75]. As a negative control, the forearm blood flow was also measured in response to an endothelium independent vasodilator (sodium nitroprusside), which showed no change after PycnogenolⓇ intake compared with the placebo group. In this study, healthy individuals were supplemented with placebo or 180 mg PycnogenolⓇ per day for 2 weeks in a double-blinded fashion [75]. This is a confirmation of the beneficial effects of PycnogenolⓇ on endothelial function.
Several studies reported that the efficiency of PycnogenolⓇ on blood vessels depends on the endothelium, as it could be abolished by administration of an endothelium-specific NO synthase inhibitor or by removing the endothelial lining [75,77]. These findings suggest that PycnogenolⓇ acts by increasing NO production in the endothelium, which in turn leads to better perfusion and blood circulation within vessels [75]. As mentioned before (1.2), SARS-CoV2 strongly affects endothelial cells, triggering an inflammation and/or coagulopathies and leading to endothelial activation and pro-thrombotic conditions. Regarding endotheliitis, PycnogenolⓇ studies offer good evidence for potential beneficial effects for patients suffering from COVID-19 by improving endothelial function.
2.3.2. Improvement of microcirculation
Insufficient microcirculation is observed in diabetes, hypertension or cardiovascular and lung diseases. PycnogenolⓇ has been shown to improve this condition by strengthening the microcirculation perfusion system [78,79]. The microcirculation in fingernails, for instance, was determined by measuring the diameter of micro-vessels, which improved in patients treated with PycnogenolⓇ compared with placebo treatment [78]. In two clinical studies, the transcutaneous PO2 and PCO2 levels as well as the flux at rest and the level of venoarteriolar response were measured to investigate the effects of PycnogenolⓇ on microcirculation in patients with microangiopathy resulting from diabetes or chronic venous insufficiency [79,80]. The PO2 levels increased, whereas the PCO2 levels decreased compared with control patients upon intake of PycnogenolⓇ for 6 weeks [79]. The flux at rest was lower than at inclusion and the level of venoarteriolar response significantly increased upon supplementation [79]. Another measure for capillary leaking is the strain-gauge-derived rate of ankle swelling, which was significantly reduced after supplementation with PycnogenolⓇ in diabetic patients with microangiopathy [81]. Tissue health is tightly connected to the strength of capillary walls. Increased capillary wall strength was demonstrated after intake of PycnogenolⓇ for 3 months in a clinical study with diabetic patients suffering from retinopathy [82]. Here, retinal oedema, assessed by measuring the retinal thickness, was significantly reduced in patients taking 150 mg PycnogenolⓇ per day. This resulted in improved visual acuity in the supplemented subjects, whereas it was unchanged in control patients. In severe cases of retinopathy, dysfunctional retinal capillaries can leak, which eventually leads to irreversible vision loss. This study suggested that PycnogenolⓇ can counteract capillary leaking by strengthening the capillary walls [82]. Capillary leak syndrome is characterised by hyperpermeable capillaries through disruption of endothelial cell-to-cell binding, which results in diffusion of blood plasma into surrounding tissues or interstitial spaces [83], [84], [85]. In most cases, acute kidney injury or nephritis and severe capillary leak syndrome are found together [84]. PycnogenolⓇ supplementation has also been shown to improve kidney function in metabolic-syndrome patients with micro-albuminuria and in hypertensive patients with early signs of renal function impairment [86,87]. Urinary albumin levels significantly decreased and kidney cortical blood flow increased in patients taking PycnogenolⓇ in addition to an ACE inhibitor, compared with subjects medicated only with the ACE inhibitor for 6 months [86,87]. Microcirculatory dysfunction accompanying endothelial problems has also been reported for COVID-19 patients [32,33]; again, PycnogenolⓇ has the potential to act favourably and bring microcirculation to normal levels.
2.3.3. Platelet reactivity
The activation and subsequent aggregation of blood platelets can lead to severe, life-threatening conditions like thrombosis, stroke or heart attack. By increasing the production of endothelial NO, PycnogenolⓇ has the ability to lower blood platelet aggregation as effectively as aspirin, without increasing the bleeding time [88,89]. In individuals with increased blood platelet activity – such as smokers – PycnogenolⓇ has been shown to act dose-dependently on platelet aggregation [88,89]. This smoke-induced platelet aggregation was reduced to the level of non-smokers after supplementation with 200 mg PycnogenolⓇ per day for 2 months [89]. This effect was not observed in healthy non-smokers; hence, PycnogenolⓇ normalised pathologically-increased platelet activity, but did not further decrease normal platelet function [89]. PycnogenolⓇ prevented platelet hyperactivity but is safe, as it did not influence bleeding time, unlike aspirin, which significantly increased the time of bleeding from 167 to 236 seconds [88]. These results suggest that PycnogenolⓇ acts on platelet aggregation as effectively as aspirin, but without increasing the risk of bleeding complications [88]. It has been confirmed in a university study that PycnogenolⓇ does not further decrease platelet activity in cardiovascular patients taking aspirin [72]. This property of PycnogenolⓇ should also be helpful in COVID-19 patients. Here, the percentage of patients with blood coagulation problems or thrombosis was relatively high (40%) [35]. One reason for this could be induced morphological changes of peripheral blood cells, such as giant platelets that have been found in COVID-19 patients [90]. Along with this, several cases of acute pulmonary embolism have been described in COVID-19 patients [91,92]. Normalising the blood platelet activity by regular intake of PycnogenolⓇ might be beneficial in these cases to prevent thrombosis and pulmonary embolism in COVID-19 patients [93,94].
2.3.4. Anti-inflammatory and antioxidative aspects
Intrusion of viruses, bacteria or other pathogens activates inflammatory cascades and pathways, like the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway, resulting in the release of inflammatory mediators such as TNF-α and the interleukins IL-1 and IL-6 [95]. In severe cases of inflammation, as observed in COVID-19 patients, this might provoke a cytokine storm or hyperinflammation syndrome [35]. During an inflammation, a number of reactive oxygen species are produced, which in turn fuel the inflammasome, leading to the secretion of interleukins [96]. The antioxidant activity of PycnogenolⓇ has been investigated in a number of clinical studies [72,[97], [98], [99], [100], [101]]. Orally administered Pycnogenol® has been shown to both increase the plasma antioxidant capacity, expressed as oxygen radical absorbance capacity [99], and decrease the plasma oxidative stress measured as plasma free radicals [102]. PycnogenolⓇ has further been shown to protect lipids from peroxidation by free radicals in elderly people and people with coronary artery disease [72,97]. The protective effect of PycnogenolⓇ on DNA oxidation was shown in a randomised, double-blind, placebo-controlled study of children with ADHD, by measuring the level of oxidised purines [100]. The anti-inflammatory activity of PycnogenolⓇ has been observed in many studies [103], [104], [105], [106]. To investigate the underlying mechanism in a context close to physiology, ex-vivo approaches have been developed, in which healthy volunteers were supplemented with PycnogenolⓇ and blood samples were taken after a specific time [104,106]. The respective serum/plasma samples contain bioactive molecules and can be used in cell culture studies as active ingredients and be compared with plasma before supplementation [104,106]. Thus, by taking into account the process of absorption, distribution and gut metabolism, the molecular pharmacological mechanisms of complex plant extracts can be investigated in a more rational way compared with in vitro studies [105]. To induce inflammation ex vivo, leucocytes are primed with endotoxins such as lipopolysaccharides, which are present on the outer membrane of Gram-negative bacteria, triggering a strong immune response [107]. In addition, the leucocytes can be stimulated with formyl-methionyl-leucyl-phenylalanine (fMLP) to activate the arachidonic acid cascade, a pathway involved in inflammation, whereby cyclooxygenase (COX) enzymes 1 and 2, and 5-lipoxygenase (5-LOX) metabolise arachidonic acid to prostaglandins, prostacyclin, thromboxanes and leukotrienes [108]. After intake of 150 mg PycnogenolⓇ per day for 5 days, the serum of volunteers decreased 5-LOX and COX-2 gene up-regulation and fMLP-enhanced leukotriene biosynthesis in an ex vivo study using polymorphonuclear leukocytes [104]. Another ex vivo study employed plasma from healthy individuals consuming 200 mg PycnogenolⓇ per day for 5 days, which was incubated with monocytes and subsequently with LPS to induce inflammation [105]. Plasma samples obtained after intake of PycnogenolⓇ statistically significantly inhibited matrix metalloproteinase 9 (MMP-9) release from human monocytes and NF-κB activation as compared with control plasma samples before supplementation [105]. The PycnogenolⓇ metabolite M1 (δ-(3,4-dihydroxy-phenyl)-γ-valerolactone), which undergoes facilitated uptake by monocytes, macrophages, erythrocytes and endothelial cells, was shown to exert direct anti-inflammatory activity by reducing iNOS (inducible nitric oxide synthase) expression and excessive nitrite production [73,105,109]. In a similar setup, statistically significant inhibition of COX-1 and COX-2 was observed with serum samples of the volunteers obtained 30 minutes after a single dose of 300 mg PycnogenolⓇ [106]. In an animal-based study, the effects of PycnogenolⓇ on ventilator-induced lung injury of rats – which generally involves excessive inflammation – were investigated [103]. The production of pro-inflammatory cytokines, such as TNF-α, IL-1β, macrophage IL-6 and MIP-2 was reduced towards normal levels through the inhibition of NF-κB activation after administration of PycnogenolⓇ. The authors suggested PycnogenolⓇ to be a potential therapeutic option for ventilator-induced injury [103]. Hence, having anti-inflammatory and antioxidative effects in addition to the beneficial effects on ventilator-induced injuries, PycnogenolⓇ presents two very important advantages regarding a COVID-19 infection. Additionally, there are hints that PycnogenolⓇ metabolites M1 (δ-(3,4-dihydroxy-phenyl)-γ-valerolactone) and M2 (δ-(3-methoxy-4-hydroxyphenyl)-γ-valerolactone) bind to zinc (2+) ions [110]. Zinc ions are known modulators of antiviral and antibacterial immunity and have inflammatory regulation abilities, which could also be beneficial in COVID-19 [111].
2.4. From administration to bioactivity
The effects of PycnogenolⓇ on endothelial health, microcirculation, platelet reactivity and inflammation have been discussed above. In addition to clinical effects, the mechanisms of action have been also investigated [73,75,109,112,113]. PycnogenolⓇ mainly consists of highly condensed procyanidins [114], and the uptake of these high molecular-weight biopolymers in the gastrointestinal tract is not possible. However, the polymers are metabolised by gut bacteria yielding small molecules, which are actually taken up in the large intestine. After oral intake of single and multiple doses of PycnogenolⓇ, catechin, ferulic acid, caffeic acid, and taxifolin, a metabolite M1 (δ-(3,4-dihydroxy-phenyl)-γ-valerolactone) and further, yet unknown compounds were detected in plasma samples of volunteers [112]. The metabolite M1 is no component of the pine bark extract, but a gut microbial metabolite, which is generated from catechin. The activity of M1 was further investigated and found to dose-dependently inhibit both iNOS (inducible nitric oxide synthase) expression and excessive nitrite production, as it is observed in inflammatory states [73]. The metabolite M1 was found to be enriched in macrophages, monocytes and endothelial cells by facilitated uptake, thus explaining the rather low plasma/serum concentrations [73]. Further analysis found M1 to be transported into erythrocytes and intracellularly conjugating to a new glutathione adduct, the function of which has yet to be elucidated [109].
3. Possible treatment and support of recovery of symptoms of a SARS-CoV2 infection
Several laboratories around the world are trying to find a vaccine against the new coronavirus SARS-CoV2; some are already in clinical evaluation [115]. As long as no effective vaccine against the rapidly spreading virus is available, other measures and treatments have to be used. Non-pharmaceutical interventions, like worldwide confinement procedures, have been dominating the months since the pandemic outbreak [116]. Severely diseased patients need invasive ventilation and treatment with drugs to decrease viral replication or partially block the dysregulated immune response [117], [118], [119], [120]. One exemplary medication to potentially treat COVID-19 symptoms is remdesivir [121]. Remdesivir is a monophosphoramidate (a nucleoside analogue) and inhibits the viral RNA-dependent RNA-polymerase, which decelerates viral replication [121,122]. This effect was already shown in the previous epidemical outbreaks of SARS-CoV1 and MERS coronavirus infections and was confirmed for SARS-CoV2 [121,122]. There are, however, also reports of clinical trials in which remdesivir did not show statistically significant benefits in COVID-19 patients [123]. By the end of June 2020, remdesivir was the first treatment against COVID-19 in the EU that was approved by the European medicine agency [124]. Very recently, in a large-scale study, dexamethasone emerged as a promising drug candidate to treat COVID-19, being the first drug to lower mortality [125]. The RECOVERY (Randomized evaluation of COVID-19 therapy) trial has found improved survival in severely ill COVID-19 patients [125]. The mortality was reduced by 35% in patients on invasive ventilation and by 20% in patients only treated with oxygen [125]. Dexamethasone is a corticosteroid with strong anti-inflammatory and immunosuppressive effects that has been used to treat various health issues for decades [126]. However, dexamethasone is also associated with considerable side effects and its immunosuppressive activities might impair the production of antibodies during recovery [127,128]. In COVID-19 patients, a significant decrease of T-lymphocytes has been connected with the severity of illness [129]. In contrast, PycnogenolⓇ supplementation seems to be able to restore immune dysfunction by improving T-cell function, thus acting as an immune modulating agent [130,131]. As PycnogenolⓇ offers antioxidant and anti-inflammatory activities and positively influences endothelial cell function as well as microcirculation and platelet reactivity, a supplementation might support the management of COVID-19 patients. Since COVID-19 seems to have severe long-term consequences for health [29,30], a sustained supplementation with PycnogenolⓇ might be helpful and should be further investigated in a clinical setting.
4. Conclusions
COVID-19 can have severe and possibly persisting consequences, including endothelial dysfunction, microcirculatory problems, coagulopathy, cytokine storm and capillary leak syndrome. In various clinical studies, PycnogenolⓇ revealed positive effects relating to conditions that are also present in SARS-CoV2 infections. PycnogenolⓇ has been found to improve endothelial health and microcirculation, normalise platelet reactivity and has also been shown to have anti-inflammatory properties. Possible additional beneficial effects of PycnogenolⓇ were hypothesised for patients infected with the new coronavirus SARS-CoV2 and those who suffer from other health problems, when also complemented with standard treatment on the first day of symptoms or infection.
Declarations
Funding: No funding.
Competing Interests: The authors declare the following financial interests/personal relationships that may be considered as potential competing interests: Franziska Weichmann is an employee of Horphag Research LTD.
Ethical Approval: Not required.
References
- 1.Gorbalenya AE, Baker SC, Baric RS, De Groot RJ, Gulyaeva AA, Haagmans BL, et al. The species and its viruses – a statement of the Coronavirus Study Group. Biorxiv (Cold Spring Harb Lab [Internet] 2020:1–15. https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1.full Available from: [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aylward B, Liang W. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) WHO-China Jt Mission Coronavirus Dis 2019. 2020;2019:16–24. (February) [Google Scholar]
- 4.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26 doi: 10.1038/s41591-020-0869-5. (May) [DOI] [PubMed] [Google Scholar]
- 6.Xu* Z, Shi* L, Wang* Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. 2020:1–3. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen* Nanshan, Zhou* Min, Dong* Xuan, Qu* Jieming, Gong Fengyun, Han Yang, Qiu Yang, Wang Jingli, Liu Ying, Wei Yuan, Xia Jia'an, Yu Ting, Xinxin Zhang LZ. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020:19–21. doi: 10.1016/S0140-6736(20)30211-7. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.jin Zhang J, X Dong, yuan Cao Y, dong Yuan Y, bin Yang Y, qin Yan Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020:1–12. doi: 10.1111/all.14238. (February) [DOI] [PubMed] [Google Scholar]
- 11.Román GC, Spencer PS, Reis J, Buguet A, El M, Faris A, et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020;414:1–12. doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain [Internet] 2020;(2020):2–37. doi: 10.1093/brain/awaa240. http://www.ncbi.nlm.nih.gov/pubmed/32637987 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;(Xx Xxxx):2–3. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngology [Internet] 2020;2 doi: 10.1007/s00405-020-05965-1. (0123456789). Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menni C, Valdes A, Freydin MB, Ganesh S, Moustafa JE-S, Visconti A, et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020 2020.04.05.20048421. [Google Scholar]
- 16.Mao L, Wang M, Chen S, He Q, Chang J, Hong C, et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. SSRN Electron J. 2020 [Google Scholar]
- 17.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao F, Meiwen T, Zheng X, Liu Y, Xiaofeng L, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020:1–3. doi: 10.1053/j.gastro.2020.02.055. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Madhavan MV., Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med [Internet] 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. http://www.nature.com/articles/s41591-020-0968-3 (July)Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int [Internet] 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross O, Moerer O, Weber M, Huber TB, Scheithauer S. COVID-19-associated nephritis: early warning for disease severity and complications? Lancet [Internet] 2020;395(10236):e87–e88. doi: 10.1016/S0140-6736(20)31041-2. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criado PR, Pagliari C, Carneiro FRO, Quaresma JAS. Lessons from dermatology about inflammatory responses in Covid-19. Rev Med Virol [Internet] 2020:e2130. doi: 10.1002/rmv.2130. http://www.ncbi.nlm.nih.gov/pubmed/32656939 (May)Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv [Internet] 2020;2:1–15. doi: 10.1136/bmj.m1985. https://www.medrxiv.org/content/10.1101/2020.04.23.20076042v1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol [Internet] 2020;3026:1–8. doi: 10.1016/S2352-3026(20)30217-9. http://www.ncbi.nlm.nih.gov/pubmed/32659214 (Dic)Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-Onset Diabetes in Covid-19. N Engl J Med. 2020:1–2. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:1–2. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes, Obes Metab. 2020:1–7. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polonikov A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect Dis. 2020;(2):8–12. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 29.Drew DA, Nguyen LH, Steves CJ, Menni C, Freydin M, Varsavsky T, et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science. 2020;368(6497):1362–1367. doi: 10.1126/science.abc0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med [Internet] 2020;26 doi: 10.1038/s41591-020-0916-2. (July)Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menni C, Sudre CH, Steves CJ, Ourselin S, Spector TD. Quantifying additional COVID-19 symptoms will save lives. Lancet. 2020;395:e107–e108. doi: 10.1016/S0140-6736(20)31281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet [Internet] 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Is COVID-19 an Endothelial Disease? Clinical and Basic Evidence. Clin Basic Evid [Internet] 2020;9:1–26. doi: 10.3390/jcm9051417. https://www.mdpi.com/2077-0383/9/5/1417 (April)Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21) doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med [Internet] 2020:6–9. doi: 10.1007/s00134-020-06059-6. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Moore MJ, Vasilieva N, Sui J. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Lett to Nat. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26 doi: 10.1038/s41591-020-0868-6. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7):1–9. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012:2012. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A Novel Angiotensin-Converting Enzyme – Related to Angiotensin 1-9. Circ Res. 2000;87:1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 42.Fehr AR, Perlman S. Methods in Molecular Biology. 2015. Coronaviruses: An Overview of Their Replication and Pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan. J Chem Inf Model. 2019;53(9):1689–1699. [Google Scholar]
- 44.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res [Internet] 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. (April)Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bikdeli B, Madhavan MV., Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. 2020;(January). [DOI] [PMC free article] [PubMed]
- 48.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimer's Dis. 2020:1–17. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamers MM, Beumer J, van der Vaart J, Knoops K, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science (80-) 2020:1–11. doi: 10.1126/science.abc1669. 1 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, et al. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun [Internet] 2020;109 doi: 10.1016/j.jaut.2020.102433. (February)Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:1–8. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol [Internet] 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv [Internet] 2020 https://www.biorxiv.org/content/biorxiv/early/2020/02/04/2020.02.03.931766.full.pdf 2020.02.03.931766. Available from: [Google Scholar]
- 56.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol [Internet] 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? the Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;2019(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue X, Mi Z, Wang Z, Pang Z, Liu H, Zhang F. High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J Invest Dermatol. 2020 doi: 10.1016/j.jid.2020.05.087. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Zeng Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. bioRxiv. 2020 [Google Scholar]
- 61.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen* N, Zhou* M, Dong* X, Qu* J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(507–513) doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1315. doi: 10.1016/S0140-6736(20)31129-6. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet [Internet] 2020;6736(20):1–8. doi: 10.1016/S0140-6736(20)31103-X. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal S, Agrawal DK. Kawasaki Disease: Etiopathogenesis and Novel Treatment Strategies. Expert Rev Clin Immunol. 2017;13(3):247–258. doi: 10.1080/1744666X.2017.1232165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Vol. 135. Circulation; 2017. pp. 927–999. [DOI] [PubMed] [Google Scholar]
- 67.Franco A, Shimizu C, Tremoulet AH, Burns JC. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity. 2010;43(4):317–324. doi: 10.3109/08916930903405891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet [Internet] 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.European Centre for Disease Prevention and Control Rapid Risk Assessment: Paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children. ECDC [Internet] 2020 https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment (May). Available from: [Google Scholar]
- 70.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA - J Am Med Assoc. 2020:1–11. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliff H. ABC scientific and clinical Monograph for Pycnogenol 2019 UPDATE. Am Bot Counc Monogr. 2019:1–46. (January) [Google Scholar]
- 72.Enseleit F, Sudano I, Périat D, Winnik S, Wolfrum M, Flammer AJ, et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: A double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. 2012;33(13):1589–1597. doi: 10.1093/eurheartj/ehr482. [DOI] [PubMed] [Google Scholar]
- 73.Uhlenhut K, Högger P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol) Free Radic Biol Med. 2012;53(2):305–313. doi: 10.1016/j.freeradbiomed.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 74.Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28(5):315–320. doi: 10.1016/j.nutres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Nishioka K, Hidaka T, Nakamura S, Umemura T, Jitsuiki D, Soga J, et al. Pycnogenol®, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens Res. 2007;30(9):775–780. doi: 10.1291/hypres.30.775. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Wei J, Tan F, Zhou S, Würthwein G, Rohdewald P. Pycnogenol®, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. 2004;74(7):855–862. doi: 10.1016/j.lfs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 77.Fitzpatrick DF, Bing B, Rohdewald PJ. Endothelium-Dependent Vascular Effects of Pycnogenol. J Cardiovasc Pharmacol. 1998;32(4):509–515. doi: 10.1097/00005344-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Shiwen Wang MD, Duanjun Tan MD, Yusheng Zhao MD, Guankai Gao MD, Xue Gao LH. The Effect of Pycnogenol® on the Microcirculation, Platelet Function and Ischemic Myocardium in Patients With Coronary Artery Diseases. Eur Bull Drug Res. 1999;7(2) [Google Scholar]
- 79.Belcaro G, Cesarone MR, Errichi BM, Ledda A, Di Renzo A, Stuard S, et al. Diabetic ulcers: Microcirculatory improvement and faster healing with Pycnogenol. Clin Appl Thromb. 2006;12(3):318–323. doi: 10.1177/1076029606290133. [DOI] [PubMed] [Google Scholar]
- 80.Belcaro G, Cerasone MR, Errichi BM, Ledda A, Di Renzo A, Stuard S, et al. Venous Ulcers: Microangiopathy improvement and faster healing with local use of Pycnogenol. Angiology. 2005;56(6):699–705. doi: 10.1177/000331970505600607. [DOI] [PubMed] [Google Scholar]
- 81.Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, et al. Improvement of diabetic microangiopathy with Pycnogenol®: A prospective, controlled study. Angiology. 2006;57(4):431–436. doi: 10.1177/0003319706290318. [DOI] [PubMed] [Google Scholar]
- 82.Steigerwalt R, Belcaro G, Cesarone MR, Di Renzo A, Grossi MG, Ricci A, et al. Pycnogenol® improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J Ocul Pharmacol Ther. 2009;25(6):537–540. doi: 10.1089/jop.2009.0023. [DOI] [PubMed] [Google Scholar]
- 83.Kawabe S, Saeki T, Yamazaki H, Nagai M, Aoyagi R, Miyamura S. Systemic capillary leak syndrome. Intern Med. 2002;41(3):211–215. doi: 10.2169/internalmedicine.41.211. [DOI] [PubMed] [Google Scholar]
- 84.Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int [Internet] 2017;92(1):37–46. doi: 10.1016/j.kint.2016.11.029. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 85.Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A. 1999;96(7):3957–3962. doi: 10.1073/pnas.96.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stuard S, Belcaro G, Cesarone MR, Ricci A, Dugall M, Cornelli U, et al. Kidney function in metabolic syndrome may be improved with Pycnogenol®. Panminerva Med. 2010;52(2 Suppl 1):27–32. [PubMed] [Google Scholar]
- 87.Cesarone MR, Belcaro G, Stuard S, Scḧnlau F, Di Renzo A, Grossi MG, et al. Kidney flow and function in hypertension: Protective effects of pycnogenol in hypertensive participants-a controlled study. J Cardiovasc Pharmacol Ther. 2010;15(1):41–46. doi: 10.1177/1074248409356063. [DOI] [PubMed] [Google Scholar]
- 88.Pütter M, Grotemeyer KHM, Würthwein G, Araghi-Niknam M, Watson RR, Hosseini S, et al. Inhibition of smoking-induced platelet aggregation by aspirin and pycnogenol. Thromb Res. 1999;95(4):155–161. doi: 10.1016/s0049-3848(99)00030-4. [DOI] [PubMed] [Google Scholar]
- 89.Araghi-Niknam M, Hosseini S, Larson D, Rohdewald P, Watson RR. Pine bark extract reduces platelet aggregation. Integr Med. 2000;2(00):73–77. doi: 10.1016/s1096-2190(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 90.Lüke F, Grube M, Lubnow M, Orsó E, Kirsten J, Poeck H, et al. Coronavirus disease 2019 induces multi-lineage, morphologic changes in peripheral blood cells. eJHaem, Br Soc Haematol. 2020:1–8. doi: 10.1002/jha2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie Y, Wang X, Yang P, Zhang S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belcaro G, Dugall M, Hu S, Feragalli B, Cotellese R, Ledda A, et al. Prevention of recurrent venous thrombosis and post-Thrombotic syndrome. Minerva Cardioangiol. 2018;66(3):238–245. doi: 10.23736/S0026-4725.18.04618-2. [DOI] [PubMed] [Google Scholar]
- 94.Gulati OP. Pycnogenol® in chronic venous insufficiency and related venous disorders. Phyther Res. 2014;28(3):348–362. doi: 10.1002/ptr.5019. [DOI] [PubMed] [Google Scholar]
- 95.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 96.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan J, Croft K, Mori T, Wesnes K, Spong J, Downey L, et al. An examination of the effects of the antioxidant Pycnogenol® on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22(5):553–562. doi: 10.1177/0269881108091584. [DOI] [PubMed] [Google Scholar]
- 98.Kashevarova AA, Nazarenko LP, Skryabin NA, Nikitina T V, Vasilyev SA, Tolmacheva EN, et al. A mosaic intragenic microduplication of LAMA1 and a constitutional 18p11.32 microduplication in a patient with keratosis pilaris and intellectual disability. Am J Med Genet A [Internet] 2018:14–15. doi: 10.1002/ajmg.a.40478. http://doi.wiley.com/10.1002/ajmg.a.40478%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/30244536 (June)Available from: [DOI] [PubMed] [Google Scholar]
- 99.Devaraj S, Vega-López S, Kaul N, Schönlau F, Rohdewald P, Jialal I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids. 2002;37(10):931–934. doi: 10.1007/s11745-006-0982-3. [DOI] [PubMed] [Google Scholar]
- 100.Chovanová Z, Muchová J, Sivoňová M, Dvořáková M, Žitňanová I, Waczulíková I, et al. Effect of polyphenolic extract, Pycnogenol®, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radic Res. 2006;40(9):1003–1010. doi: 10.1080/10715760600824902. [DOI] [PubMed] [Google Scholar]
- 101.Ďuračková Z, Trebatický B, Novotný V, Žitňanová I, Breza J. Lipid metabolism and erectile function improvement by Pycnogenol®, extract from the bark of Pinus pinaster in patients suffering from erectile dysfunction - A pilot study. Nutr Res. 2003;23(9):1189–1198. [Google Scholar]
- 102.Belcaro G, Hu S, Cesarone MR, Dugall M. A controlled study shows daily intake of 50 mg of French Pine Bark Extract (Pycnogenol®) lowers plasma reactive oxygen metabolites in healthy smokers. Minerva Med. 2013;104(4):439–446. [PubMed] [Google Scholar]
- 103.Xia YF, Zhang JH, Xu ZF, Deng XM. Pycnogenol, a compound isolated from the bark of pinus maritime mill, attenuates ventilator-induced lung injury through inhibiting NF-κB-mediated inflammatory response. Int J Clin Exp Med. 2015;8(2):1824–1833. [PMC free article] [PubMed] [Google Scholar]
- 104.Canali R, Comitato R, Schonlau F, Virgili F. The anti-inflammatory pharmacology of Pycnogenol® in humans involves COX-2 and 5-LOX mRNA expression in leukocytes. Int Immunopharmacol [Internet] 2009;9(10):1145–1149. doi: 10.1016/j.intimp.2009.06.001. Available from: http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 105.Grimm T, Chovanová Z, Muchová J, Sumegová K, Liptáková A, Ďuračková Z, et al. Inhibition of NF-κB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol) J Inflamm. 2006;3:1–6. doi: 10.1186/1476-9255-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schäfer A, Chovanová Z, Muchová J, Sumegová K, Liptáková A, Ďuračková Z, et al. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol) Biomed Pharmacother. 2006;60(1):5–9. doi: 10.1016/j.biopha.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 107.Raetz CRH, Whitfield C. Lipopolysaccharide Endotoxins Endotoxins as Activators of Innate Immunity. Annu Rev Biochem. 2008;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanna VS, Hafez EAA. Synopsis of arachidonic acid metabolism: A review. J Adv Res [Internet] 2018;11:23–32. doi: 10.1016/j.jare.2018.03.005. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurlbaum M, Mülek M, Högger P. Facilitated Uptake of a Bioactive Metabolite of Maritime Pine Bark Extract (Pycnogenol) into Human Erythrocytes. PLoS One. 2013;8(4):1–10. doi: 10.1371/journal.pone.0063197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol) Free Radic Biol Med. 2004;36(6):811–822. doi: 10.1016/j.freeradbiomed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 111.Skalny AV., Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review) Int J Mol Med. 2020;19:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grimm T, Skrabala R, Chovanová Z, Muchová J, Sumegová K, Liptákova A, et al. Single and multiple dose pharmacokinetics of maritime pine bark extract (Pycnogenol) after oral administration to healthy volunteers. BMC Clin Pharmacol. 2006;6:1–12. doi: 10.1186/1472-6904-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim YJ, Kang KS, Yokozawa T. The anti-melanogenic effect of pycnogenol by its anti-oxidative actions. Food Chem Toxicol. 2008;46(7):2466–2471. doi: 10.1016/j.fct.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol®), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40(4):158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 115.World Health Organization . Draft landscape of COVID-19 candidate vaccines - 29 June 2020. Who [Internet]; 2020. pp. 1–7.https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines Available from: [Google Scholar]
- 116.Lai S, Ruktanonchai NW, Zhou L, Prosper O, Luo W, Floyd JR, et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature [Internet] 2020 doi: 10.1038/s41586-020-2293-x. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hua J, Qian C, Luo Z, Li Q, Wang F. Invasive mechanical ventilation in COVID-19 patient management: the experience with 469 patients in Wuhan. Crit Care [Internet] 2020;24(1):348. doi: 10.1186/s13054-020-03044-9. https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03044-9 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet [Internet] 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gaborit BJ, Bergmann JF, Mussini C, Arribas JR, Behrens G, Walmsley S, et al. Plea for multitargeted interventions for severe COVID-19. Lancet Infect Dis. 2020;3099(20):30312. doi: 10.1016/S1473-3099(20)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe [Internet] 2020;27(6) doi: 10.1016/j.chom.2020.04.009. 992-1000.e3. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 — Preliminary Report. N Engl J Med. 2020:1–12. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 122.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):1–15. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet [Internet] 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. Available from: http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.EMA First COVID-19 treatment recommended for EU authorisation. Eur Med Agency [Internet] 2020 Available from: https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation#:∼:text=Remdesivir is the first medicine,data as they become available. [Google Scholar]
- 125.Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. medRxiv. 2020 [Google Scholar]
- 126.Entringer S. Dexamethasone Monograph [Internet]. The American Society of Health-System Pharmacists. Available from: https://www.drugs.com/monograph/dexamethasone.html
- 127.Giles AJ, Hutchinson MKND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):1–13. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Theoharides TC, Conti P. Dexamethasone for COVID-19? Not So Fast. J Biol Regul Homeost Agents. 2020;34(3) doi: 10.23812/20-EDITORIAL_1-5. [DOI] [PubMed] [Google Scholar]
- 129.Xu B, Fan C, Wang A, Zou Y, Yu Y, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.012. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee J, Nam DE, Kim OK, Lee MY. Pycnogenol attenuates the symptoms of immune dysfunction through restoring a cellular antioxidant status in low micronutrient-induced immune deficient mice. Nutr Res Pract. 2014;8(5):533–538. doi: 10.4162/nrp.2014.8.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu FJ, Zhang YX, Lau BHS. Pycnogenol enhances immune and haemopoietic functions in senescence-accelerated mice. Cell Mol Life Sci. 1998;54(10):1168–1172. doi: 10.1007/s000180050245. [DOI] [PMC free article] [PubMed] [Google Scholar]