Abstract
Context:
Few studies have been done for correlating asthma severity, IgE level, and spirometry results with high-resolution computed tomographic (HRCT) findings in allergic bronchopulmonary aspergillosis (ABPA).
Aims:
This prospective observational study was conducted to correlate asthma severity, IgE level, and spirometry results with HRCT findings in ABPA.
Settings and Design:
Prospective observational.
Subjects and Methods:
Fifty consecutive adult patients with asthma and positive specific IgE (>0.35 kUA/L) to Aspergillus fumigatus were recruited from October 2015 to July 2017. Asthma severity, IgE levels, and spirometry results were correlated with HRCT score, bronchiectasis score, air trapping segments, and low-attenuation lung volume on inspiratory CT and expiratory CT.
Statistical Analysis Used:
One way ANOVA, Spearman's correlation coefficients.
Results:
Asthma severity showed a significant positive correlation with HRCT score and bronchiectasis score. MEF pre and postbronchodilator values showed a significant negative correlation with HRCT score, bronchiectasis score, and percentage expiratory volumes -851 to -950 HU voxels. FEV1 prebronchodilator value showed a significant negative correlation with percentage expiratory volume -851 to -950 HU voxels and percentage expiratory volume -851 to -1024 HU voxels. Specific IgE antibody level showed a significant positive correlation with bronchiectasis score.
Conclusions:
Asthma severity, specific IgE level, and MEF values showed a good correlation with HRCT findings. The restrictive pattern is common on spirometry in patients of ABPA. In addition to central bronchiectasis, peripheral bronchial and small airway involvement was an important finding in ABPA. Expiratory HRCT may reveal air trapping in patients having no abnormality on inspiratory CT.
Keywords: Allergic bronchopulmonary aspergillosis, asthma, bronchiectasis, high-resolution computed tomographic, spirometry
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity reaction to Aspergillus fumigatus colonization of the airway without tissue invasion, mostly occurring in a patient of asthma and cystic fibrosis.[1,2] The prevalence of ABPA is reported to be 1–2% in patients with asthma, 7–14% in steroid-dependent asthma, and 2–15% in cystic fibrosis.[3] ABPA causes airway inflammation and eosinophilic infiltration leading to bronchiectasis and ultimately pulmonary fibrosis. Agarwal et al. have proposed a new diagnostic criterion for allergic bronchopulmonary aspergillosis in 2013 for ABPA complicating asthma ISHAM working group.[4]
HRCT chest is essential for the disease staging, monitoring the treatment response, and for assessing the prognosis. Spirometry is commonly used for assessing the changes in pulmonary function in ABPA; however, limited literature is available regarding its correlation with HRCT findings. This study was conducted to correlate asthma severity, IgE level, and spirometry results with HRCT findings in ABPA patients. Role of expiratory CT for detection of small airway involvement in ABPA was also evaluated.
Subjects and Methods
Subjects: Consecutive adult patients diagnosed with clinical asthma and positive specific IgE (>0.35 kUA/L) for Aspergillus fumigatus referred for CT scan from pulmonary medicine department were prospectively recruited in the study from October 2015 to July 2017. The study was conducted after obtaining ethical clearance from the institutional ethics committee on April 6, 2016 (2016-68-MD-90). Informed consent was obtained from all patients.
Clinical Evaluation: The clinical features, IgE level, and recent spirometry findings (within 2 weeks of CT scan) were recorded. Patients were categorized as mild, moderate, or severe asthmatic as per the Global Initiative for Asthma (GINA) guidelines.[5]
Pulmonary Function Test: Spirometry was done on Ganshorn Medizin Electronics, Germany equipment. Following values were recorded: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and mean forced expiratory flow between 25% and 75% of FVC (MEF25–75%). Spirometry was done before and after bronchodilator therapy.
Image acquisition: Multidetector computed tomography (MDCT) thorax was done on a 128-row CT scanner (Somatom Definition AS plus; Siemens Healthcare, Germany) in the supine position and HRCT lung images were reconstructed. A non-contrast MDCT of thorax was done in maximum inspiration with following acquisition parameters: tube voltage, 120 kV; tube current 100–250 mAs depending on dose modulation; rotation time, 0.5 s; slice collimation, 128 × 0.6 mm; slice width/reconstruction increment, 1.0/1.0 mm mediastinal window, 0.6/0.6 mm lung window; reconstruction kernel, B31f medium smooth mediastinal window, B70f very sharp lung window; reconstruction window, W 400, C40, mediastinal window, and W 1500, C-500 lung window. A low-dose MDCT after prolong expiration was done next using 80–100 mAs with rest of parameters same as inspiratory scan. Radiation dose was approximately 5–7 mSv for inspiratory CT and 3 millisieverts for expiratory CT. Image analysis was done by a radiologist having 9 years of experience in chest radiology.
Image Analysis: Images were analyzed for presence and degree of bronchiectasis, the number of segments involved by bronchiectasis, axial distribution of bronchiectasis, bronchial wall thickening, mucoid impaction, centrilobular nodules, mosaic perfusion, consolidation/atelectasis, and presence of bullae and emphysema. Presence of high-attenuation mucus, lymphadenopathy, fibrosis, interlobular septal thickening, ground-glass opacities, medium size nodular opacities, and main pulmonary artery diameter was also recorded. After completing the evaluation, a composite HRCT score was assigned as per Table 1 with a maximum score of 25. Bronchiectasis score was calculated by adding scores related to bronchiectasis only (severity, extent, axial distribution, and bronchial wall thickening; maximum score 10).[6]
Table 1.
Criteria used for scoring on HRCT thorax in patients with allergic bronchopulmonary aspergillosis (Modified and adapted from Webb WR, Muller NL, Naidich DP. High-Resolution CT of the Lung. 5th ed..)[5]
| Category | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Severity of bronchiectasis | Absent | Mild (Luminal diameter greater than adjacent blood vessel) | Moderate (Lumen two to three times the diameter of the vessel) | Severe (Lumen more than three times the diameter of the vessel) |
| Extent of bronchiectasis | Absent | 1-5 segments | 6-9 segments | >9 segments |
| Axial distribution of bronchiectasis | Absent | Inner third | Up to middle third | Into the outer third |
| Bronchial wall thickening | Absent | Present (more than 0.5X compared to adjacent pulmonary artery) | ||
| Extent of mucus plugging | Absent | 1-5 segments | 6-9 segments | >9 segments |
| Extent of centrilobular nodules | Absent | 1-5 segments | 6-9 segments | >9 segments |
| Severity of mosaic perfusion | Absent | 1-5 segments | > 5 segments | |
| Severity of collapse/ consolidation | Absent | Subsegmental | Segmental or lobar | |
| Severity of emphysema | Absent | 1-5 segments | > 5 segments | |
| Severity of bullae | Absent | unilateral not more than 4 | bilateral not more than 4 | more than 4 |
HRCT: High-resolution computed tomography
Quantitative analysis of inspiratory and expiratory lung volumes was done by manually tracing the lung margins on volume software (Syngo, Siemens). Trachea and major bronchi were excluded. The total volume of the lung was calculated using the threshold value of -500 to -1024 HU. Percentage of the low-attenuation lung on the inspiratory scan was calculated by using the following cutoff thresholds; -851 to -950 HU (% Insp Vol -851 to -950 HU) and -951 to -1024 HU (% Insp Vol -951 to -1024 HU) [Figure 1]. Percentage of the low-attenuation lung on the expiratory scan was calculated by using the following cut off thresholds; -851 to -950 HU (% Exp vol -851 to -950 HU) and -851 to -1024 HU (% Exp vol -851 to -1024 HU) [Figure 2A and B]. The ratio of lung volume of -851 to -950 HU voxels from inspiratory to expiratory was also calculated (Ratio Exp Insp -851 to -950 HU). Segments of the lung affected by air trapping were recorded on expiratory CT [Figure 2C and D]. Patients having less than 500 cc decrease in lung volume on expiration were not considered for expiratory volumetric analysis and air trapping.
Figure 1.
The number of patients included for scoring and quantitative volumetric CT analysis
Figure 2 (A-D).
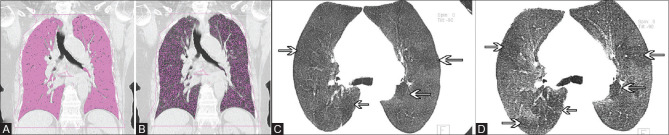
A 70-year-old woman, a case of allergic bronchopulmonary aspergillosis with mild persistent asthma, total IgE level 3117.7 IU/mL and specific IgE to A. fumigatus of 0.97 kUA/L. Spirometry was suggestive of the restrictive pattern (FEV 1 Pre 82%, FEV1 Post 82%, FVC Pre 74%, FVC post 75%, MEF pre 53%, and MEF post 52%). (A) Coronal HRCT thorax image shows the calculation of total lung volume by using the threshold value of -500 to -1024 HU (1783 cc). Thresholding only picks up aerated lung tissue (pink areas) and leaves non-aerated tissue, pulmonary vessels, etc. The central airways have also been excluded. (B) Coronal HRCT thorax image shows the calculation of low-attenuation lung volume by using the threshold value of -851 to -950 HU. By using these threshold values only low-attenuation areas of lung are picked up (pink dots). Low-attenuation expiratory lung volume was 432 c.c. (C) Inspiratory HRCT thorax axial minimum intensity projection image reveals subtle mosaic perfusion areas (arrows). (D) Expiratory HRCT thorax axial minimum intensity projection image confirms the presence of air trapping areas (arrows)
Statistical analysis
Descriptive statistics for individual variables were expressed in the form of mean and standard deviation. One-way ANOVA was used for the correlation of asthma severity with HRCT findings. Spearman correlation coefficients were calculated for correlation of IgE level and spirometry results with HRCT findings. The volume of the low-attenuation lung was also correlated with asthma severity, IgE level, and spirometry results. Total 8 HRCT variables were used for correlation; HRCT score, bronchiectasis score, air trapping segments, % Insp vol -851 to -950 HU, % Insp vol -951 to -1024 HU, % Exp vol -851 to -950 HU, % Exp vol -851 to -1024 HU, and Ratio Exp/Insp -851 to -950 HU. The statistical software package (SPSS, version 17; SPSS, Chicago, IL, USA) was used for analysis.
Results
Total 50 patients were included in the study; however, two patients were excluded from analysis as one was having nonspecific interstitial pneumonia-like, and other was having organize pneumonia-like pattern on HRCT [Figure 1]. The clinical characteristics and laboratory findings are as described in Table 2. Around 43 patients were on inhaled steroids and long-acting β2 agonist and additionally, 6 of these patients were also on systemic steroids. Three patients were on short-acting β2 agonist only. Spirometry findings are described in Table 3.
Table 2.
Clinical characteristics and laboratory findings in 48 patients with allergic bronchopulmonary aspergillosis
| Parameter | Value |
|---|---|
| Age (years) ±SD | 45.43±14.89 |
| Sex ratio M:F | 30:18 |
| Cough | 48 (100%) |
| Expectoration | 26 (54.16%) |
| Hemoptysis | 2 (4.16%) |
| History of ATT | 9 (18.75%) |
| Smokers | 7 (14.58%) |
| Severity of Asthma (GINA classification) | |
| Mild intermittent | 20 (41.6%) |
| Mild persistent | 21 (43.75%) |
| Moderate persistent | 6 (12.50%) |
| Severe persistent | 1 (2.08%) |
| AEC (SD) | 797.20±1160.06 |
| AEC >500 | 17 (35.42%) |
| IgE (IU/mL) | 3122.83±3374.73 |
| IgE >1000 IU/mL | 37 (77.08%) |
| Specific IgE (kUA/L) | 10.25±20.26 |
SD: Standard Deviation, ATT: Anti-tubercular treatment, GINA: Global Initiative for Asthma, AEC: Absolute Eosinophil Count, IgE: Immunoglobulin E, IU/mL: International Units per millilitre, kUA/L: Kilo units of allergen per litre
Table 3.
Spirometry findings in 48 patients with allergic bronchopulmonary aspergillosis
| Spirometry | Value |
|---|---|
| FEV1 prebronchodilator (percent predicted) | 54.77±17.23 |
| FEV1 postbronchodilator (percent predicted) | 57.00±16.56 |
| FVC prebronchodilator (percent predicted) | 59.77±15.74 |
| FVC postbronchodilator (percent predicted) | 62.44±15.77 |
| MEF prebronchodilator (percent predicted) | 35.56±18.31 |
| MEF postbronchodilator (percent predicted) | 38.52±20.92 |
| Obstructive pattern | |
| Mild | 5 (10.4%) |
| Moderate | 5 (10.4%) |
| Severe | 9 (18.7%) |
| Restrictive pattern | 25 (52.1%) |
| Normal | 4 (8.3%) |
FEV1: Forced expiratory volume over 1.0 second, FVC: Forced vital capacity, MEF: Mean forced expiratory flow between 25% and 75% of FVC, %: Percentage
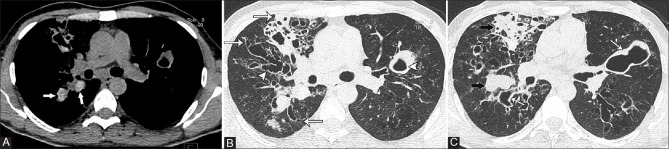
Out of a total of 48 patients, 31 had bronchiectasis while 17 had no bronchiectasis. The severity of bronchiectasis was mild in 12, moderate in 8, and severe in 11 patients. The extent of involvement was 1–5 segments in 3, 6–9 segments in 13, and more than 9 segments in 15 patients. Axial distribution of bronchiectasis was inner third lung fields in 2, up to middle third in 13, and extending into the outer third in 16 patients [Figure 3]. Mostly peripheral bronchiectasis was less prominent and extension of the central bronchiectasis; however, in two cases, peripheral bronchiectasis was more prominent [Figure 4]. Bronchial wall thickening was noted in 13 patients.
Figure 3 (A-C).
A 30-year-old male, a case of allergic bronchopulmonary aspergillosis with moderate asthma, total IgE level 850.7 IU/mL, and specific IgE 20.9 kUA/L. Spirometry shows moderate obstructive pattern (FEV1 Pre 60%, FEV1 Post 56%, FVC Pre 65%, FVC post 75%, MEF pre 57%, and MEF post 32%). (A) High-attenuation mucus (white arrow) is seen. (B) CT images reveal central (arrowheads) as well as peripheral bronchiectasis (arrows). (C) Multiple areas of mucus plugging (black arrow) and bronchial wall thickening (white arrow) are present
Figure 4 (A and B).
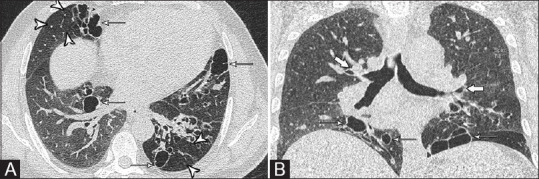
A 52-year-old male, a case of allergic bronchopulmonary aspergillosis with mild persistent asthma, total IgE 1251.9 IU/mL, and specific IgE 0.36 kUA/L. Spirometry showed moderate restrictive pattern (FEV1 Pre 59%, FEV1 Post 63%, FVC Pre 53%, FVC post 49%, MEF pre 66%, and MEF post 71%). (A) Areas of peripheral bronchiectasis (thin arrows) with mosaic perfusion (arrowheads) are seen. (B) Coronal reformatted image shows relative sparing of central bronchi (thick arrows)
Mucus plugging was seen in 15 patients, including 3 patients with high-attenuation mucus (HAM). The extent of mucus plugging was 1–5 segments in 13 and more than 9 segments in 2 patients. Centrilobular nodules were seen in 17 patients. The extent of centrilobular nodules was 1-5 segments in 13 patients and more than 9 segments in 4 patients. Mosaic perfusion was noted in 14 patients, involving 1–5 segments in 5 patients, and more than 5 segments in 9 patients. Collapse or consolidation was sub-segmental in 13 patients and segmental or lobar in 2 patients. Emphysema was noted in 3 patients; involving 1–5 segments in 2 and more than 5 segments in 1 patient. Bullae were observed in 7 patients; unilateral not more than four in number in 5 patients and bilateral not more than four in 2 patients. Out of the 17 patients without bronchiectasis, 2 had centrilobular nodules, 3 had mosaic perfusion, 2 had collapse/consolidation, 1 had collapsed with bullae, and 1 had mosaic perfusion with mucus plugging. Inspiratory HRCT chest was normal in 8 patients; however, 4 out of these patients showed air trapping on the expiratory scan [Figure 5]. Mean HRCT severity score was 6.7 (range 0–17; SD ± 5.1). Mean bronchiectasis score was 4.7 (range 0–10; SD ± 3.8).
Figure 5 (A and B).
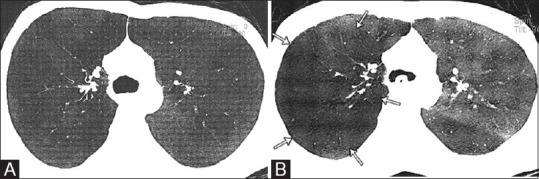
A 28-year-old male, a case of allergic bronchopulmonary aspergillosis with mild persistent asthma, total IgE 12765.1 IU/mL, and specific IgE 4.92 kUA/L. Spirometry showed restrictive pattern (FEV1 Pre 48%, FEV1 Post 52%, FVC Pre 46%, FVC post 50%, MEF pre 42%, MEF post 49%). (A) Axial CT minimum intensity projection image shows the normal appearance of lung parenchyma on inspiratory CT without any bronchiectasis. (B) Air trapping is appreciated on expiratory CT (arrows)
Fibrotic opacities were seen in 18 patients. Areas of septal thickening were noted in 5 patients and ground-glass opacities in 4 patients. Few medium size well-defined nodules were seen in 5 patients (>5 mm diameter), which were calcified in 3 patients. Mediastinal lymphadenopathy was observed in 7 patients. Mean pulmonary artery diameter was 24.3 mm (range 20–32 mm; SD ± 2.8). Minimal pericardial effusion with right chamber dilatation was observed in one patient (MPA = 28 mm). Left ventricular hypertrophy was noted in one patient.
Inspiratory volumetric analysis was done in 47 patients as one patient could not do proper inspiration. Expiratory volumetric imaging and air trapping analysis were done in 31 patients as 17 patients could not do proper expiration. Mean total inspiratory volume was 3593.9 cc (range 1983.7–6086.9cc; SD ± 1008.9). Mean percentage of voxels -851 to -950 HU on the inspiratory scan was 28.7% (range 19.7–36.7%). Mean percentage of voxels -951 to -1024 HU on the inspiratory scan was 27.7% (range 11.9–40.7%). Mean total expiratory volume was 2222.2 cc (range 529.5–4896.3 cc; SD ± 951.9) in 31 patients. Mean Percentage of voxels -851 to -950 HU on the expiratory scan was 20.2% (range 3.5–40.5%). Mean Percentage of voxels -851 to -1024 HU on the expiratory scan was 38.5% (range 4.8–67.3%). Mean ratio of expiratory to inspiratory voxels of -851 to -950 HU in 30 patients was 0.4% (range 0.02 to 1).
In 31 patients who could perform expiratory CT, air trapping was noted in 18 patients involving a mean number of 5.1 segments (range 1–13 segments). Five patients had air trapping on expiratory scan in same areas showing mosaic perfusion on the inspiratory scan. Two patients showed additional segments of air trapping on expiratory HRCT along with air trapping in areas with mosaic perfusion. In 11 patients air trapping was seen on expiratory scan without any appreciable mosaic perfusion on the inspiratory scan [Figure 4]. The result of the correlation analysis of clinical severity, IgE level, and spirometry findings with HRCT findings is shown in Tables 4 and 5.
Table 4.
Correlation of asthma severity with HRCT thorax findings in patients with allergic bronchopulmonary aspergillosis
| GINA 1 (Mild intermittent) | GINA 2 (Mild persistent) | GINA 3, 4 (Moderate, Severe) | P | |
| HRCT Score (n=48) | 2.95±3.45 | 8.09±4.10 | 13.0±3.10 | <0.01 |
| Bronchiectasis Score (n=48) | 1.70±2.71 | 6.28±3.16 | 8.28±1.79 | <0.01 |
| Airway trapping segments (n=31) | 1.27±1.79 | 3.31±3.43 | 5.25±5.56 | NS |
| % Insp vol -851 to -950 HU (n=47) | 28.39±4.06 | 29.27±4.40 | 27.53±2.90 | NS |
| % Insp vol -951 to -1024 HU (n=47) | 25.87±7.95 | 28.63±8.17 | 30.47±4.87 | NS |
| % Exp vol -851 to -950 HU (n=31) | 15.83±6.80 | 22.58±7.44 | 22.62±5.44 | NS |
| % Exp vol -851 to -1024 HU (n=31) | 31.17±15.18 | 42.46±13.54 | 43.12±10.78 | NS |
| Ratio Exp/Insp-851 to -950 HU (n=30) | 0.36±0.23 | 0.49±0.23 | 0.56±0.18 | NS |
GINA: Global Initiative for Asthma, n: Number of patients, NS: Not significant, %: Percentage, Insp: Inspiratory, Vol: Volume, HU: Hounsfield unit (CT scan), Exp: Expiratory, P-value <0.05 is taken as significant and have been marked in bold
Table 5.
Correlation of spirometry, total and specific IgE levels with HRCT thorax findings in patients with Allergic bronchopulmonary aspergillosis
| FEV1 pre | FEV1 post | FVC pre | FVC post | MEF pre | MEF post | Total IgE | Specific IgE | |
|---|---|---|---|---|---|---|---|---|
| HRCT Score (n=48) | -0.221 (.131) | -0.207 (.158) | -0.066 (.657) | 0.024 (.871) | -0.384* (.007) | -0.383* (.007) | 0.012 (.934) | 0.277 (.057) |
| Bronchiectasis Score (n=48) | -0.149 (.311) | -0.164 (.264) | -0.032 (.830) | 0.056 (.708) | -0.329* (.022) | -0.393* (.006) | 0.029 (.845) | 0.313* (.03) |
| Airway trapping segments (n=31) | -0.123 (.511) | -0.156 (.402) | -0.022 (.908) | -0.069 (.711) | -0.181 (.330) | -0.267 (.147) | 0.315 (.084) | 0.299 (.102) |
| % Insp Vol-851 to -950 HU (n=47) | -0.017 (.911) | -0.001 (.992) | 0.090 (.549) | 0.097 (.516) | -0.124 (.406) | -0.098 (.512) | -0.112 (.452) | -0.008 (.958) |
| % Insp Vol-951 to -1024 HU (n=47) | -0.112 (.455) | -0.096 (.520) | 0.020 (.896) | 0.077 (.605) | -0.210 (.156) | -0.258 (.080) | -0.290* (.048) | 0.048 (.751) |
| % Exp vol-851 to -950 HU (n=31) | -0.378* (.036) | -0.306 (.904) | -0.277 (.132) | -0.178 (.338) | -0.385* (.032) | -0.364* (.044) | -0.116 (.535) | -0.024 (.900) |
| % Exp vol-851 to -1024 HU (n=31) | -0.357* (.049) | -0.292 (.112) | -0.347 (.056) | -0.264 (.152) | -0.160 (.391) | -0.141 (.450) | -0.268 (.145) | -0.176 (.344) |
| Ratio Exp/Insp-851 to -950 HU (n=30) | -0.310 (.096) | -0.269 (.151) | -0.328 (.077) | -0.218 (.246) | -0.167 (.379) | -0.180 (.342) | -0.027 (.887) | -0.065 (.731) |
Pre: Prebronchodilator, post: Postbronchodilator, *: Significant correlation coefficient (P), n: Number of patients, %: Percentage, Insp: Inspiratory, Vol: Volume, HU: Hounsfield unit (CT scan), Exp: Expiratory, P-value<0.05 considered significant and marked in bold
MEF pre and postbronchodilator showed a significant negative correlation with HRCT score, bronchiectasis score and percentage expiratory volumes -851 to -950 HU voxels. FEV1 prebronchodilator value showed a significant negative correlation with percentage expiratory volume -851 to -950 HU voxels and percentage expiratory volume -851 to -1024 HU voxels. Specific IgE antibody level showed a significant positive correlation with bronchiectasis score while total IgE antibody level showed a significant positive correlation with percentage inspiratory volume -951 to -1024 HU.
Discussion
HRCT plays a key role in identifying the lung changes associated with ABPA.[7,8,9] Incidence of ABPA is high in asthma clinics of tertiary care institutes as in a study by Nath et al. wherein the incidence of ABPA in asthma clinic was found to be 21.7%.[10] In patients with asthma, bronchiectasis involving three or more lobes, presence of centrilobular nodules, and mucoid impaction highly suggest the possibility of ABPA.[8] In our study, 64.6% of the patient had bronchiectasis with or without other lung findings. HRCT was completely normal in 8.3% patients while 27% of patients had lung changes such as centrilobular nodules, mosaic perfusion, collapse/consolidation, mucus plugging, air trapping, and so on without the presence of bronchiectasis. As high as 78–100% prevalence of bronchiectasis has been described in patients of ABPA.[11,12] However, with the availability of serological tests and HRCT scan, many patients are now identified at an early stage without the presence of bronchiectasis referred to as serological ABPA.[13,14] Petterson et al. also suggested that the possibility of ABPA should not be dismissed in patients without bronchiectasis if clinical findings are suggestive.[1] Although historically central bronchiectasis with peripheral sparing has been described in ABPA, many reports now mention the extension of bronchiectasis into peripheral portion in 26%–39% lobes.[15,16,17,18] We have also observed peripheral bronchiectasis in 51.6% of patients (16/31) in addition to central bronchiectasis.
In patients with ABPA, the mosaic pattern on HRCT may be due to the concomitant small airways disease.[18] In our study, 22.9% of patients, air trapping on expiratory scan without any appreciable mosaic perfusion on the inspiratory scan. Arakawa et al., have also shown air trapping on expiratory scans in patients with no lung abnormality on inspiratory scans, mostly in association with bronchiolitis obliterans and asthma.[19] The presence of air trapping on expiratory scan with normal-appearing lung on inspiratory scan suggests that small airway involvement may be present without larger airway abnormality.
A statistically significant positive correlation of asthma severity was noted with HRCT score and bronchiectasis score. Although no scoring has been employed in previous studies, a good correlation of asthma severity and relapses has been shown with HRCT findings.[13] The number of air trapping segments and ratio expiratory/inspiratory lung volume of -851 to -950 HU voxels increased with disease severity; however, this correlation was not statistically significant. Quantification of air trapping using lung density on volumetric inspiratory and expiratory HRCT has been used for the estimation of airway impairment in chronic obstructive pulmonary disease.[20,21] The relative volume of lung below -950 HU on inspiratory scan corresponds to emphysema and below -856 HU on expiratory scan corresponds to air trapping.[21] Our observations suggest that HRCT accurately reflects the lung changes associated with ABPA. Larger airway changes are directly recognized on HRCT while small airway involvement is indicated by air trapping and a high proportion of low-attenuation lung volume.
Raised level of A. fumigatus specific IgE is known to be a characteristic finding of ABPA.[4] In our study, a significant positive correlation was noted between specific IgE level and bronchiectasis score. Correlation of serological severity with HRCT findings has been described in ABPA, with HAM being the most consistent marker.[13] As HAM was present in only 3 patients in our study, we could not correlate it with a specific IgE level. As no significant correlation was observed between specific IgE and HRCT score, this suggests that probably HRCT findings other than bronchiectasis do not determine serological severity. Agarwal R et al., also observed that HRCT findings other than central bronchiectasis and HAM probably represents the fibrotic, burnt-out phase of the disease and does not determine serological severity.[13]
In our study, the most common pattern seen on spirometry was restrictive (52.1%) while the obstructive pattern was seen in 39.7%. A clinically significant correlation was found between MEF pre and postbronchodilator values with HRCT score, bronchiectasis score and percentage of low-attenuation lung volume (-851 to -950 HU) on the expiratory scan. This may imply that MEF values are important in assessing the severity of ABPA. A significant negative correlation of FEV1 prebronchodilator value was also noted with the percentage of low-attenuation lung volume (-851 to -950 HU, -851 to -1024 HU) on the expiratory scan. Spirometry may show partially reversible airflow obstruction in mild or early disease whereas fixed airflow obstruction and lung volume reduction may be seen in progressive disease due to interstitial changes.[1] However, in our study, we have observed the restrictive pattern in many patients who had only mosaic perfusion or air trapping in the absence of other HRCT changes. Mean FEV1 prebronchodilator (% predicted) was 54.77% in our study. Angus also noted a mean FEV1 value of 49.4% in ABPA patients.[8] Agarwal et al., have described obstructive changes on spirometry in ABPA patients.[13] Kumar has also observed obstructive changes on spirometry, which were more severe in patients having central bronchiectasis along with other radiological features compared to those having serological ABPA or ABPA with central bronchiectasis.[22] The restrictive pattern was not described by Agarwal or Kumar. Although the results are encouraging, there are many limitations to our study. We have not excluded smokers and those on steroids, and this might influence the results and also our sample size was small.
Conclusion
In conclusion, HRCT chest is an essential tool in the assessment of the severity of ABPA and shows good correlation with asthma severity, specific IgE level, and MEF values on spirometry. Our observations suggest that although there is predominant central bronchiectasis in ABPA, peripheral bronchial and small airway involvement are important finding. Expiratory HRCT may reveal air trapping in patients with no abnormality on inspiratory CT; however, it does provide much additional information. The restrictive pattern is common on spirometry in patients of ABPA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc. 2010;7:237–44. doi: 10.1513/pats.200908-086AL. [DOI] [PubMed] [Google Scholar]
- 2.Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin Dev Immunol 2011. 2011:843763. doi: 10.1155/2011/843763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RK, Chandr A, Gautam PB. Allergic bronchopulmonary aspergillosis--A clinical review. J Assoc Physicians India. 2012;60:46–51. [PubMed] [Google Scholar]
- 4.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850–73. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma. Global Initiative for Asthma Management and Prevention. 2014 [Google Scholar]
- 6.Webb WR, Muller NL, Naidich DP. High-Resolution CT of the Lung. 5th ed. Wolters Kluwer Health; 2015. Airway Diseases; p. 569. [Google Scholar]
- 7.Agarwal R. Allergic bronchopulmonary aspergillosis: Lessons for the busy radiologist. World J Radiol. 2011;3:178–81. doi: 10.4329/wjr.v3.i7.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus RM, Davies ML, Cowan MD, McSharry C, Thomson NC. Computed tomographic scanning of the lung in patients with allergic bronchopulmonary aspergillosis and in asthmatic patients with a positive skin test to Aspergillus fumigatus. Thorax. 1994;49:586–9. doi: 10.1136/thx.49.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward S, Heyneman L, Lee MJ, Leung AN, Hansell DM, Muller NL. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol. 1999;173:937–42. doi: 10.2214/ajr.173.4.10511153. [DOI] [PubMed] [Google Scholar]
- 10.Nath A, Khan A, Hashim Z, Patra JK. Prevalence of Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma at a tertiary care center in North India. Lung India. 2017;34:150–4. doi: 10.4103/0970-2113.201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panchal N, Bhagat R, Pant C, Shah A. Allergic bronchopulmonary aspergillosis: The spectrum of computed tomography appearances. Respir Med. 1997;91:213–9. doi: 10.1016/s0954-6111(97)90041-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaur M, Sudan DS. Allergic Bronchopulmonary Aspergillosis (ABPA)-The High Resolution Computed Tomography (HRCT) chest imaging scenario. J Clin Diagn Res. 2014;8:Rc05–7. doi: 10.7860/JCDR/2014/8255.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R, Khan A, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A. An alternate method of classifying allergic bronchopulmonary aspergillosis based on high-attenuation mucus. PLoS One. 2010;5:e15346. doi: 10.1371/journal.pone.0015346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal R, Garg M, Aggarwal AN, Saikia B, Gupta D, Chakrabarti A. Serologic allergic bronchopulmonary aspergillosis (ABPA-S): Long-term outcomes. Respir Med. 2012;106:942–7. doi: 10.1016/j.rmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Prasad R, Garg R, Sanjay, Shukla AD. Allergic bronchopulmonary aspergillosis: A review of 42 patients from a tertiary care center in India. Lung India. 2009;26:38–40. doi: 10.4103/0970-2113.48895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar A, Mukherjee A, Ghoshal AG, Kundu S, Mitra S. Occurrence of allergic bronchopulmonary mycosis in patients with asthma: An Eastern India experience. Lung India. 2010;27:212–6. doi: 10.4103/0970-2113.71949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–8. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Khan A, Garg M, Aggarwal AN, Gupta D. Pictorial essay: Allergic bronchopulmonary aspergillosis. Indian J Radiol Imaging. 2011;21:242–52. doi: 10.4103/0971-3026.90680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakawa H, Webb WR. Air trapping on expiratory high-resolution CT scans in the absence of inspiratory scan abnormalities: Correlation with pulmonary function tests and differential diagnosis. AJR Am J Roentgenol. 1998;170:1349–53. doi: 10.2214/ajr.170.5.9574614. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Watanabe N, Nakajima Y. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008;190:762–9. doi: 10.2214/AJR.07.2820. [DOI] [PubMed] [Google Scholar]
- 21.Mascalchi M, Camiciottoli G, Diciotti S. Lung densitometry: Why, how and when. J Thorac Dis. 2017;9:3319–45. doi: 10.21037/jtd.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R. Mild, moderate, and severe forms of allergic bronchopulmonary aspergillosis: A clinical and serologic evaluation. Chest. 2003;124:890–2. doi: 10.1378/chest.124.3.890. [DOI] [PubMed] [Google Scholar]