Abstract
Introduction
The role of MerTK, a member of the Tyro3-Axl-MerTK family of receptor tyrosine kinase, in the immune response to radiotherapy (RT) is unclear. We investigated immune- mediated tumor control following RT in murine models of colorectal and pancreatic adenocarcinoma using MerTK wild-type and knock-out hosts, and whether inhibition of MerTK signaling with warfarin could replicate MerTK knock-out phenotypes.
Methods
Wild-type and MerTK−/− BALB/c mice were grafted in the flanks with CT26 tumors and treated with CT-guided RT. The role of macrophages and CD8 T cells in the response to radiation were demonstrated with cell depletion studies. The role of MerTK in priming immune responses after RT alone and with agonist antibodies to the T cell costimulatory molecule OX40 was evaluated in a Panc02-SIY model antigen system. The effect of warfarin therapy on the in-field and abscopal response to RT was demonstrated in murine models of colorectal adenocarcinoma. The association between warfarin and progression free survival for patients treated with stereotactic ablative radiotherapy (SABR) for early stage non-small lung cancer (NSCLC) was evaluated in a multi-institutional retrospective study.
Results
MerTK−/− hosts had better tumor control after RT compared to wild-type mice in a macrophage and CD8 T cell-dependent manner. MerTK−/− mice showed increased counts of tumor antigen-specific CD8 T cells in the peripheral blood after tumor-directed RT alone and in combination with agonist anti-OX40. Warfarin therapy phenocopied MerTK’−/− for single-flank tumors treated with RT, and improved abscopal responses for RT combined with anti-CTLA4. Patients on warfarin therapy when treated with SABR for NSCLC had higher progression free survival rates compared to non-warfarin users.
Conclusions
MerTK inhibits adaptive immune responses after SABR. As warfarin inhibits MerTK signaling, and phenocopies genetic deletion of MerTK in mice, warfarin therapy may have beneficial effects in combination with SABR and immune therapy in patients with cancer.
Keywords: Cancer, radiotherapy, immunotherapy, warfarin
Introduction
Ionizing radiotherapy (RT) is an effective and efficient means to control tumor growth. Evidence supports a role for adaptive immune mediated clearance of residual disease after RT in order to achieve tumor cure.1,2 RT also holds promise to combine with immune therapy to control disease outside the treatment field.3–5
Myeloid cells infiltrate tumors after RT and commonly assume an immunosuppressive phenotype and thwart adaptive immune-mediated killing of remaining cancer cells.6 As macrophages infiltrate irradiated tumors, they encounter dead and dying cancer cells which are an important source of tumor antigen to prime anti-tumor immune responses.7 However, macrophages are not competent at cross-presentation of exogenous antigen to MHC class I, and do not traffic to tumor-draining lymph nodes. Thus, cancer antigen taken up by macrophages cannot generate new or expand existing CD8 T cell responses. MerTK, a member of the Tyro3-Axl-MerTk family of receptor tyrosine kinase, is expressed primarily on macrophages, and is important for sensing and phagocytosis of apoptotic cells. MerTK activation initiates a signaling pathway that engenders a macrophage-mediated immunosuppressive tumor environment that can limit adaptive immune killing of residual tumor cells.8,9 Thus, MerTK-mediated phagocytosis of irradiated cancer cells may be an important mechanism by which irradiated tumors escape immune control. Importantly, deletion of host MerTK has been shown to improve control of tumor growth with radiation8.
Warfarin is used as an anticoagulant to prevent thrombotic and thromboembolic disease. Warfarin inhibits a vitamin K dependent post-translational modification of several coagulation factors that inhibits enzyme complex formation on phosphatidylserine exposed on the outer membrane leaflet of activated platelets. Gas6 and Protein S are vitamin K-dependent endogenous ligands for the Tyro3-Axl-MerTK family of receptors. These ligands function as a phagocytic signal by binding to phosphatidylserine exposed on the outer membrane leaflet of apoptotic cells. Warfarin has been shown to inhibit MerTK and Axl signaling in vitro and exhibit in vivo efficacy in preclinical studies.10–15
We hypothesized that warfarin would enhance adaptive immune control of tumors following RT by preventing MerTK interaction with dying cells. Results from our preclinical studies demonstrate; that MerTK inhibits the adaptive immune control of tumors, warfarin is an effective treatment to improve CD8 T cell-mediated tumor control following RT, and warfarin improves distant tumor control following RT and immunotherapy. Finally, we demonstrate that patients incidentally treated with warfarin while receiving ablative RT for early stage lung cancer exhibit improved progression free survival. These experiments demonstrate a novel intervention to improve patient outcomes following radiation and to improve responses to RT and immune therapy combinations.
Materials and Methods
Animals and cell lines
BALB/c, C57BL/6 mice were obtained from The Jackson Laboratory. BALB/c MerTK−/− mice and C57BL/6 MerTK−/− mice were generated as described previously.8 Animal protocols were approved by the Institutional Animal care and Use Committee (Animal Welfare Assurance No. A39313–01).
The CT26 murine colorectal carcinoma cell line was obtained from ATCC (Manassas, VA). Panc02-SIY murine pancreatic adenocarcinoma cell line were kindly provided by Dr. Ralph Weichselbaum (University of Chicago, Chicago, IL). Species identity checks on these murine cell lines were performed with murine-specific MHC antibodies and were tested for contamination within the past six months using a Mycoplasma Detection Kit (SouthernBiotech, Birmingham, Alabama).
Radiation therapy of tumors
When flank tumors reached an average diameter of 5 mm, mice were randomized to warfarin (1.25 mg/L in drinking water) or no drug (control). Two days later, mice were randomized to a single fraction of radiation (12.5 Gy) or no further treatment. In vivo CT- guided RT was delivered using a Small Animal Radiation Research Platform (SARRP, XStrahl, Gulmay Medical, Suwanee, GA) at 220kV to an isocenter in the tumor, with beam angles designed to minimize dose to normal tissues. Dosimetry was performed using Murislice software (XStrahl). RT was delivered to cells in culture using a cesium source.
Antibodies and reagents
Depleting anti-CD8p (250 pg, BE0223 - BioXCell), anti-CSF1 (500 pg, BE0204- BioXCell) were given by intraperitoneal (i.p.) injection one-day prior to radiotherapy and repeated every five days for a maximum of three treatments. Agonist anti-OX40 antibody was a kind gift from Dr. Andrew Weinberg (EACRI, Portland, OR) and given i.p. one-day after radiotherapy at a dose of 250 pg.16 Warfarin (Coumadin, Bristol- Meyers-Squibb) was purchased from the hospital pharmacy. Antagonist anti-CTLA4 (250 pg, BE0164 - BioXCell) was given i.p. five days prior to radiotherapy.
Fluorescently conjugated flow cytometry antibodies CD8-PerCP Cy5.5 (53.6.7), CD4- FITC (HIS51) were purchased from Ebioscience (San Diego, CA), and PE-conjugated Kb- SIYRYYGL pentamers were from Prolmmune (Sarasota, FL).
Bone-marrow derived macrophages
Bone marrow from wild-type and MerTK−/− BALB/c mice was cultured in RPMI-1640 containing 10% fetal bovine serum and colony stimulating factor-1 (CSF-1, 40 ng/mL) for five days. Bone marrow derived macrophages (BMDM) were then co-cultured with CT26 cells that had been pretreated with zero or twenty Gy. Twenty-four hours later, cocultures were treated with lipopolysaccharide (20 ng/mL final concentration) or no additional treatment. Supernatants were collected after an additional 48 hours and analyzed with a multiplex bead assay (Life Technologies) and read on a Luminex 100 array reader (Luminex Corporation). Cytokine concentrations were calculated according to a standard curve.
Blood testing
Whole blood from euthanized mice was collected by venipuncture directly into sodium citrate (3.2% w/v) at a ratio of 9:1 v/v. Platelet poor plasma was obtained from citrated blood after centrifugation and stored in −80o C.17 Thawed plasma was recalcified and protimes measured on a KC4 coagulation analyzer (Trinity Biotech) after recalcification to 8.3 mM and mixing with thromboplastin (Dade Innovin®, Siemens Healthcare Diagnostics).
Quantification of antigen-specific cell numbers in peripheral blood was measured using a whole blood assay.18 Blood was stained directly with fluorescent antibody cocktails along with PE-conjugated Kb- SIYRYYGL pentamers. AccuCheck fluorescent beads (Invitrogen) were added to each sample before lysing the red blood cells with BD FACS lysing solution (BD Biosciences) and samples analyzed on a BD LSRII flow cytometer. Cell concentrations were determined by comparing cellular events to bead events.
Response to stereotactic ablative radiotherapy in patients receiving warfarin treatment
A bi-institutional retrospective analysis was performed on patients with early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy (SABR) at two independent institutions from September 2006 to June 2018. Patient variables including age, sex, comorbidities, tumor size and treatment variables including biologically effective dose for a/p=10 (BED10) were analyzed when available. Freedom from progression of disease was measured from the time between completion of SABR and date of diagnosis of metastasis to regional lymph nodes and/or distant sites in accordance with institutional review board policies.
Statistical analysis
Data were analyzed using Prism (GraphPad Software, La Jolla, CA). Individual data sets were compared using the Student’s t test, while analyses across multiple groups were performed with ANOVA with individual groups assessed with Tukey’s comparison. Overall and progression free survival of groups was compared using Kaplan-Meier method and analyzed with the log-rank analysis.
Results
Loss of MerTK permits radiation-mediated tumor cure via macrophages and CD8 T cells
Mice lacking the macrophage-specific gene MerTK have enhanced responses to radiotherapy.8 We hypothesized that loss of MerTK prevented suppressive differentiation of tumor macrophages, leaving a macrophage population to differentiate into pro-inflammatory phenotypes and support adaptive immune control of residual cancer cells.
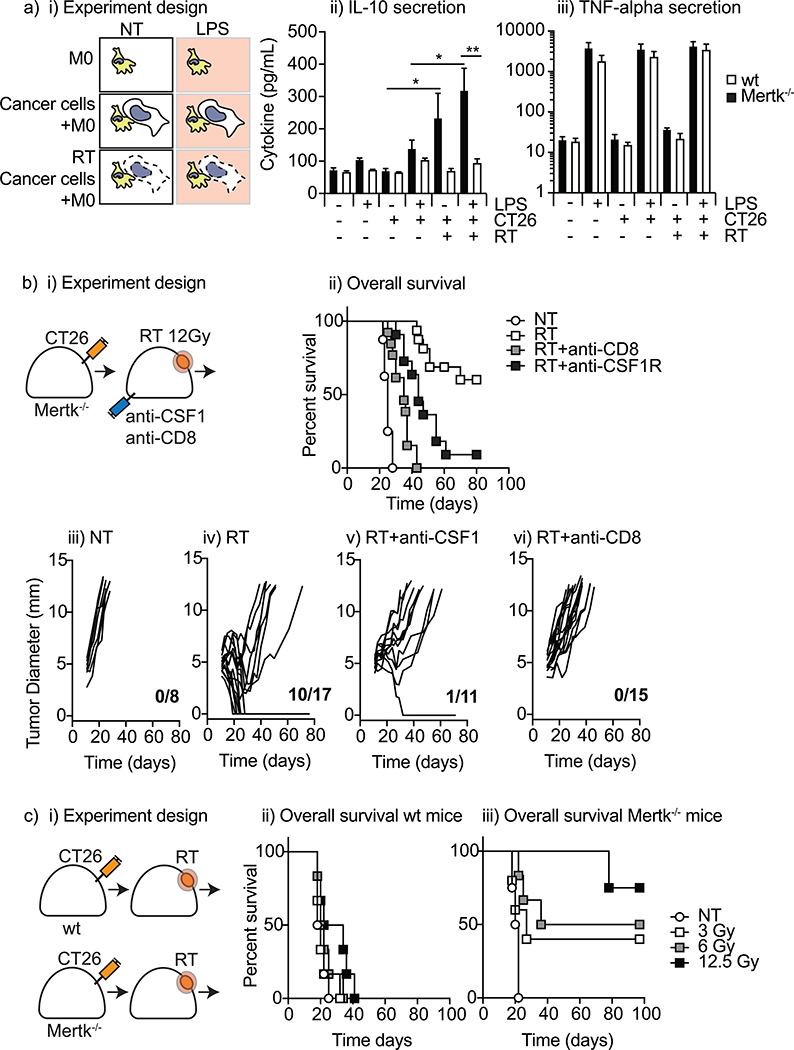
To test this, we co-cultured BMDM from wild-type and MerTK−/− hosts with untreated or irradiated CT26 cancer cells for 72 hours with or without additional LPS stimulation (Figure 1ai). We determined functional macrophage polarization by collecting supernatants and analyzing cytokine secretion profiles. BMDM from wild-type BALB/c and MerTK−/−BALB/c mice exhibited similar basal levels of IL-10 secretion when in monoculture, which was unaffected by stimulation with the TLR4 ligand LPS. Adding irradiated CT26 cells to wild-type macrophages led to a significant increase in IL-10 secretion, which did not occur in MerTK−/− macrophages (Figure 1aii). LPS treatment led to even more IL-10 secretion in the wild-type BMDM co-cultured with irradiated cells but did not affect IL-10 secretion of MerTK−/− BMDM. Interestingly, macrophage TNFa secretion was not affected by dying cells or the expression of MerTK_(Figure 1 aiii), contrary to previous data that used 4T1 cells combined with the RAW 264.7 monocyte cell line. 8 These data demonstrate that macrophage exposure to dying cells results in IL-10 secretion in a MerTK-dependent manner.
Figure 1. MerTK inhibits tumor control by radiation.
a) i) Experimental design, ii) IL-10, and iii) TNF-alpha cytokine concentrations in the supernatant from coculturing bone-marrow derived macrophages from wild-type and MerTK’−/− hosts with irradiated or untreated CT26 cancer cells for 24 hours before stimulating with LPS or no further stimuli. Data represents combined results from two independent experiments. b) i) Tumor growth assays with selective immune cell depletion. bii) Survival curves for MerTK’−/− hosts grafted with CT26 tumors and subjected to RT or no further treatment with selective depletion of immune cell populations. Individual tumor growth kinetics for CT26 tumors grafted on MerTK’−/− hosts on a BALB/c background grafted with CT26 tumors and given iii) no further treatment (NT), iv) a single dose of radiation to 12.5 Gy (RT), or v) depletion of CD8 T cells (RT/aCD8) or vi) macrophages (RT/aCSF1) one day prior to localized radiation to the tumor. Data represents the combined results from at least two independent experiments. c) i) Experimental design and Survival curves for ii) wild-type and iii) MerTK−/− hosts grafted with CT26 tumors and subjected to RT at 0, 3, 6 or 12.5 Gy. *p<0.05; **p<0.01;
To determine whether macrophages promoted tumor control in MerTK−/− mice we tested the effect of macrophage depletion on tumor cure following RT. Macrophage depletion using anti-CSF1R or anti-CSF1 has been shown to improve tumor control following RT by depleting suppressive macrophage populations, but does not result in tumor cure.19,20 If MerTK drives suppressive macrophage differentiation, it is possible that macrophage depletion in mice lacking MerTK would have no effect. Immune competent wild-type or MerTK−/− mice were grafted with CT26 tumors and treated with CT-guided radiation to the tumor (Figure 1 bi). As anticipated, MerTK−/− exhibited improved local control following RT (MS=25 days vs unmet, p<0.0001) (Figure 1bii). An additional group of mice received anti-CSF1 starting 1 day prior to RT to deplete macrophages. Importantly, loss of macrophages decreased tumor control following RT in MerTK−/− (MS=44 days, p<.005 vs RT) (Figures 1 bii, 1bv). These data demonstrate that if macrophages are prevented from undergoing immunosuppressive differentiation following tumor irradiation, they can play a positive role in clearance of residual disease. To determine if improved tumor cure rates after radiotherapy was dependent upon adaptive immune responses, MerTK−/− mice were depleted of CD8 T cells starting 1 day prior to RT. Loss of CD8 T cells resulted in no tumor cures following radiotherapy and a significant decrease in overall survival (MS=35 days, p<0.0001 vs RT) (Figures 1 bii, 1bvi). Thus, in mice where macrophages cannot respond to dying cells through MerTK, both macrophages and CD8 T cells contribute to tumor cure.
There is continuing debate regarding the optimal radiation dose to activate host-antitumor immune responses and to combine with immune therapies. To determine whether MerTK impacted responses at lower doses of RT, we evaluated overall survival in wild-type or MerTK−/− mice following a single fraction of 12.5 Gy radiotherapy down to 3 Gy (Figure 1ci). We found that while lower doses of RT had no impact on survival in wild-type mice (Figure 1cii), even a 3 Gy dose of RT led to tumor cure in a fraction of MerTK−/− mice (Figure 1 ciii). These data support a role for targeting MerTK across a broad range of radiation doses.
Host MerTK suppresses the generation of tumor antigen specific CD8 T cells and reduces the number of long-term survivors in a model antigen tumor model of pancreatic adenocarcinoma
To further investigate the role of MerTK on CD8 T cell control of tumors following radiotherapy, we used Panc02 tumors expressing the SIY model antigen where we are able to track tumor antigen-specific T cells in the circulation following RT.18
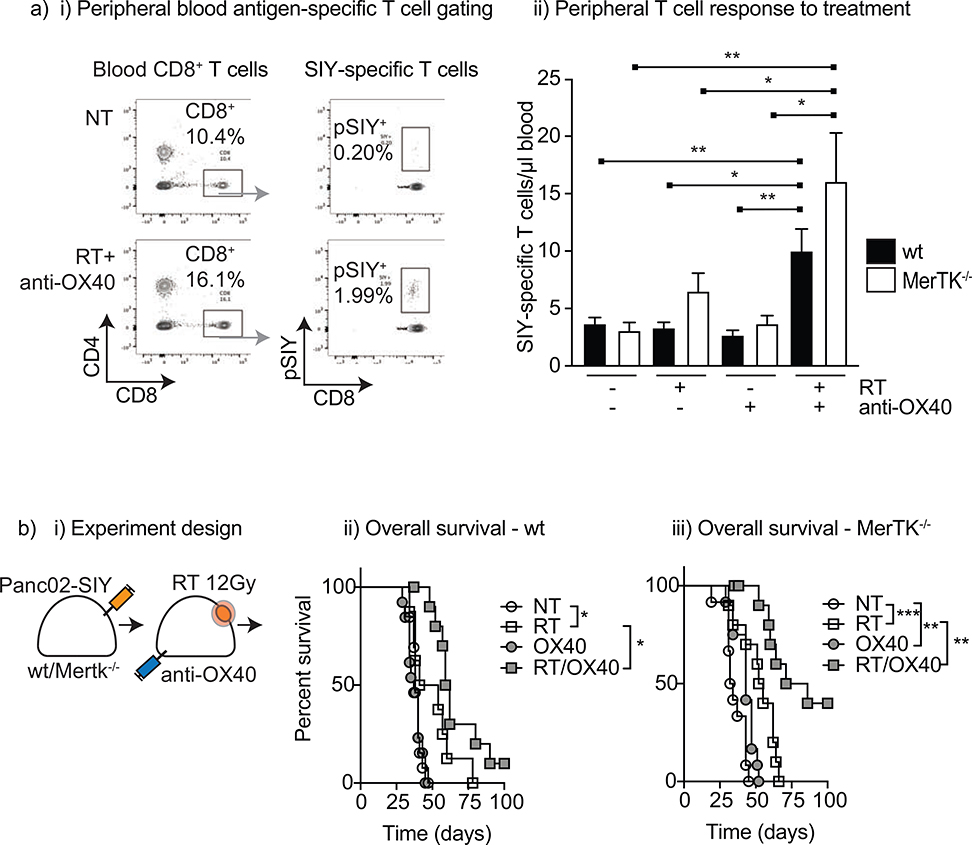
Panc02-SIY tumors were grafted in the flank of wild-type and MerTK−/− mice, allowed to grow to 5 mm before given a single dose of radiotherapy (12.5 Gy x1) or no treatment. Six days after RT, peripheral blood was collected and stained for tumor antigen-specific CD8 T cells using an SIY-MHC pentamer (Figure 2a). In untreated mice, low counts of pentamer-SIY positive CD8 T cells were detected in the peripheral blood that were not different between wild-type and MerTK−/− mice (mean =3.7 and 2.7/pL blood for wild-type and MerTK−/−, respectively) (Figure 2aii). Following RT, no increase in pSIY+CD8+ T cells were detected in wild-type mice, but there was a moderate increase in MerTK−/− hosts after RT (mean = 6.5/pL blood, p=0.051) (Figure 2aii). Both wild-type and MerTK−/− hosts achieved a survival advantage following RT, but MerTK did not influence survival after RT in this model (MS = 47.5 vs 53.5 days, p=0.84) (Figures 2bii and 2biii). Thus, the T cell response generated after RT was insufficient to generate local control, and we tested additional approaches to enhance anti-tumor T cell responses.
Figure 2. MerTK negatively regulates immune priming by radiation.
(a) Wild-type and MerTK−/− C57BL/6 mice were grafted in the R flank with Panc02-SIY tumors and allowed to grow to 5 mm diameter before subjecting to a single dose of RT (12.5 Gy), anti-OX40 (250 pg) or both. (ai) Gating strategy used for enumeration of pentamer-SIY specific CD8 T cells. (aii) Absolute number of peripheral blood SIY- specific CD8 T cells five days after RT. (b) i) Experimental design and Survival curves for ii) wild-type and iii) MerTK−/− mice grafted with Panc02-SIY tumors and treated with 0 or 12.5 Gy RT, anti-OX40 or both. Data represents combined results from three independent experiments. *p<0.05, **p<0.01, ***p<0.001
Agonist antibodies targeting OX40 are known to increase the number and function of tumor antigen-specific T cells in preclinical models21,22, and the combination of anti- OX40 and RT has demonstrated synergy.16,21 In mice given anti-OX40 alone, no increase in peripheral blood SlY-specific CD8 T cells were seen (mean = 3.1 and 3.7/pL blood for wild-type and MerTK−/−, respectively (Figure 2a). Combining RT with anti-OX40 resulted in a significant increase in SIY-CD8 T cells for both wild-type (mean=10/ pL, p=0.0085 vs untreated) and MerTK−/− mice (mean = 16/pL blood, p=0.0092 vs untreated). Importantly, the combination of RT and anti-OX40 led to improvement in tumor control and improvement in survival for both wild-type and MerTK−/− mice (Figure 2b). Median survival was not statistically different between wild-type and MerTK−/− hosts treated with RT and anti-OX40 (p=0.11), but an increase in tumor cures were seen in the MerTK−/− hosts compared to wild-type. These data demonstrate that loss of MerTK increased the generation of tumor antigen-specific T cell responses following RT and can combine with immune therapies to improve tumor control.
Warfarin administration in combination with RT drives T cell control of tumors
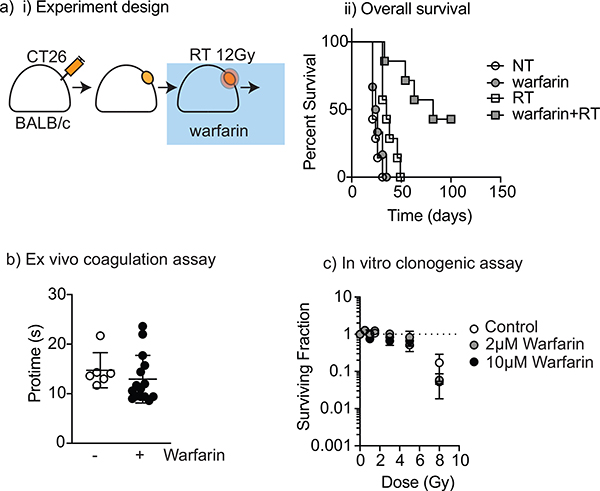
Warfarin has been demonstrated to interrupt Tyro3-Axl-MerTK signaling in vitro.10,15 In animal experiments, Gas6 signaling has been inhibited by adding warfarin to drinking water.11 This Gas6-disrupting dose is significantly below the threshold for coagulative effects.23 We hypothesized that warfarin could provide a therapeutic alternative to MerTK knockout, resulting in improved responses following RT. To test this hypothesis, warfarin was added to drinking water (1.25 mg/L) of wild-type mice bearing CT26 tumors and refreshed every 3–5 days for 14 days. CT26 tumors were implanted in wild-type BALB/c mice as above, and mice were randomized to receive control treatment or warfarin starting two days prior to RT. Non-irradiated mice given warfarin exhibited a marginal survival advantage (35 days vs 27 days for control, p=0.02, Figure 3a), but all tumors showed progression without further treatment. A single dose of irradiation led to 8/9 tumor cures in mice treated concurrently with warfarin and significantly increased overall survival compared to control mice (p<0.01, Figure 3aii).
Figure 3. Warfarin improves tumor control with radiotherapy at subtherapeutic doses without influencing radiation sensitivity.
(a) BALB/c mice were grafted with CT26 tumors and the tumors allowed to grow to 5 mm diameter before randomizing to warfarin and/or a single dose of ionizing radiotherapy 12.5 Gy and followed for tumor growth and host survival. i) Experimental design; ii) Overall survival. (b) BALB/c mice were grafted with CT26 tumors and subsequently randomized to warfarin (1.25 mg/L) or control drinking water for two weeks before harvesting platelet poor plasma and measuring protime. (c) CT26 cells were cultured with 0, 2 or 10 pM warfarin and subjected to radiation doses from 0 to 8 Gy, and clonogenic cells measured by counting formed colonies 5 days later.
To determine whether the effect of adding warfarin was due to changes in blood coagulation, mouse plasma was harvested, and coagulation testing performed by measuring the time needed to clot plasma when reconstituted with thromboplastin (protime). Mice subjected to warfarin at 1.25 mg/L did not exhibit different protimes from untreated mice, demonstrating that at 1.25 mg/L, warfarin administration was subtherapeutic with respect to anticoagulation (Figure 3b). However, further studies are needed to determine if there is a dose threshold for warfarin in the preclinical model. Similarly, to determine whether warfarin treatment altered the radiosensitivity of cancer cells distinct from any immune effect, CT26 growing in vitro were left untreated or treated with warfarin and tested for the radiosensitivity using a clonogenic assay. Warfarin treatment did not significantly affect the response of the cancer cells to RT (Figure 3c). These data are consistent with warfarin treatment affecting the tumor response to radiation via an immune mechanism.
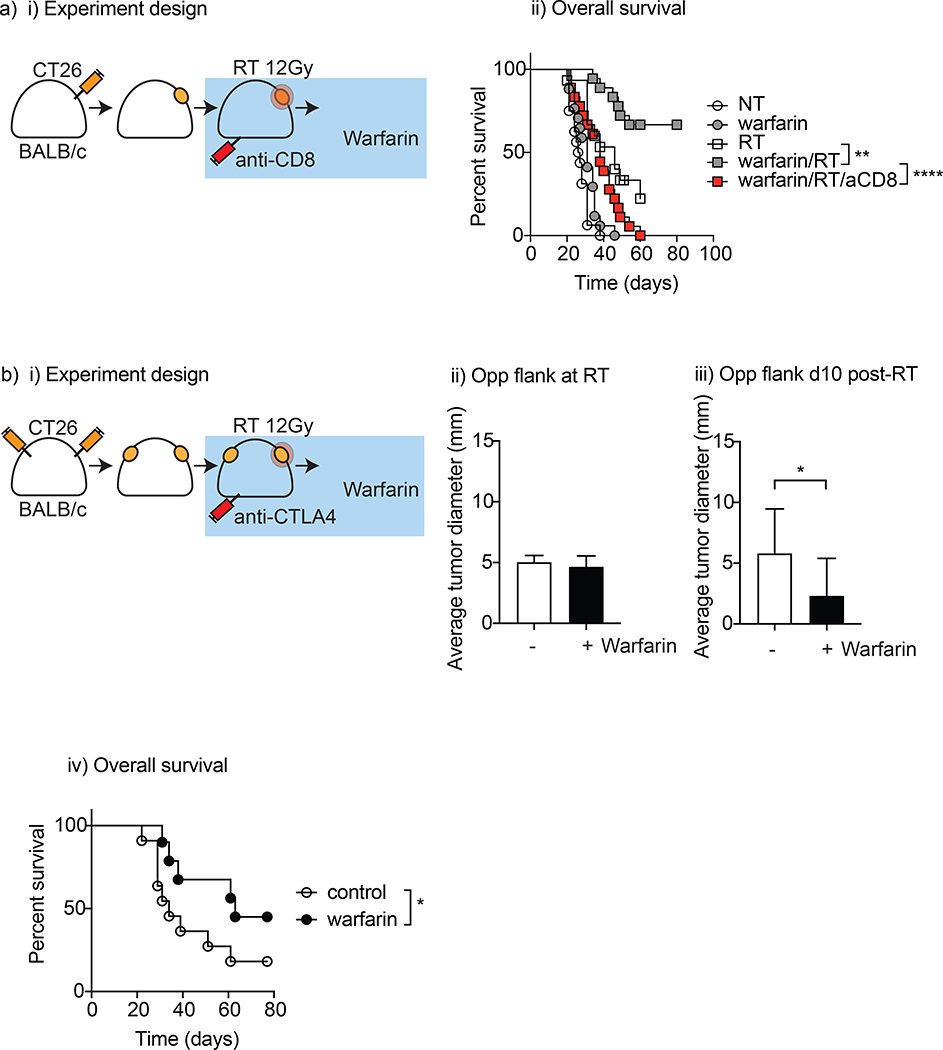
To determine whether tumor cure following RT and warfarin treatment was dependent on adaptive immune responses, we depleted CD8 T cells prior to RT (Figure 4ai). CD8 T cell depletion significantly decreased tumor control following warfarin and RT treatment (p<0.001), and eliminated tumor cures (Figure 4aii). These data demonstrate that warfarin treatment promoted tumor cure in wild-type mice via CD8 T cells, replicating the effect seen in MerTK−/− mice.
Figure 4. Warfarin promotes adaptive immune-mediated local and abscopal tumor control after radiation therapy.
(a) BALB/c mice were grafted with CT26 tumors which were allowed to grow to 5 mm before randomizing to warfarin (1.25mg/L) or control drinking water, CD8-depleting antibody 1-day prior to RT 12.5 Gy or no further treatment. i) Experimental design; ii) Overall survival. (b) i) BALB/c mice were grafted with CT26 tumors in both flanks, treated with anti-CTLA4 and tumors allowed to grow to 5 mm diameter before randomizing to warfarin (1.25 mg/L) or control drinking water before treating the R flank with RT (8Gy X3 on consecutive days); (bii) non-irradiated tumor measurements after RT and (biii) ten days later and (biv) corresponding survival curves. *p<0.05
Prior experiments have demonstrated that the combination of hypofractionated RT and anti-CTLA4 antibodies could control both local and distant tumors.24 Since warfarin is able to improve CD8 T cell control of tumors following RT, we tested whether warfarin could permit improved control of distant disease. To test this, we used a dual flank tumor model with CT26 tumors established simultaneously on both flanks. Mice were treated with a-CTLA4 before treating the right flank tumor with RT (8Gy x 3) which results in local tumor cure in this model and improved abscopal responses over irradiation with a large single fraction.18 Mice were randomized to receive control or warfarin treatment starting 2 days prior to RT. The contralateral, non-irradiated flank was monitored for tumor regression. On the last day of contralateral flank irradiation, the non-irradiated tumors from both control and warfarin-treated mice were not significantly different (Figure 4bii). Ten days later, the average size of the distant tumor had increased in the control group (diameter 5.8 +/− 1.1 mm) but decreased in the warfarin treated group (diameter 2.3 +/− 0.97 mm, p<0.05) (Figure 4biii). As expected, RT plus anti-CTLA4 resulted in cure of both tumors in a proportion of mice, but the addition of warfarin significantly improved overall survival in this model (63 days vs 34 days, p<0.05) (Figure 4biv). These data demonstrate that warfarin added to checkpoint inhibitors and RT improved control of distant disease.
Warfarin is associated with improved progression free survival in humans treated with SBRT for early stage non-small cell lung cancer
Warfarin is commonly prescribed clinically for thromboembolic prevention in the setting of atrial fibrillation or heart valve replacement in addition to treatment and prevention of deep vein thrombosis and pulmonary embolism, as well as after myocardial infarction. Warfarin use is not a contraindication to radiotherapy.
The recommended treatment for inoperable early-stage NSCLC is SABR. SABR, like surgery, achieves excellent control of the treated tumor, but a minority of patients develop regional and distant recurrences after treatment independent of irradiated tumor control, presumably from subclinical metastases that were not detected at the time of treatment. Promoting an immune response after SABR could potentially eliminate subclinical disease leading to improved progression free survival. We hypothesized that patients with non-small cell lung cancer (NSCLC) would include a substantial proportion incidentally receiving warfarin treatment, and that patients treated with SABR for early stage NSCLC while on warfarin would show a favorable PFS response to SABR.
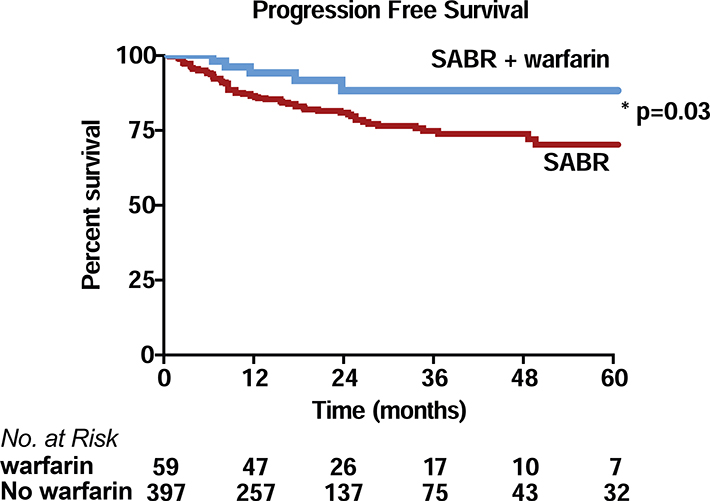
To test this, records from 456 patients treated with SABR at two distinct institutions were analyzed for warfarin use at the time of treatment. Patient, treatment and tumor characteristics are shown in Table 1. In our analysis, 59 (13%) were on warfarin at the time of SABR. Warfarin users were significantly older than non-warfarin users, but tumor and treatment characteristics were similar between groups. Local control (LC) was excellent and not different between warfarin users and non-warfarin users (3 year LC 96.1% vs 93.3%, p=0.64). Overall survival was similar (33 vs 39 mos., p=0.58), but warfarin users had increased progression free survival compared to non-users (Figure 5, p=0.03).
Table 1.
Characteristics of the Patients and SABR
| Characteristic | warfarin (n=59) | no warfarin (n = 397) | P-value |
|---|---|---|---|
| Age (yr) | 75.2 ± 7.8 | 72.4 ± 10.1 | 0.04 |
| Follow-up (mo) | 28.4 ± 22.2 | 22.9 ± 20.5 | 0.054 |
| Sex - male (%) | 89.5 | 85.5 | |
| Current smoker - no (%) | 9 (26.5) | 112 (47.9) | |
| COPD - no. (%) | 28 (82.4) | 165 (70.5) | |
| CAD - no. (%) | 19 (55.9) | 69 (29.5) | |
| Diabetes - no. (%) | 1 (2.9) | 39 (16.7) | |
| CHF - no. (%) | 10 (29.4) | 31 (13.2) | |
| Previous malignancy - no. (%) | 15 (44.1) | 65 (27.8) | |
| Tumor size - cm | 2.3 ± 1.1 | 2.2 ± 0.9 | 0.33 |
| cT1a - no. (%) | 5 (8.5) | 22 (5.5) | |
| cT1b- no. (%) | 19 (32.2) | 167 (42.1) | |
| cT 1 c- no. (%) | 22 (37.2) | 135 (34.0) | |
| cT2a- no. (%) | 6 (10.2) | 50 (12.6) | |
| cT2b- no. (%) | 4 (6.8) | 9 (2.3) | |
| cT3+ - no. (%) | 2 (3.4) | 8 (2.0) | |
| Not available - no (%) | 1 (1.7) | 6 (1.5) | |
| Radiation Dose - BED10 | 110 ± 12.6 | 112± 16.3 | 0.34 |
| Warfarin weekly dose (mg) – median (range) | 31.25 (8 – 105) | NA | |
| INR – median (range) | 2.23 (1.12 – 5.01) | NA |
Figure 5.
Progression (combined regional and distant) free survival for 59 warfarin users and 397 non-warfarin users with cT1–2N0 non-small cell lung cancers treated with stereotactic body radiotherapy (SABR) for curative intent at two different institutions.
Discussion
We demonstrate that targeting MerTK enhances control of tumors following RT, via a mechanism that depends on macrophages and CD8 T cells. The addition of warfarin to RT and a-CTLA4 improves control of tumors outside the treatment field. Importantly, patients on warfarin treatment have a favorable response to RT, suggesting that similar mechanisms may occur in patients. These data identify a novel intervention to improve immune control of tumors following RT, which may partner well with existing combination immune therapy and RT approaches to improve cancer control.
Warfarin is an old drug with a rich history of idiopathic anti-neoplastic activity in preclinical studies, with no clear agreement on how warfarin exhibits antineoplastic effects.25,26 Preclinical studies have shown some precedent that immunomodulation and anti-neoplastic effects of warfarin in vivo is mediated by macrophages.27 Multiple phase I/II studies have suggested that patients on warfarin exhibit improved outcome to conventional cancer therapies However, warfarin has exhibited mixed results in prospective phase III studies, and a lack of dose-dependent effects of warfarin on cancer outcomes has stymied further investigations as warfarin trials were designed primarily for its anticoagulant effects.32,33 Our data suggests that an immune mechanism can be activated with warfarin treatment at levels that do not impact coagulation, therefore this drug can be applied for immune effects at lower doses than for anticoagulation. However, the optimal dose range in patients remains to be determined.
We describe a novel mechanism of antineoplastic efficacy of warfarin when used in combination with radiotherapy as a promoter of anti-tumor adaptive immune responses. Thus, as these past studies were not focused on exploiting immune mechanisms to promote systemic cancer control, or ablative doses of radiotherapy, a re-evaluation of warfarin in combination with SABR and or immune therapies is warranted. As part of these studies, the impact of warfarin dose on patient outcomes should be evaluated through a larger retrospective analysis, and there is rationale for a prospective study at a lower effective dose that includes immune monitoring to evaluate immune responses to patient tumors. Importantly, warfarin exhibits these effects in vivo at sub-therapeutic anticoagulation levels, and fixed-dosing of warfarin has been shown to be safe in patients with cancer and should alleviate the concern that warfarin leads to increased risk of hemorrhage in patients with cancer.34
Importantly, these data demonstrate that by targeting a blood coagulation pathway we can improve immune control of tumors. This mechanism is indirect and is dependent on tumor-associated macrophages, with CD8 T cells providing the final effector pathway. These data are impactful since tumor-associated macrophages are broadly considered a negative force and multiple strategies are in development and clinical testing to deplete these cells or prevent their entry to tumors following treatment. However, we show that in MerTK−/− mice, macrophages are critical to support anti-tumor immunity and their depletion results in loss of activity. This has not been determined in warfarin treated mice, and although we expect similar results, it is possible that warfarin also affects other cells by impacting Gas6 and protein S ligation of Tyro3 or Axl. Thus, macrophages can be converted to provide a critical support for T cell antitumor immunity through targeting this pathway. Since starting this work, a recent paper has demonstrated that antibodies blocking MerTK can combine with anti-PD1 therapy to improve control of local and distant tumors in preclinical models.35 However, in these models, anti-MerTK combined with RT was not more effective than radiation alone, and the addition of anti-PD1 was needed to prolong survival, though this did not result in tumor cures.35 It is possible that the difference may relate to drug penetration or target coverage in the tumor environment, or pharmacodynamic differences of sustained post-translational inhibition with warfarin. Coagulation Factor X is also dependent on post-translational vitamin K-dependent processes for its functionality and is readily inhibited by warfarin, though at higher doses than Gas6. A role for myeloid cell expression of coagulation factor X in the tumor microenvironment to inhibit adaptive immune responses has being demonstrated.36 Thus, warfarin may have additional MerTK unrelated effects in the tumor microenvironment that promote adaptive immune responses following radiotherapy. At a time with intense interest in promoting systemic cancer control with combination immunotherapy and RT, warfarin treatment has the potential to be an extremely cost effective adjuvant to immunotherapy to improve patient outcomes and deserves further study.37
Acknowledgments
Supported in part by: NIH R01CA182311 (MJG), NIH R01CA208644 (MRC), NIH R21AI126151 (MRC), RSNA RR1972 (GWT), NIH RO1HL101972 and R01GM116184 (OJTM)
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gough MJ & Crittenden MR Immune system plays an important role in the success and failure of conventional cancer therapy. Immunotherapy 4, 125–128, doi: 10.2217/imt.11.157 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Lee Y et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595, doi: 10.1182/blood-2009-02-206870 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demaria S et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 11, 728–734 (2005). [PubMed] [Google Scholar]
- 4.Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC & Demaria S In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 33, 7415–7422, doi: 10.1016/j.vaccine.2015.05.105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh JW et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation- Related Abscopal Responses. Cancer Immunol Res, doi: 10.1158/2326-6066.CIR-18-0793 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crittenden MR et al. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PloS one 7, e39295, doi: 10.1371/journal.pone.0039295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tormoen GW, Crittenden MR & Gough MJ The TAM family as a therapeutic target in combination with radiation therapy. Emerging Topics in Life Sciences 1, 493–500, doi: 10.1042/ETLS20170066 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crittenden MR et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 7, 78653–78666, doi: 10.18632/oncotarget.11823 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dransfield I, Zagorska A, Lew ED, Michail K & Lemke G Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell Death Dis 6, e1646, doi: 10.1038/cddis.2015.18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng K et al. Requirement of Gamma-Carboxyglutamic Acid Modification and Phosphatidylserine Binding for the Activation of Tyro3, Axl, and Mertk Receptors by Growth Arrest-Specific 6. Front Immunol 8, 1521, doi: 10.3389/fimmu.2017.01521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirane A et al. Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis. Cancer Res 75, 3699–3705, doi: 10.1158/0008-5472.CAN-14-2887-T (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waizenegger JS et al. Role of Growth arrest-specific gene 6-Mer axis in multiple myeloma. Leukemia 29, 696–704, doi: 10.1038/leu.2014.236 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Paolino M et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512, doi: 10.1038/nature12998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagita M et al. Gas6 regulates mesangial cell proliferation through Axl in experimental glomerulonephritis. Am J Pathol 158, 1423–1432, doi: 10.1016/S0002-9440(10)64093-X (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwar A et al. Mer tyrosine kinase (MerTK) promotes macrophage survival following exposure to oxidative stress. J Leukoc Biol 86, 73–79, doi: 10.1189/jlb.0608334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young KH et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 11, e0157164, doi: 10.1371/journal.pone.0157164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tormoen GW, Recht O, Gruber A, Levine RL & McCarty OJ Phosphatidylserine index as a marker of the procoagulant phenotype of acute myelogenous leukemia cells. Phys Biol 10, 056010, doi: 10.1088/1478-3975/10/5/056010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crittenden MR et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Scientific reports 8, 7012, doi: 10.1038/s41598-018-25482-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 73, 2782–2794, doi: 10.1158/0008-5472.CAN-12-3981 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiao SL et al. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer immunology research 3, 518–525, doi: 10.1158/2326-6066.CIR-14-0232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough MJ et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother 33, 798–809, doi: 10.1097/CJI.0b013e3181ee7095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen SM et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol 37, 524–532, doi: 10.1053/j.seminoncol.2010.09.013 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerch C et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke 39, 3397–3404, doi: 10.1161/strokeaha.108.517482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewan MZ et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 5379–5388, doi: 10.1158/1078-0432.CCR-09-0265 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilgard P & Thornes RD Anticoagulants in the treatment of cancer. Eur J Cancer 12, 755–762 (1976). [DOI] [PubMed] [Google Scholar]
- 26.Bobek V & Kovarik J Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother 58, 213–219, doi: 10.1016/j.biopha.2003.11.007 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Maat B Selective macrophage inhibition abolishes warfarin-induced reduction of metastasis. Br J Cancer 41, 313–316, doi: 10.1038/bjc.1980.46 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zacharski LR et al. Effect of warfarin on survival in small cell carcinoma of the lung. Veterans Administration Study No. 75. JAMA 245, 831–835 (1981). [PubMed] [Google Scholar]
- 29.Thornes RD et al. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol 120 Suppl, S32–34 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakchbandi W, Muller H, Singer MV, Lohr JM & Nakchbandi IA Prospective study on warfarin and regional chemotherapy in patients with pancreatic carcinoma. J Gastrointestin Liver Dis 17, 285–290 (2008). [PubMed] [Google Scholar]
- 31.Beg MS et al. Impact of Concurrent Medication Use on Pancreatic Cancer Survival-SEER-Medicare Analysis. Am J Clin Oncol 41, 766–771, doi: 10.1097/COC.0000000000000359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zacharski LR et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer 53, 2046–2052 (1984). [DOI] [PubMed] [Google Scholar]
- 33.Maurer LH et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. J Clin Oncol 15, 3378–3387, doi: 10.1200/JCO.1997.15.11.3378 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Levine M et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 343, 886–889, doi: 10.1016/s0140-6736(94)90008-6 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Caetano MS et al. Triple Therapy with MerTK and PD1 Inhibition plus Radiotherapy Promotes Abscopal Antitumor Immune Responses. Clin Cancer Res, doi: 10.1158/1078-0432.CCR-19-0795 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graf C et al. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci Immunol 4, doi: 10.1126/sciimmunol.aaw8405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tormoen GW, Crittenden MR & Gough MJ Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol 3, 520–526, doi: 10.1016/j.adro.2018.08.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]