Abstract
The design of injectable hydrogel systems addresses the growing demand for minimally invasive approaches towards local and sustained delivery of therapeutics. We developed a class of hyaluronic acid (HA) hydrogels that forms through non-covalent guest-host interactions, which undergo disassembly (shear-thinning) when injected through a syringe and then rapidly re-assemble (self-healing) when shear forces are removed. The unique properties enable the use of this hydrogel system for numerous applications, such as injection in vivo including with cells and therapeutic molecules, or as a bioink in 3D-printing applications. Here, we describe the synthesis route for functionalizing HA with either adamantane (guest) via controlled esterification or with β-cyclodextrin (host) through amidation. The controlled grafting of β-cyclodextrin to HA includes the preparation of an aminated β-cyclodextrin (6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin). We next illustrate the optional modification of the HA derivatives with methacrylates for secondary covalent crosslinking and fluorophores for imaging, exemplified by the fluorescent derivatization of adamantane functionalized HA, useful toward subsequent in vitro and in vivo hydrogel imaging. HA polymers are designed rationally with controlled modifications to prepare hydrogels with tailored properties, and this procedure takes 3-4 weeks to complete. We detail the preparation and characterization of the guest-host hydrogels, including assessment of the rheological properties, erosion and biomolecule release in vitro. We furthermore demonstrate strategies for the encapsulation of cells in vitro and procedures for assessing in vivo hydrogel degradation through image-based quantification of fluorescently derivatized materials.
Keywords: polymer chemistry, rheology, hydrogel, cell delivery, 3D printing
INTRODUCTION
Injectable materials have great potential towards the development of minimally-invasive therapeutics1. Hydrogels are of particular interest, as the hydrophilic polymer structure imparts a biomimetic microenvironment, which is used either to tailor cellular interactions or for the delivery of cells and biologically relevant molecules2,3. Many injectable hydrogels are designed to form in situ through covalent bond formation, which is usually obtained via photo-4,5 or redox-initiated6 polymerizations or addition-crosslinking reactions7. Although covalent crosslinking typically leads to the formation of mechanically stable structures, there are limitations due to the irreversibility of their crosslinks. For example, care must be taken to control the rate of crosslinking – too slow may lead to material dispersion upon injection and too rapid may clog the injection device.
In contrast, non-covalent interactions are also used to assemble injectable hydrogels, where assembly is through physical interactions, including hydrogen bonds, electrostatics, and hydrophobic attraction8. Recent developments in supramolecular chemistry have facilitated the development of an increasing number of biologically inspired hydrogels9-11. Most of these hydrogels show viscous flow under shear stress (i.e., shear-thinning) and recovery when the applied stress is removed (i.e., self-healing). Indeed, the potential of these materials toward minimally-invasive implantation has been recently leveraged toward numerous applications in the biomedical field12. However, many of these systems rely upon non-specific interactions or the development of long-range order, resulting in prolonged recovery times (on the order of minutes to hours) following shear-thinning. This may limit their suitability as injectable hydrogels, because the material components or encapsulated cargo may diffuse away from the injection site prior to self-healing. Additionally, several systems are based on highly engineered polypeptides or recombinant proteins. While these systems are highly effective toward precise materials engineering, it may be challenging for many laboratories to produce the quantities of raw material required for high-throughput assays or clinical translation13.
To overcome these challenges, systems with precise interactions of complementary association domains have been developed14. In particular, guest-host (GH) interactions based on the physical bonding of a macrocyclic host cavitand and a complementary guest molecule have been investigated. The specific molecular recognition of a guest by its host and the rapid association of the GH complex enable precise control over the assembly, shear-thinning, and re-assembly processes. These unique properties of GH materials have been exploited for various biomedical applications15-17.
Development of the protocol
Harnessing the specificity of GH interactions, we developed a self-assembling hydrogel based on the hydrophobic interactions of adamantane (Ad, guest) and β-cyclodextrin (CD, host). The GH hydrogel is comprised of hyaluronic acid (HA) as the polymer backbone18, which is modified with either the host molecule (CD) or the complementary guest molecule (Ad). The route toward polymer preparation described here utilizes controlled functionalization of HA and results in guest and host polymers with reproducible modifications and the potential towards scalable synthesis.
The supramolecular GH associations disassemble when ejected through a syringe (shear-thinning) and rapidly re-assemble (self-healing) upon cessation of shear. Building upon previous fundamental work of such systems19-21, we have extensively studied the tunability of the properties of this GH hydrogel system (e.g. bulk relaxation and erosion behaviors)22.
We have demonstrated the versatility of this hydrogel platform towards multiple biomedical applications (Fig. 1). Toward the design of minimally-invasive strategies for treating disease, we employed the injectable hydrogel as a delivery system for therapeutic agents and cells in vitro and in vivo (Fig. 1). We have applied this primarily to applications in the repair of cardiac tissue after myocardial infarction23-25 and for the delivery of anti-inflammatory agents into the kidney capsule26,27.
Figure 1∣.
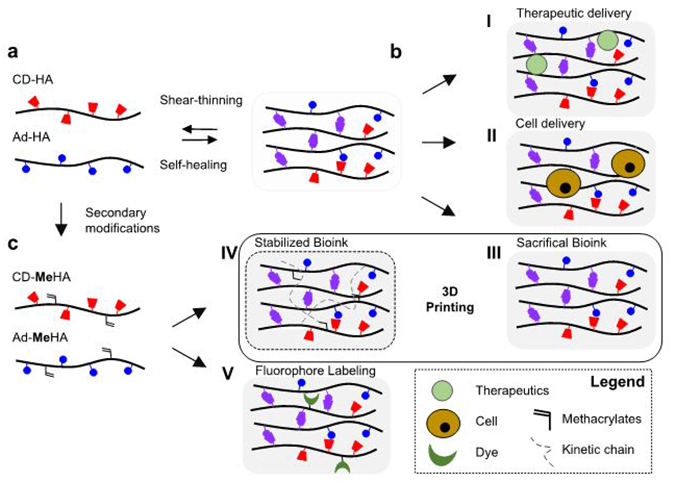
Overview of the guest-host hydrogel platform. (a) Schematic illustration of hyaluronic acid (HA) modified with β-cyclodextrin (CD-HA, red) and adamantane (Ad-HA, blue) with the assembly (self-healing, purple) and disassembly (shear-thinning) of the guest-host complex. (b) Encapsulation and delivery of (I) therapeutics (e.g. proteins and therapeutic molecules) and (II) cells (e.g. endothelial progenitor cells) for in vivo delivery. (c) Methacrylation of CD-HA and Ad-HA to obtain CD-MeHA and Ad-MeHA polymers, which facilitate hydrogel formation by both (III) physical crosslinking and (IV) through secondary crosslinking of methacrylates upon UV light exposure, including towards 3D printing applications. CD-MeHA derivatives can also be used for (V) additional peptide-conjugated fluorophore coupling to track hydrogel degradation.
Naturally, the dynamic nature of supramolecular interactions imparts relatively weak mechanical properties on formed hydrogels; thus, we introduced secondary covalent crosslinking to stabilize the network to yield improved mechanical strength24,28,29. The unique capacity of the GH hydrogels for extrusion with rapid self-healing furthermore permitted their use as a bioink for spatially controlled 3D printing, where secondary crosslinking via selective photopolymerization allowed fabrication of complex 3D structures (Fig. 1). Additionally, we developed a facile strategy to label the GH hydrogel components, which allows for visualizing and tracking material degradation in vitro22 as well as in vivo24,26. Thus, using the CD and Ad functionalized HA polymers, we designed a platform that combines ease of injection, tunability of the hydrogel’s physical and biochemical properties, and the capacity for thorough characterization.
Applications
The hydrogel platform has potential for a wide range of applications, as the material concept is generalizable and addresses the challenges of minimally-invasive delivery, retention of the injected material, and secondary stabilization. This encompasses several tissue-specific therapies, including those where mechanical robustness is desirable, such as for cardiac tissue repair. To date, this approach has been used to synthesize injectable hydrogels based on HA with or without secondary modifications. However, the chemistry of GH modifications is quite diverse and can also be applied for other macromolecular backbones, such as chitosan,20 poly(ethylene glycol),30 and polyethylenimine31 to achieve a broad range of backbone functionalities, as well as with other GH pairs15.
Beyond the formation of injectable hydrogels for minimally-invasive delivery, GH hydrogels provide controlled release of therapeutic cargo, such as encapsulated proteins or growth factors. This is shown by the sustained release of encapsulated interleukin 10 (IL-10) and anti-transforming growth factor β (anti-TGFβ), which is concurrent with the gradual erosion of the GH hydrogels in vitro (Fig.2). To demonstrate the therapeutic potential of the hydrogel system, we injected the Cy7.5 labeled GH hydrogels into subcutaneous pockets of mice and monitored the gradual erosion of GH material over 28 days (Fig.2). We extended this application for the treatment of chronic kidney disease, which commonly progresses into renal dysfunction and failure26. This approach exemplifies a model where the local release of therapeutic agents is desired to improve efficacy and reduce systemic side effects. Here, we injected the GH hydrogels below the renal capsule of mice, following chronic injury, to permit the diffusive release of encapsulated IL-10 and anti-TGFβ32,33. Resultant from the therapy, macrophage infiltration was reduced, indicating reduced inflammatory response to injury and attenuation of the progression toward renal failure (Fig.2). We further investigated the GH hydrogel system for controlled delivery of small hydrophobic molecules, including the pharmaceutical drugs doxorubicin and doxycycline, and peptides containing tryptophan residues34. The inclusion of either the pharmaceutical or amino acid side chain as a guest with the CD containing hydrogel provided tunable release through varying the guest affinity in the system.
Figure 2∣.
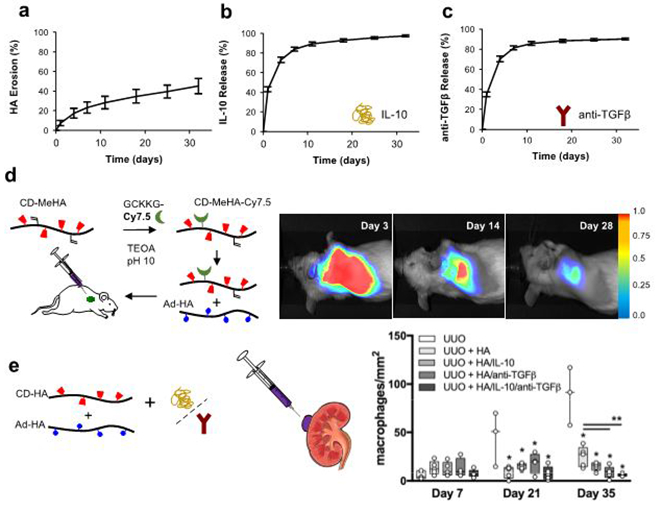
Application of GH hydrogels for local delivery of therapeutics. Assessment of in vitro and in vivo hydrogel degradation and release. (a) Erosion profile of GH hydrogels demonstrated in vitro stability and gradual degradation over 32 days, which was associated with the sustained release of (b) IL-10 and (c) anti-TGFβ (n=3, mean ± SD). (d) Near-infrared labeling of GH hydrogels enabled non-invasive optical imaging and quantification of subcutaneous hydrogel degradation, 25, 636-644 (2015), copyright Wiley-VCH Verlag GmbH & Co. KGaA. (e) GH hydrogels containing IL-10 (5 μg) and anti-TGFβ (0.1 mg) were injected under the renal capsule of the kidney of wild-type BALB/c mice after unilateral ureteral obstruction (UUO). Quantitative analysis of immunohistochemistry for macrophage infiltration (CD68 expression) confirmed anti-inflammatory effects of GH hydrogel injection (UUO+HA) and GH hydrogels containing IL-10 and anti-TGFβ (UUO + IL-10/anti-TGFβ) or either IL-10 or anti-TGFβ after 21 and 35 days. Statistical significance is indicated relative to untreated UUO (*) or UUO + GH hydrogel (**) controls (p<0.05, n≥3, median with minimum to maximum). All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee of the University of Pennsylvania. a,b,c,e adapted from Rodell et al., Copyright (2015), with permission from Elsevier.
While these results underline injectable GH hydrogels as powerful platforms for drug delivery, injectable hydrogels are also being developed for delivering cells. Cell delivery is often challenging due to limited retention at the injection site, particularly in tissues that are highly vascularized and undergo continual dynamic motion, such as cardiac tissue35,36. We employed the cell-protective capacity of the shear-thinning hydrogel for the delivery of endothelial progenitor cells (EPCs) toward the repair of ischemic myocardium after infarction (Fig. 3)23. Retention of injected EPCs into the infarcted myocardial tissue was significantly increased for EPCs encapsulated into GH hydrogels compared to EPC treatment alone. Moreover, the therapy resulted in significantly increased vascularization, improved ventricular function and reduced scar formation (Fig. 3), which represent the main targets for the repair of ischemic myocardium after infarction. This approach demonstrates the use of GH hydrogels toward cell-based therapies by improving cell retention.
Figure 3∣.
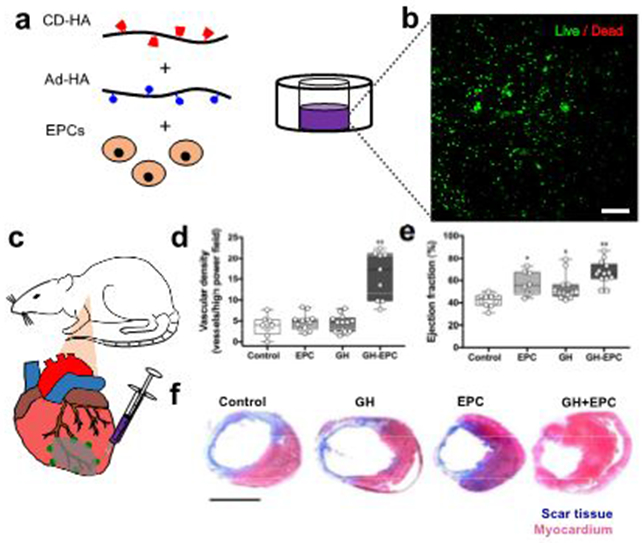
Application of GH hydrogels as injectable cell carriers. (a) Encapsulation of rat endothelial progenitor cells (EPCs) in GH hydrogels. (b) Confocal microscopy of EPCs (calcein and ethidium staining) confirmed high viability after encapsulation in GH hydrogels (4% wt/vol), scale bar 200 μm. (c) Intramyocardial injection of EPCs encapsulated in GH hydrogels after ligation of the left anterior descending (LAD) coronary artery in male adult Wistar rats. Significant enhancement of (d) vascularization and (e) left ventricular function as shown by the ejection fraction confirmed the efficacy of GH hydrogels in cellular retention and improved perfusion. Statistical significance is indicated relative to untreated LAD (*), LAD + EPCs (**) controls (p<0.05, n≥8, median with minimum to maximum). Additionally, (f) Trichrome staining of myocardial cross-sectional images showed less scar formation (blue) and preserved myocardium (red) 4 weeks after treatment with GH, EPCs alone and GH+EPCs. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee of the University of Pennsylvania. d,e,f reprinted and adapted from Gaffey et al., copyright (2015), with permission from Elsevier. Scale bar 5 mm.
Toward the design of complex multiscale structures, we developed a 3D printing technique where the shear-thinning hydrogel ink is directly printed into the 3D space of a second self-healing “support” hydrogel (Fig. 4)28. This concept is unique within the field, particularly as the supramolecular assembly of GH hydrogels simultaneously enables both the printability of the “bioink” and the resulting deformation of the “support” hydrogel to accommodate the extruded material. High viability of encapsulated cells in the support as well as in the extruded material show promise toward the design of multicellular structures (Fig. 4). This approach may also be useful to investigate cellular behavior within various 3D-topographies that can be extruded into hydrogels.
Figure 4∣.
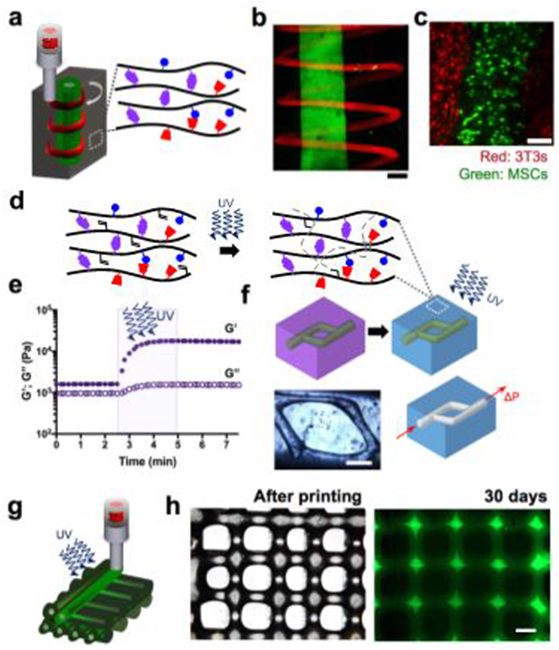
Direct 3D bioprioting of GH hydrogels and secondary stabilization for fabrication of complex 3D structures. (a) Schematic illustration of the extrusion of two differently labeled guest-host hydrogel inks (red and green) into an unlabeled guest-host support hydrogel (gray). (b) Confocal images show a filament of a fluorescein-labeled ink with a continuous spiral of a second, rhodamine-labeled ink extruded into an unlabeled support gel. (c) The printing process enables the patterning of multicellular structures shown by printing MSCs (green) into a support gel containing 3T3 fibroblasts (red), scale bars 200 μm, (d) Secondary stabilization of either of the GH hydrogels was accomplished by modifying the Ad-HA and CD-HA with methacrylates (20% modification). Mechanical stabilization with (e) an increase in G’ is achieved by photocrosslinking in the presence of UV light and a radical photoinitiator (0.05% lrgacure 2959). (f) The secondary stabilization was used within the support guest-host hydrogel to introduce channels by removal of the unstabilized gels, scale bars 500 μm. Adv Mater 27, 5075-5079 (2015), copyright Wiley-VCH Verlag GmbH & Co. KGaA. (g) Schematic illustration of the extrusion of methacrylated GH hydrogel ink and subsequent stabilization with UV exposure in the presence of lrgacure 2959. (h) The UV-stabilization process allows for printing of multilayered structures with high spatial fidelity as shown by the phase contrast top view image, which maintains the grid-structure for more than 30 days in aqueous culture medium; adapted with permission from Ouyang et al., copyright (2016) American Chemical Society. Scale bar 500 μm.
We further employed the concept of secondary crosslinking toward the design of complex structures. Here we introduced methacrylates into the CD-HA and Ad-HA derivatives (Fig. 4). UV photocrosslinking was then used to covalently stabilize either the support or the extruded material after printing the construct28. This approach allowed the removal of the non-covalently crosslinked components either by flow applied through a needle (physical disassembly of the reversible bonds) or introduction of excess soluble CD (dissociation by competitive binding). These treatments yielded complex 3D geometries composed of the covalently stabilized hydrogel. Alternatively, we applied photopolymerization at the time of extrusion in a layer-by-layer printing process to maintain the structural integrity of printed GH hydrogels (Fig. 4)29. While the reversible GH interactions enable the printing of circular filaments, the stability of the printed layers is limited by the relaxation of the supramolecular bonds. However, the secondary covalent UV photocrosslinking step allowed preservation of the printed structures over multiple layers. With this strategy we addressed the challenges of designing a hydrogel bioink that is printable but also exhibits the mechanical stability to support the printed structures29. As such, we fabricated multi-layered GH structures with high spatial fidelity and stability in vitro (Fig. 4).
Limitations
The protocol described here presents a versatile platform for the design of injectable and self-healing HA hydrogels with unique physical properties. However, due to the reversible nature of the crosslinking mechanism, this class of hydrogels has inherently weak mechanical properties, which limits its application for mechanically demanding tissues. Also, relatively high erosion rates of GH materials may restrict investigations toward in vitro cell behavior. However, by introducing additional functional groups to the HA, the formation of a secondary covalent network can stabilize the constructs28,29 and may facilitate a better understanding of cell dynamics and matrix remodeling. Although much of the secondary covalent crosslinking has been induced with ultraviolet light, visible blue light exposure can also be used, such as with lithium acylphosphinate (LAP) as a photoinitiator37. Toward higher stability and retention of GH hydrogels upon injection in vivo, we implemented secondary Michael-addition, which affords slow secondary covalent crosslinking in situ24. We also addressed the low mechanical strength of GH hydrogels by forming double network hydrogels where the interpenetration of the supramolecular GH network with a covalently formed HA network results in a self-healing and tough hydrogel through network entanglement and tethering38. Finally, the applications of the described GH hydrogel system are limited to injecting and delivering an encapsulated cargo. As such, advances in GH complexation demonstrate strategies to add biofunctionality, such as cell-adhesive peptides39,40 and proteolytic sequences41. These approaches could leverage GH hydrogels as a platform to investigate the impact of dynamic biophysical and biochemical signals on cell behavior.
Experimental design
HA polymer synthesis.
In this protocol, several HA polymers are synthesized to form GH hydrogels with and without secondary covalent crosslinks. A step-by-step protocol with characterization of the desired products is provided for the following polymers.
Methacrylated HA (MeHA). The methacrylation of HA is based on previously established protocols42,43. HA or MeHA is then converted to a tetrabutylammonium (TBA) salt by ion exchange44 for further synthetic modification.
CD-HA and CD-MeHA. The water-soluble β-cyclodextrin (β-CD) is aminated via intermediate tosylation of one of the primary hydroxyl groups to obtain 6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin (CD-HDA). CD-HA or CD-MeHA is then synthesized by the reaction of CD-HDA with the TBA salt of either HA or MeHA, respectively, with benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP) as coupling reagent in anhydrous dimethyl sulfoxide (DMSO) (Fig. 5).
Ad-HA and Ad-MeHA. Ad-HA and Ad-MeHA are synthesized by reacting the TBA salt of HA or MeHA, respectively, with 1-adamantane acetic using 4-(dimethylamino)pyridine (DMAP) and di-tert-butyl dicarbonate (BOC2O) in DMSO (Fig. 5).
Figure 5∣.
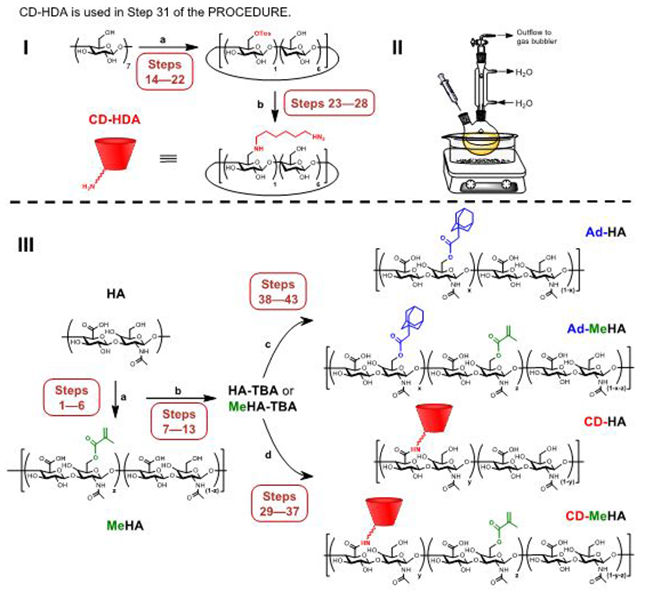
Guest and host polymer synthesis schemes (I) Synthetic route for preparation of aminated β-cyclodextrin (CD-HDA). Reagents and conditions are as follows. Reaction (a): deionized water, p-toluenesulfonyl chloride, sodium hydroxide (NaOH), 0°C → room temperature; b: N,N-Dimethylformamide (DMF), 1,6-hexanediamine (HDA), 80°C. (II) The reaction setup for the amination reaction, where the dry round-bottomed flask containing a stir bar is charged by the tosylated intermediate, purged by nitrogen, and DMF and HDA are separately added via syringe through a rubber septa. (III) Synthetic route for preparation of guest and host polymers, including optional methacrylation. Reagents and conditions are as follows: Reaction (a) deionized water, methacrylic anhydride, NaOH, 0°C, pH 7.5-8.5; (b) deionized water, Dowex-100 resin, tetrabutylammonium hydroxide (TBA-OH); See Supplementary Figure 4 for the chemical structure of the TBA salt of HA and MeHA; (c) anhydrous dimethyl sulfoxide (DMSO), 1-adamantane acetic acid, di-tert-butyl dicarbonate (BOC2O), 4-(dimethylamino)pyridine (DMAP), 45°C; (d) anhydrous DMSO, aminated cyclodextrin, benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP).
The products are purified by excessive dialysis, lyophilized for storage, and then rehydrated and used to form hydrogels.
Fluorescent derivatization.
To enable direct visualization of GH hydrogels, we describe derivatization of methacrylated Ad-HA with a fluorescent GCKKG-Cy7.5 peptide. Using standard solid peptide synthesis, the peptide is synthesized off a glycinol 2-chlorotrityl resin with FMOC protected amino acids, and the amine terminus is reacted with the free acid of Cy7.5. Subsequently, the peptide is cleaved from the resin with trifluoracetic acid, triisopropylsilane and water. This example shows facile near-IR labeling to monitor hydrogel degradation in vivo24. Yet, this strategy may be generalized to allow for coupling of various dyes to methacrylated Ad-HA or MeHA, such as 5,6-carboxyfluorescein and rhodamine, which may be used for in vitro imaging of the hydrogels28,29.
Hydrogel formation.
The two hydrogel components (e.g. Ad-HA and CD-HA) are individually dissolved and mixed in an Eppendorf tube (Fig. 6a Supplementary Video 1). The preformed hydrogel is then briefly centrifuged to remove entrapped air and transferred into the back of a syringe for injection (Fig. 6b, Supplementary Video 2). Preformed hydrogels can also be injected into customized acrylamide molds, which can be filled with a buffer reservoir (1 mL) on top of the constructs (Fig. 6b, Supplementary Video 3). We recommend this technique for in vitro characterization of GH hydrogels, such as hydrogel erosion and molecule release studies.
Figure 6∣.
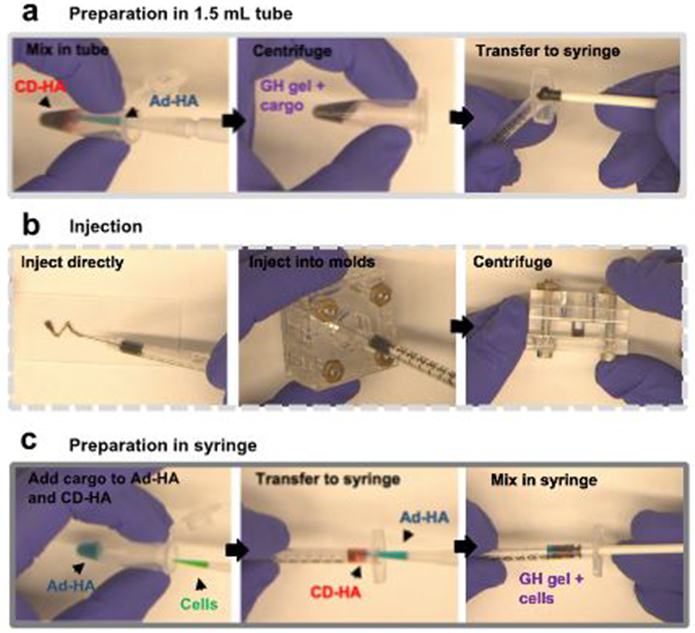
Guest-host hydrogel preparation and injection. Prepare CD-HA and Ad-HA solutions in stoichiometric equivalence in PBS and (a) portion the therapeutic cargo between the two stock solutions. Mix the two components in a 1.5 mL tube, briefly centrifuge to remove entrapped air and transfer the pre-formed hydrogel into the back of a syringe. (b) To inject the hydrogel, insert back the plunger and press firm. For hydrogel erosion and molecule release studies, inject 30 ml hydrogel into the depression (5 mm diameter 6 mm height) of customized acrylamide molds. Briefly centrifuge to level the hydrogel surface, and fill the molds with a buffer reservoir directly on top of the hydrogels. (c) For cell encapsulation, portion the cell suspension between the two stock solutions. Load a syringe with the GH gel by adding the CD-HA solution to the back of the syringe, add the Ad-HA while continuously mixing the two components, and gently mix it.
Rheological properties.
We recommend that rheological examination of GH hydrogels is performed to characterize their shear-thinning, gel recovery and mechanical properties (Fig. 7). We typically perform an oscillatory frequency sweep (0.01-100 Hz; 1% strain) to show the frequency dependence of the storage (G’) and loss modulus (G”) (Supplementary Fig. 1a). When the material is then covalently crosslinked, e.g. for stabilizing the bioink, this frequency dependence is reduced (Supplementary Fig. 1b). An oscillatory strain sweep (0.01-100% strain; 10 Hz) of GH gels can be performed to determine the yield stress at which the hydrogel becomes fluid-like (Fig. 7b). In addition, cyclic deformation measurements (100% and 1% strain; 10 Hz) show rapid recovery of GH hydrogels to initial properties when transitioning from high to low strain conditions, e.g. following injection (Fig. 7c). The initial biomechanical properties, including crosslink density and network architecture, can be tuned by varying the concentration of the individual polymers and the extent of Ad-functionalization22. In general, hydrogels with a stoichiometric balance of Ad and CD functional groups enable the most robust constructs. However, for the release of small molecules that exhibit affinity for CD, various stoichiometric ratios may be desired. Increasing the amount of available CD (1 Ad : 2 CD ratio) increases the sustained release of molecules with affinity for CD34.
Figure 7∣.

Guest-host assembly and recovery mechanism. (a) Macroscopic image of the injection of a guest-host (GH) hydrogel into PBS demonstrates the injectability and rapid recovery of the GH hydrogels, scale bar 500 μm. (b) Strain sweep with G’ (filled symbols) and G” (open symbols) shows the shear yielding of GH hydrogels with increasing strain, (c) Corresponding recovery of a GH hydrogel undergoing cyclic deformation of 1% (low, non-shaded areas) and 100% (high, shaded areas) strain at 10 Hz with G’ (purple continuous line) and G” (purple dotted line).
Hydrogel erosion and biomolecule release.
We perform in vitro characterizations in customized acrylamide molds (Fig. 6b, Supplementary Video 3). To quantify GH hydrogel degradation, we describe a colorimetric assay based on the amount of released uronic acid, which is a component of HA. This procedure is based on a previously described carbazole reaction42 and can be easily adapted for use with a broad range of HA-materials. For release of encapsulated cargo, we describe quantification of fluorescence intensities of fluorescein-conjugated bovine serum albumin (FITC-BSA) as a model biomolecule22. This strategy can be generalized to examine release of other fluorescently labeled therapeutics or proteins. Further, we describe the characterization of in vivo hydrogel degradation using quantitative fluorescence imaging of subcutaneously injected hydrogels. The method is generalizable to material implantation in numerous tissues, including after renal and Intramyocardial injection24,26.
Cell-encapsulation and in vitro cell culture.
For cell encapsulation, the preparation and injection of GH hydrogels necessitates careful handling to ensure encapsulated cells do not undergo shear-induced damage. To address this challenge, we assemble the hydrogel components in the back of a syringe where they are gently mixed (Fig. 6c, Supplementary Video 4). This strategy reduces the shear that cells may experience while handling the hydrogel. For in vitro cell culture experiments, we recommend the use of a well-in-well culture system to ensure facile media changes without disrupting the GH hydrogel construct. We designed a mold made of Acrylonitrile-Butadiene-Styrene (ABS), which fits into a 24-well plate and can be used to fabricate agarose well-in-well molds (Supplementary Fig. 2). Agarose represents an ideal material for preparing such molds as cells do not adhere to the agarose surface, which prevents cells from excessive aggregation or proliferation at the gel-gel interface.
MATERIALS
REAGENTS
!CAUTION When using the chemicals described in this protocol, always wear suitable personal protective equipment, including lab coat, gloves, safety goggles, and where indicated face shield, respirator and thermo-insulated gloves. Follow the institutional and governmental safety guidelines and refer to the appropriate materials safety data sheets. All synthesis steps should be performed within a chemical fume hood.
Preparation of adamantane modified MeHA/HA and ß-cyclodextrin modified MeHA/HA
Sodium hyaluronic acid, 66-99 kDa (Lifecore, cat. no. HA-60K; store under nitrogen at −20 °C). CRITICAL Other molecular weights of sodium hyaluronic acid can be used for this protocol. However, this affects the viscosity during synthesis and properties of resulting hydrogels.
Methacrylic anhydride (MA, Sigma Aldrich, cat. no. 276685; store under nitrogen at 4 °C). !CAUTION Contact with methacrylic anhydride can cause skin irritation, eye damage and respiratory irritation. Wear a face shield and respirator.
Sodium hydroxide (NaOH, Sigma Aldrich, cat. no. S8045 store at RT). !CAUTION Sodium hydroxide can cause skin corrosion and serious eye damage. Avoid any direct contact.
Dowex resin (Sigma Aldrich, cat. no. 217506, store at RT). !CAUTION Dowex is acute toxic and causes skin and eye irritations and presents organ toxicity after single exposure. Wear a respirator.
Tetrabutylammonium hydroxide solution 40% (w/v) in water (TBA-OH, Sigma Aldrich cat. no. 178780, store at RT). !CAUTION Tetrabutylammonium hydroxide can cause skin corrosion and serious eye damage. Avoid any direct contact and wear face shield and respirator.
1-adamantane acetic acid (Acros Organics, cat. no. 331560010, store at RT). !CAUTION 1-adamantane acetic acid causes skin irritation and serious eye irritation, and may cause respiratory irritation if inhaled.
4-(Dimethylamino)pyridine (DMAP, Sigma Aldrich, cat. no. 29224, store dry at RT). !CAUTION 4-(Dimethylamino)pyridine can cause severe skin burns and eye damage. Wear a face shield and respirator.
Anhydrous dimethyl sulfoxide (DMSO, Sigma Aldrich, cat. no. 276855, store at RT). !CAUTION Always wear a face shield.
Di-tert-butyl dicarbonate (BOC2O, Sigma Aldrich, cat. no. 361941, store under nitrogen at 4 °C). !CAUTION Di-tert-butyl dicarbonate is a flammable and causes serious eye damage and is fatal if inhaled. Always wear a respirator and face shield.
Acetone (Fisher Scientific, cat. no. A18-20, store at RT). !CAUTION Acetone is flammable, may cause irritation of respiratory tract and vapors may cause dizziness. Keep it away from heat and hot surfaces.
p-toluenesulfonyl chloride (TCI America, cat. no. T0272, store dry at RT). !CAUTION p-toluenesulfonyl chloride is highly corrosive and may cause severe skin burns and eye damage. It should be directly weighted out in the reaction vessel. Avoid any direct contact and wear a face shield.
Acetonitrile (Sigma Aldrich, cat. no. 271004, store at RT). !CAUTION Acetonitrile is flammable, irritating to eyes and may cause skin and respiratory tract irritation. Wear a face shield and respirator when handling.
Diethyl ether (Sigma Aldrich cat. no. 296082, store at RT). !CAUTION Diethyl ether is flammable, may cause respiratory irritation if inhaled and skin dryness if exposed repeatedly. Wear a respirator and face shield.
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP, Sigma Aldrich, cat. no. 226084, store under nitrogen at 4 °C). !CAUTION Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate is flammable, acute toxic, and causes specific organ toxicity upon single exposure.
β-cyclodextrin (CD, TCI America cat. no. C0900, store under nitrogen at 4 °C)
Ammonium chloride (Fisher Scientific cat. no. A661-500, store at RT). !CAUTION Ammonium chloride is harmful if swallowed and may cause skin and respiratory tract irritation.
N,N-Dimethylformamide (DMF, Sigma Aldrich cat. no. 227056, store at RT). !CAUTION N,N-Dimethylformamide is flammable, acute toxic, can cause serious eye irritation and is harmful if inhaled. Always wear respirator and a face shield.
Liquid nitrogen. !CAUTION Contact with liquid nitrogen may cause serious freezing injuries. When handling liquid nitrogen, always wear thermo-insulated gloves, a full-face shield and laboratory coat.
1,6-hexanediamine (HDA, TCI cat. no. D0095, store at RT). !CAUTION 1,6-hexanediamine is harmful in contact with skin and if swallowed, can cause severe skin burns and eye damage.
Sodium chloride (Sigma Aldrich cat. no. S9888, store at RT).
Deuterium oxide (D2O, Sigma Aldrich cat. no. 151882, store at RT in a desiccator).
Dimethyl sulfoxide-d6 (DMSO-d6, Sigma Aldrich cat. no. 151874, store at RT in a desiccator).
Preparation of CD-MeHA-Cy7.5
Cyanine7.5 carboxylic acid (Lumiprobe cat. no. 46090, store at −20 °C).
FMOC protected amino acids (EMD Millipore, Novabiochem®, Fmoc-Cys(Trt)-OH (cat. no. 852008), Fmoc-Gly-OH (cat. no. 852001), Fmoc-Lys(Boc)-OH (cat. no. 852012), store at −20 °C).
Glycinol 2-chlorotrityl resin (EMD Millipore, Novabiochem®, cat. no. 856088, store at −20 °C).
2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU, EMD Millipore, Novabiochem®, cat. no. 851006, store at −20 °C). !CAUTION HBTU causes eye and skin irritation, and respiratory tract irritation if inhaled.
Trifluoroacetic acid (Sigma Aldrich cat. no. 302031, store at RT). !CAUTION Trifluoroacetic acid can cause severe skin burns and eye damage, and is harmful by inhalation.
Triisopropylsilane (Sigma Aldrich cat. no. 233781, store at RT). !CAUTION Triisopropylsilane is flammable. Wear face shield and respirator.
Triethanolamine (Sigma Aldrich cat. no. 90279, store at 4 °C). !CAUTION Triethanolamine causes eye irritation, and may cause skin and respiratory tract irritation.
α-Cyano-4-hydroxycinnamic acid (Sigma Aldrich cat. no. 70990, store at RT). !CAUTION α-Cyano-4-hydroxycinnamic acid is harmful if swallowed and causes skin and serious eye irritation.
Dichloromethane (Sigma Aldrich cat. No. 66738, store at RT). !CAUTION Dichloromethane causes skin and serious eye irritation, and may cause respiratory irritation.
Gel preparation, erosion and release studies
Sulfuric acid (Sigma Aldrich, cat. no. 339741). !CAUTION Sulfuric acid is highly corrosive and can cause serious eye damage. Wear face shield and respirator.
Sodium tetraborate decahydrate (Sigma Aldrich, cat. no. S9640, store at RT).
Carbazole (Sigma Aldrich, cat. no. C5132, store at RT).
Ethanol (100%, Fisher Scientific cat. no. 04-355-223, store at RT). !CAUTION Ethanol is flammable, and can cause moderate eye and skin irritation.
Hyaluronidase from bovine testes (Sigma Aldrich, cat. no. 3506, store at −20 °C).
Adult mice (e.g. C57BL/6, JAX) !CAUTION All experiments involving live mice must conform to relevant institutional and national regulations. All animal procedures that we describe here conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee of the University of Pennsylvania.
Cell encapsulation
Human mesenchymal stem cells (MSCs, e.g. Lonza cat. No. PT-2501, store in liquid nitrogen) !CAUTION These cells should be routinely checked to ensure that they are authentic and not contaminated with mycoplasma.
Minimum Essential Media (α-MEM, Thermo Fisher Scientific, cat. no. 11095080, store at 4 °C).
Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, cat. no. 11885084, store at 4°C).
Fetal bovine serum (FBS, Thermo Fisher Scientific cat. no. 1437028, store at −20 °C).
Penicillin streptomycin, 10.000 U/ml (Life Technologies cat. no. 15140-122, store at −20 °C).
Gentamycin (Life Technologies cat. no. 15710-064, store at 4 °C).
Phosphate-Buffered Saline (Life Technologies cat. no. 10010023, store at 4 °C).
Trypsin (Life Technologies, cat. no. 15090-046, store at 4 °C).
Agarose (Sigma Aldrich, cat. no. A9539, store at RT)
Live/Dead Viability/Cytotoxicity Kit for mammalian cells (e.g. Thermo Fisher Scientific, cat. no. L3224, store at −20 °C).
EQUIPMENT
Preparation of adamantane modified MeHA/HA and β-cyclodextrin modified MeHA/HA
Heated stir plate (e.g. RCT Basic, IKA cat. no. 0003810001).
1,2 and 3-neck round-bottom flasks, assorted sizes, with a magnetic stir bar.
Rubber septa (Sigma Aldrich cat. no. Z553980).
pH meter (e.g. Fisher Scientific™ accumet ™ cat. no. AB150BCERT).
Cannula 20 gauge, (e.g. Chemglass cat. no. A-0519-08).
Glass pipettes with dropper bulbs.
Beaker with a minimum of 3 L volume.
Separatory funnel (e.g. Chemglass cat. no. CG-1742-04).
Dialysis tubing (6000-8000 MW cut-off regenerated cellulose, e.g. SpectrumLabs Spectra/Por 1 cat. no. 132670) and tubing closures (e.g. SpectrumLabs cat. no. 142734).
Schlenk line with vacuum line, cold trap and oil-pump.
Lyophilizer (e.g.Thermo Fisher Scientific Labconco™ 7387020).
Nuclear magnetic resonance spectrometer (NMR, e.g. Bruker DMX360).
MALDI-tof mass spectrometry (e.g. Applied Biosystem Voyager 6030).
Solid phase peptide synthesizer (e.g. PS3 automated peptide synthesizer Protein Technologies).
Mechanical characterization of CD/Ad-HA hydrogels
Rheometer with cone-and-plate geometry (e.g. TA Instruments AR2000; 20 mm diameter cone with 59 min 42 s cone angle).
Gel preparation, release studies and cell encapsulation
Tissue culture hood (e.g. Thermo Fisher Scientific 1300 Series Class II, Type A2 Biological Safety Cabinet).
CO2 cell culture incubator set to 5% CO2 and 37°C (e.g. Thermo Fisher Scientific Heracell™ VIOS).
Microwave
Epifluorescent microscope (e.g. Olympus BX51).
Confocal microscope (e.g. Leica TCS SP5).
In vivo fluorescence imager (e.g. Pearl® Impulse, LI-COR)
Plate reader (e.g. Tecan Infinite M200).
Hemocytometer (e.g. Hausser Scientific cat. no. 0630010).
Water bath set to 37°C.
Sterile, individually wrapped 0.5 mL syringes 1/2cc with attached needle 27Gx1/2” (e.g. BD cat. no. 305620).
Filter 0.22 μm (e.g. EMD Millipore cat. no. SLGP033RS).
Sterile 24- well plates for 3D cell culture (e.g. Corning Costar Sigma Aldrich, cat. no. CLS3527).
Glass tubes (e.g. Fisher Scientific cat. no. 14-961-27).
Disposable plastic cuvettes (e.g. Fisher Scientific cat. no. 14-955-127).
96-well black plates with clear bottom for absorbance and fluorescent measurements with a plate reader (e.g. Corning Costar Sigma Aldrich, cat no. CLS3631).
UV-lamp (e.g. EXFO OmniCure Series 1000 with a 320-390 filter).
Other laboratory equipment
Sterile 1.5-, 2-, 15- and 50-mL conical (micro)centrifuge tubes
Glass beaker, 200–500 L
Spatula
Forceps
Micropipettes and tips
Disposable Pasteur glass pipettes
Pasteur pipette rubber bulbs
Sterile syringes and needles
Fridge set to 4 °C.
Freezer set to −20 °C.
Deep freezer set −80 °C.
Standard laboratory bench-top refrigerated centrifuge for 50 mL conical tubes (e.g. Thermo Fisher Scientific cat. no. 75004521).
Microcentrifuge (e.g. Eppendorf®Refrigerated Microcentrifuge, model 5417R).
Vortex Mixer (e.g. Fisher Scientific cat. no. 02-215-414) and tube holder (e.g. Fisher Scientific cat. no 11-676-363).
REAGENT SETUP
Matrix solution for MALDI-tof mass spectrometry Dissolve 10 mg α-cyano-4-hydroxycinnamic acid in 1 mL MiliQ water/acetonitrile (50/50). Matrix solution can be stored for least 1 week protected from light at RT.
Triethanolamine buffer (50 mM) for synthesis of Ad-MeHA-Cy7.5 Dissolve 0.746 g triethanolamine in 100 mL PBS and adjust to pH 10. CRITICAL Triethanolamine should be prepared fresh at the time of the synthesis.
Polymer stock solutions for gel formation Determine the average disaccharide molecular weight (MW) for CD-HA (MWCD-HA) and Ad-HA (MWAd-HA) components based on the modification of HA (%CD, %Ad respectively). Given the desired weight fraction (wt) and total gel volume (VGH), determine the total mass of polymer (mGH). Along with the ratio of Ad to CD in the gel (RatioAd/CD), determine the mass of CD-HA (mCD-HA) and Ad-HA (mAd-HA) with the following equations:
Dissolve CD-HA and Ad-HA individually in PBS at the desired concentration, shaking overnight to ensure complete dissolution. Polymer concentrations ranging from 2.5 to 10% (wt/vol) have been utilized to successfully assemble hydrogels22. For high concentrations (>5% (wt/vol)), sonication may be required. CRITICAL Polymer stock solutions should be prepared fresh the day before each experiment.
Solutions for uronic acid assay Prepare sodium tetraborate decahydrate/sulfuric acid solution with 1.9 g sodium tetraborate decahydrate in 200 mL concentrated sulfuric acid in a glass vessel and stir until fully dissolved. CRITICAL Prepare at least 1 mL per sample, adjusting the volume according to the number of samples to be assayed. !CAUTION Sulfuric acid is highly corrosive. Avoid any direct contact. Sodium tetraborate decahydrate/sulfuric acid solution can be stored for least 1 month at RT. Prepare 0.125% carbazole solution in absolute ethanol with 12.5 mg carbazole dissolved in 10 mL ethanol in a 20 mL glass vial and stir until fully dissolved. Cover the vial with aluminum foil to avoid photodegradation. CRITICAL Carbazole solution should be prepared at the time of the assay. Prepare at least 30 μl solution per sample, adjusting the volume according to the number of samples to be assayed.
Human MSCs Expand cells in α-MEM, 16.7% FBS, 1% L-glutamine, 1% penicillin/streptomycin. This medium can be stored for 1 week at 4 °C.
Solution for Live/Dead staining Add 0.5 μl calcein AM (2 μM ) and 2 μl (4 μM) ethidium homodimer-1 to 1 mL serum-free DMEM and allow it to warm to 37 °C. Protect vial from light. CRITICAL Live/Dead solution should be prepared fresh at the time of the assay. Prepare at least 300 μl per sample, adjusting the volume according to the number of samples to be assayed.
PROCEDURE
Preparation of methacrylated HA TIMING 4-8 h, 7 days dialysis.
CRITICAL Ensure that all glassware and laboratory equipment is clean to avoid chemical contamination.
Dissolve HA at 2% (wt/vol) in deionized water in a three-neck bottom flask with a magnetic stir bar. Stir rigorously for 20 min at RT to facilitate complete HA dissolution. Place the round bottom flask containing the solution in an ice bath and fix a pH meter probe into the solution through one of the neck of the round bottom flask.
While stirring, adjust the pH of the HA solution to 8.5 by 1.0 M NaOH. Add 0.75 mL MA (viscous liquid) dropwise per g HA and 10% desired modification and adjust the pH to 8.5 repeatedly during the MA addition. The reaction will be opaque and white due to the emulsion of methacrylic anhydride. Maintain the pH of the reaction at 7.5-8.5 during 4 h on ice.
CRITICAL Ensure adequate stirring to generate an emulsion during MeHA synthesis, as insufficient stirring leads to phase separation and low modification.
!CAUTION Use a glass pipette when handling MA and sodium hydroxide, as organic solvents and alkali may corrode plastic pipette tips.
CRITICAL STEP The degree of modification of MeHA can be varied by changing the ratio of MA to HA43. For a desired degree of modification (X) determine the volume of MA (VMA) per g HA with the following equation, where MWHA is the disaccharide MW and ρMA is the density of MA:
Alternatively, complete conversion (i.e. 100% modification) can be achieved by running the reaction at pH 8.5-9.5 with 2.8 mL MA per g HA for 4 h and repeating (additional 2.8 mL MA per g HA for 4 h, without intermediate purification)37.
-
3.
After the reaction, stir vigorously overnight at RT. Transfer the solution to dialysis tubing. Dialyze against distilled water for 7 days, changing water twice daily.
?TROUBLESHOOTING
CRITICAL STEP MA and methacrylic acidic byproducts are cytotoxic and have to be removed by dialysis. Ensure vigorous stirring of the MeHA solution overnight as it allows the unreacted MA to hydrolyze and dialyze out more easily.
-
4.
After dialysis, transfer solution to 50 mL tubes and freeze them at −80 °C.
PAUSE POINT Samples can be stored at −80°C for at least 1 month.
-
5.
Lyophilize sample until the polymer is fully dry (3-4 days). To maintain a barrier, cover the 50 mL conical tubes with gauze and seal with rubber bands before lyophilization. Ensure that samples do not thaw when transferred to the lyophilizer. Once dry, store polymers under nitrogen at −20°C to avoid absorption of water and hydrolysis of HA.
PAUSE POINT Lyophilized MeHA can be stored for at least one year at −20°C.
-
6.
Characterize the degree of modification using 1H NMR with D2O as the solvent (5 mg MeHA / 0.7 mL D2O).
?TROUBLESHOOTING
Preparation of tetrabutylammonium salts of HA and MeHA (HA-TBA and MeHA-TBA)44. TIMING 2h, 3 days lyophilization.
-
7.
Dissolve 3.0 g HA (or MeHA) in 150 mL deionized water in a 250 mL one-neck round-bottom flask equipped with a magnetic stir bar.
-
8.
Add 9.0 g Dowex 50Wx8 to the HA solution and continue stirring for 30 min at RT.
CRITICAL the reaction may be scaled by adjusting the amount of HA/MeHA while maintaining a 2.0% w/v solution and a 3x weight ratio of Dowex/HA.
!CAUTION Hydrogen form Dowex is a strong acid. Avoid any direct contact, always wear appropriate personal protective equipment including gloves, safety goggles, and laboratory coat.
-
9.
Collect the HA from the resin by vacuum filtration using a No.1 filter paper.
-
10.
Prepare 1:1 (20%) and 1:5 (8%) dilutions of tetrabutylammonium hydroxide (TBA-OH, 40%) against deionized water.
-
11.
Titrate HA solution by 40% TBA-OH to pH 5, by 20% TBA-OH to pH 7, and by 8% TBA-OH to a pH of 7.02-7.05.
CRITICAL Slowly add TBA-OH as the pH of the solution can change rapidly near neutral pH.
-
12.
Transfer HA solution to 50 mL tubes, freeze at −80°C and lyophilize (see step 5).
-
13.
Confirm product and degree of modification using 1H NMR and D2O as a solvent.
PAUSE POINT Lyophilized HA-TBA and MeHA-TBA samples can be stored under nitrogen for at least one year at −20 °C.
Preparation of CD-HA TIMING Part 1 (CD-Tos), 2 days; part 2 (CD-HDA), 4 days; part 3 (CD-HA), 11 days.
Part 1: Synthesis of 6-o-monotosyl-6-deoxy-β-cyclodextrin (CD-Tos)45
CRITICAL Ensure that all glassware and laboratory equipment is clean to avoid chemical contamination.
Protocol is described for 50 g CD and can be scaled accordingly (reactions from 10 g – 100 g CD have been successfully performed in our laboratory).
-
14.
Suspend 50 g (44 mmol) CD in 300 mL MiliQ water in a one-neck round-bottom flask and cool solution in an ice bath. The solution will remain cloudy white due to limited CD solubility.
-
15.
Dissolve 10.07 g (53 mmol) p-toluenesulfonyl chloride in 25 mL acetonitrile under stirring.
-
16.
Gradually add the p-toluenesulfonyl chloride solution to the CD solution over 10 min under stirring. Use a cannula to transfer the p-toluenesulfonyl chloride solution to the CD solution. Alternatively, add the solution through a needle using a 50 mL glass syringe. Maintain on ice for 2 h and continue stirring vigorously.
CRITICAL To achieve the desired pressure difference for cannulation, place the flask containing p-toluenesulfonyl chloride under nitrogen pressure, vent the flask of the CD solution, and connect with cannula (Supplementary Fig. 5). Ensure adequate stirring of the CD solution to avoid precipitation during the reaction.
-
17.
Remove the ice bath. Dissolve 5.3 g (133 mmol) sodium hydroxide in 20 mL deionized water and add dropwise to CD solution over 10 min. Continue stirring at RT for 30 min. The solution will turn slightly yellowish and become clearer.
!CAUTION Use a glass pipette when handling sodium hydroxide, as alkali solutions may corrode plastic pipette tips.
-
18.
Add 40 g (748 mmol) ammonium chloride to the solution to adjust pH to 9. Add additional ammonium chloride in 5 g increments until the pH is stable between 8.5-9. Flocculation of the CD-Tos should occur.
!CAUTION Ensure adequate stirring and complete dissolution of ammonium chloride before continuing ammonium chloride addition.
-
19.
Precipitate the CD-Tos product: Separate the solution into 50 mL conical tubes and cool on ice for 15 min. Centrifuge the tubes at 3200 g for 2 min at 4°C and decant the supernatant. Resuspend the crude product in 40 mL deionized water (per tube) by vortexing, cool on ice for 15 min, and centrifuge at 3200 g for 2 min at 4°C. Repeat this step twice.
CRITICAL For best yields, ensure the solution is completely cooled on ice before centrifugation.
CRITICAL Wash the reaction vessel with deionized water to collect the entire product.
-
20.
Wash the CD-Tos product: Resuspend product in 40 mL ice-cold acetone (per tube) by vortexing, cool on ice for 15 min, and centrifuge at 3200 g for 2 min at 4 °C. Repeat this step twice. Resuspend product in 40 mL diethylether (per tube) and centrifuge at 3200 g for 2 min at 4 °C. Repeat this step.
-
21.
Evaporate organic residues by vacuum pressure overnight to obtain a dry CD-Tos product.
?TROUBLESHOOTING
CRITICAL Vacuum drying should be carried out in a fume hood using a cold trap filled with liquid nitrogen. To maintain a barrier, cover the 50 mL conical tubes with gauze and seal with rubber bands.
!CAUTION Wear goggles, thermally insulated gloves, and long sleeved laboratory coat to avoid direct contact of liquid nitrogen with eyes and skin.
-
22.
Confirm the CD-Tos product using 1H NMR and DMSO-d6 as a solvent.
?TROUBLESHOOTING
PAUSE POINT CD-Tos can be stored under nitrogen at −20°C for at least 6 months.
Part 2: Synthesis of 6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin (CD-HDA)46
-
23.
Equip a dry three-neck-bottom flask with 10 g (8.8 mmol) CD-Tos and connect to a Liebig condenser in-line with a gas bubbler (Supplementary Fig. 5). Purge by nitrogen, add 50 mL DMF via syringe, and stir at RT until CD-Tos is fully dissolved.
CRITICAL To ensure that glassware is dry, place vessels in a drying-oven at 125°C for 24 h. Remove glassware from oven and stopper immediately.
!CAUTION Be careful when handling hot glass and allow to cool to RT before use.
-
24.
Melt HDA to 60°C in an oil bath and add 40 g (34 mL, 344 mmol) to the CD-Tos solution via syringe. The solution will turn yellowish. Allow the reaction to stir for 18 h at 80°C in an oil bath prior to cooling to RT.
CRITICAL Transfer the HDA solution quickly to ensure it does not solidify in the syringe.
-
25.
Precipitate the CD-HDA product: Prepare 50 mL conical tubes with 45 mL ice-cold acetone and add 5 mL of the reaction solution to precipitate. Centrifuge at 3200 g for 2 min at 4°C. Decant and re-dissolve the precipitate in 5 mL DMF (per tube) by vortexing. Precipitate by adding 45 mL of ice-cold acetone (per tube) and centrifuge at 3200 g for 2 min at 4°C. Repeat this step three times.
?TROUBLESHOOTING
CRITICAL The crude product will agglomerate as a pellet during centrifugation, which requires vigorous vortexing to re-dissolve in DMF. Increase the volume of DMF and acetone accordingly if full dissolution cannot be achieved.
-
26.
Wash the product by resuspending in 45 mL cold acetone (per tube) and centrifuge at 3200 g for 2 min at 4 °C. Resuspend in 40 mL ice-cold diethyl ether (per tube) and centrifuge at 3200 g for 2 min at 4 °C. Repeat the diethyl ether washing step two times.
CRITICAL To reduce the amount of tubes to allow for easier drying and collection of the final product, combine multiple fractions while washing by diethyl ether.
-
27.
Evaporate organic residues by vacuum pressure for 48 h to obtain a dry CD-HDA as white powder (see step 21).
-
28.
Confirm the CD-HDA product using 1H NMR and DMSO-d6 as a solvent
PAUSE POINT CD-HDA can be stored under nitrogen at −20°C for at least 6 months.
Part 3: Synthesis of CD-HA and CD-MeHA
-
29.
Allow HA-TBA to warm up to RT. The synthesis of CD-MeHA follows the analogous protocol.
CRITICAL Methacrylation of HA cannot be performed following modification by CD, as concurrent methacrylation of CD is anticipated.
CRITICAL Keep HA-TBA in desiccator, as it is hydroscopic and will adsorb water from the atmosphere.
-
30.
Equip a dry one-neck round bottom flask with a magnetic stir bar and stopper.
CRITICAL It is essential to perform this reaction with exclusion of moisture as the presence of water reduces the coupling efficiency of the reaction.
-
31.
Add 2.5 g (3.43 mmol, 1 eq.) HA-TBA, 2.96 g (4.86 mmol, 0.5 eq.) CD-HDA to the flask. Purge with nitrogen before adding 125 mL anhydrous DMSO (2% wt/vol) via cannulation. Stir at 350 rpm until fully dissolved.
-
32.
Add 1.06 g (4.86 mmol, 0.5 eq.) BOP into a separate dried round-bottom flask, purge with nitrogen, and add 20 mL DMSO via cannulation. Once BOP is fully dissolved, transfer to the HA-TBA/ CD-HDA solution via cannulation and stir for 3 h at RT.
-
33.
Quench reaction with 10 mL cold deionized water and transfer to dialysis tubing.
-
34.
Dialyze for 5 days at RT and change water twice daily. During the first three days add sodium chloride (NaCl) (1.5 g per liter dialysate) to force dialysis of the TBA salt.
-
35.
Remove insoluble by-products from the BOP coupling reaction by vacuum filtration, and return to dialysis for additional 5 days.
-
36.
Freeze at −80°C and lyophilize (see step 5).
-
37.
Confirm product and degree of modification using 1H NMR and D2O as a solvent.
?TROUBLESHOOTING
PAUSE POINT Lyophilized CD-HA and CD-MeHA samples can be stored under nitrogen for at least one year at −20 °C.
Preparation of Ad-HA TIMING 10-15 days.
-
38.
Allow HA-TBA to warm up to RT (see step 29). The synthesis of Ad-MeHA follows the analogous protocol.
-
39.
Equip a one-neck round bottom flask with a magnetic stir bar and stopper. Add 2.5 g (3.5 mmol, 1 eq.) HA-TBA, 2.04 g (10.5 mmol, 0.75 eq.) DMAP and 0.32 g (2.63 mmol, 3 eq.) 1-adamantane acetic acid to the flask and purge with nitrogen before adding 125 mL anhydrous DMSO (2% wt/vol) to the vessel via cannulation. Stir at 350 rpm for 20 min, or until the HA-TBA is fully dissolved.
CRITICAL Secure the stopper with a copper wire to prevent the stopper from becoming dislodged due to slight pressure when heated during the reaction.
CRITICAL It is essential to perform this reaction with exclusion of moisture as the presence of water reduces the coupling efficiency of the reaction.
-
40.
Melt BOC2O in a 37°C water bath and add 0.35 mL (0.41 eq.) to the reaction with a plastic syringe. Purge the reaction vessel with nitrogen and stir for 20 h at 45°C in an oil bath.
CRITICAL The degree of modification can be controlled by adjusting the quantity of BOC2O, where modifications of 20-50% have been achieved in our laboratory.
-
41.
Let the reaction mixture cool to RT, add 10 mL cold deionized water to quench the reaction, and transfer to dialysis tubing.
-
42.
Dialyze for 3 days at RT and change water twice daily.
CRITICAL STEP Precipitation of excess adamantane is expected and can be removed by either precipitation (option A) or filtration of the product followed by extensive dialysis (option B).
- Precipitation
- Add 0.95 g NaCl to the reaction solution (0.75 g/100 mL).
- Prepare 1.25 L ice-cold acetone (1 L per 100 mL) and stir at 500 rpm.
- Add the Ad-HA dropwise with a separation funnel.
- Stop stirring and allow the precipitate to settle (approximately 10 min).
- Decant excess acetone.
- Partition remaining acetone and precipitated product into 50 mL conical tubes.
- Centrifuge at 3200 g for 2 min and decant acetone.
- Re-dissolve product in 100 mL deionized water by vortexing, and return the product to dialysis for 5 days. Change water twice daily.
- Filtration
- Remove the precipitate from the solution by vacuum filtration using a No. 1 filter paper.
- Return the filtrate to dialysis for an additional 12 days. Change water twice daily.
-
43.Freeze at −80°C and lyophilize (see step 5).
-
44.Confirm product and degree of modification using 1H NMR and D2O as a solvent.
?TROUBLESHOOTING
PAUSE POINT Lyophilized Ad-HA and Ad-MeHA samples can be stored under nitrogen for at least one year at −20 °C.
Ad-HA fluorescent derivatization TIMING Part 1, variable ca. 20 h; Part 2, 9 days.
CRITICAL We describe the coupling of the near-infrared fluorescent Cy7.5-peptide to Ad-HA for in vivo monitoring of GH hydrogel degradation24. Alternatively, various dyes can be coupled to the GCKKG peptide to visualize GH hydrogels, including 5,6-carboxyfluoresceine and rhodamine following an analogous protocol28.
Part 1: Synthesis of GCKKG-Cy7.5 peptide
CRITICAL Synthesis of fluorescent peptide GCKKG-Cy7.5 is performed through standard solid phase peptide synthesis with glycinol 2-chlorotrityl resin (78 mg, nominal 0.31 mmol/g loading), and FMOC protected amino acids and HBTU (each 4-fold excess to resin).
-
45.
React the amine terminus with the free acid of Cy7.5 (50 mg, 3-fold excess to resin) by substitution of the fluorophore for the terminal amino acid.
-
46.
Cleave the peptide from the resin by stirring against 2.5 mL 95% trifluoroacetic acid, 2.5% triisopropylsilane, and 2.5% deionized water for 2 hours. Filter to remove resin and wash by dichloromethane.
-
47.
Recover the product by precipitation from 40 mL cold diethyl ether and wash three times by cold diethyl ether, with product recovery by centrifugation (3200 g, 3 min). Evaporate organic residues by vacuum pressure for 24 h (see step 21). Re-dissolve the peptide in MilliQ water, freeze at −80 °C, and lyophilize (see step 5).
-
48.
Verify the product by MALDI-tof mass spectrometry, using 1 mg per 1 mL matrix solution.
PAUSE POINT The dry GCKKG-Cy7.5 peptide can be stored under nitrogen for at least one year at −20 °C.
Part 2: Synthesis of Ad-MeHA-Cy7.5
CRITICAL Synthesis of CD-MeHA follows an analogous procedure. However, we recommend modification of Ad-MeHA to avoid potential interaction between CD and the fluorophore, which may hamper purification by dialysis.
-
49.
Dissolve 75 mg (0.15 mmol, 1.0 eq.) Ad-MeHA in 20 mL triethanolamine buffer (pH 10, see Reagent Setup).
-
50.
Dissolve 8.44 mg (7.24 μmol, 0.036 eq.) GCKKG-Cy7.5 peptide in 1 mL deionized water and add dropwise to the Ad-MeHA solution.
CRITICAL When using other fluorescently labeled peptides, adjust the quantity of peptide added accordingly due to change in molecular weight.
-
51.
Stir at RT for 2 h.
-
52.
Dialyze the solution for 5 days at RT, freeze at −80°C and lyophilize (see step 5).
-
53.
Verify the absorbance and emission spectra of the product in dilute aqueous solution (approximately 0.05 mg/mL) using a spectrophotometer. The Cy7.5 fluorescent polymer displays absorbance in the 650-850nm range (λmax abs 800 nm) and strong fluorescence in the near-infrared range (λmax em 808 nm).
?TROUBLESHOOTING
PAUSE POINT Lyophilized HA-Cy7.5 samples can be stored under nitrogen for at least one year at −20°C.
Characterization of GH hydrogels
Rheological properties TIMING 2-3 h.
-
54.
Prepare stock solutions of polymer by dissolving Ad-HA and CD-HA individually in PBS at the desired concentration (see Reagent Setup). Mix components in a 1.5 mL tube and briefly centrifuge to remove entrapped air (Fig. 6, Supplementary Video 1).
-
55.
Prepare the rheological testing equipment with a suitable cone and plate geometry (e.g. 20 mm diameter).
CRITICAL Measurements of in situ photopolymerization require a rheometer that is equipped with a UV curing system to monitor secondary covalent crosslinking. For photopolymerization, prepare polymers in PBS containing 0.05% Irgacure2959 (I2959).
-
56.
Use an appropriate scoopula to transfer ca. 100 μl of the pre-formed GH hydrogel onto the peltier plate.
CRITICAL Adjust the amount of sample based on the diameter of the upper plate and the selected gap size.
-
57.
Lower the upper plate to an appropriate gap size (e.g. 27 μm, specified for the geometry used).
CRITICAL Ensure correct loading on the plate by slightly overfilling and trimming off excess samples from the sides. Use of a solvent trap is recommended to prevent dehydration during characterization.
-
58.Characterize the rheological properties of GH hydrogels (option A) or alternatively, examine in situ photopolymerization of CD-MeHA and Ad-MeHA hydrogels (option B).
- GH hydrogel rheological properties
- Determine the shear-yielding of GH hydrogels by performing a strain sweep (0-100%) at 10 Hz (Fig. 7b).
- Investigate the recovery of the material after cyclic deformation by alternating strains of 1% and 100% (Fig. 7c).
- Measure the frequency dependence of GH hydrogels by running a frequency sweep from 0-100 Hz at 1% strain Supplementary Fig. 1a).
CRITICAL Dependent upon hydrogel composition, yield strain may vary. Frequency sweeps should be conducted at a strain below the yield point.
- In-situ photopolymerization
- Investigate the mechanical stabilization of Ad-MeHA and CD-MeHA by performing a time sweep (10 Hz and 1% strain), exposing the sample to UV light (10 mW/cm2) (Fig. 4e).
- Measure the frequency dependence of the covalently crosslinked hydrogels by running a frequency sweep from 0-100 Hz at 1% strain (Supplementary Fig. 1b).
?TROUBLESHOOTING
HA polymer release and erosion profile TIMING variable; assay, 3-4 h.
-
59.
Prepare hydrogels (see step 54) and load into a syringe (Supplementary Video 2).
-
60.
Inject the desired volume (e.g. 25 μL) of the hydrogel into the depression (5 mm diameter) of an acrylamide mold and briefly centrifuge to remove entrapped air and ensure a flat hydrogel surface, and cover hydrogels with 1 mL PBS and allow them to erode at 37 °C. (Fig. 6, Supplementary Video 3).
-
61.
Remove and collect the PBS at specified time points, replacing the buffer with fresh PBS.
-
62.
At the desired end point of the study, recover the remaining hydrogel by degrading the hydrogels in 1 mg/ mL hyaluronidase, incubating overnight at 37 °C.
-
63.
Determine the release of polymer with an uronic acid assay (see Reagent Setup). To obtain a standard curve dissolve 4 mg pristine HA in 1 mL PBS and perform serial dilutions (2, 1, 0.5, 0.25, 0.125, 0.062 and 0.031 mg/mL) and 0 mg/mL as a blank control.
-
64.
Vortex the tube containing the sample, and add 30 μl of the sample to the bottom of a 5 mL glass tube.
CRITICAL If a low concentration of HA is expected, increase the sensitivity of the assay by increasing the volume to 50 μl for all samples.
-
65.
Add 1 mL ice-cold sodium tetraborate decahydrate/sulfuric acid solution to each sample.
-
66.
Incubate the samples at 100°C for 10 min.
-
67.
Cool the samples on ice, add 30 μL of carbazole solution, and lightly vortex.
CRITICAL Avoid any water getting into the sample tubes, as reaction with chlorinated water will result in a color change (blue/green) which will alter absorption readings during subsequent data collection.
-
68.
Incubate samples at 100°C for 15 min and then cool on ice.
-
69.
Transfer samples into plastic cuvettes for absorbance measurement, using the 0 mg/mL standard to blank the instrument before recording sample absorbance at 525 nm.
!CAUTION To ensure accurate measurement, avoid handling cuvettes by the light-penetrating surfaces.
-
70.
Determine the sample HA concentration of each sample by comparison to linear fit performed on the standards. For cumulative release, normalize to the total HA content of all samples, including hydrogel content recovered by hyaluronidase degradation (step 70).
Release of encapsulated therapeutic cargo TIMING variable; assay, 3 h.
CRITICAL We describe the quantification of FITC-labeled BSA as a model biomolecule22. Alternatively, the release of various molecules, including antibodies, proteins, peptides, and small molecule therapeutics follows similar protocols26,34.
-
71.
Prepare stock solutions of polymer by dissolving Ad-HA and CD-HA individually in PBS at the desired concentration (see step 54).
CRITICAL To yield the desired final concentration, reserve an appropriate volume (e.g. 10%) for the addition of the therapeutic cargo.
-
72.
Add the therapeutic cargo at an appropriate concentration to the CD-HA and Ad-HA stock solutions (e.g. 0.1% wt/vol fluorescein isothiocyanate conjugated bovine serum albumin, FITC-BSA) and use these solutions to prepare the hydrogels (step 57). Load hydrogels into a syringe (step 63), transfer hydrogels into acrylamide molds (step 63), and perform release analogous to HA erosion (steps 63-65).
-
73.
Measure the release of FITC-BSA by determining the fluorescence intensity of the conjugated fluorophores relative to a standard curve of FITC-BSA with concentrations of 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.78 μg/mL in PBS, using PBS as blank control.
-
74.
Transfer 250 μL of each solution to a 96 well plates for fluorescence-spectroscopy. Determine fluorescence intensities at 525 nm with an excitation wavelength of 490 nm by using a plate reader.
-
75.
Determine the sample FITC-BSA concentration of each sample by comparison to linear fit performed on the standards. For cumulative release, normalize to the total content of all samples, including that recovered by hyaluronidase degradation.
CRITICAL Protect samples from light during storage and handling.
In vivo hydrogel erosion TIMING Part 1, 2 h; part 2, 1-2 h initial implantation; part 3, variable.
CRITICAL For in vivo applications, materials should be prepared under sterile conditions. Work in a class II biological safety hood and use sterile instruments. Clean all working surfaces with 70% (vol/vol) ethanol before use. All animal procedures should be conducted with appropriate regulatory board permissions and comply with the Guide for the Care and Use of Laboratory Animals.
Part 1: Preparation of GH hydrogel components
-
76.
Prepare sterile polymer stock solutions: Sterilize the desired amounts of dry Cy7.5 derivatized Ad-HA and CD-HA components by placing them beneath a germicidal ultraviolet lamp for 60 min in a class II biological safety cabinet. Dissolve the components individually in sterile PBS at the desired concentration and shake the polymer solutions overnight to ensure complete dissolution of the polymer (see Reagent Setup).
-
77.
Prepare hydrogels (see step 54) and load into a sterile syringe.
Part 2: Subcutaneous implantation
-
78.
Induce anesthesia with 1-2% inhaled isoflurane.
-
79.
Image the whole animal in a prone position (λex/λem = 785/820 nm) to provide baseline assessment of background auto-fluorescence.
-
80.
Inject the desired hydrogel volume (e.g. 25 μL) subcutaneously in the hind flank.
Part 3: Quantitative fluorescence imaging
-
81.
Repeat imaging post-injection and at subsequent specified time points.
CRITICAL Hair will significantly attenuate the fluorescent signal. Removal of hair by standard techniques around the injection site prior to initial injection and subsequent imaging time points is required.
-
82.
Use software provided for the imaging system and integrate the fluorescent signal over identically sized regions of interest covering the entire fluorescent signal.
-
83.
Correct the background to pre-injection values (step 79) and normalized to the peak fluorescence signal of the cohort.
?TROUBLESHOOTING
Cell encapsulation and viability in GH hydrogels TIMING Part 1, 2 h; part 2, 2-3 h; part 3, 3 h; part 4, 3 h.
CRITICAL For cell culture applications, work in a class II biological safety hood and use sterile instruments. Clean all working surfaces with 70% (vol/vol) ethanol before use.
Part 1: Preparation of GH hydrogel components
-
84.
Prepare sterile polymer stock solutions: Sterilize the desired amounts of dry Ad-HA and CD-HA components by placing them beneath a germicidal ultraviolet lamp for 60 min in a class II biological safety cabinet. Dissolve the components individually in sterile PBS at the desired concentration and shake the polymer solutions overnight to ensure complete dissolution of the polymer (see Reagent Setup).
CRITICAL 20% of the final volume should be reserved for resuspending the cell pellet, to yield the desired final concentration.
Part 2: Fabrication of agarose well-well molds
-
85.
Sterilize the customized plastic mold (Supplementary Fig. 2a) by spraying it with 70% (v/v) ethanol in a class II biological safety cabinet.
CRITICAL Ensure that the plastic mold has fully dried before proceeding.
-
86.
Dissolve 1.5 g agarose in 50 mL PBS by microwaving the agarose solution for 1 min at 600 Watt.
!CAUTION Wear thermo-insulated gloves when handling the agarose containing glassware.
-
87.
Pipette 2 mL of agarose into each well of a sterile 24 well plate, avoiding introduction of air bubbles. Cover the 24 well plate with the customized plastic mold (Supplementary Fig. 2b) and secure with rubber bands. Let it cool to RT for at least 60 min to ensure that agarose is fully solidified.
-
88.
Gently remove the plastic mold from the well plate and rinse agarose containing wells twice with 1 mL sterile PBS per well (Supplementary Fig. 2c)
-
89.
Add 0.5 mL sterile PBS to each well and transfer to a cell culture incubator at 37°C while preparing the hydrogel and processing the cells.
Part 3: Encapsulation of cells in GH hydrogels
-
90.
Harvest human MSCs or the cells of choice (e.g. EPCs or 3T3 fibroblasts) by detaching them from cell culture plastic (see Reagent Setup). Resuspend the cell pellet in a suitable volume of medium to determine the cell concentration using a hemocytometer.
-
91.
Calculate the cell suspension volume for the desired cell number and transfer to a sterile 2 mL microcentrifuge tube. Pellet the cells by centrifuging at 300 g for 5 min.
CRITICAL STEP The cell concentration depends on the cell type and its application. A typical cell density for the encapsulation of human MSCs is 5x106/mL.
-
92.
Aspirate the media from the cell pellet, resuspend with 20% PBS of the final gel volume and separate to the Ad-HA and CD-HA stock solution. Ensure homogeneous cell dispersion by gently pipet mixing.
CRITICAL The CD-HA solutions can be viscous, depending on the polymer concentration. Alternatively, use a positive-displacement pipette for mixing with the cell suspension.
!CAUTION Be very gentle when handling the cell-polymer solutions to ensure low shear-stress during mixing.
-
93.
Combine the two component solutions by gentle mixing in a 1.5 mL tube, briefly centrifuge, and transfer to the syringe barrel using an adequate scoopula (Supplementary Video 1 and 2). Alternatively, components may be combined directly in a 1 mL syringe barrel (Supplementary Video 4).
CRITICAL STEP We recommend mixing the GH components in the back of a syringe to reduce unnecessary shear forces that may be experienced by the cells during hydrogel transfer to the syringe.
-
94.
Depress plunger slowly and inject the gel into the agarose wells. Add 0.5 mL growth medium and incubate in a culture incubator at 37°C.
Part 4: Assessment of cell morphology and viability
CRITICAL STEP To visualize morphology and viability of encapsulated cells, perform fluorescent staining of live samples protected from light and take images of the hydrogels using confocal laser scanning microscopy (CLSM).
-
95.
Remove media from the wells and add 300 μl Live/Dead staining solution per sample in a 24 well-plate (see Reagent Setup).
-
96.
Incubate for 30 min in a culture incubator at 37°C. Aspirate the staining solution and replace with 0.5 mL serum-free media
-
97.
Image the samples with a CLSM with a 10/20x objective. Quantify cell viability by counting cells that stain positive for live and dead stains using ImageJ or CellProfiler.
?TROUBLESHOOTING
TIMING
MeHA synthesis, 12 days
HA-TBA/MeHA-TBA synthesis, 3-4 days
CD-HDA synthesis, 6 days
CD-HA/CD-MeHA synthesis, 10-11 days
Ad-HA/Ad-MeHA synthesis, 12 days
Dye-coupling to Ad-MeHA, 10-11 days
Rheological characterization, 1-3 h
Hydrogel erosion, variable erosion times; polymer quantification assay, 4 h
Molecule release, variable release times; FITC-assay, 3 h
In vivo hydrogel erosion, material preparation, 2 h; initial implantation, 1-2 h; quantitative imaging, 1h.
Cell encapsulation, material preparation, 2 h; agarose molds, 2-3 h; encapsulation, 3 h; Live-Dead assay, 3 h
ANTICIPATED RESULTS
Analytical data
Methacrylated HA
The extent of methacrylation depends on the pH, reaction time and ratio of MA to HA, and up to 100% of methacrylation can be achieved. The product should be recovered as a spongy white solid, with typical yields of approximately 70%. 1H NMR (300MHz, D2O) integration of the vinyl groups (δ=5.8 (1 H) and δ=6.25 (1 H)) relative to the HA backbone δ=3.20-4.20 (10 H); see Supplementary Fig. 3. For troubleshooting, see step 6.
Tetrabutylammonium salts of HA and MeHA
The product should be recovered as a spongy white solid.1H NMR (D2O) HA-TBA δ =0.9-1.04 (t, 12H), 1.33-1.47 (m, 8H), 1.62-1.76 (m, 8H), 2.1 (s, 3H), 3.13-3.3 (m, 8H), 3.30-4.20 (10 H); see Supplementary Fig. 4a. MeHA-TBA with additional vinyl groups (δ=5.8 (1 H) and δ=6.25 (1 H); see Supplementary Fig. 4b. All products derived from this intermediate (CD-HA, CD-MeHA, Ad-HA, and Ad-MeHA should be examined to confirm sufficient dialysis for elimination of the TBA salt.
CD-HA
6-o-monotosyl-6-deoxy-β-cyclodextrin (CD-Tos)
The product should be recovered as a white powder, where yields (2.5 g, 21.1%; 20 g, 22.2%; 50 g, 17.8%) and product characterization are consistent regardless of reaction scale and are consistent with literature reports45. 1H NMR (300MHz, DMSO-d6) δ = 2.42 (s, 3H), 3.12−3.80 (m, overlaps with HOD), 4.12–4.40 (m, 6H), 4.77 (s, 2H), 4.83 (s, 5H), 5.60–6.05 (br s, 14H), 7.43 (d, 2H), 7.75 (d, 2H); see Supplementary Fig. 6. For troubleshooting, see step 22 and Supplementary Fig. 10.
6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin (CD-HDA)
The overall yield of this step is 74.7± 7.4% (n = 3) for CD-HDA (white powder). 1H NMR (DMSO-d6) δ = 1.14–1.61 (m, 12H), 3.12–3.45 (m, overlaps with HOD), 3.48–3.78 (m, 28H), 4.28–4.56 (br s, 6H), 4.83 (s, 7H), 5.59–5.88 (br s, 14H), see Supplementary Fig. 7.
CD-HA and CD-MeHA
The product should be recovered as a spongy white to off-white solid, with typical yields of approximately 70%. The modification of HA is approximately 30% for CD-HA and CD-MeHA, respectively, where conduction of parallel syntheses yielding identical modifications (30.2% and 30.3%) serves to validate the reaction reproducibility, regardless of prior methacrylation. 1H NMR (D2O) based on the integration of the hexane linker δ = 1.35–1.85 (12H) relative to the methyl singlet of HA δ =2.1 (3H); see Supplementary Fig. 8a. For CD-MeHA, methacrylate modification is assumed to be conserved throughout the reaction and used to integrate the hexane linker δ = 1.35–1.85 (12H); see Supplementary Fig.8b. For troubleshooting, see step 37.
Ad-HA
The product should be recovered as a spongy white solid, with typical yields of approximately 70%. The modification of HA repeat units is 32.5% and 32.8% for Ad-HA and Ad-MeHA respectively. 1H NMR (D2O) based on integration of the ethyl multiplet of adamantane (δ = 1.50–1.85, 12H) relative to the HA backbone (δ = 3.20–4.20, 10H); see Supplementary Fig. 9. For troubleshooting, see step 44.
GCKKG-Cy7.5 peptide for Ad-HA fluorescent derivatization
The GCKKG-Cy7.5 peptide is verified by MALDI-tof and the expected mass of is 1165 Da.
Characterization of GH hydrogels
The rheological properties, erosion and release profiles of GH hydrogels largely depend on the hydrogel composition. By using the recommended stoichiometric equivalence of CD-HA and Ad-HA, estimated storage modulus for a 3.5% (wt/vol) hydrogel is 1000 Pa (10 Hz, 1% strain, see Supplementary Fig. 1a). Higher polymer concentrations increase the moduli of the hydrogel. Similarly, increasing the polymer concentration decreases hydrogel erosion and sustains the release of encapsulated therapeutic cargo22. Upon secondary photo-crosslinking, the extent of methacrylation and the UV exposure time control the number of covalent bonds (i.e. increase of G’) of the polymer network (see Supplementary Fig. 1b). In vivo, peak fluorescence intensity is typically observed within 24 hours post-injection and subsequent degradation proceeds over the course of approximately 30 days for a 3.5% (wt/vol) hydrogel injected subcutaneously24.
Towards applications of GH hydrogels as injectable cell carrier, cell viability is >95% before and >90% after injection in vitro (18 gauge needle). For troubleshooting, see step 97.
Supplementary Material
TABLE 1.
TROUBLESHOOTING
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3 | Cloudy MeHA solution | Insufficient dialysis. | Change water twice daily until the MeHA solution turns clear. |
| Solid particles are present | Formation of MA or methacrylic acid precipitates. | Filtrate the solution (#1 filter) before freezing and lyophilisation. | |
| 6 | Low modification (methacrylation) | Insufficient emulsification and phase separation of MA (step 2). | Use stir bar of sufficient size with a powerful stirrer to ensure good dispersion of MA. |
| Acidic pH while adding MA, will form methacrylic acid and thus not react with HA (step 2). | Ensure immediate pH adjustment when adding MA. Keep pH between 8.5–7.5, do not let it drop under 7.5. |
||
| 21 | Low yield of CD-Tos |
p-toluenesulfonyl chloride is of poor quality. p-toluenesulfonyl chloride is added to fast (step 16). |
Use freshly opened p-toluenesulfonyl chloride. Ensure low nitrogen pressure when using a cannula. Alternatively, add dropwise via syringe over approximately 10 minutes. |
| Poor precipitation water and acetone washes (steps 19 & 20). | Keep water, acetone, and samples on ice between the washing steps and centrifuge at 4°C. | ||
| 22 | High residual water peak (see Supplementary Fig. 10 as example) | Incomplete drying (step 21). | Repeat vacuum drying overnight to remove residual water. Purge sample with nitrogen before storage. |
| 25 | Cloudy acetone supernatant | Poor precipitation. | Ensure low amounts of DMF (ca. 5 mL) to dissolve pellet during the washing steps. |
| 37 | Low yields of CD modification on HA | Residual water quenches anhydrous reaction (steps 30-32). | Ensure that glassware is completely dry and purged with nitrogen before starting the reaction. |
| Pre-weigh HA(MeHA)-TBA before storage under nitrogen (step 30) to avoid long exposure to the atmosphere before starting CD-HA synthesis. Sufficiently purge by dry nitrogen prior to the reaction. | |||
| 44 | Low degree of Ad modification | Residual water quenches anhydrous reaction (steps 39 & 40). | Ensure that glassware is completely dry and purged with nitrogen before starting the reaction. |
| Pre-weigh HA(MeHA)-TBA before storage under nitrogen (step 39) to avoid long exposure to the atmosphere before starting CD-HA synthesis. Sufficiently purge by dry nitrogen prior to the reaction. | |||
| 53 | Low fluorescence intensities | High fluorophore modification of Ad-HA causes self-quenching of the coupled dye (step 50). | Titrate the ratio of labeled and un-labeled polymer components within the GH hydrogel. |
| 58 | Technical replicates are inconsistent | GH hydrogel sample is underfilled (step 56). | Ensure sufficient filling of the sample and trim excess material from all sides of the upper plate. |
| 83 | Low fluorescence Intensity | Over-labeling with Cy7.5 causes quenching of the fluorophore (step 76) | Titrate the ratio of the Cy7.5-labeled polymer with the non-labeled analogue (ca. 1:10). |
| 97 | Low cell viability (see Supplementary Fig. 11 as example). | Air bubbles are entrapped inside the sample (step 93). | Make sure to centrifuge the pre-formed hydrogel and avoid rupture of the sample when transferring (see Supplementary Video 1 and 2). Alternatively, mix GH components in the back of a syringe (see Supplementary Video 4) |
ACKNOWLEDGEMENTS
The authors are grateful for financial support from the National Science Foundation (DMR Award 1610525), an NIH/NIAMS training grant (T32-AR007132; M.H.C.) a Postdoctoral Fellowship (C.L.) from the IBSA Foundation, Switzerland, and predoctoral Fellowships (C.B.R., M.H.C.) and an Established Investigator Award (J.A.B) from the American Heart Association.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Li J & Mooney DJ Designing hydrogels for controlled drug delivery. Nature Reviews Materials 1, 16071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdick JA & Murphy WL Moving from static to dynamic complexity in hydrogel design. Nat Commun 3, 1269 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Drury JL & Mooney DJ Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337–4351 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Ifkovits JL & Burdick JA Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng 13, 2369–2385 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Nguyen KT & West JL Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 23, 4307–4314 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Ifkovits JL et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A 107, 11507–11512 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin R et al. Synthesis and characterization of hyaluronic acid–poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 6, 1968–1977 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Lehn J-M Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew Chem Int Ed Engl 29, 1304–1319 (1990). [Google Scholar]
- 9.Tibbitt MW, Rodell CB, Burdick JA & Anseth KS Progress in material design for biomedical applications. Proc Natl Acad Sci U S A 112, 14444–14451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H & Heilshorn SC Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv Mater 27, 3717–3736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber MJ, Appel EA, Meijer EW & Langer R Supramolecular biomaterials. Nat Mater 15, 13–26 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Guvendiren M, Lu HD & Burdick JA Shear-thinning hydrogels for biomedical applications. Soft Matter 8, 260–272 (2012). [Google Scholar]
- 13.DiMarco RL & Heilshorn SC Multifunctional Materials through Modular Protein Engineering. Adv Mater 24, 3923–3940 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Lu HD, Charati MB, Kim IL & Burdick JA Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials 33, 2145–2153 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Rodell CB, Mealy JE & Burdick JA Supramolecular Guest-Host Interactions for the Preparation of Biomedical Materials. Bioconjug Chem 26, 2279–2289 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Yang X et al. Self-healing polymer materials constructed by macrocycle-based host-guest interactions. Soft Matter 11, 1242–1252 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Ma X & Zhao Y Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chemical Reviews 115, 7794–7839 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Highley CB, Prestwich GD & Burdick JA Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 40, 35–40 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Charlot A & Auzély-Velty R Synthesis of Novel Supramolecular Assemblies Based on Hyaluronic Acid Derivatives Bearing Bivalent β-Cyclodextrin and Adamantane Moieties. Macromolecules 40, 1147–1158 (2007). [Google Scholar]
- 20.Charlot A, Auzély-Velty R & Rinaudo M Specific Interactions in Model Charged Polysaccharide Systems. The Journal of Physical Chemistry B 107, 8248–8254 (2003). [Google Scholar]
- 21.Semenov A, Charlot A, Auzély-Velty R & Rinaudo M Rheological properties of binary associating polymers. Rheologica Acta 46, 541–568 (2007). [Google Scholar]
- 22.Rodell CB, Kaminski AL & Burdick JA Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 14, 4125–4134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaffey AC et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg 150, 1268–1276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodell CB et al. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv Funct Mater 25, 636–644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodell CB et al. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Circ Cardiovasc Interv 9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodell CB, Rai R, Faubel S, Burdick JA & Soranno DE Local immunotherapy via delivery of interleukin-10 and transforming growth factor beta antagonist for treatment of chronic kidney disease. J Control Release 206, 131–139 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Soranno DE et al. Delivery of interleukin-10 via injectable hydrogels improves renal outcomes and reduces systemic inflammation following ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 311, F362–372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Highley CB, Rodell CB & Burdick JA Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater 27, 5075–5079 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Ouyang L, Highley CB, Rodell CB, Sun W & Burdick JA 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater Sci Eng 2, 1743–1751 (2016). [DOI] [PubMed] [Google Scholar]
- 30.van de Manakker F, Kroon-Batenburg LM, Vermonden T, van Nostrum CF & Hennink WE Supramolecular hydrogels formed by β-cyclodextrin self-association and host–guest inclusion complexes. Soft Matter 6, 187–194 (2010). [Google Scholar]
- 31.Wang LL et al. Injectable, Guest-Host Assembled Polyethylenimine Hydrogel for siRNA Delivery. Biomacromolecules 18, 77–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soranno DE, Lu HD, Weber HM, Rai R & Burdick JA Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J Biomed Mater Res A 102, 2173–2180 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyajima A et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58, 2301–2313 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Mealy JE, Rodell CB & Burdick JA Sustained small molecule delivery from injectable hyaluronic acid hydrogels through host-guest mediated retention. J Mater Chem B Mater Biol Med 3, 8010–8019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche ET et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials 35, 6850–6858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick JA, Mauck RL & Gerecht S To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 18, 13–15 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Caliari SR et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci Rep 6, 21387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodell CB, Dusaj NN, Highley CB & Burdick JA Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv Mater 28, 8419–8424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boekhoven J, Rubert Pérez, C. M., Sur, S., Worthy, A. & Stupp, S. I. Dynamic Display of Bioactivity through Host–Guest Chemistry. Angew Chem Int Ed Engl 52, 12077–12080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Frith J & Cooper-White JJ Modulation of stem cell adhesion and morphology via facile control over surface presentation of cell adhesion molecules. Biomacromolecules 15, 43–52 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Rodell CB, Wade RJ, Purcell BP, Dusaj NN & Burdick JA Selective Proteolytic Degradation of Guest–Host Assembled, Injectable Hyaluronic Acid Hydrogels. ACS Biomater Sci Eng 1, 277–286 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Burdick JA, Chung C, Jia X, Randolph MA & Langer R Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6, 386–391 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smeds KA & Grinstaff MW Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res A 54, 115–121 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Sahoo S, Chung C, Khetan S & Burdick JA Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules 9, 1088–1092 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNaughton M, Engman L, Birmingham A, Powis G & Cotgreave IA Cyclodextrin-Derived Diorganyl Tellurides as Glutathione Peroxidase Mimics and Inhibitors of Thioredoxin Reductase and Cancer Cell Growth. J. Med. Chem 47, 233–239 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Kaya E & Mathias LJ Synthesis and characterization of physical crosslinking systems based on cyclodextrin inclusion/host-guest complexation. J Polym Sci A Polym Chem 48, 581–592 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


