Abstract
The ecdysteroid and sesquiterpenoid pathways control growth, developmental transition, and embryogenesis in insects. However, the function of orthologous genes and the cross-talk between both pathways remain largely uncharacterized in non-insect arthropods. Spook (Spo) and Juvenile hormone acid o-methyltransferase (Jhamt) have been suggested to function as rate-limiting factors in ecdysteroid and sesquiterpenoid biosynthesis, respectively, in insects. In this study, we report on the functions of Spo and Jhamt and the cross-talk between them in embryos of the branchiopod crustacean Daphnia magna. Spo expression was activated at the onset of gastrulation, with the depletion of Spo transcript by RNAi resulting in developmental arrest at this stage. This phenotype could be partially rescued by supplementation with 20-hydroxyecdysone, indicating that Spo may play the same role in ecdysteroid biosynthesis in early embryos, as reported in insects. After hatching, Spo expression was repressed, while Jhamt expression was activated transiently, despite its silencing during other embryonic stages. Jhamt RNAi showed little effect on survival, but shortened the embryonic period. Exposure to the sesquiterpenoid analog Fenoxycarb extended the embryonic period and rescued the Jhamt RNAi phenotype, demonstrating a previously unidentified role of sesquiterpenoid in the repression of precocious embryogenesis. Interestingly, the knockdown of Jhamt resulted in the derepression of ecdysteroid biosynthesis genes, including Spo, similar to regulation during insect hormonal biosynthesis. Sesquiterpenoid signaling via the Methoprene-tolerant gene was found to be responsible for the repression of ecdysteroid biosynthesis genes. It upregulated an ortholog of CYP18a1 that degrades ecdysteroid in insects. These results illuminate the conserved and specific functions of the ecdysteroid and sesquiterpenoid pathways in Daphnia embryos. We also infer that the common ancestor of branchiopod crustaceans and insects exhibited antagonism between the two endocrine hormones before their divergence 400 million years ago.
Introduction
Two endocrine hormones, ecdysteroid and sesquiterpenoid, play important roles in arthropod physiology, development, and phenotypic plasticity [1–5]. Thus, they are believed to have contributed to the evolution of a diverse range of life strategies in arthropods, making them exceptionally abundant and ecologically diverse [6]. The high conservation of genes in the ecdysteroid and sesquiterpenoid biosynthesis pathways across arthropod species (Chelicerata, Myriapoda, Crustacea, and Insecta) is indicative of the vital roles of these two hormones in this clade [7]. The mutagenesis and knockdown of ecdysteroid and sesquiterpenoid pathway genes in insects have demonstrated their functions not only in larva and adults, but also in embryos [8–11].
Ecdysteroid biosynthesis genes in embryos have been widely studied in multiple insect species, including Drosophila melanogaster and Bombyx mori. Ecdysteroids are supplied maternally into eggs as conjugated ecdysteroid. During early embryogenesis, ecdysteroid-phosphate phosphatase (EPPase) converts the conjugated ecdysteroid into an active form, 20-hydroxyecdysone (20E) [12]. When embryos and larva have de novo biosynthesis capability, they synthesize ecdysteroids from cholesterol. The conversion of cholesterol to active ecdysteroids (ecdysteroidogenesis) requires a series of hydroxylation reactions involving multiple enzymes, mostly of the P450 families, including Noppera-bo (Nobo), Neverland (Nvd), Non-molting glossy/shroud (Sro), Spook (Spo), Spookier (Spok), Cyp6t3, Phantom (Phm), Disembodied (Dib), Shadow (Sad), and Shade (Shd) [13]. Genes encoding these enzymes (except Nvd, Cyp6t3, and Spok) are collectively termed as the Halloween genes [13–15]. Mutants of these genes exhibit common phenotypes that result from abnormally low ecdysteroid titers, including a poorly differentiated embryonic cuticle, dorsal closure failure, defective midgut morphogenesis, and eventually embryonic lethality [8, 16, 17].
The sesquiterpenoid hormone is synthesized from acetyl-CoA through the mevalonate pathway. The early steps of the mevalonate pathway to produce farnesyl pyrophosphate (FPP) are conserved among arthropods. The conversion of FPP into its final form of sesquiterpenoid, however, varies among taxa [18, 19]. In chelicerates and some crustaceans, methyl farnesoate (MF) has been reported as the final product of the sesquiterpenoid pathway, while in the majority of insect species, the final product of the sesquiterpenoid pathway is Juvenile Hormone III (JH III) [20, 21]. The rate-limiting reaction of sesquiterpenoid hormone biosynthesis has been thought to be the final conversion into JH III or MF via S-adenosyl-methyltransferase (SAM)-dependent methylation by juvenile hormone acid methyltransferase (Jhamt) [11, 18]. In the hemimetabolous insect Blattella germanica, knockdown of Jhamt resulted in impaired hatchability [22]. In the holometabolous insect B. mori, a biallelic mutation of Jhamt also reduced hatchability [23]. In both cases, the phenotypic changes produced by modulation of Jhamt were not severe and in B. mori, hatchability could be improved by dechorionating the eggshell. Eventually, mutant embryos were able to grow into larvae, indicating that this hormone is dispensable in the embryonic development of insects.
Recently, cross-talk between these two hormones has been found participate in the regulation of each other’s biosynthesis in D. melanogaster larvae. Through its response genes, JH reduced the size of prothoracic gland (PG), the portion of ring glands that synthesize ecdysteroid [24]. Meanwhile, the ecdysteroid signaling cascade inhibits the expression of JH biosynthesis genes in the corpora allata (CA) [24]. The antagonism between these two hormones is a key element in the progression of metamorphosis [24]. However, the functions of the biosynthetic genes for these two hormones and the cross-talk between pathways remain to be studied in non-insect arthropod species.
The branchiopod crustacean Daphnia magna is not only the most closely related crustacean to insects, but is also the only crustacean with a sequenced genome that can be easily manipulated [25]. Its draft genome sequence and transcriptome data are publicly available [26–28]; moreover, gene manipulation techniques, including RNAi, TALEN, and CRISPR/Cas9, have been established in this organism [29–35]. In a nutrient rich environment, D. magna produces parthenogenetic eggs and increases its population asexually. Its embryos show typical direct development [36]. Hatching of D. magna embryos occurs when appendage segmentation ends [37]. In insects, this stage seems to be consistent with timing of blastokinesis [38]. When the hatched embryos become juveniles, they molt to attain their complete morphology, with structures including setae of the second antennae and a tail spine. The hatched embryo subsists on its own yolk supply, similarly to later stages of the hemimetabolan embryo, or pronymph [39]. Previous reports have identified the presence of homologs of genes involved in ecdysteroid and sesquiterpenoid biosynthesis and signaling in Daphnia genomes [40–43]. The ecdysteroid biosynthesis pathway had been partly analyzed by the knockdown of Neverland (Nvd) and Shade (Shd) [44, 45], suggesting that both genes play roles in de novo ecdysteroid synthesis during embryogenesis. Functional analysis related to the sesquiterpenoid biosynthesis genes and signaling has also been conducted. The analysis of Jhamt in D. pulex confirmed its role in MF biosynthesis and suggested that it has a role in male offspring production in adults [43].
In this study, we analyzed the function of Spo and Jhamt during the progression of D. magna embryogenesis. We confirmed the role of Spo in ecdysteroid biosynthesis in early embryos. We present evidence of the potential function of Jhamt and sesquiterpenoid in the regulation of embryonic timing of D. magna. Moreover, Jhamt/sesquiterpenoid controls genes that are involved in ecdysteroid metabolism.
Results
Cloning and characterization of Spo and Jhamt
We performed a TBLASTN search using the amino-acid sequences of Spo and Jhamt obtained from several insect species against the D. magna genome database (http://arthropods.eugenes.org/EvidentialGene/daphnia/daphnia_magna/). One ortholog of Spo was located at scaffold 02011 in the database. The full sequence of the Spo cDNA was determined using 5′ and 3′ RACE. Comparing the Spo cDNA to genome sequences revealed that it contains 3 exons and 2 introns, spanning 4,191 bp in the genome (Fig 1A). The Spo coding sequence (CDS) was found to encode a 591-residue protein with a molecular weight of 66 kDa (S1A Fig). Multiple alignment with fruit fly (D. melanogaster), silkworm (B. mori), and red flour beetle (Tribolium castaneum) orthologs revealed that D. magna Spo contains typical CYP450 characteristic domains: Pro/Gly cluster, WxxxR, ExxR, PERF motif, and heme loop (S1B Fig).
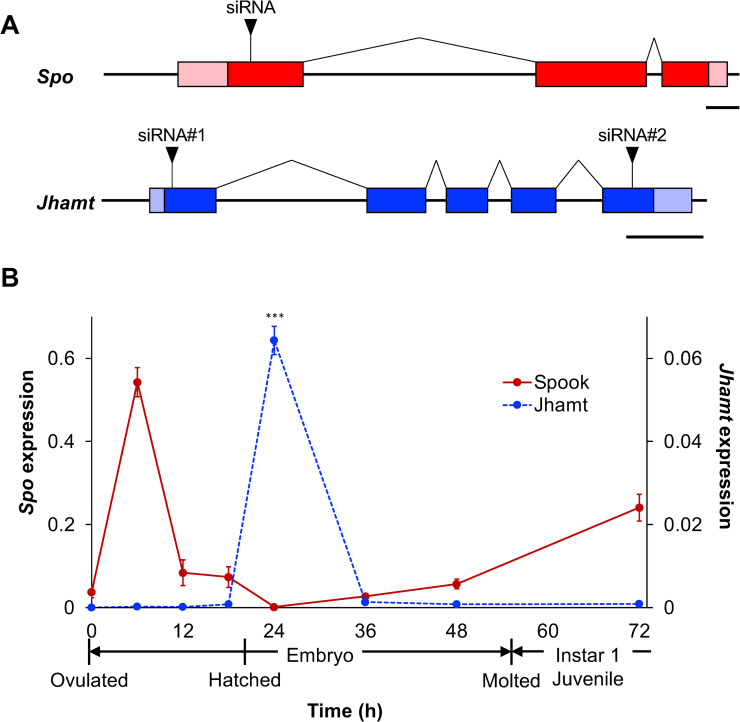
Fig 1. Gene structures and temporal expression patterns of Spo and Jhamt during embryogenesis.
(A) Schematics of Spo (red) and Jhamt (blue) genes. Coding sequences (CDSs) are shown in dark-colored boxes and untranslated regions (UTRs) are indicated in light-colored boxes. siRNA target sites are denoted by black triangles. Scale bar, 250 nt. (B) Expression of Spo (red) and Jhamt (blue) during embryogenesis quantified by qRT-PCR. Timing of hatching and molting to instar 1 juveniles are shown under the x-axis. Expression levels are normalized to those of the reference gene ribosomal L32. Values are means. Error bars represent SD (N = 3). ***p<0.001 (Student’s t-test).
The Jhamt gene was found at scaffold 00915. We sequenced a full-length the Jhamt cDNA, revealing that this gene spans 1,767 bp and consists of 5 exons and 4 introns (Fig 1A). It encodes a protein of 270 residues with a molecular weight of 31 kDa (S2A Fig). We identified a putative S-adenosyl-L-methionine (SAM) domain located in the N-terminus that is characteristic of the methyltransferase protein family.
Spo is essential for the progression of early embryogenesis
We first measured Spo gene expression levels using qRT-PCR. Expression peaked at 6 h after ovulation (hao) before abruptly dropping to its lowest level shortly after hatching at 24 hao. Expression then steadily increased throughout the remainder of embryogenesis (Fig 1B).
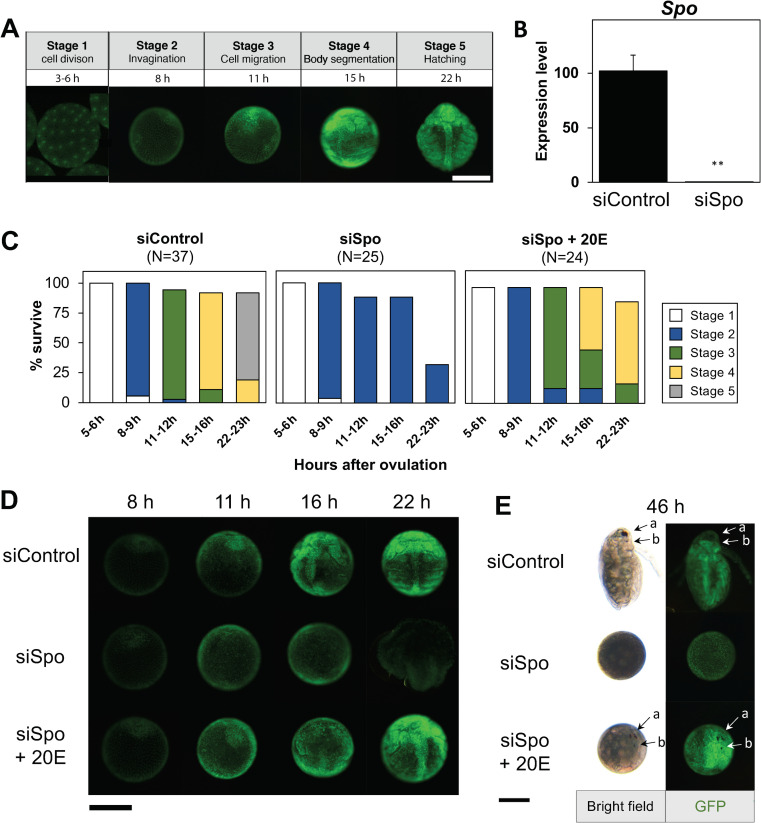
To investigate the role of Spo, we performed loss of function analysis using RNA interference (RNAi). To easily track its developmental progression in the embryo stage, we utilized a previously established transgenic Daphnia that ubiquitously expresses an H2B-GFP fusion protein [30]. We determined a staging parameter as previously described [30, 37] (Fig 2A). Cleavage and blastula formation occurred during the first 6 hao (Stage 1). Shortly after, the appearance of an invagination pit was indicative of gastrulation (Stage 2). Following gastrulation, cell mass migration proceeded, with the formation of three germ layers (Stage 3). At around 15 hao, the formation of thoracic appendages and a pair of secondary antennae was observed (Stage 4). Hatching occurred around 22 hao (Stage 5).
Fig 2. Spo is essential during early embryogenesis in Daphnia magna.
(A) Developmental stages of D. magna during early embryogenesis at 23°C as observed by GFP fluorescence in an H2B-GFP transgenic line. (B) Expression levels of Spo in siControl and siSpo embryos at 6 h assessed by qRT-PCR. Values are means. Error bars represent SD (N = 3). **p<0.01 (Student’s t-test). (C) Developmental progression and survival rate of siControl and siSpo with or without exposure to 1 μM 20-hydroxyecdysone (20E). Bars denote the developmental stages described in (A): stage 1 (white), stage 2 (blue), stage 3 (green), stage 4 (yellow), stage 5 (gray). (D) Representative images of embryos injected with siControl, siSpo, and siSpo rescued by 20E. Embryos were imaged by GFP fluorescence. Representative photographs from different stages are combined into a single image. (E) Retardation of embryo developmental progression. Compound eye (a) and naupliar eye (b) development observed at 46 h after ovulation. Scale bar, 200 μm.
We initially confirmed that siRNA-Spo significantly reduced Spo transcripts at 6 hao (Fig 2B). Embryos injected with siRNA-Spo showed no developmental differences relative to siRNA-ctrl-injected embryos until stage 2, during cleavage and cell mass migration (Fig 2C and 2D). Subsequently, all of the injected embryos showed a retardation in development and failed to proceed beyond stage 2 (Fig 2C and 2D; siSpo), in contrast to control embryos (Fig 2C and 2D; siControl). This developmental arrest was consistent with the EcR RNAi phenotype observed in our previous study [46].
To demonstrate the role of Spo in ecdysteroid biosynthesis in D. magna embryogenesis, we attempted to rescue the phenotype of Spo RNAi embryos by supplementing the culture medium with 20E. As expected, 20E-exposed Spo RNAi embryos progressed beyond stage 2 (Fig 2C and 2D; siSpo+20E). Around 70% of rescued embryos progressed to stage 4 (Fig 2C; siSpo+20E). Of the rescued embryos, 10% developed further and formed naupliar and compound eyes after 46 hao (Fig 2E) but did not become juveniles. These results demonstrate that Spo is essential for early embryogenesis, likely via ecdysteroid biosynthesis.
Jhamt prevents precocious embryonic development
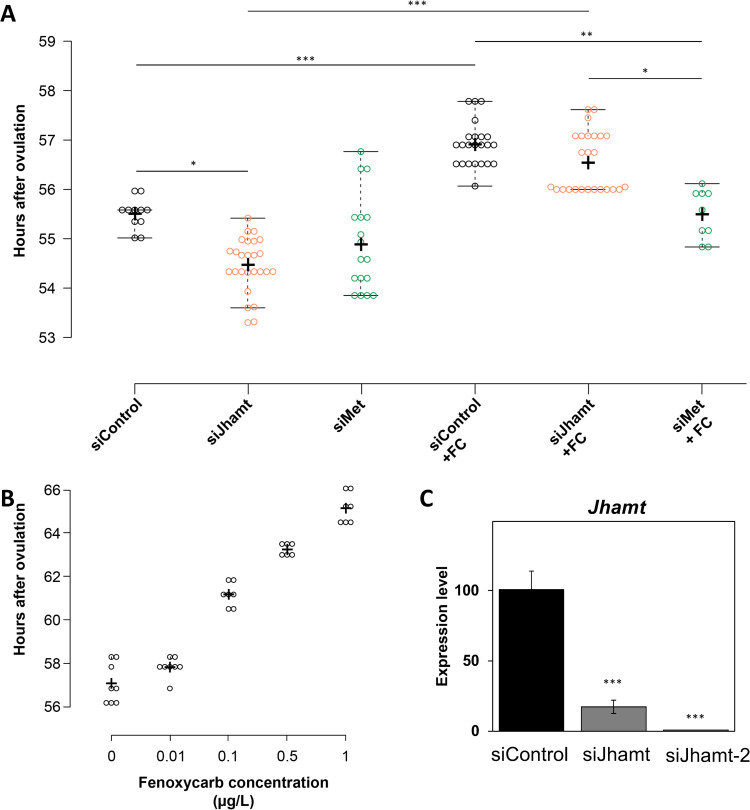
In contrast to Spo, Jhamt mRNA was rarely detected during embryogenesis, with the exception of a significant expression surge at 24 hao (Fig 1B). To determine the function of Jhamt during embryogenesis, we injected eggs with siRNA-Jhamt. However, no significant difference in hatching or survival was observed in Jhamt RNAi embryos, suggesting that Jhamt loss of function has little effect on embryo survival (Table 1). Because sesquiterpenoids are known as “status quo” hormones in insects, we also examined the timing of molting after the end of embryogenesis. siRNA-Jhamt-injected embryos showed a significant reduction during the embryonic period compared with the control (Fig 3A; siJhamt), suggesting that sesquiterpenoid biosynthesis represses the precocious transition of embryos into the juvenile stage.
Table 1. RNAi inhibition of sesquiterpenoid pathway genes.
| siRNA | Total injected eggs | Relative hatchability (%) | Relative survivability (%) |
|---|---|---|---|
| siControl | 104 | 100± 13 | 100± 19 |
| siJhamt | 133 | 105 ± 3a | 102 ± 11b |
| siMet | 95 | 105 ± 5c | 86 ± 4 d |
Values are means ± SD.
ap = 0.30
bp = 0.43
cp = 0.29
dp = 0.84 (Welch’s t-test).
Fig 3. Jhamt knockdown shortens Daphnia magna embryonic period.
(A) Embryonic periods (hours after ovulation, or hao) of siControl, siJhamt, and siMet embryos with or without fenoxycarb. *p<0.05; **p<0.01; ***p<0.001 (Kruskal-Wallis followed by Dunn post-hoc test; p-values adjusted using the Benjamini-Hochberg procedure). (B) Embryonic period of embryos exposed to different concentrations of fenoxycarb. (C) Expression levels of Jhamt in 24 h embryos injected with siControl and siJhamt as measured by qRT-PCR. Values are means. Error bars represent SD (N = 3). **p<0.01 (Student’s t-test).
To elucidate the regulatory role of sesquiterpenoid signaling in embryos, we silenced Met, which codes for the sesquiterpenoid receptor. Consistent with siRNA-Jhamt, Met RNAi had little effect on embryo survivability (Table 1). There were similarities with the Jhamt RNAi embryos during embryogenesis, although there was no significant difference between the control and Met RNAi treatment (Fig 3A; siMet). We also examined the effect of exposure to the sesquiterpenoid analog Fenoxycarb (FC), on this phenotype. FC increased the length of the embryonic period in both control and Jhamt siRNA-injected embryos (Fig 3A; siControl+FC, siJhamt+FC) in a FC concentration-dependent manner (Fig 3B). In contrast, FC treatment of siRNA-Met injected embryos did not significantly elongate the embryonic period (Fig 3A; siMet+FC), possibly due to the silencing of sesquiterpenoid signaling. A reduction in the expression level of the Jhamt transcript was observed upon siRNA-Jhamt injection (Fig 3C). These results suggest that Jhamt plays an important role in sesquiterpenoid biosynthesis and the regulation of the embryonic period.
Sesquiterpenoid signaling regulates the expression of ecdysteroid metabolism genes in hatched embryos
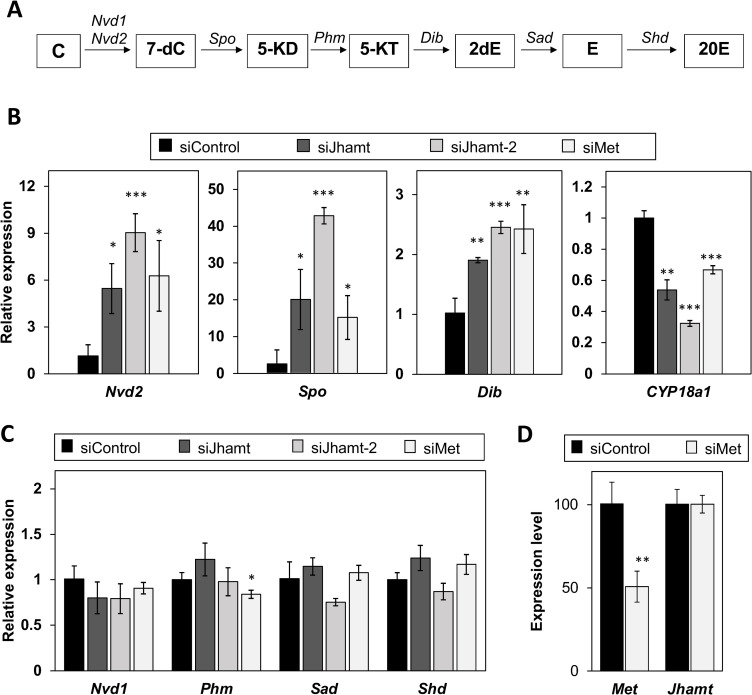
Unexpectedly, significant upregulation of Jhamt expression was found to be coincided with low levels of Spo transcript expression in hatched embryos at 24 hao (Fig 1B). This antagonistic pattern of Spo and Jhamt expression suggested the hypothesis of cross-talk between ecdysteroid and sesquiterpenoid biosynthesis at this stage. To investigate this hypothesis, we measured Spo expression in Jhamt RNAi embryos, observing a significant increase at 24 hao after siRNA injection (Fig 4B). We confirmed the specificity of this Spo activation using a different Jhamt-targeting siRNA, siRNA-Jhamt-2 (Fig 4B). We analyzed the expression of other genes functioning in ecdysteroidogenesis pathways in insects (Fig 4A, 4B and 4C) and found that Nvd2 and Dib were upregulated (Fig 4B and 4C). Furthermore, we measured the expression of Cyp18a1, which is responsible for the degradation of ecdysteroid [45, 47]. In contrast to ecdysteroidogenesis genes, Cyp18a1 expression was reduced by Jhamt RNAi (Fig 4D).
Fig 4. Jhamt knockdown upregulates expression of ecdysteroid biosynthesis genes at 24 hao.
(A) Predicted ecdysteroid biosynthesis pathway in Daphnia magna. C: cholesterol; 7-dC: 7-dehydrocholesterol; 5-KD: 5β-ketodiol; 5-KT: 5β-ketotriol; 2dE: 2-deoxyecdysone; E: ecdysone; 20E: 20-hydroxyecdysone. Nvd: Neverland; Spo: Spook; Phm: Phantom; Dib: Disembodied; Sad: Shadow; Shd: Shade. (B) Expression of Nvd2, Spo, Dib, and Cyp18a1 at 24 h in siControl, siJhamt and siMet embryos at 24 h after siRNAt as measured by qRT-PCR. (C) Expression levels of other ecdysteroidogenesis genes in siControl, siJhamt, and siMet embryos. Expression levels are normalized to those of ribosomal L32, then shown relative to expression levels of siRNA-ctrl. Values are means. Error bars represent SD (N = 3). *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test).
We also examined the effect of Met RNAi on genes related to ecdysteroid metabolism. Consistent with the effect of siRNA-Jhamt injection, mRNA levels of Spo, Nvd2, and Dib (Fig 4B) were increased, while Cyp18a1 expression was decreased, with no effect on the other genes (Fig 4C). The level of Jhamt mRNA expression was not affected by Met knockdown (Fig 4D), suggesting that sesquiterpenoid signal transduction regulates ecdysteroid biosynthesis and degradation, respectively. Taken together, our results suggest cross-talk between the sesquiterpenoid and ecdysteroid metabolic pathways in hatched embryos.
Discussion
The endocrine hormones ecdysteroid and sesquiterpenoid are known to regulate developmental transition, growth duration, and embryogenesis in insects. Recently, genome sequencing studies have revealed that genes related to ecdysteroid and sesquiterpenoid biosynthesis are highly conserved, indicating the importance of these two hormones across arthropod species [7]. However, their functions have not yet been elucidated in non-insect arthropods, which has prevented a deeper understanding the functional similarity and diversity of genes related to these hormonal pathways. In this study, we reported on the functional analysis of potential rate-limiting genes Spo and Jhamt, and cross-talk between ecdysteroid and sesquiterpenoid biosynthesis pathways in a branchiopod crustacean. A combination of RNAi and hormone administration indicated that Spo and Jhamt play roles in ecdysteroidogenesis and sesquiterpenoid biosynthesis, respectively, during embryogenesis. We also found evidence of cross-talk between the ecdysteroid and sesquiterpenoid pathways.
The expression pattern and functional analysis of Spo confirmed its indispensable role in ecdysteroidogenesis during D. magna embryogenesis. The Spo transcript was highly expressed during early embryonic stages and peaked at 6 hpo, which coincides with the onset of gastrulation [30], indicating that ecdysteroid is required for embryonic cell differentiation. Spo expression in the D. magna embryo showed a similar expression pattern to that in the early embryo of D. melanogaster, where Spo has been found to be expressed during pre-cellular blastoderm stage, prior to gastrulation [17]. In D. magna, the RNAi-mediated knockdown of Spo resulted in embryonic arrest during gastrulation. This phenotype is consistent with Halloween gene mutants previously reported in insects [16, 17, 48, 49], suggesting its conserved role in embryonic ecdysteroid biosynthesis. Interestingly, Spo RNAi embryos showed no developmental differences before 8 hpo, which includes the processes cleavage and early gastrula formation (Fig 2B and 2C). This suggests that embryos utilized maternally supplied ecdysteroid conjugates instead of performing de novo ecdysteroidogenesis before gastrulation. Indeed, in a previous study, we found that the expression of EPPase dropped sharply after 6 hpo, which was consistent with the activation of Spo [50]. This pattern suggests that Spo begins to take over ecdysteroidogenesis after the depletion of maternally supplied ecdysteroid or EPPase. Spo RNAi embryos supplemented with 20E resumed development, although hatching was never achieved (Fig 2C, 2D and 2E). In Drosophila, the Spo mutant phenotype could be completely rescued by the administration of 20E, eventually progressing to adulthood [17]. This is possible because in Drosophila and other dipteran species, Spo is only expressed during embryogenesis, while another paralog, Spookier (Spok), takes over ecdysteroidogenesis in later stages [17, 51]. Since D. magna only has one ortholog of Spo, 20E supplementation may not be sufficient to completely rescue embryogenesis.
The knockout and knockdown of Jhamt are known to affect development in insects. In a previous study, Jhamt and Met RNAi resulted in impaired hatchability of embryos in the hemimetabolous insect Blattella Germanica [22]. In the holometabolous insect B. mori, Jhamt knockout was performed using TALENs. The resulting biallelic mutation did not have any effect on embryonic development in homozygous Jhamt mutants generated by crossing heterozygous adults, but led to a decreased embryo hatching rate [23]. These results suggest that Jhamt plays a role in hatchability in insects. In D. magna, neither Jhamt nor Met RNAi severely affected hatching of embryos or the survival of juveniles, suggesting that sesquiterpenoid signaling in D. magna embryogenesis is less important than it is in insect embryos. This may be due to the short duration of Jhamt expression in the Daphnia mid-embryonic stage compared with a longer duration from mid- to late embryogenesis in B. germanica and B. mori. The Met RNAi phenotype in this study was different from that observed in a previous study, in which embryos injection with long, double-stranded RNA targeting Met arrested embryogenesis [42]. Although the reason for this contradiction is unclear, this embryonic-lethal phenotype was neither evaluated by injecting another non-overlapping Met dsRNA nor by a rescue experiment. In D. pulex, Jhamt was reported to be expressed during the juvenile and adult molting cycles [43, 52]. Moreover, in Daphnia, sesquiterpenoid has been found to regulate phenotypic plasticity in predator defense [5, 53, 54] and environmental sex determination [55, 56]. Therefore, the role(s) of sesquiterpenoid in Daphnia may change in later life stages, as observed in insects [57]. To disrupt Jhamt function completely, we should generate and cross heterozygous D. magna mutants. However, the hatchability and genetic stability of embryos produced by sexual reproduction is low due to the accumulation of deleterious homozygous mutations, as this species typically undergoes parthenogenetic reproduction, preventing us from more precisely evaluating the function of Jhamt in hatching. To overcome this problem, a conditional knockout method will need to be developed in D. magna in the future.
Our results indicated that Jhamt and sesquiterpenoid signaling via Met play a role in the repression of precocious development, as their knockdown reduced the duration of the embryonic period, while supplementation with a sesquiterpenoid analog elongated embryogenesis. This phenotype is in concordance with the well-known effect of sesquiterpenoid, a “status quo” action. In contrast, a B. mori Jhamt mutant showed an increased embryonic period, while a sesquiterpenoid analog-exposed cricket showed precocious embryonic development, demonstrating that D. magna may have uniquely co-opted sesquiterpenoid signaling for the timing of embryogenesis.
A possible advantage of the repression of precocious embryonic development may be the synchronization of embryogenesis with ovarian development. In D. magna, once eggs are laid inside the mother’s brood chamber, the embryos develop in this chamber until their first juvenile instar stage. Interestingly, embryogenesis is almost perfectly synchronized with the maturation of the mother’s ovaries. Shortly after the release of the first instar juveniles from the brood chamber, the mother molts and lays the next batch of eggs. A shortening of embryonic development leads to the earlier release of juveniles from the brood chamber. In contrast, when embryonic development is delayed and embryos are kept longer, they will be released together with the mother’s carapace, possibly increasing the risk of predation.
The antagonistic relationship between ecdysteroid and sesquiterpenoid biosynthesis has been previously reported in insects [24, 58]. A recent study on the Drosophila ring gland showed that JH induces Kr-h1 expression, whose gene product inhibits ecdysteroid biosynthesis by suppressing EcR/USP and ecdysone-induced early transcription factors (TFs) (Broad complex, E75, and E73), resulting in reduced PG size and subsequently the inhibition of metamorphosis. Vice versa, 20E via EcR/USP action suppress the JH biosynthesis genes Jhamt and HMG-CoA reductase (Hmgcr) in CA, thereby lowering the JH level and allowing the larva to proceed to metamorphosis [24]. In this study, we provide evidence of antagonism between the ecdysteroid and sesquiterpenoid biosynthesis mechanisms during D. magna embryogenesis (Fig 5). Interestingly, we also observed a significant downregulation of Cyp18a1, which is responsible for ecdysteroid inactivation [45, 47] (Fig 4B). To the best of our knowledge, this study is the first to demonstrate a correlation between ecdysteroid inactivation and the antagonistic action of sesquiterpenoid, indicating that sesquiterpenoid plays a broader role in the regulation of ecdysteroid metabolism. The regulation of ecdysteroidogenesis by sesquiterpenoid is likely to be orchestrated by a sesquiterpenoid signaling cascade, since the Met knockdown caused similar ecdysteroid biosynthesis gene upregulation. It will be important to clarify which downstream components of sesquiterpenoid signaling inhibit ecdysteroid biosynthesis genes in Daphnia. In contrast, in this study, we were unable to determine the role of ecdysteroid in the regulation of sesquiterpenoid biosynthesis or metabolism in D. magna since the currently available approaches for the impairment of ecdysteroid signaling or biosynthesis resulted in embryonic lethality during early embryogenesis [45, 46]. Nevertheless, our results suggest that ecdysteroid and sesquiterpenoid hormonal cross-talk was established before the evolutionary divergence of Daphnia from insects over 400 million years ago.
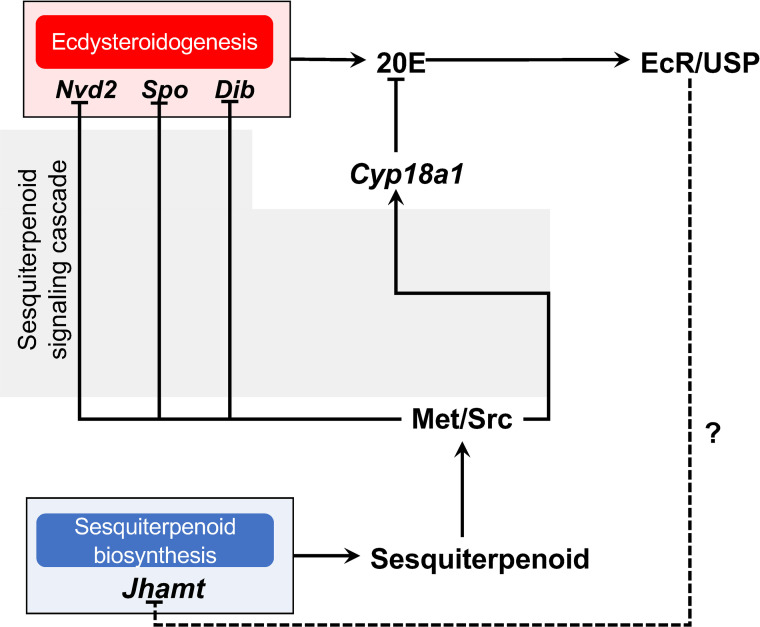
Fig 5. Schematic diagram of proposed ecdysteroid and sesquiterpenoid cross-talk in Daphnia magna embryo.
Solid lines indicate direct interactions whereas dashed line indicates possible interaction. Sesquiterpenoid through sesquiterpenoid signaling cascade downregulates ecdysteroidogenesis gene expression, hence suggesting antagonistic relationship between sesquiterpenoid and ecdysteroid.
This study demonstrates the roles and cross-talk of ecdysteroid and sesquiterpenoid biosynthesis during embryogenesis in a branchiopod crustacean, D. magna. Similar to insects, ecdysteroidogenesis is necessary for early embryogenesis in D. manga. Sesquiterpenoids regulate the expression of ecdysteroid metabolism genes, including Spo, and repress precocious embryonic development. We anticipate that this work will contribute for furthering our understanding of the evolution and diversity of arthropod endocrinology.
Materials and methods
Daphnia strains and transgenic line
All daphnids were raised under the following conditions: 80 neonates (under 24 h) were transferred to 5 L medium and cultured at 22–24°C, under a constant light/dark photoperiod of 16 h/8 h. Artificial Daphnia Medium (ADaM) was used as the culture medium and prepared using reverse osmosis (RO) water, as reported previously [59]. Daphnids were fed daily with a 100 μL suspension of 8×109 cells/mL Chlorella vulgaris (Oitamedakabiyori, Oita, Japan) and 15 μL suspension of 0.15 g/mL baker’s yeast (Marusan Pantry, Ehime, Japan) during the first week. Upon reaching reproductive age, their offspring were removed once per day and fed daily with a 200 μL suspension of 8×109 cells/mL chlorella and 30 μL suspension of 0.15 g/mL baker's yeast.
The wildtype strain (NIES clone) was obtained from the National Institute of Environmental Studies (NIES, Tsukuba, Japan). We utilized previously established transgenic Daphnia that express a H2B-GFP fusion protein under Daphnia magna elongation factor 1-α (Ef1α) promoter/enhancer [30] to visualize the progression of embryogenesis.
RNA isolation and purification
Daphnia (adults or embryos) were collected in 2-mL tubes, immediately frozen in liquid nitrogen, and homogenized using a MicroSmash MS-100 machine (TOMY, Tokyo, Japan) in the presence of Sepasol-RNA I reagent (Nacalai Tesque, Kyoto, Japan), according to the manufacturer’s instructions. Extracted total RNA was further purified using phenol-chloroform extraction and ethanol precipitation. Purified total RNA was dissolved in RNase free water (Invitrogen, Carlsbad, USA) and stored at –80°C until further use.
Cloning and sequencing of Spo and Jhamt transcripts
Adult Daphnia (115 inds) were collected and subjected to total RNA extraction according to the above-mentioned procedure. Beforehand, eggs were removed from the brood chamber. Polyadenylated RNA was purified from 500 μg of total RNA using the PolyATtract mRNA Isolation System (Promega Corporation, Tokyo, Japan) and used for 5′ and 3′ Rapid Amplification of cDNA Ends (RACE) using the GeneRacer (Invitrogen, Carlsbad, USA) and SMARTer RACE cDNA Amplification (Clontech Laboratories, Mountain View, WI, USA) kits, respectively. The primers used for cDNA amplification are listed in S1 Table. PCR was performed using KOD+ DNA Polymerase (Toyobo, Osaka, Japan). PCR products were verified by agarose gel electrophoresis, purified, cloned using a Zero Blunt TOPO PCR Cloning Kit (Invitrogen, Carlsbad, USA), and sequenced. The nucleotide sequences were submitted to the DNA Data Bank of Japan (DDBJ) website, with accession numbers LC547945 (Spo) and LC547946 (Jhamt).
Quantitative RT-PCR
Embryos were collected at 0, 6, 12, 18, 24, 36, 48, and 72 h after ovulation. These timepoints correspond to specific embryonic developmental landmarks described in [30, 37, 60]. Samples were collected in three biological replicates and subjected to total RNA isolation as described in the preceding section. Synthesis of cDNA was performed using a random primer from 1 μg of the purified total RNA with the SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, USA). The absence of genomic DNA (gDNA) contamination was confirmed as described previously [61]. PCR was performed in an Mx3005P (Stratagene, La Jolla, CA, USA) instrument using the Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, USA) with primers listed in S1 Table. PCR amplification was performed in triplicate under the following conditions: 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Primer specificity was confirmed by analyzing dissociation curves. Expression levels of each gene were normalized against those of the ribosomal L32 gene.
RNAi and microinjection
Small interfering RNAs for Spo and Jhamt were designed using the Block-iT RNAi designer (http://www.invitrogen.com/rnaidesigner.html). The siRNA sequences were: siRNA-Spo (5′ CCGUCUUCUUGCGAUCAAAA 3′); siRNA-Jhamt#1 (5′ GGACUUCGGUUGUGGUGAU 3′); siRNA-Jhamt#2 (5′ GGCACCAUCUGCAGAUGAA 3′). Two nucleotides dTdT were added to the 3′ end of the siRNA strand. Two additional siRNAs, one containing a random sequence (5′ GGUUAAGCCGCCUCACAU 3′) (siRNA-scrambled) [50], and another one targeting the Escherichia coli MalE gene (siRNA-MalE) were used as controls. Microinjection was performed according to an established protocol [29]. Briefly, freshly ovulated eggs from 2–3-week-old Daphnia were collected and placed in ice-cold M4 medium [62] containing 80 mM sucrose (M4-Sucrose). Specific siRNA samples for each experiment were mixed with 5 μM AlexaFluor 568 fluorescent dye (Invitrogen, Carlsbad, USA) or Lucifer Yellow dye (Invitrogen, Carlsbad, USA) as an injection marker. After injection, intact eggs were transferred and cultured individually inside 96-well plates filled with 100 μL M4-Sucrose at 23°C. Injected embryos were collected in three biological replicates and subjected to total RNA isolation as described above. Ten micrograms of yeast tRNA (Ambion) were added to each sample as carrier RNA. Synthesis of cDNA was performed using a random primer with a PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Shiga, Japan). Gene expression levels were evaluated by qPCR using the primer pairs shown in S1 Table.
Rescue with 20-hydroxyecdysone (20E) and Fenoxycarb (FC)
Shortly after siRNA injection, intact eggs were transferred into 100 μL M4-Sucrose containing 0.01% dimethylformamide (DMF) as control, 1 μM (500 μg/L) 20-hydroxyecdsone (Tokyo Chemical Industry, Tokyo, Japan), or 33 pM (0.01 μg/L) Fenoxycarb (Wako Pure Chemical, Osaka, Japan) in 0.01% DMF. Embryos were incubated at 23°C in the dark. At designated time points, development was observed microscopically.
Fluorescence photography and image quantification
Fluorescence micrographs of embryos were acquired with a Leica DC500 CCD digital camera mounted on a Leica M165FC fluorescence microscope (Leica Microsystem, Mannheim, Germany). GFP-expressing embryos were imaged using a GFP2 filter.
Statistical analysis
Differences between datasets were calculated using Student’s t-test or Welch’s t-test. For multiple comparisons, differences were assessed using Kruskal-Wallis analysis followed by the Dunn post-hoc test. P-values were further adjusted using the Benjamini-Hochberg procedure. All calculations were performed using R 3.6.2 for MacOS software (R Foundation for Statistical Computing).
Supporting information
(A) Comparison of Daphnia magna (Dmagna) Spo protein length with those of Daphnia pulex (Dpulex), Drosophila melanogaster Spo (DmelSpo), Drosophila melanogaster Spookier (DmelSpok), Tribolium castaneum (Tcastaneum), and Bombyx mori (Bmori). Accession numbers are provided in S2 Table. Percentages indicate identities. (B) Multiple alignment of Dmagna, Dpulex, Dmel (Spo), Dmel (Spok), Tcastaneum, and Bmori Spo proteins. Asterisks, semicolons, and dots indicate conserved, strongly similar, and weakly similar residues, respectively. The positions of characteristic P450 motifs are indicated by black boxes under the red characters.
(TIF)
(A) Comparison of Daphnia magna (Dmagna) Jhamt protein length with those of Daphnia pulex (Dpulex), Drosophila melanogaster (Dmel), Tribolium castaneum (Tcastaneum), and Bombyx mori (Bmori). Percentages indicate identities. Accession numbers are provided in S2 Table. (B) Multiple alignment of Dmagna, Dpulex, Dmel, Tcastaneum, and Bmori Jhamt proteins. Asterisks, semicolons, and dots indicate conserved, strongly similar, and weakly residues, respectively. Putative S-adenosyl-L-methionine (SAM) binding sites are indicated by red boxes.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
YK gratefully acknowledges the support of the Frontier Research Base for Global Young Researchers, Osaka University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
HW: 17H01880 18H04619 YK: 19H05423 20H04923 Kato Yasuhiko; 18H04884. from Japan Society for Promotion of Science, KAKENHI, Japan https://www.jsps.go.jp/english/index.html.
References
- 1.Truman JW, Riddiford LM. Endocrine Insights into the Evolution of Metamorphosis in Insects. Annu Rev Entomol. 2002;47: 467–500. 10.1146/annurev.ento.47.091201.145230 [DOI] [PubMed] [Google Scholar]
- 2.Truman JW. The Evolution of Insect Metamorphosis. Curr Biol. 2019;29: R1252–R1268. 10.1016/j.cub.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 3.Jindra M, Palli SR, Riddiford LM. The Juvenile Hormone Signaling Pathway in Insect Development. Annu Rev Entomol. 2013;58: 181–204. 10.1146/annurev-ento-120811-153700 [DOI] [PubMed] [Google Scholar]
- 4.Jindra M, Bellés X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11: 39–46. 10.1016/j.cois.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 5.Miura T. Juvenile hormone as a physiological regulator mediating phenotypic plasticity in pancrustaceans. Dev Growth Differ. 2019;61: 85–96. 10.1111/dgd.12572 [DOI] [PubMed] [Google Scholar]
- 6.Jockusch EL, Smith FW. Hexapoda: Comparative Aspects of Later Embryogenesis and Metamorphosis BT—Evolutionary Developmental Biology of Invertebrates 5: Ecdysozoa III: Hexapoda. In: Wanninger A, editor. Vienna: Springer Vienna; 2015. pp. 111–208. 10.1007/978-3-7091-1868-9_3 [DOI] [Google Scholar]
- 7.Qu Z, Kenny NJ, Lam HM, Chan TF, Chu KH, Bendena WG, et al. How Did Arthropod Sesquiterpenoids and Ecdysteroids Arise? Comparison of Hormonal Pathway Genes in Noninsect Arthropod Genomes. Genome Biol Evol. 2015;7: 1951–1959. 10.1093/gbe/evv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert LI. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Molecular and Cellular Endocrinology. 2004. pp. 1–10. 10.1016/j.mce.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34: 1256–1260. 10.1042/BST0341256 [DOI] [PubMed] [Google Scholar]
- 10.Silva Gunawardene YIN, Tobe SS, Bendena WG, Chow BKC, Yagi KJ, Chan SM. Function and cellular localization of farnesoic acid O-methyltransferase (FAMeT) in the shrimp, Metapenaeus ensis. Eur J Biochem. 2002;269: 3587–3595. 10.1046/j.1432-1033.2002.03048.x [DOI] [PubMed] [Google Scholar]
- 11.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci U S A. 2003;100: 11986–11991. 10.1073/pnas.2134232100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonobe H, Yamada R. Ecdysteroids during early embryonic development in silkworm Bombyx mori: metabolism and functions. Zoolog Sci. 2004;21: 503–16. 10.2108/zsj.21.503 [DOI] [PubMed] [Google Scholar]
- 13.Niwa YS, Niwa R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev Growth Differ. 2016;58: 94–105. 10.1111/dgd.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert LI, Rybczynski R, Warren JT. Control and Biochemical Nature of the Ecdysteroidogenic Pathway. Annu Rev Entomol. 2002;47: 883–916. 10.1146/annurev.ento.47.091201.145302 [DOI] [PubMed] [Google Scholar]
- 15.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2006;36: 188–199. 10.1016/j.ibmb.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Chávez VM, Marqués G, Delbecque JP, Kobayashi K, Hollingsworth M, Burr J, et al. the Dm dib gene controls late embryonic morphogenesis and codes for a P450 enzyme that regulates embryonic ecdysone levels.pdf. 2000;4126: 4115–4126. [DOI] [PubMed] [Google Scholar]
- 17.Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol. 2006;298: 555–570. 10.1016/j.ydbio.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 18.Bellés X, Martín D, Piulachs M-D. the Mevalonate Pathway and the Synthesis of Juvenile Hormone in Insects. Annu Rev Entomol. 2005;50: 181–199. 10.1146/annurev.ento.50.071803.130356 [DOI] [PubMed] [Google Scholar]
- 19.Noriega FG. Juvenile Hormone Biosynthesis in Insects: What Is New, What Do We Know, and What Questions Remain? Int Sch Res Not. 2014;2014: 1–16. 10.1155/2014/967361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyakawa H, Toyota K, Sumiya E, Iguchi T. Comparison of JH signaling in insects and crustaceans. Curr Opin Insect Sci. 2014;1: 81–87. 10.1016/j.cois.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Sin YW, Kenny NJ, Qu Z, Chan KW, Chan KWS, Cheong SPS, et al. Identification of putative ecdysteroid and juvenile hormone pathway genes in the shrimp Neocaridina denticulata. Gen Comp Endocrinol. 2015;214: 167–176. 10.1016/j.ygcen.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Nicolas A, Belles X. Juvenile hormone signaling in short germ-band hemimetabolan embryos. Development. 2017;144: 4637–4644. 10.1242/dev.152827 [DOI] [PubMed] [Google Scholar]
- 23.Daimon T, Uchibori M, Nakao H, Sezutsu H, Shinoda T. Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc Natl Acad Sci U S A. 2015;112: E4226–E4235. 10.1073/pnas.1506645112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Li K, Gao Y, Liu X, Chen W, Ge W, et al. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc Natl Acad Sci. 2017;115: 201716897 10.1073/pnas.1716897115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwentner M, Combosch DJ, Pakes Nelson J, Giribet G. A Phylogenomic Solution to the Origin of Insects by Resolving Crustacean-Hexapod Relationships. Curr Biol. 2017;27: 1818–1824.e5. 10.1016/j.cub.2017.05.040 [DOI] [PubMed] [Google Scholar]
- 26.Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, et al. The ecoresponsive genome of Daphnia pulex. Science (80-). 2011;331: 555–561. 10.1126/science.1197761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsini L, Orsini L, Gilbert D, Podicheti R, Jansen M, Brown JB. Data Descriptor: Daphnia magna transcriptome by RNA-Seq across 12 environmental stressors. 2016; 1–15. 10.1038/sdata.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BY, Choi BS, Kim MS, Park JC, Jeong CB, Han J, et al. The genome of the freshwater water flea Daphnia magna: A potential use for freshwater molecular ecotoxicology. Aquat Toxicol. 2019;210: 69–84. 10.1016/j.aquatox.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 29.Kato Y, Shiga Y, Kobayashi K, Tokishita SI, Yamagata H, Iguchi T, et al. Development of an RNA interference method in the cladoceran crustacean Daphnia magna. Dev Genes Evol. 2011;220: 337–345. 10.1007/s00427-011-0353-9 [DOI] [PubMed] [Google Scholar]
- 30.Kato Y, Matsuura T, Watanabe H. Genomic Integration and Germline Transmission of Plasmid Injected into Crustacean Daphnia magna Eggs. PLoS One. 2012;7: 0–6. 10.1371/journal.pone.0045318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Törner K, Nakanishi T, Matsuura T, Kato Y, Watanabe H. Optimization of mRNA design for protein expression in the crustacean Daphnia magna. Mol Genet Genomics. 2014;289: 707–715. 10.1007/s00438-014-0830-8 [DOI] [PubMed] [Google Scholar]
- 32.Naitou A, Kato Y, Nakanishi T, Matsuura T, Watanabe H. Heterodimeric TALENs induce targeted heritable mutations in the crustacean Daphnia magna. Biol Open. 2015;4: 364–369. 10.1242/bio.20149738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi T, Kato Y, Matsuura T, Watanabe H. TALEN-mediated knock-in via non-homologous end joining in the crustacean Daphnia magna. Sci Rep. 2016;6: 1–7. 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai H, Nakanishi T, Matsuura T, Kato Y, Watanabe H. CRISPR/Cas-mediated knock-in via nonhomologous end-joining in the crustacean Daphnia magna. PLoS One. 2017;12: 1–12. 10.1371/journal.pone.0186112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi T, Kato Y, Matsuura T, Watanabe H. TALEN-mediated homologous recombination in Daphnia magna. Sci Rep. 2015;5: 1–10. 10.1038/srep18312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olesen J. On the ontogeny of the Branchiopoda (Crustacea): Contribution of development to phylogeny and classification. Crustacean Issues Vol 15 2004. pp. 217–269. [Google Scholar]
- 37.Mittmann B, Ungerer P, Klann M, Stollewerk A, Wolff C. Development and staging of the water flea Daphnia magna (Straus, 1820; Cladocera, Daphniidae) based on morphological landmarks. Evodevo. 2014;5. 10.1186/2041-9139-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panfilio KA. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev Biol. 2008;313: 471–491. 10.1016/j.ydbio.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999;401: 447–452. 10.1038/46737 [DOI] [PubMed] [Google Scholar]
- 40.Kato Y, Kobayashi K, Oda S, Tatarazako N, Watanabe H, Iguchi T. Cloning and characterization of the ecdysone receptor and ultraspiracle protein from the water flea Daphnia magna. J Endocrinol. 2007;193: 183–194. 10.1677/JOE-06-0228 [DOI] [PubMed] [Google Scholar]
- 41.Rewitz KF, Gilbert LI. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: Evolutionary implications. BMC Evol Biol. 2008;8: 1–8. 10.1186/1471-2148-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, Oda S, et al. A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Commun. 2013;4: 1856–1857. 10.1038/ncomms2868 [DOI] [PubMed] [Google Scholar]
- 43.Toyota K, Miyakawa H, Hiruta C, Furuta K, Ogino Y, Shinoda T, et al. Methyl farnesoate synthesis is necessary for the environmental sex determination in the water flea Daphnia pulex. J Insect Physiol. 2015;80: 22–30. 10.1016/j.jinsphys.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 44.Sumiya E, Ogino Y, Miyakawa H, Hiruta C, Toyota K, Miyagawa S, et al. Roles of ecdysteroids for progression of reproductive cycle in the fresh water crustacean Daphnia magna. Front Zool. 2014;11: 1–12. 10.1186/1742-9994-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumiya E, Ogino Y, Toyota K, Miyakawa H, Miyagawa S, Iguchi T. Neverland regulates embryonic moltings through the regulation of ecdysteroid synthesis in the water flea Daphnia magna, and may thus act as a target for chemical disruption of molting. J Appl Toxicol. 2016;36: 1476–1485. 10.1002/jat.3306 [DOI] [PubMed] [Google Scholar]
- 46.Adhitama N, Matsuura T, Kato Y, Watanabe H. Monitoring ecdysteroid activities using genetically encoded reporter gene in Daphnia magna. Mar Environ Res. 2018;140: 375–381. 10.1016/j.marenvres.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 47.Rewitz KF, Yamanaka N, O’Connor MB. Steroid Hormone Inactivation Is Required during the Juvenile-Adult Transition in Drosophila. Dev Cell. 2010;19: 895–902. 10.1016/j.devcel.2010.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petryk A, Warren JT, Marqués G, Jarcho MP, Gilbert LI, Kahler J, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100: 13773–13778. 10.1073/pnas.2336088100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, et al. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 2004;279: 35942–35949. 10.1074/jbc.M404514200 [DOI] [PubMed] [Google Scholar]
- 50.Asada M, Kato Y, Matsuura T, Watanabe H. Early embryonic expression of a putative ecdysteroid-phosphate phosphatase in the water flea, daphnia magna (Cladocera: Daphniidae). J Insect Sci. 2014;14: 1–6. 10.1093/jis/14.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumann I, Kenny N, Hui J, Hering L, Mayer G. Halloween genes in panarthropods and the evolution of the early moulting pathway in Ecdysozoa. R Soc Open Sci. 2018;5 10.1098/rsos.180888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyakawa H, Imai M, Sugimoto N, Ishikawa Y, Ishikawa A, Ishigaki H, et al. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol. 2010;10 10.1186/1471-213X-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyakawa H, Gotoh H, Sugimoto N, Miura T. Effect of juvenoids on predator-induced polyphenism in the water flea, daphnia pulex. J Exp Zool Part A Ecol Genet Physiol. 2013;319: 440–450. 10.1002/jez.1807 [DOI] [PubMed] [Google Scholar]
- 54.Oda S, Kato Y, Watanabe H, Tatarazako N, Iguchi T. Morphological changes in Daphnia galeata induced by a crustacean terpenoid hormone and its analog. Environ Toxicol Chem. 2011;30: 232–238. 10.1002/etc.378 [DOI] [PubMed] [Google Scholar]
- 55.Olmstead AW, Leblanc GA. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool. 2002;293: 736–739. 10.1002/jez.10162 [DOI] [PubMed] [Google Scholar]
- 56.Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere. 2003;53: 827–833. 10.1016/S0045-6535(03)00761-6 [DOI] [PubMed] [Google Scholar]
- 57.Jindra M. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Philos Trans R Soc B Biol Sci. 2019;374: 20190064 10.1098/rstb.2019.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ono H. Ecdysone differentially regulates metamorphic timing relative to 20-hydroxyecdysone by antagonizing juvenile hormone in Drosophila melanogaster. Dev Biol. 2014;391: 32–42. 10.1016/j.ydbio.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 59.Klüttgen B, Dülmer U, Engels M, Ratte HT. Adam, an Artificial Freshwater for the Culture of Zooplankton. Water Res. 1994;28: 743–746. 10.1016/0043-1354(94)90157-0 [DOI] [Google Scholar]
- 60.Sagawa K, Yamagata H, Shiga Y. Exploring embryonic germ line development in the water flea, Daphnia magna, by zinc-finger-containing VASA as a marker. Gene Expr Patterns. 2005;5: 669–678. 10.1016/j.modgep.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 61.Kato Y, Perez CAG, Mohamad Ishak NS, Nong QD, Sudo Y, Matsuura T, et al. A 5′ UTR-Overlapping LncRNA Activates the Male-Determining Gene doublesex1 in the Crustacean Daphnia magna. Curr Biol. 2018;28: 1811–1817.e4. 10.1016/j.cub.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 62.Elendt B, Bias W. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing: Effects of the optimization of culture conditions on life history parameters of Daphnia magna. Water Res. 1990;24: 1157–1167. 10.1016/0043-1354(90)90180-E [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Comparison of Daphnia magna (Dmagna) Spo protein length with those of Daphnia pulex (Dpulex), Drosophila melanogaster Spo (DmelSpo), Drosophila melanogaster Spookier (DmelSpok), Tribolium castaneum (Tcastaneum), and Bombyx mori (Bmori). Accession numbers are provided in S2 Table. Percentages indicate identities. (B) Multiple alignment of Dmagna, Dpulex, Dmel (Spo), Dmel (Spok), Tcastaneum, and Bmori Spo proteins. Asterisks, semicolons, and dots indicate conserved, strongly similar, and weakly similar residues, respectively. The positions of characteristic P450 motifs are indicated by black boxes under the red characters.
(TIF)
(A) Comparison of Daphnia magna (Dmagna) Jhamt protein length with those of Daphnia pulex (Dpulex), Drosophila melanogaster (Dmel), Tribolium castaneum (Tcastaneum), and Bombyx mori (Bmori). Percentages indicate identities. Accession numbers are provided in S2 Table. (B) Multiple alignment of Dmagna, Dpulex, Dmel, Tcastaneum, and Bmori Jhamt proteins. Asterisks, semicolons, and dots indicate conserved, strongly similar, and weakly residues, respectively. Putative S-adenosyl-L-methionine (SAM) binding sites are indicated by red boxes.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.