Abstract
Severe bleeding after cardiothoracic surgery with cardiopulmonary bypass (CPB) is associated with increased morbidity and mortality in adults and children. Fibroblast Growth Factor-2 (FGF-2) and Vascular Endothelial Growth Factor-A (VEGF-A) induce hemorrhage in murine models with heparin exposure. We aim to determine if plasma and urine levels of FGF-2 and VEGF-A in the immediate perioperative period can identify children with severe bleeding after CPB. We performed a prospective, observational biomarker study in 64 children undergoing CPB for congenital heart disease repair from June 2015 - January 2017 in a tertiary pediatric referral center. Primary outcome was severe bleeding defined as ≥ 20% estimated blood volume loss within 24-hours. Independent variables included perioperative plasma and urinary FGF-2 and VEGF-A levels. Analyses included comparative (Wilcoxon rank sum, Fisher’s exact, and Student’s t tests) and discriminative (receiver operator characteristic [ROC] curve) analyses.
Forty-eight (75%) children developed severe bleeding. Median plasma and urinary FGF-2 and VEGF-A levels were elevated in children with severe bleeding compared to without bleeding (preoperative: plasma FGF-2 = 16[10–35] vs. 9[2–13] pg/ml; urine FGF-2= 28[15–76] vs. 14.5[1.5–22] pg/mg; postoperative: plasma VEGF-A = 146[34–379] vs. 53 [0–134] pg/ml; urine VEGF-A = 132 [52–257] vs. 45[0.1–144] pg/mg; all p < 0.05). ROC curve analyses of combined plasma and urinary FGF-2 and VEGF-A levels discriminated severe postoperative bleeding (AUC: 0.73–0.77) with mean sensitivity and specificity above 80%. We conclude that the perioperative plasma and urinary levels of FGF-2 and VEGF-A discriminate risk of severe bleeding after pediatric CPB.
Keywords: Cardiopulmonary bypass surgery, heparin, Fibroblast Growth Factor 2, bleeding
Background
Severe postoperative bleeding (SB) is associated with increased morbidity and mortality in children undergoing cardiothoracic surgery (CTS) with cardiopulmonary bypass (CPB).1–3 Postoperative bleeding after CPB can result in hemodynamic instability necessitating volume resuscitation, blood product replacement, vasoactive administration, extra-corporeal life support (ECLS), and mediastinal exploration.2 The hemostatic derangement in this context is multifactorial and related to underlying cardiac anatomy, procedure-specific data, and existing comorbidities.3–7 Furthermore, exposure to CPB may result in unbalanced production of inflammatory cytokines and activation of complement, coagulation, and fibrinolytic pathways precipitating SB in the postoperative period.4–7
Fibroblast Growth Factor-2 (FGF-2) is a heparin binding growth factor released into circulation by injured endothelial cells.8–11 Previous studies demonstrate high levels of circulating FGF-2 result in lethal bleeding in mice treated with heparin-like drugs.12,13 Hemorrhage in this model occurs only in combination of both heparin exposure and FGF-2-mediated release of inflammatory cytokines, matrix metalloproteinases, and Vascular Endothelial Growth Factor-A (VEGF-A). While FGF-2 and VEGF-A levels may be elevated in critical illness11,14 and in neonates with congenital heart disease15, it is unclear if exposure to CPB and heparin is associated with SB in children undergoing CTS. We aim to determine whether perioperative plasma and urinary FGF-2 and VEGF-A discriminate children who experience SB after CPB.
Results.
Descriptive and postoperative outcomes data Sixty-four children met study criteria of which 48 (75%) experienced postoperative SB and 16 (25%) did not. General sample and study cohort demographic and surgical data are listed in Table 1.
Table 1.
General demographics, characteristics, and surgical data of the patients with and without severe postoperative bleeding.
| Patient Characteristics | Severe Bleeding (n=48) | No Severe Bleeding (n=16) | P-value |
|---|---|---|---|
| Gender ratio, male:female | 2:1 | 1:1 | 0.25 |
| Age, median months (IQR) | 2.5 (0.3–15.1) | 89.9 (39.1–126.6) | <0.01 |
| Weight, median kg (IQR) | 4.3 (3.2–9.9) | 18.5 (13.3–39.8) | <0.01 |
| STAT, median category (IQR) | 2.5 (2–4) | 1 (1–2) | <0.01 |
| Cyanotic CHD, n (%) | 31 (64.6%) | 6 (37.5%) | 0.08 |
| Intraoperative steroids, n (%) | 21 (43.8%) | 3 (18.8%) | 0.08 |
| Surgical times, median min (IQR) | |||
| Postoperative anticoagulation, n (%) | 15 (31.3%) | 6 (37.5%) | 0.76 |
Children with SB were younger (median age 2.5 [0.3–15.1] vs 89.9 [39.1–126.6] months), had increased median STAT category (2.5 [2–4] vs 1 [1–2]), longer median CPB times (126 [81–179] vs 71[56–95] minutes), and greater frequency of deep hypothermic circa arrest (39.6% vs 0%, all p <0.01). No differences were observed in gender ratio, frequency of cyanotic heart disease, intraoperative steroid administration, or postoperative anticoagulation strategy among those with and without SB.
General postoperative outcomes are reported in Table 2. Children who experienced SB had a greater hospital LOS (17 vs. 4 days), postoperative LOS (11 vs. 4 days), postoperative VIS (12 [5–15] vs. 1.5 [0–5]), fluid overload (11 [4–18.8] vs. 2 [0–4.7] %), frequency of delayed sternal closure (19% vs 0%), and exposure to neuromuscular blockade (43.8% vs 0%, all p < 0.01). No differences were observed in index mortality or incidence of acute renal failure among children with and without SB.
Table 2:
Secondary outcomes for children with and without severe postoperative bleeding.
| Patient Characteristics | Severe Bleeding (n=48) | No Severe Bleeding (n=16) | P-value |
|---|---|---|---|
| Vasoactive inotropic score, (IQR) | 12 (5–15) | 1.5 (0–5) | <0.01 |
| Length of stay, day (IQR) | |||
| Fluid overload data | |||
| Acute kidney injury, n (%) | 8 (16.7%) | 2 (12.5%) | >0.99 |
| Continuous Renal Replacement Therapy, n (%) | 1 (2.1%) | 0 (0%) | >0.99 |
| Neuromuscular blockade, n (%) | 21 (43.8%) | 0 (0%) | <0.01 |
| Delayed Sternal closure, n (%) | 19 (39.6%) | 0 (0%) | <0.01 |
| Surgical chest exploration, n (%) | 6 (12.5%) | 0 (0%) | 0.32 |
| Mortality, n (%) | 3 (6.3%) | 0 (0%) | 0.56 |
Postoperative bleeding data.
The median 24-hour postoperative thoracostomy output (36.7 [27–64.1 vs 12.3 [9–14.2] ml/kg) and blood product transfusions volume (10.7 [0–25.6] vs. 0 mL/kg, all p <0.01) were greater in children experiencing SB than those without SB. Children with SB had >20% EBV of thoracostomy output in 73% of cases by 10-hours. In the first 24-hours postoperative period, median EBV in children with SB was 45.9 (33.7–80.2) % of which 64% required rescue blood product transfusion.
Perioperative FGF-2 and VEGF-A levels.
Preoperative FGF-2 and VEGF-A plasma levels (shown in Figure 1) were elevated in children with SB as compared to controls (FGF-2 = 16 [10–35] vs 9 [2–13] pg/ml, p<0.01) and VEGF-A = 146 [34–379] vs 53 [0 −134] pg/ml, p = 0.02), Preoperative urinary FGF-2 and VEGF-A were also higher in children who developed SB (FGF-2 = 28 [15–76] vs 14.5 [1.5–22] pg/mg UCr, p = 0.02) and VEGF-A = 132 [52–257] vs. 45 [0.1–144] pg/mg UCr; p = 0.02). Higher plasma FGF-2 and VEGF-A were noted preoperatively in children with cyanotic CHD compared to those with acyanotic CHD (FGF-2 = 23 [10–35.7] vs 10 [0.1–22] pg/ml, p = 0.05) and VEGF-A = 127.5 [43–374] vs 64 [5.7–173] pg/ml, p =0.03). Postoperative plasma FGF-2 and VEGF-A trended but were not significantly different in children with SB as compared to controls (FGF-2 = 16 [9.2–32] pg/ml vs 11 [0.1–20.5] pg/ml, p = 0.11) and VEGF-A = 251 [59–405] pg/ml vs 91 [14–235] pg/ml; p = 0.06). Postoperative urinary FGF-2 and VEGF-A were higher in children with SB as compared to controls (FGF-2 = 29.5 [15–127] vs 12 [0.1–19] pg/mg UCr) and VEGF-A = 233 (81–468) vs 47 (16–140) pg/mg UCr; all p < 0.01).
Figure 1:
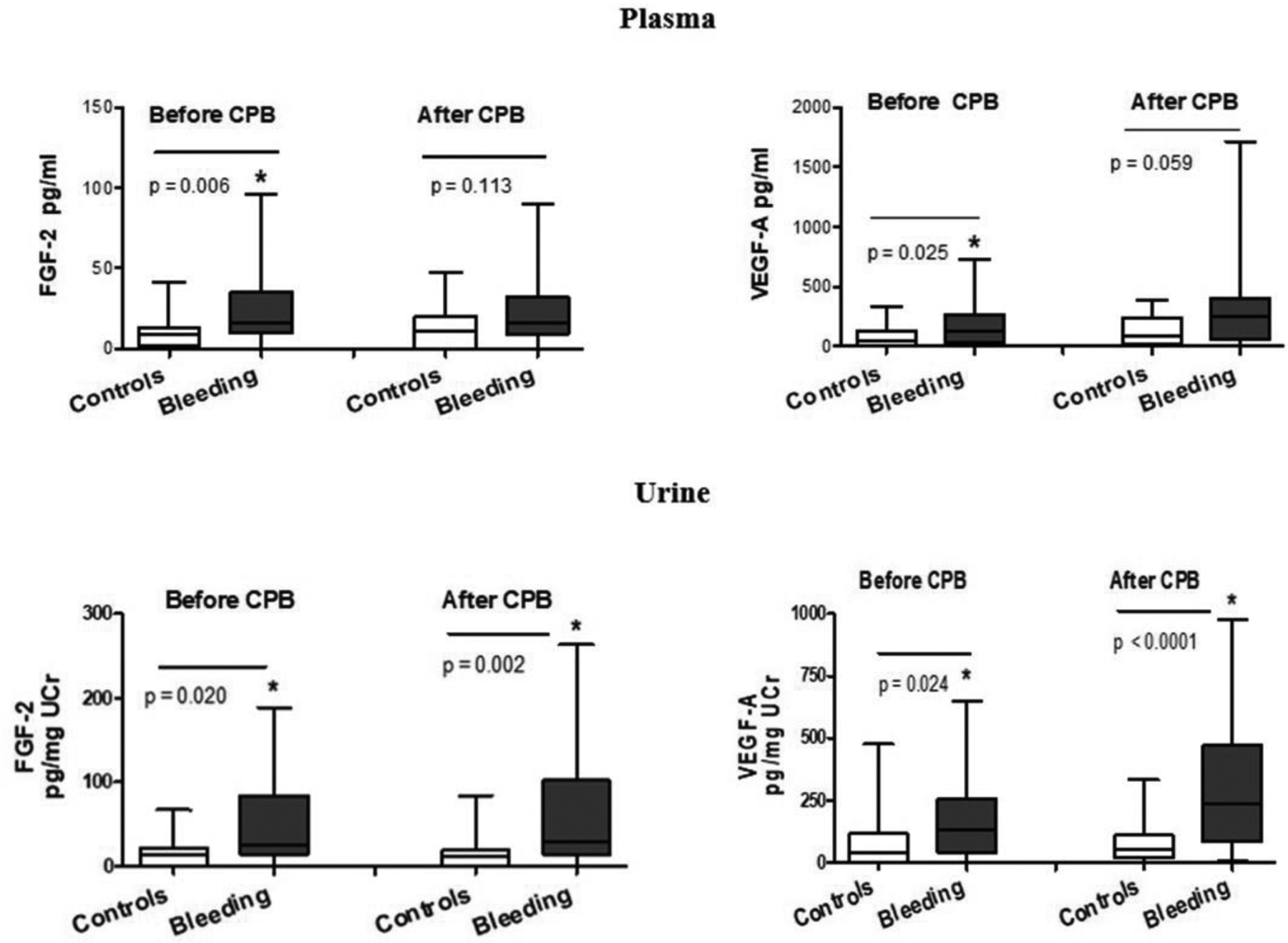
Plasma and urinary levels of Fibroblast Growth Factor-2 and Vascular Endothelial Growth Factor-A before and after cardiopulmonary bypass in children with critical congenital heart surgery with and without severe postoperative bleeding.
Preoperative plasma FGF-2 and VEGF-A in children < 2 years of age were comparable to older children (FGF- 2 = 15 [10–30.5] vs 10.6 [3.6–26.5], p = 0.420) and VEGF-A = 125 [38.5 −316.5] vs 97.5 [7–234], p = 0. 174). After adjusting for children with AKI, differences in the urinary FGF-2 and VEGF-A levels in those with and without SB persisted (preoperative FGF-2 = 31 [14.2–78] vs 13 [0.1–22] pg/mg UCr, p = 0.01; preoperative VEGF-A = 134 [56–258] vs 54 [0.1–149] pg/mg UCr; p = 0.05; Postoperative FGF-2 = 27 [11–123] vs 13.5 [0.3–22.7] pg/mg UCr, p = 0.02; VEGF-A 235 [79–470] vs 92 [0.1–210] pg/mg UCr; p = 0.01).
ROC curve analyses.
Both plasma and urinary preoperative FGF-2 and VEGF-A were individually discriminatory for SB after CPB.
Combined ROC curve analysis of plasma and urinary FGF-2 and VEGF-A threshold values (shown in Table 4) demonstrated above average sensitivity and specificity to distinguish SB after CPB.
Table 4:
Threshold values obtained via receiver operator characteristic curve analyses combining pre- and postoperative Fibroblast Growth Factor-2 and Vascular Endothelial Growth Factor-A levels in children undergoing cardiopulmonary bypass for repair of critical congenital heart disease. Plasma (pg/mL); Urine (pg/mg UCr)
| Biomarker combination | Threshold Values | AUC | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| FGF-2 | VEGF-A | ||||
| Plasma, before CPB, FGF-2 + VEGF-A | >9 | >57 | 0.76 | 84% | 60% |
| Urine, before CPB, FGF-2 + VEGF-A | >22 | >67 | 0.77 | 70% | 83% |
| Plasma, after CPB, FGF-2 + VEGF-A | >7 | >235 | 0.73 | 82% | 80% |
| Urine after CPB, FGF- 2 + VEGF-A | >19 | >47 | 0.77 | 91% | 67% |
Preoperatively, combined plasma FGF-2 and VEGF-A yielded 84% sensitivity, while urinary levels 83% specificity. Postoperatively, plasma levels yielded 80% sensitivity and specificity, while urinary values yielded specificity of 91%. Threshold values associated with SB were > 2 SD above the normal values adjusted for age.11,14,15
Exploratory risk assessment.
Univariate logistic regression analyses of age, STAT category, and plasma or urinary preoperative FGF-2 and VEGF-A levels yielded significant associations to SB postoperatively (all p < 0.05). As STAT itself accounts for age, patient weight, procedure complexity, prior cardiothoracic operations, and other preoperative factors, it was included as a covariate for multiple regression modeling. A multivariant logistic regression analysis adjusting for STAT category of preoperative plasma FGF-2 confirmed a relationship with SB, p < 0.01 (FGF-2, coefficient 0.07, standard error 0.0311, p = 0.02; and STAT, coefficient 1.340, standard error 0.459, p < 0.01). Associations persisted after adjusting for DHCA as a potential confounder (data not shown). When adjusting for age and STAT, combined plasma and urinary FGF-2 and VEGF-A could discriminate SB after CPB (sensitivity and specificity ~90%).
Discussion
Heparin-binding growth factors released into the circulation of children with CHD15 regulate angiogenesis, vascular tone, and blood pressure.16–19 Their role in the pathogenesis of postoperative bleeding in children subjected to CPB has not been previously explored. Our study identified elevated perioperative plasma and urinary levels of FGF-2 and VEGF-A that are associated with SB in the immediate post-CPB period. These data suggest angiogenic factors in combination with known clinical variables may be evaluated preoperatively as a biomarker profile to assess risk of SB postoperatively.
The pathogenesis of bleeding in children subjected to CTS and CPB is multifactorial. A complex system of feedback loops triggered by CTS and exposure to the extracorporeal circuit results in systemic inflammatory dysregulation and hemostatic changes. These events are induced both directly by the activation of complement and adhesion of platelets or inflammatory cells to the extracorporeal circuit and indirectly by factors released from injured endothelial cells, including FGF-2 and VEGF-A.20–22 We postulate FGF-2 and VEGF-A are markers of endothelial injury and vascular leakage and contribute to increased capillary permeability and bleeding.
Our findings are consistent with results of previous studies demonstrating elevated plasma FGF-2 and VEGF-A in patients with cyanotic CHD.23,24 Children with cyanotic CHD can develop formation of collateral vessels that might be induced, at least partially, by hypoxia15 via concurrent synthesis and release of FGF-2 and VEGFA-A25 Abnormal blood vessel proliferation in children with cyanotic CHD can increase morbidity and mortality causing left to right shunting, pulmonary “steal” from systemic blood flow, and bleeding during surgical manipulation. VEGF-A and FGF-2 regulate vascular tone and blood pressure.18,19 These studies suggest an assessment of angiogenic growth factors may be of utility in children with CHD undergoing CTS with CPB.
Several angiogenic growth factors are released into the circulation of children with CHD during CPB surgery that play a role maintaining the integrity of vasculature.15,26 In neonates, high serum levels of VEGF-A correlate with the degree of capillary leak, while other parameters such as birth weight and CPB time were less discriminatory.26 High serum Angiopoietin-2 / Angiopoietin-1 ratio, considered a marker of endothelial injury, correlate with adverse outcomes in children following CPB.27 Angiopoeitin-2, an angiogenic cytokine released by endothelial cells, is activated by VEGF-A, hypoxia, thrombin, and shear stress28 and induces vascular permeability changes via phosphorylation of myosin light chain (MLC) kinase and TNF-α.28–30
In contrast, angiopoietin-1 has anti-inflammatory activity inhibiting NFκB activation and endothelial cell adhesion molecules.28 Our research group noted angiopoietin-1 prevents lethal intestinal bleeding induced by FGF-2 and heparin-like drugs in a murine model.13 The beneficial effects of angiopoietin-1 appear to be related to the reduction of FGF-2-induced release of metalloproteinases and VEGF-A.13 Further studies are warranted to clarify the exact mechanisms by which FGF-2 precipitate bleeding complications when children are exposed to heparin-like drugs.
Serum obtained from children after CPB decrease the resistance of endothelial cell monolayers exposed to non-pulsatile and pulsatile flow conditions31, as well as the transendothelial electrical resistance of cultured human dermal and pulmonary microvascular endothelial cells.32 We found that FGF-2 and VEGF-A, in combination with heparin, increases the permeability of cultured endothelial cells acting through Rho-A and Src signaling pathways.33 Inappropriate levels of angiogenic factors may alter the morbidity and mortality of children undergoing CBP surgery by increasing their vascular permeability. As such, they may represent not only candidate biomarkers, but an approach to novel therapeutic intervention.
Combined plasma and urinary FGF-2 and VEGF-A levels improved the discriminatory capacity of the biomarker profile to assess risk of SB in children undergoing CPB surgery. This could be related to the ability of heparin, infused during CPB, to release FGF-2 bound to endothelial cells34, therefore altering FGF-2 levels. Alternatively, collection of partially hemolyzed plasma samples after surgery may increase measurement variability via ELISA assays, a problem avoided by urine sampling. As postoperative urinary FGF-2 and VEGF-A were highly associated with SB, and FGF-2 and VEGF-A are removed from circulation by heparan sulfate proteoglycans in renal glomeruli and systemic endothelial cells34, we speculate our findings are a reflection of systemic release of heparin binding growth factors facilitated by heparin and renal accumulation during CPB. Urinary FGF-2 and VEGF-A remained significant after adjusting for AKI.35 As urine may be collected with minimal risk, this matrix of biomarker sampling may provide a more practical approach in the hemodynamically fragile child with CHD after CPB.
Limitations.
Data reported are a pilot dataset and, therefore, underpowered. A high proportion of children in our sample met SB definition relative to controls and the control group represented older children with lower STAT category. The definition of bleeding severity in children has recently come under scrutiny, but our definition used in this study is consistent with moderate-severe bleeding in recent literature.36,37 Young children with high STAT categories were independently associated with SB. Nonetheless, preoperative plasma FGF-2 or VEGF-A in children younger < 2 years of age were not significantly different from levels in older children. Threshold values on ROC analyses were significantly higher than reported in otherwise healthy young children11,14,15 After adjusting for STAT, FGF-2 and VEGF-A yielded persistent associations with SB.
Conclusions.
Preoperative plasma and urinary FGF-2 and VEGF-A levels are elevated in children who develop SB after CTS with CPB. These findings suggest that angiogenic factors, in combination with other clinical risk factors, are reliable biomarkers to predict SB after CPB. Specifically, FGF-2 may play a direct role in the pathogenesis of bleeding complications induced by heparin exposure. Recognition of elevated preoperative plasma and urinary FGF-2 and VEGF-A levels may allow for anticipation of predicted SB. Future research is planned for drug development for children with elevated levels of FGF-2 and VEGF-A to minimize their physiologic effects that contribute to SB after CPB.
Methods
Study design.
We performed a prospective, observational cohort study in children 0 to 18 years of age undergoing CTS with CPB between June 2015 to January 2017. All subjects were admitted to the cardiac intensive care unit (CICU) in a university-affiliated, tertiary pediatric referral center. This 26-bed CICU performs approximately 450 cases on CPB annually. Patients were excluded for prematurity < 36 weeks gestational age and preexisting renal, hepatic, oncologic, hematologic, or connective tissue disorders. The study was reviewed and approved by an Institutional Review Board.
Sample Collection.
Baseline plasma and urine samples were obtained in the operating room prior to heparin exposure. Postoperatively, samples were collected within one hour of CICU admission. Plasma samples were drawn from an arterial line, placed in a citrate tube, and centrifuged within one hour of collection. Urine samples were obtained via bladder catheterization. Plasma and urine samples were centrifuged at 4,000 x g for 5 minutes and stored in 1-mL aliquots at −80°C until processed. Both FGF-2 and VEGF-A were measured with commercially available ELISA Quantikine Kits (R&D Systems, Minneapolis, MN) as previously described.38 Inter- and intra-assay coefficient of variation for FGF-2 and VEGF-A were 3–9% and 2–8%, respectively. Urinary levels were expressed as a ratio of urinary creatinine (UCr) concentration, in pg per mg of UCr. All measurements were made in duplicate and investigators performing assays were blinded to sample source and clinical outcomes.
Study Definitions and Outcomes.
The primary study outcome was SB defined as ≥ 20% estimated blood volume (EBV) loss within 24-hours of operation via thoracostomy tube. Independent variables were perioperative plasma and urinary FGF-2 and VEGF-A levels. Secondary outcomes included vasoactive-inotropic scores (VIS), hospital length of stay (LOS), CICU LOS, type and volume of blood product transfusion, percent fluid overload, acute kidney injury (AKI), and index mortality. Fluid overload was assessed using the equation: ([preoperative weight–daily weight]/preoperative weight) x100%. We defined AKI according to Kidney Disease Improving Global Outcome (KDIGO) criteria.38,39 Surgical risk stratification was assessed via Society of Thoracic Surgeons – European Association for Cardiothoracic Surgeons (STAT) category.
Statistical Analysis.
Descriptive data are reported as proportions (percentages), means ± standard deviation (SD), or median with inter-quartile ranges (IQR). Groups with and without SB were assessed using Fisher’s exact tests for categorical variables, Student’s t-tests for normally distributed continuous variables, and Wilcoxon rank sum tests for non-normally distributed continuous variables. Receiver-operator characteristic (ROC) curve analyses were used to determine FGF-2 and VEGF-A threshold values using Youden’s index to discriminate children with SB. Exploratory logistic regression analyses were performed to determine how FGF-2 and VEGF-A performed together and assess relative contributions of covariates.40 Type I error was set 0.05. Analyses were completed using Stata© v15.1 (StataCorp, College Station, TX) and Prism v6 (GraphPad Holdings, San Diego, CA).
Figure 2:
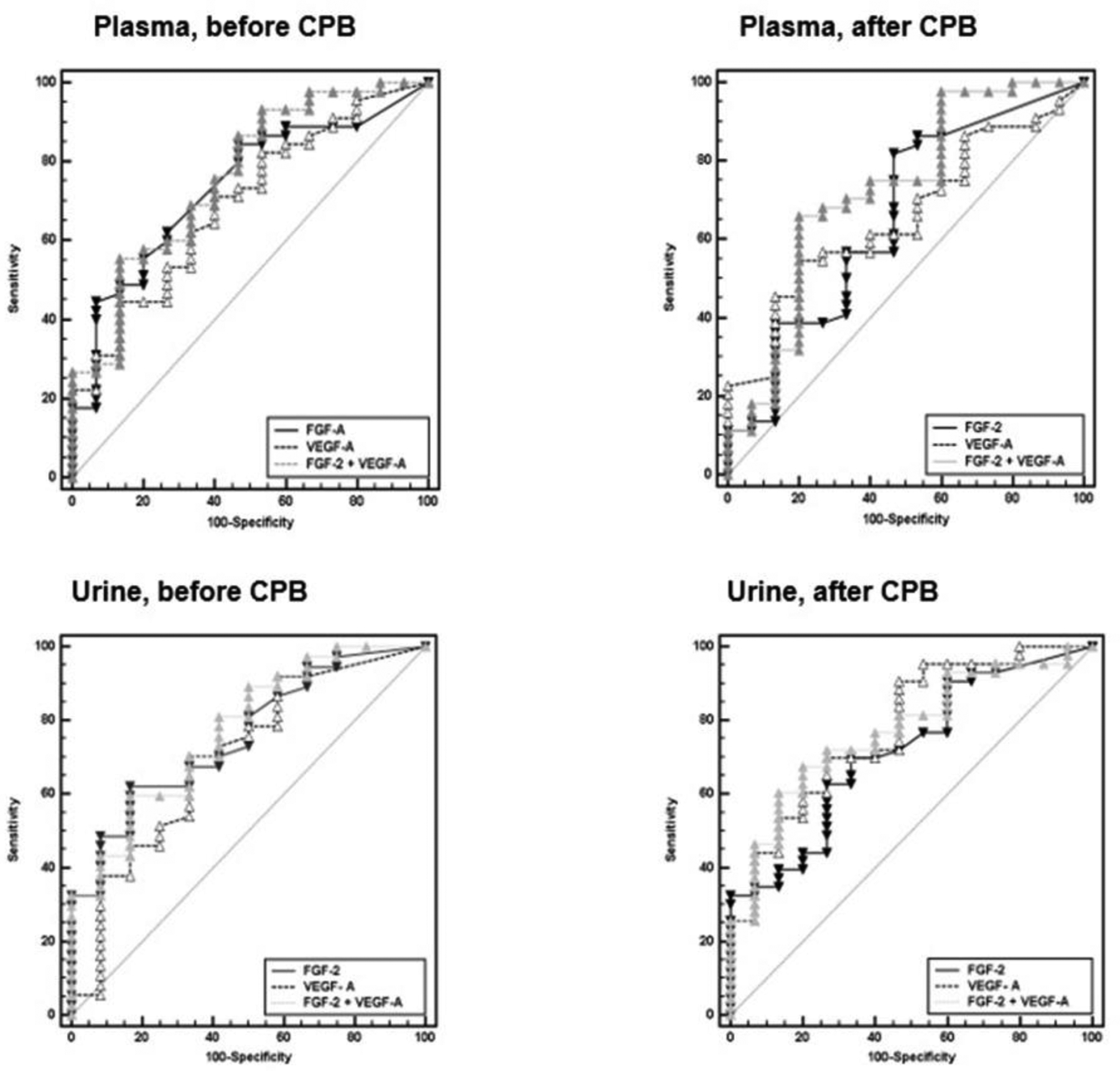
Receiver operator characteristic curve analyses of Fibroblast Growth Factor-2 and Vascular Endothelial Growth Factor-A before and after cardiopulmonary bypass on the primary outcome of severe postoperative bleeding.
Table 3.
Cumulative thoracostomy output (TO) and estimated blood volumes (EBV) recorded at 1, 2, 10, and 24-hours postoperatively.
| Postoperative timing | Patient Characteristics | Severe Bleeding (n=48) | No Severe Bleeding (n=16) | P-value |
|---|---|---|---|---|
| Hour 1 | TO, mL/kg/hr (IQR) | 8.8 (5.7–13.8) | 4 (2.5–5) | <0.01 |
| Hour 2 | TO, mL/kg/hr (IQR) | 5.4 (3.8–9.4) | 2.6 (2.1–3.2) | 0.01 |
| Hour 10 | TO, mL/kg/hr (IQR) | 2 (1.6–4.2) | 0.8 (0.7–1.1) | <0.01 |
| Hour 24 | TO, mL/kg/hr (IQR) | 1.6 (1.1–2.7) | 0.5 (0.4–0.6) | <0.01 |
Acknowledgments.
This study was supported by National Institutes of Health Grants R01 HL 102497 and R01 DK049419
References
- 1.Despotis G, Avidan M, Eby C. Prediction and management of bleeding in cardiac surgery. J Thromb Haemost. 2009;7 Suppl 1:111–117. [DOI] [PubMed] [Google Scholar]
- 2.Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76(9):1680–1697. [PubMed] [Google Scholar]
- 3.Guay J, Rivard GE. Mediastinal bleeding after cardiopulmonary bypass in pediatric patients. Ann Thorac Surg. 1996;62(6):1955–1960. [DOI] [PubMed] [Google Scholar]
- 4.Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48(1 Suppl):2S–30S. [DOI] [PubMed] [Google Scholar]
- 5.Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55(2):552–559. [DOI] [PubMed] [Google Scholar]
- 6.Kilbridge PM, Mayer JE, Newburger JW, et al. Induction of intercellular adhesion molecule-1 and E-selectin mRNA in heart and skeletal muscle of pediatric patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;107(5):1183–1192. [PubMed] [Google Scholar]
- 7.Wilson I, Gillinov AM, Curtis WE, et al. Inhibition of neutrophil adherence improves postischemic ventricular performance of the neonatal heart. Circulation. 1993;88(5 Pt 2):II372–379. [PubMed] [Google Scholar]
- 8.Gospodarowicz D, Ferrara N, Schweigerer L, et al. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987;8(2):95–114. [DOI] [PubMed] [Google Scholar]
- 9.Klagsbrun M, D’Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7(3):259–270. [DOI] [PubMed] [Google Scholar]
- 10.Andres G, Leali D, Mitola S, et al. A pro-inflammatory signature mediates FGF2-induced angiogenesis. J Cell Mol Med. 2009;13(8B):2083–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray P, Acheson D, Chitrakar R, et al. Basic fibroblast growth factor among children with diarrhea-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2002;13(3):699–707. [DOI] [PubMed] [Google Scholar]
- 12.Jerebtsova M, Wong E, Przygodzki R, et al. A novel role of fibroblast growth factor-2 and pentosan polysulfate in the pathogenesis of intestinal bleeding in mice. Am J Physiol Heart Circ Physiol. 2007;292(2):H743–750. [DOI] [PubMed] [Google Scholar]
- 13.Jerebtsova M, Das JR, Tang P, et al. Angiopoietin-1 prevents severe bleeding complications induced by heparin-like drugs and fibroblast growth factor-2 in mice. Am J Physiol Heart Circ Physiol. 2015;309(8):H1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray PE, Liu XH, Xu L, et al. Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol. 1999;13(7):586–593. [DOI] [PubMed] [Google Scholar]
- 15.Starnes SL, Duncan BW, Kneebone JM, et al. Vascular endothelial growth factor and basic fibroblast growth factor in children with cyanotic congenital heart disease. J Thorac Cardiovasc Surg. 2000;119(3):534–539. [DOI] [PubMed] [Google Scholar]
- 16.Eliceiri BP, Paul R, Schwartzberg PL, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–924. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Bunting S. Vascular endothelial growth factor, a specific regulator of angiogenesis. Curr Opin Nephrol Hypertens. 1996;5(1):35–44. [DOI] [PubMed] [Google Scholar]
- 18.Tassi E, Lai EY, Li L, et al. Blood Pressure Control by a Secreted FGFBP1 (Fibroblast Growth Factor-Binding Protein). Hypertension. 2018;71(1):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger JP. Vascular endothelial growth factor inhibitors and hypertension: a central role for the kidney and endothelial factors? Hypertension. 2009;54(3):465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBride WT, Armstrong MA, Crockard AD, et al. Cytokine balance and immunosuppressive changes at cardiac surgery: contrasting response between patients and isolated CPB circuits. Br J Anaesth. 1995;75(6):724–733. [DOI] [PubMed] [Google Scholar]
- 21.Himeno W, Akagi T, Furui J, et al. Increased angiogenic growth factor in cyanotic congenital heart disease. Pediatr Cardiol. 2003;24(2):127–132. [DOI] [PubMed] [Google Scholar]
- 22.Seghaye MC, Grabitz RG, Duchateau J, et al. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112(3):687–697. [DOI] [PubMed] [Google Scholar]
- 23.Dirix LY, Vermeulen PB, Pawinski A, et al. Elevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patients. Br J Cancer. 1997;76(2):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baghdady Y, Hussein Y, Shehata M. Vascular endothelial growth factor in children with cyanotic and acyanotic and congenital heart disease. Arch Med Sci. 2010;6(2):221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogi E, Wu T, Namiki A, et al. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90(2):649–652. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamov D, Erez E, Tamariz M, et al. Plasma vascular endothelial growth factor level is a predictor of the severity of postoperative capillary leak syndrome in neonates undergoing cardiopulmonary bypass. Pediatr Surg Int. 2002;18(1):54–59. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano JS Jr., Lahni PM, Bigham MT, et al. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 2008;34(10):1851–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12(2):235–239. [DOI] [PubMed] [Google Scholar]
- 29.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314(2):738–744. [DOI] [PubMed] [Google Scholar]
- 31.Koning NJ, Overmars MA, van den Brom CE, et al. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br J Anaesth. 2016;116(2):223–232. [DOI] [PubMed] [Google Scholar]
- 32.Pierce RW, Zahr RA, Kandil S, et al. Sera From Children After Cardiopulmonary Bypass Reduces Permeability of Capillary Endothelial Cell Barriers. Pediatr Crit Care Med. 2018;19(7):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das JR, Gutkind JS, Ray PE. Circulating Fibroblast Growth Factor-2, HIV-Tat, and Vascular Endothelial Cell Growth Factor-A in HIV-Infected Children with Renal Disease Activate Rho-A and Src in Cultured Renal Endothelial Cells. PLoS One. 2016;11(4):e0153837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whalen GF, Shing Y, Folkman J. The fate of intravenously administered bFGF and the effect of heparin. Growth Factors. 1989;1(2):157–164. [DOI] [PubMed] [Google Scholar]
- 35.Wai K, Soler-Garcia AA, Perazzo S, et al. A pilot study of urinary fibroblast growth factor-2 and epithelial growth factor as potential biomarkers of acute kidney injury in critically ill children. Pediatr Nephrol. 2013;28(11):2189–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nellis ME, Tucci M, Lacroix J, et al. Bleeding Assessment Scale in Critically Ill Children (BASIC): Physician-Driven Diagnostic Criteria for Bleeding Severity. Crit Care Med. 2019;47(12):1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karam O, Nellis ME, Zantek ND, et al. Criteria for Clinically Relevant Bleeding in Critically Ill Children: An International Survey. Pediatr Crit Care Med. 2019;20(3):e137–e144. [DOI] [PubMed] [Google Scholar]
- 38.Soler-Garcia AA, Rakhmanina NY, Mattison PC, et al. A urinary biomarker profile for children with HIV-associated renal diseases. Kidney Int. 2009;76(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lex DJ, Toth R, Cserep Z, et al. A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg. 2014;97(1):202–210. [DOI] [PubMed] [Google Scholar]
- 40.Gupta C, Massaro AN, Ray PE. A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr Nephrol. 2016;31(7):1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]


