Abstract
1. Host behaviour is known to influence disease dynamics. Additionally, hosts often change their behaviours in response to pathogen detection to resist and avoid disease. The capacity of wildlife populations to respond to pathogens using behavioural plasticity is critical for reducing the impacts of disease outbreaks. However, there is limited information regarding the ability of ectothermic vertebrates to resist diseases via behavioural plasticity.
2. Here, we experimentally examine the effect of host behaviour on ranaviral infections, which affect at least 175 species of ectothermic vertebrates. We placed metamorphic (temporal block 1) or adult (block 2) Southern toads (Anaxyrus terrestris) in thermal gradients, tested their temperature preferences before and after oral inoculation by measuring individual-level body temperature over time, and measured ranaviral loads of viral-exposed individuals.
3. We found significant individual-level variation in temperature preference and evidence for behavioural fever in both metamorph and adult A. terrestris during the first two days after exposure. Additionally, we found that individual-level change in temperature preference was negatively correlated with ranaviral load and a better predictor of load than average temperature preference or maximum temperature reached by an individual. In other words, an increase in baseline temperature preference was more important than simply reaching an absolute temperature.
4. These results suggest that behavioural fever is an effective mechanism for resisting ranaviral infections.
Keywords: Thermoregulation, behavioural fever, amphibian declines, ranavirus, disease ecology, thermal biology
Graphical Abstract:

Introduction
Pathogens can impose strong selective pressures on their hosts, driving the evolution of host behaviours that reduce disease risk (Moore 2002, Han, Bradley et al. 2008). Additionally, many hosts cope with pathogens via phenotypic plasticity, modulation of trait values within the lifespan of the host (Agrawal 2001). For example, some ectothermic hosts can respond to pathogen exposure by exhibiting a behavioural fever, which is an acute increase in temperature preference (Tpref) (Kluger, Kozak et al. 1998). The slightly warmer environmental temperatures chosen by the host after pathogen exposure are presumably more favorable for the host than the pathogen (Reynolds, Casterlin et al. 1977, Burns, Ramos et al. 1996, Ouedraogo, Goettel et al. 2004). Fever is primarily considered a method for improving host immune function by stimulating immunological defenses (Evans, Repasky et al. 2015). However, fever has also been proposed as a mechanism for directly killing or slowing pathogen growth with heat (Richards-Zawacki 2009). Understanding how phenotypic plasticity, such as behavioural fever, can limit infection and lower disease risk is crucial for predicting how hosts might be affected by emerging diseases in a changing climate. This is especially true for ectothermic hosts, which cannot regulate body temperature independent of the environment and are especially sensitive to climatic abnormalities.
Studies demonstrating that ectothermic hosts use behavioural fever as an effective method of pathogen resistance, defined as a host strategy that limits or inhibits infection (Roy and Kirchner 2000), are limited (Adamo and Lovett 2011, de Roode and Lefèvre 2012). Here, we test for an effective behavioural fever response to a ranavirus in an amphibian. Ranavirus is an emerging, widespread viral disease caused by viruses in the genus Ranavirus. It has caused mass mortality events in amphibian hosts contributing to amphibian population declines in last few decades (Chinchar 2002, Brunner and Yarber 2018). Ranaviruses are also known to cause frequent infection in other ectothermic vertebrates, such as in fishes and reptiles (Chinchar 2002, Duffus, Waltzek et al. 2015). Some of these viruses are even capable of transmission across taxa, or interclass transmission (Brunner, Schock et al. 2004, Bandín and Dopazo 2011). Hosts likely encounter ranaviruses frequently because of their broad distributions and lack of host specificity (Miller, Gray et al. 2011), and thus ranaviruses might impose strong selective pressures on hosts.
In fact, mounting evidence suggests that many hosts are adapting immunological and behavioural strategies to combat ranaviral infections (Whittington, Philbey et al. 1994, Parris, Davis et al. 2004, Teacher, Garner et al. 2009, Duffus, Waltzek et al. 2015). For example, larval amphibians appear to prefer warmer temperatures while infected with ranaviruses (Parris, Davis et al. 2004). However, this study did not measure temperature preference before exposure and therefore could not differentiate between preexisting differences in Tpref and differences caused by exposure to ranavirus. Additionally, this study did not measure ranaviral load or prevalence, so there is no evidence that the apparent difference in Tpref is effective at resisting infection. Other studies have shown that experimentally warming ranavirus-infected hosts can reduce host mortality and viral loads (Rojas, Richards et al. 2005, Echaubard, Leduc et al. 2014) despite successful ranviral growth in culture at these higher temperatures (Chinchar 2002). In these studies, the warming was induced by the experimenters, not the hosts, and exceeded the magnitude and duration of most behavioural fever responses. Additionally, these studies only tested for temperature effects in larval amphibians. Hence, it is unclear if hosts more generally respond to ranaviral infections with behavioural fever and whether fever is effective at reducing ranaviral loads within hosts.
Here, we test whether metamoprhic and adult Southern toads, Anaxyrus terrestris, adjust their preferred temperature after infection with ranavirus and whether any change in temperature preference reduces ranaviral load within hosts. To accomplish these goals, we exposed A. terrestris to a ranaviral isolate in thermal gradients ranging in temperature from 12–33 °C (Sauer, Sperry et al. 2016) to assess individual Tpref before and after exposure to this virus. We also measured viral load within individual hosts to assess whether variation in Tpref affected virus replication.
Materials and Methods
Animal husbandry
Adult and metamorphic A. terrestris were collected from Hillsborough County, Florida from sites where ranavirus has not previously been detected. Individuals were maintained in individual containers (23.5 × 16.8 × 10 cm or 11.7 dia. X 13.5 cm, respectively) on top of folded paper towels soaked with artificial spring water (Cohen, Neimark et al. 1980). These toads were held in a laboratory maintained between 24–25°C with a 12h photoperiod for at least two weeks before the start of the experiment. The toads were fed mineral-dusted crickets ad libitum until the start of the experiment and their containers and paper towels were changed weekly. Only adult individuals were tested for ranavirus before exposure (see Ranaviral exposures and quantification for details) as metamorphic A. terrestris were too small to safely test using the oral swabbing methods described below.
Experimental design
Experimental design was based on a prior experiment testing for behavioural fever in amphibians to the chyrid fungus and thus we only briefly describe the methods here; see Sauer, Fuller et al. (2018) for additional details on the design. Experiments were conducted in Tampa, Florida with two temporal blocks, the first being conducted with metamorphs and the second with adults. In each experiment, we first measured baseline non-infected temperature preference (baseline Tpref) in thermal gradient apparatuses (see next paragraph for the frequency of these measurements). These apparatuses were previously shown to provide variation in temperature that is independent of humidity and which does not confound amphibian and prey temperature preferences (see Fig. S1 and supplemental methods, and Sauer et al. (2016) for thermal gradient construction and validation details). The apparatuses provided an ecologically relevant ambient temperature range of 12 to 33°C (Fritts, Grodsky et al. 2015, Sauer, Sperry et al. 2016, Sauer, Fuller et al. 2018). After measuring baseline Tpref, individuals were split into two groups of similar mean body masses and baseline Tpref (N = 53), 1) a sham-exposed control group (metamorph: n = 12; adult n = 14) and 2) a ranavirus-exposed treatment group (metamorph: n = 13; adult n = 14). While individual-level body mass was consistent across treatments within a block, body mass was much smaller in the metamorph (mean mass = 0.48 g ± 0.05 SE) than adult block (mean mass = 17.70 g ± 1.42 SE).
Throughout the experiment, temperature measurements were taken each day, every four hours, between 10:00h and 22:00h (two during the lighted part of the photoperiod and two during the dark) at the center of each animal’s dorsum (Rowley and Alford 2007) (Extech® High Temperature IR Thermometer; accuracy: ±2% < 932°F). The only exception is that these measurements were not taken during feeding periods, during which all individuals were fed 10 live crickets in containment bags to prevent crickets from moving freely within the thermal gradient (see Sauer, Sperry et al. (2016) and Figure S4 for more details). Temperature measurements were taken for four days before ranaviral or sham exposure. The mean Tpref of those four days for each individual toad is referred to hereafter as the baseline Tpref ( in equations). Temperature measurements were taken daily for two weeks (metamorph block) or four days (adult block) after ranaviral or sham exposure. We shortened our time between exposure and sampling for the adult block after discovering that the metamorphs were uninfected two weeks after exposure to ranavirus.
Ranaviral exposures and quantification
We used a ranavirus strain isolated from infected wood frogs in Michigan with 99% similarity to Frog Virus 3 (GenBank Accession number: PRJNA504607). We cultured the virus on fathead minnow cells and Eagle’s minimum essential media containing 5% fetal bovine serum (MEM) to a titer of 3.6×105 plaque-forming units (PFU) mL−1. The virus was stored at −80°C until used in the experiments. Before exposure, virus stock was thawed and homogenized and then each individual was dosed orally with 77 µL of the virus (2.8×104 PFU) or the same volume of a sham inoculum of MEM. The individuals from the metamorph block were euthanized two weeks after ranavirus exposure and dissected to remove spleen, kidney, and liver for quantification of ranaviral loads. The individuals in the adult block were sampled prior to exposure, to ensure they were not already infected, then sampled again four days after exposure by inserting and twirling a sterile swab in their mouth for thirty seconds and then freezing these swabs at −80°C (Allender, Mitchell et al. 2013). Non-destructive sampling via oral swab was preferred because it allowed us the option to sample individuals multiple times. However, we had to destructively sample metamorphic individuals because they were too small to swab orally.
Ranaviral DNA was extracted from each metamorph and adult sample using the Quiagen DNeasy Blood and Tissue protocol (Qiagen, Inc., Valencia, CA). To determine viral load, we used qPCR methods based on Forson and Storfer (2006), with a 250-bp fragment of the major capsid protein (MCP) gene used as a standard (gBlocks® plasmid-based standards; Integrated DNA Technologies, Skokie, IL). The qPCR reaction mixture contained 10 μL TaqMan® Universal Master Mix (Applied Biosystems, Foster City, CA), 0.6 μL of primer (Thermo Fisher Scientific, Waltham, MA), 0.625 μL of TaqMan® TAMRA florescent probe (Applied Biosystems, Foster City, CA), and 6.275 μL of nuclease-free water per well with 2.5 μL of either DNA sample, standard, or nuclease-free water added to each well. All samples and standards were run in duplicate and thus, an individual-level load represents the mean DNA copies of these two subsamples. The duplicates agreed in all but one instance where one of the two did not amplify. For that individual we ran a third subsample, which did amplify and used the mean of the two positive subsamples.
Data analysis
All statistics were conducted with R 3.4.0 (Team 2017). To test for repeatability in baseline Tpref within individuals and variation in baseline Tpref among individuals, we conducted a one-way repeated measures ANOVA (stats package, aov function). This analysis tested whether baseline Tprefs of individuals varied significantly across days (main effect of day) and whether they varied among individuals (among-individual variance). Using the ANOVA table from this analysis, we calculated repeatability or the variance explained by individual-level behaviour: (; where MSW is the within-group variance component and MSA is the among-groups variance component (Lessells and Boag 1987).
To test for behavioural fever, we conducted multiple two-factor (treatment and time) repeated measures linear mixed effects models with individual treated as a random effect (lme4 package, lmer function) followed by log-likelihood ratio tests to determine significance (car package, Anova function). For each model, we paired baseline Tpref with each post-exposure day Tpref (time; one model for each post exposure day) and looked for an interaction between treatment (ranavirus-exposed or sham-exposed) and time on the z-score of ΔTpref. Change in Tpref (ΔTpref) is calculated as:
where is the temperature preference for individual i at time point j, and is the mean temperature preference of all baseline time points for individual i. Before statistical analysis, we transformed our data to standardized deviations away from the mean of the ΔTpref of all individuals in a day. This ensured that temperature preferences were independent of any change in room temperature over time and that we were comparing Tprefs between treatments within rather than across days. A significant interaction between treatment and time would mean that the two treatments behaved differently after ranaviral or sham exposure.
To test whether individual-level Tpref affects ranaviral load (log-transformed ranaviral DNA copies divided by mass of the individual), we conducted multiple linear regressions (stats package, glm function; normal error distribution). Each analysis tested for an effect of one of three metrics for body temperature on ranaviral load during a specified time interval. Our three metrics for body temperature were: 1) ΔTpref (), 2) mean Tpref (mean of the four body temperature measurements per day), and 3) maximum Tpref (maximum of the four body temperature measurements per day). We conducted an analysis for each of the first four days after exposure (one model per independent variable, per day; 12 total models) as well as pooled across all four days (one model per independent variable; 3 total models). Additionally, we tested for an effect of baseline Tpref on ranavial load to check for underlying differences in susceptibility that happened to be correlated with baseline Tpref.
Results
There was no mortality during either block of this experiment. Before ranaviral exposure, we were able to detect consistency in the baseline Tpref of individuals (repeatability: r > 0.98; Fig. S2) and variation in baseline temperature preferences among individuals (all blocks and treatments combined; main effect of individual on baseline Tpref: F52,1537 = 7.60, P < 2.0×10−16). A. terestris metamorphs and adults did not have significantly different mean Tprefs (F1,51 = 3.90, P = 0.54) and together their mean Tpref was 23.07 °C ± 0.66 SE. For both temporal blocks, we found evidence of behavioural fever after ranaviral exposure (effect of the interaction between treatment and time; metamorphs: day 1 χ2 = 22.0, P < 0.001 and day 3 χ2 = 5.70, P = 0.017; adults: day 2 χ2 = 5.51, P = 0.019; Fig 1 & Table S1). One day after exposure, metamorphs exposed to ranavirus increased their preferred temperature by 3.52 °C ± 0.78 SE relative to controls (Fig. 1A). Two days after exposure, adults exposed to ranavirus significantly increased their preferred temperature by 1.43 °C ± 0.59 SE relative to controls (Fig. 1B). We did not test whether the magnitude of behavioural fever between metamorphs and adults were different, because the two life stages were not tested simultaneously.
Figure 1 |.
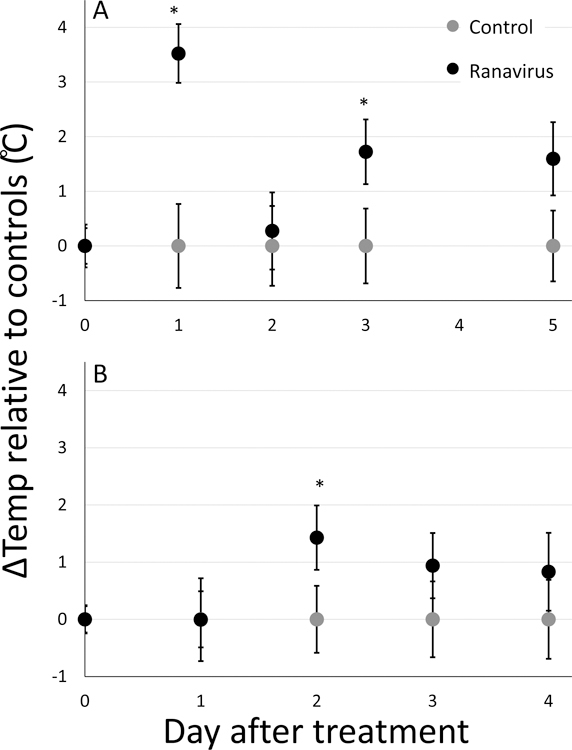
Difference between the daily mean for each treatment group and sham exposed (control) treatments () in change in individual-level temperature preference () through time for a) metamorphic and b) adult Anaxyrus terrestris toads. Where is the temperature preference for individual i at time point j and is the mean temperature preference of all control animals from time point j. Asterisks denote time points where the exposed and sham-exposed groups differ significantly (P < 0.05) error bars represent ±1 SE.
For metamorphs, we were unable to detect ranavirus in sampled toads two weeks after exposure. For adults, we intentionally sampled after four days rather than 14 days in the hopes that we would detect ranavirus before it was cleared. We detected ranavirus in 92.9% of the adult toads. We found that ΔTpref (t11 = −4.89, P < 0.001; Fig. 2B), mean Tpref (t11 = −3.11, P = 0.01; Fig. S3), and max Tpref (t11 = −3.1, P = 0.004; Fig. S4) two days after exposure were all associated negatively with ranaviral load four days after exposure. Additionally, ΔTpref on day one, three, and four post-exposure were also significant negative predictors of ranaviral load on day four (t11 = −3.19, P = 0.009; t11 = −2.42, P = 0.03; t11 = −3.78, P = 0.003, respectively; Fig. 2). Finally, we found a positive effect of mean ΔTpref (t11 = −4.77, P < 0.001) and mean Tpref (t11 = −2.42, P = 0.03) on ranaviral load but no effect of overall max Tpref (t11 = −1.23, P = 0.25), and baseline Tpref (t11 = 1.04, P = 0.32) on ranaviral load.
Figure 2|.
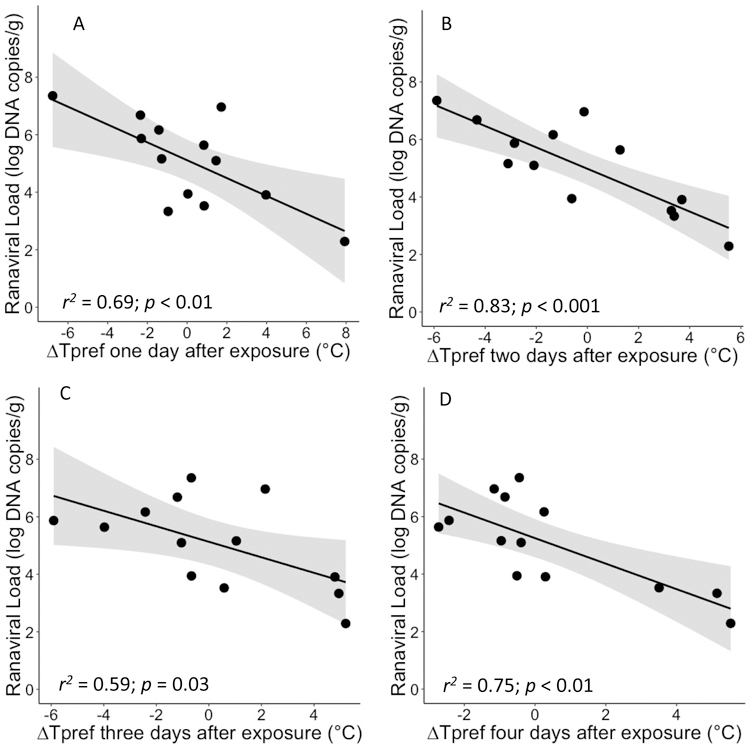
Relationship between change in individual-level temperature preference () A) one, B) two, C) three, and D) four days after ranaviral exposure and ranaviral loads on adult Anaxyrus terrestris. Where is the temperature preference for individual i at time point j and is the mean temperature preference of individual i prior to ranaviral exposure. Frogs that exhibited the greatest increase in Tpref had the lowest ranaviral abundance (t11 = −4.89, P < 0.001). The shaded gray area represents the 95% confidence band.
Discussion
We set out to determine whether A. terrestris responded to ranaviral exposure with behavioural fever and whether fever facilitated ranaviral resistance by limiting pathogen loads. By measuring the thermal preference of individuals in thermal gradients both before and after exposure, we found that A. terrestris individuals responded to ranaviral exposure with behavioural fever. We also demonstrated that individual-level change in Tpref during the first two days after exposure was the greatest predictor of ranaviral load in adult toads. Individuals that increased their Tpref the most had the lowest ranaviral loads. These results suggest that behavioural fever appears to be effective at resisting ranaviral infections.
We demonstrated that variation in baseline Tpref among individuals before ranaviral exposure was greater than the variation in baseline Tpref within individuals (Fig. S2). In other words, individuals showed consistency in their preferred temperature through time and individual toads exhibited different preferred temperatures. However, once exposed to ranavirus, we found that individuals moved from their baseline Tpref to warmer locations. For A. terrestris metamorphs, this behavioural fever response peaked one day after exposure while for adults, it peaked two days after exposure (Fig. 1). These differences might be partly because of large differences in body size. As both blocks were exposed to the same dose, the dose per g was higher in the metamorph than adult block. Additionally, given that there is a negative relationship between body size and metabolic rates and processes, immune and fever responses to ranavirus might have been triggered more quickly in the smaller-bodied metamorphs than adults (Garner, Rowcliffe et al. 2011, Rohr, Civitello et al. 2018).
We did not sample metamorphic A. terrestris for ranavirus until 14d after exposure, and by that time, no individuals were infected. Thus, we do not discuss the effects of thermoregulatory behavour on ranaviral loads in the metamorph block. For adult A. terrestris, which were sampled for ranavirus 4d after exposure, there was a significant negative correlation between ranaviral load four days post exposure and ΔTpref, mean Tpref, and maximum Tpref during the second day post exposure (Fig. 2). We also found that ΔTpref was negatively correlated with ranaviral loads across all four days post exposure. Thus, an increase in temperature, regardless of an individual’s baseline Tpref, helped to reduce ranaviral infection. We did not find the same overall effect of mean Tpref or maximum Tpref on ranaviral load; in fact, maximum Tpref was the worst predictor of ranaviral load of the three measurements (ΔTpref, mean Tpref, and maximum Tpref). This result supports the hypothesis that the main purpose of fever is to increase the immune system’s efficiency by raising body temperature to promote both innate and adaptive immunity (Evans, Repasky et al. 2015, Rakus, Ronsmans et al. 2017, Boltana, Aguilar et al. 2018), not to maximize absolute preferred temperature within the range that a host can tolerate. Though fever or increased temperature can slow or stop pathogen growth directly for some host-pathogen systems (Anderson, Blanford et al. 2013, Sauer, Fuller et al. 2018), any increase in body temperature from baseline Tpref should benefit the host. Behavioural fever should be beneficial to the host even if the pathogen tends to grow better in warmer temperatures; as is the case for ranaviruses and many other viral and bacterial pathogens, to which fever is a common method of resistance (Evans, Repasky et al. 2015). Assuming the temperature increase falls within the bounds of the host’s thermal performance breadth (Evans, Repasky et al. 2015, Cohen, Venesky et al. 2017, Sauer, Fuller et al. 2018). For example, some ectothermic host species that are adapted to cooler climates cannot tolerate temperature increases when infected with ranavirus (Bayley, Hill et al. 2013, Brand, Hill et al. 2016) and other pathogens (Thomas and Blanford 2003, Cohen, Civitello et al. 2018).
While we do not have any load data for metamorphic A. terrestris, the ranaviral load results from the adults suggests that behavioural fever increased A. terrestris resistance to ranavirus. Interestingly, previous studies have shown that ranavirus grows in vitro and in vivo up to temperatures even higher than those reached here by feverish A. terrestris (Chinchar 2002, Ariel, Nicolajsen et al. 2009). Thus, the decrease in ranaviral load associated with behavioural fever might be the result of a host-mediated mechanism and not a direct effect of increased temperature. Behavioural fever might be one way that ectothermic hosts resist ranaviral infections, but it is unclear how widespread that response is given the paucity of studies examining behavioural fever as a mechanism for resistance to ranaviruses (Parris, Davis et al. 2004), especially if the fairly heat-tolerant A. terrestris exhibits thermal regulatory behaviour that is not representative of more cold-adapted species (Sauer, Fuller et al. 2018). Nevertheless, assuming that behavioural fever is in part used as a method of improving immune efficiency and not simply as a method of heat-killing the pathogen, this strategy could be employed more broadly by ectothermic hosts than a strategy of simply reaching an absolute temperature to heat-kill ranavirus. That is, ectothermic hosts with critical thermal maxima lower than that of the pathogen should still benefit from behavioural fever as method to improve immunological resistance, assuming the appropriate temperatures are available and the host does not have a very small thermal safety margin. However, there are likely costs of behavioural fever that might be balanced against its benefits (Sheldon and Verhulst 1996, Lochmiller and Deerenberg 2000). For example, behavioural fever can increase predation risk in ectothermic hosts if it requires hosts to increase activity or leave refugia (Bundey, Raymond et al. 2003, Parris, Davis et al. 2004, Han, Bradley et al. 2008, Otti, Gantenbein-Ritter et al. 2012, Todd, Jodrey et al. 2016).
In summary, both metamorphic and adult A. terrestris responded to ranaviral exposure with behavioural fever. Additionally, we found that adult A. terrestris were successful at reducing their viral loads by increasing their body temperature after exposure. Hence, for A. terrestris, behavioural fever is a successful method of ranaviral resistance, a host strategy for limiting or inhibiting infection (Roy and Kirchner 2000). Our results support the idea that behavioural fever is primarily used as a method of improving immunological resistance rather than simply damaging pathogens with heat (Evans, Repasky et al. 2015). Thus, behavioural fever could be an effective mechanism of resistance used by ectothermic hosts that are less heat tolerant than the infecting pathogen. More experimental work is needed to determine how widespread the use of behavioural fever as a method of pathogen resistance is in ectothermic vertebrates. Understanding how ectothermic hosts rapidly respond to ranaviruses and other emerging pathogens and how changes to environmental temperature affect these host-parasite interactions is crucial given that ectotherms are increasingly experiencing population declines and die-offs due, in part, to increases in emerging diseases that often appear to be exasperated by abnormal temperatures (Harvell, Altizer et al. 2009, Raffel, Romansic et al. 2013, Grayfer, Edholm et al. 2015).
Supplementary Material
Acknowledgments
We thank A. Bhairo, S. Glazer, G. Goldring, M. Mallikarachchi, K. Medina, M. Rios, B. Schrickel, A. Styf, and K. Surbaugh for their assistance in all aspects of the laboratory work and maintenance of experiments. In addition, we thank A. Brace and V. Wuerthner for their tips on viral quantification methods and J. Cohen and L. Sackett for their helpful comments on the manuscript. Funds were provided by grants to J.R.R. from the Department of the Army, South Florida-Caribbean Cooperative Ecosystems Studies Unit, National Science Foundation (EF-1241889 and DEB-1518681), the National Institutes of Health (R01GM109499, R01TW010286-01), the US Department of Agriculture (2009-35102-0543), and the US Environmental Protection Agency (CAREER 83518801).
Footnotes
Data Accessibility: The data supporting the results are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.gc6p546.
Ethical Statement: An. terrestris were collected under permit with the Florida Fish and Wildlife Conservation Commission. Experimental methods were approved by the University of South Florida International Animal Care and Use Committees (W IS00000548).
References
- Adamo SA and Lovett MM (2011). “Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis.” Journal of Experimental Biology 214(12): 1997–2004. [DOI] [PubMed] [Google Scholar]
- Agrawal AA (2001). “Phenotypic plasticity in the interactions and evolution of species.” Science 294(5541): 321–326. [DOI] [PubMed] [Google Scholar]
- Allender MC, Mitchell MA, McRuer D, Christian S and Byrd J (2013). “Prevalence, clinical signs, and natural history characteristics of frog virus 3-like infections in eastern box turtles (Terrapene carolina carolina).” Herpetological Conservation and Biology 8(2): 308–320. [Google Scholar]
- Anderson RD, Blanford S, Jenkins NE and Thomas MB (2013). “Discriminating fever behavior in house flies.” PLoS One 8(4): e62269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel E, Nicolajsen N, Christophersen M-B, Holopainen R, Tapiovaara H and Jensen BB (2009). “Propagation and isolation of ranaviruses in cell culture.” Aquaculture 294(3–4): 159–164. [Google Scholar]
- Bandín I and Dopazo CP (2011). “Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates.” Veterinary research 42(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley AE, Hill BJ and Feist SW (2013). “Susceptibility of the European common frog Rana temporaria to a panel of ranavirus isolates from fish and amphibian hosts.” Diseases of Aquatic Organisms 103(3): 171–183. [DOI] [PubMed] [Google Scholar]
- Boltana S, Aguilar A, Sanhueza N, Donoso A, Mercado L, Imarai M and Mackenzie S (2018). “Behavioral Fever Drives Epigenetic Modulation of the Immune Response in Fish.” Frontiers in immunology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Hill RD, Brenes R, Chaney JC, Wilkes RP, Grayfer L, Miller DL and Gray MJ (2016). “Water temperature affects susceptibility to Ranavirus.” EcoHealth 13(2): 350–359. [DOI] [PubMed] [Google Scholar]
- Brunner JL, Schock DM, Davidson EW and Collins JP (2004). “Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus.” Ecology 85(2): 560–566. [Google Scholar]
- Brunner JL and Yarber CM (2018). “Evaluating the Importance of Environmental Persistence for Ranavirus Transmission and Epidemiology.” Advances in Virus Research [DOI] [PubMed]
- Bundey S, Raymond S, Dean P, Roberts S, Dillon R and Charnley A (2003). “Eicosanoid involvement in the regulation of behavioral fever in the desert locust, Schistocerca gregaria.” Archives of Insect Biochemistry and Physiology: Published in Collaboration with the Entomological Society of America 52(4): 183–192. [DOI] [PubMed] [Google Scholar]
- Burns G, Ramos A and Muchlinski A (1996). “Fever response in North American snakes.” Journal of Herpetology 30(2): 133–139. [Google Scholar]
- Chinchar V (2002). “Ranaviruses (family Iridoviridae): emerging cold-blooded killers.” Archives of virology 147(3): 447–470. [DOI] [PubMed] [Google Scholar]
- Cohen J, Venesky M, Civitello DJ, McMahon T, Roznik B and Rohr J (2017). “The thermal mismatch hypothesis explains outbreaks of an emerging infectious disease.” Ecology Letters 20(2): 184–193. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Civitello DJ, Venesky MD, McMahon TA and Rohr JR (2018). “An interaction between climate change and infectious disease drove widespread amphibian declines.” Global change biology [DOI] [PubMed]
- Cohen LM, Neimark H and Eveland L (1980). “Schistosoma mansoni: response of cercariae to a thermal gradient.” The Journal of parasitology 66(2): 362–364. [PubMed] [Google Scholar]
- de Roode JC and Lefèvre T (2012). “Behavioral immunity in insects.” Insects 3(3): 789–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffus ALJ, Waltzek TB, Stöhr AC, Allender MC, Gotesman M, Whittington RJ, Hick P, Hines MK and Marschang RE (2015). Distribution and Host Range of Ranaviruses. Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates Gray MJ and Chinchar VG. Cham, Springer International Publishing: 9–57. [Google Scholar]
- Echaubard P, Leduc J, Pauli B, Chinchar VG, Robert J and Lesbarreres D (2014). “Environmental dependency of amphibian–ranavirus genotypic interactions: evolutionary perspectives on infectious diseases.” Evolutionary applications 7(7): 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SS, Repasky EA and Fisher DT (2015). “Fever and the thermal regulation of immunity: the immune system feels the heat.” Nature Reviews Immunology 15(6): 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forson DD and Storfer A (2006). “Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum.” Ecological Applications 16(6): 2325–2332. [DOI] [PubMed] [Google Scholar]
- Fritts S, Grodsky S, Hazel D, Homyack J, Castleberry S and Moorman C (2015). “Quantifying multi-scale habitat use of woody biomass by southern toads.” Forest Ecology and Management 346: 81–88. [Google Scholar]
- Garner TW, Rowcliffe JM and Fisher MC (2011). “Climate change, chytridiomycosis or condition: an experimental test of amphibian survival.” Global Change Biology 17(2): 667–675. [Google Scholar]
- Grayfer L, Edholm E-S, Andino FDJ, Chinchar VG and Robert J (2015). Ranavirus host immunity and immune evasion. Ranaviruses, Springer: 141–170. [Google Scholar]
- Han BA, Bradley PW and Blaustein AR (2008). “Ancient behaviors of larval amphibians in response to an emerging fungal pathogen, Batrachochytrium dendrobatidis.” Behavioral Ecology and Sociobiology 63(2): 241–250. [Google Scholar]
- Harvell D, Altizer S, Cattadori IM, Harrington L and Weil E (2009). “Climate change and wildlife diseases: when does the host matter the most?” Ecology 90(4): 912–920. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR and Soszynski D (1998). “Role of fever in disease.” Annals of the new York Academy of Sciences 856(1): 224–233. [DOI] [PubMed] [Google Scholar]
- Lessells C and Boag PT (1987). “Unrepeatable repeatabilities: a common mistake.” The Auk: 116–121.
- Lochmiller RL and Deerenberg C (2000). “Trade-offs in evolutionary immunology: just what is the cost of immunity?” Oikos 88(1): 87–98. [Google Scholar]
- Miller D, Gray M and Storfer A (2011). “Ecopathology of ranaviruses infecting amphibians.” Viruses 3(11): 2351–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J (2002). Parasites and the behavior of animals, Oxford University Press. [Google Scholar]
- Otti O, Gantenbein-Ritter I, Jacot A and Brinkhof MW (2012). “Immune response increases predation risk.” Evolution: International Journal of Organic Evolution 66(3): 732–739. [DOI] [PubMed] [Google Scholar]
- Ouedraogo RM, Goettel MS and Brodeur J (2004). “Behavioral thermoregulation in the migratory locust: a therapy to overcome fungal infection.” Oecologia 138(2): 312–319. [DOI] [PubMed] [Google Scholar]
- Parris MJ, Davis A and Collins JP (2004). “Single-host pathogen effects on mortality and behavioral responses to predators in salamanders (Urodela: Ambystomatidae).” Canadian Journal of Zoology 82(9): 1477–1483. [Google Scholar]
- Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD and Rohr JR (2013). “Disease and thermal acclimation in a more variable and unpredictable climate.” Nature Climate Change 3(2): 146. [Google Scholar]
- Rakus K, Ronsmans M and Vanderplasschen A (2017). “Behavioral fever in ectothermic vertebrates.” Developmental & Comparative Immunology 66: 84–91. [DOI] [PubMed] [Google Scholar]
- Reynolds WW, Casterlin ME and Covert JB (1977). “Febrile responses of aquatic ectotherms to bacterial pyrogens.” American Zoologist 17(4): 903–903. [Google Scholar]
- Richards-Zawacki CL (2009). “Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs.” Proceedings of the Royal Society of London B: Biological Sciences: rspb20091656 [DOI] [PMC free article] [PubMed]
- Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B and Dell AI (2018). “The complex drivers of thermal acclimation and breadth in ectotherms.” Ecology letters 21(9): 1425–1439. [DOI] [PubMed] [Google Scholar]
- Rojas S, Richards K, Jancovich JK and Davidson EW (2005). “Influence of temperature on Ranavirus infection in larval salamanders Ambystoma tigrinum.” Diseases of aquatic organisms 63(2–3): 95–100. [DOI] [PubMed] [Google Scholar]
- Rowley JJL and Alford RA (2007). “Non-contact infrared thermometers can accurately measure amphibian body temperatures.” Herpetological Review 38: 308–316. [Google Scholar]
- Roy B and Kirchner J (2000). “Evolutionary dynamics of pathogen resistance and tolerance.” Evolution 54(1): 51–63. [DOI] [PubMed] [Google Scholar]
- Sauer E, Sperry J and Rohr J (2016). “An efficient and inexpensive method for measuring long-term thermoreulatory behavior of terrestrial and semi-terrestrial organisms.” Thermal Biology 60: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer EL, Fuller RC, Richards-Zawacki CL, Sonn J, Sperry JH and Rohr JR (2018). “Variation in individual temperature preferences, not behavioural fever, affects susceptibility to chytridiomycosis in amphibians.” Proc. R. Soc. B 285(1885): 20181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer EL, Sperry JH and Rohr JR (2016). “An efficient and inexpensive method for measuring long-term thermoregulatory behavior.” Journal of Thermal Biology 60: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC and Verhulst S (1996). “Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology.” Trends in ecology & evolution 11(8): 317–321. [DOI] [PubMed] [Google Scholar]
- Teacher AG, Garner TW and Nichols RA (2009). “Population genetic patterns suggest a behavioural change in wild common frogs (Rana temporaria) following disease outbreaks (Ranavirus).” Molecular ecology 18(15): 3163–3172. [DOI] [PubMed] [Google Scholar]
- Team, R. D. C. (2017). R: A language and environment for statistical computing Vienna, Austria, R Foundation for Statistical Computing. [Google Scholar]
- Thomas MB and Blanford S (2003). “Thermal biology in insect-parasite interactions.” Trends in Ecology & Evolution 18(7): 344–350. [Google Scholar]
- Todd G, Jodrey A and Stahlschmidt Z (2016). “Immune activation influences the trade-off between thermoregulation and shelter use.” Animal behaviour 118: 27–32. [Google Scholar]
- Whittington R, Philbey A, Reddacliff G and Macgown A (1994). “Epidemiology of epizootic haematopoietic necrosis virus (EHNV) infection in farmed rainbow trout, Oncorhynchus mykiss (Walbaum): findings based on virus isolation, antigen capture ELISA and serology.” Journal of Fish Diseases 17(3): 205–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


