Abstract
BACKGROUND
Androgen deprivation therapy (ADT) is frequently used in the treatment of prostate cancer (PC) worldwide. Variable testosterone (T) recovery profiles after ADT cessation have been cited.
AIM
To evaluate T recovery after cessation of ADT.
METHODS
We reviewed our institutional prospectively maintained database of PC patients who received ADT. Serum early morning total T (TT) levels, collected at baseline and periodically after ADT cessation, were analyzed. Patient age, baseline T level, duration of ADT, presence of diabetes and sleep apnea were selected as potential predictors of T recovery. Three metrics of T recovery after 24m of ADT cessation were analyzed: return to non-castrate level (TT>50 ng/dl), return to normal (T>300ng/dl) and return back to baseline level (BTB). Multivariable time-to-event analysis (Cox proportional hazards), chi-square test, logistic regression model and Kaplan-Meier curve were performed to define impact of the above predictors on time and chance of T recovery.
OUTCOMES
Time and chance of T recovery to non-castrate level (TT>50 ng/dl), return to normal (T>300 ng/dl) and return back to baseline level (BTB).
RESULTS
307 men with a mean age of 65±8 years were included. Mean duration of ADT was 17±25 months and median follow-up was 31±35 months. Mean TT values were: at baseline 379ng/dl and at >24 months 321ng/dl. At 24 months after cessation of ADT, 8% men remained at castrate level, 76% returned to TT >300ng/dl and 51% had returned BTB. Lower baseline T levels (TT < 400ng/dl) and ADT duration >6m were associated with a lower likelihood of recovery to normal TT at 24 months. Age over 65 years and receiving ADT for >6m were significantly associated with a slower T recovery.
CLINICAL IMPLICATIONS
T recovery after ADT is not certain and may take longer than expected. Considering the range of side effects of low T, we believe that these findings must be discussed with patients before initiating such therapies.
STRENGTHS AND LIMITATIONS
Our strengths consisted on relatively large database, long follow up and clinically meaningful endpoints. Limitations included the retrospective design of the study.
CONCLUSION
T recovery rates after ADT cessation vary according to patient age, ADT duration and baseline T levels. Approximately a quarter of patients failed to normalize their TT level and one tenth of men remained at castrate levels 24 months after ADT cessation.
Keywords: Testosterone Deficiency, Androgen Deprivation Therapy, Castration, Prostate Cancer, Testosterone Recovery
INTRODUCTION
Prostate cancer (PC) is the most common non-skin malignancy diagnosed in men, with more than 180.000 men being diagnosed in 2016.1 In addition to that, PC burden is expected to increase with aging of the global population and by 2030, 1.7 million new cases worldwide and 499,000 new deaths are expected.2
Androgen deprivation therapy (ADT), defined as the use of medications to lower serum testosterone to castrate levels, is utilized in men with metastatic PC, men with intermediate-high risk disease undergoing radiation therapy, and investigationally, as neoadjuvant therapy for men with high risk disease undergoing radical prostatectomy.3, 4
Testosterone (T) is a major homeostatic hormone involved in numerous bodily functions including sexual, physical, cognitive and metabolic processes. Low levels are associated with bone mineral density loss, glycemic control issues and premature cardiovascular events. 5, 6
Despite the intention to provide only temporary effect, there is an increasing body of literature suggesting that testosterone recovery after ADT cessation may be only partial and may take prolonged periods of time.7–10 T recovery after ADT has been defined in a heterogeneous fashion in the literature with definitions such as return to non-castrate level 11, return to normal level 9, 10 (variably defined) being most commonly used while return to baseline T levels is rarely reported. 7, 12 Overall, T recovery rates are highly variable (7–96%) due to factors such as heterogeneity in the definition of recovery, duration of ADT exposure, length of follow-up and patient age. 9, 10
Our aim in this study was to evaluate T recovery profiles after cessation of ADT in PC patients, analyzing time and chance of recovery back to baseline, above 300ng/dl and chance to be left at castrate levels (<50ng/sl).
PATIENTS & METHODS
Study Population
Following institutional review board approval (IRB16–459), we retrospectively reviewed a prospectively maintained database of PC patients who received ADT at our institution. Inclusion criteria included: availability of baseline and serial post-ADT total testosterone (TT) laboratory data commencing ≥6 months after cessation of ADT; ADT treatment details; and patient demographics and comorbidities. We excluded men without a baseline TT level and men with a baseline T <50 ng/dl.
Definitions of Testosterone Recovery
Serum early morning total T (TT) levels were measured using liquid chromatography-tandem mass spectrometry (LCMS) at baseline and periodically after ADT cessation. Assessment schedules of T labs were planned for each patient by the physician at time of ADT cessation and were not random or based on previous lab results, though some labs may have been missed or skipped due to patient non-compliance. Three metrics of TT recovery at the 24 month of ADT cessation (±3 months, 21–27 months) time-point were analyzed: normalization (N), defined as any post-ADT cessation TT level >300 ng/dl within the first 27 months after ADT cessation; back to baseline (BTB), defined as any TT measure at least as high as the individual’s baseline measure, as measured between 21–27 months after ADT cessation; and return to non-castrate level (CL), defined as a TT>50 ng/dl measured at any time point ≥24 months after ADT cessation. For normalization, analysis was restricted to men with normal baseline TT (>300 ng/dl).
Predictors of Testosterone Recovery
In the analyses, we selected five parameters as potential predictors of T recovery, patient age at baseline (pre-ADT), duration of ADT exposure, baseline T level, prevalent Diabetes, and prevalent Obstructive Sleep Apnea (O.S.A.). These parameters were chosen based on prior literature and due to the fact that they are usually available for clinicians during their discussion with a patient.
Statistical Analysis
Cox proportional hazards regression models were used to assess the impact of predictors on time to recovery. These models are appropriate in this situation as observation times varied by participant, and observation times are assumed independent of the TT value. We tested for significant violations of the proportional hazards assumption using a time-predictor interaction term and assessing Schoenfeld residuals. Results from such models were reported as hazard ratios. We estimated Cox proportional hazards models with each of the five single predictors and again as a single model for each outcome with adjustment for all potential covariates. Global 24-month recovery rates were assessed for each outcome as percentages, and confidence intervals estimated using a binomial approximation. Unadjusted effects of patient age, ADT duration, baseline T level, and prevalent Diabetes and sleep apnea were assessed using Chi-square tests. Logistic regression models were then fitted for each outcome with full adjustment for the five predictors. Recovery rates to normal TT (>300 ng/dl), based on level of each predictor, were calculated using Kaplan-Meier (KM) analyses. All statistical analyses were conducted in SAS™ software (version 9.4) with proportional hazards modeling done using SAS Proc PHREG.
RESULTS
Patient Population
Post-ADT cessation testosterone laboratory data were available for 1,641 men, only 307 of whom had an eligible baseline (pre-ADT) value and were included in the analysis. This represents a failure to assess baseline TT levels in 81% of patients, but a sample size of 307 still provides 80% power to detect effect sizes as small as d=0.32 in t-test comparisons with equal sample sizes and h=0.32 in comparisons of proportions. Patients included in the final sample had higher rates of HTN (p = 0.03), HLD (p = 0.03), and sleep apnea (p = 0.07) than those without a baseline T level, but were comparable on other demographic characteristics such as age. The mean age of the 307 patients was 65±8 (44–89) years, with 166 (54%) having hypertension, 37 (12%) diabetes mellitus and 22 (7%) sleep apnea (Table 1). Race distribution of our study population was 86% white, 10% black and the remaining either did not answer or marked other races. Primary prostate cancer treatment was radical prostatectomy (RP) in 10%, radiation therapy (RT) in 31%, RP + RT in 55% and primary ADT in 5%. The majority of patients (71%) received multiple types of ADT agents, 94% of them received a GnRH agonist, 17% received GnRH antagonist and 10% received other types of ADT at some point. Mean duration of ADT exposure was 17±25 (0.5–146) months. Distribution of ADT exposure was: <6 months 47%; 6–12 months 19%; 12–24 months 15%; >24 months 20%. Median duration of follow-up was 31.0 (IQ range: 14.9–60.6) post-ADT cessation. Mean baseline TT value was 379±148 ng/dl.
Table 1.
Patient Demographics & Comorbidities (N = 307)
| Characteristic | % | Characteristic | % |
|---|---|---|---|
| Age, mean (SD) | 8 | Sleep Apnea | 7 |
| Race | CAD | 13 | |
| White | 86 | Stroke | 1 |
| Black | 10 | Primary Treatment | |
| Asian | 2 | RP | 10 |
| Other | 1 | RT | 31 |
| Unknown/ not reported | 2 | RP + RT | 55 |
| Ethnicity | Primary ADT | 5 | |
| Hispanic | 3 | ADT Agents | |
| Non Hispanic | 51 | GnRH agonist | 94 |
| Unknown/ not reported | 46 | GnRH antagonist | 17 |
| Smoking Status | Other | 10 | |
| Current | 10 | ADT Exposure | |
| Former | 44 | <6 months | 47 |
| Never | 34 | 6 – 12 months | 19 |
| Unknown/ not reported | 12 | 12 – 24 months | 15 |
| HTN | 54 | 24+ months | 20 |
| DM | 12 | ||
| HLD | 50 |
HTN = Hypertension; HLD = Hyperlipidemia; DM= Diabetes Mellitus; GnRH= Gonadotropin-Releasing Hormone; ADT= Androgen Deprivation Therapy; CAD = Coronary Artery Disease
Testosterone Recovery
Lab data were categorized according to time from cessation of ADT exposure, and means calculated across all participants. Mean TT values recovered over time, with a mean of 216±205 ng/dl at 6–12 months post ADT cessation, and 275±241 ng/dl, 292±199 ng/dl, and 321±193 ng/dl at 12–18 months, 18–24 months, and >24m, respectively.
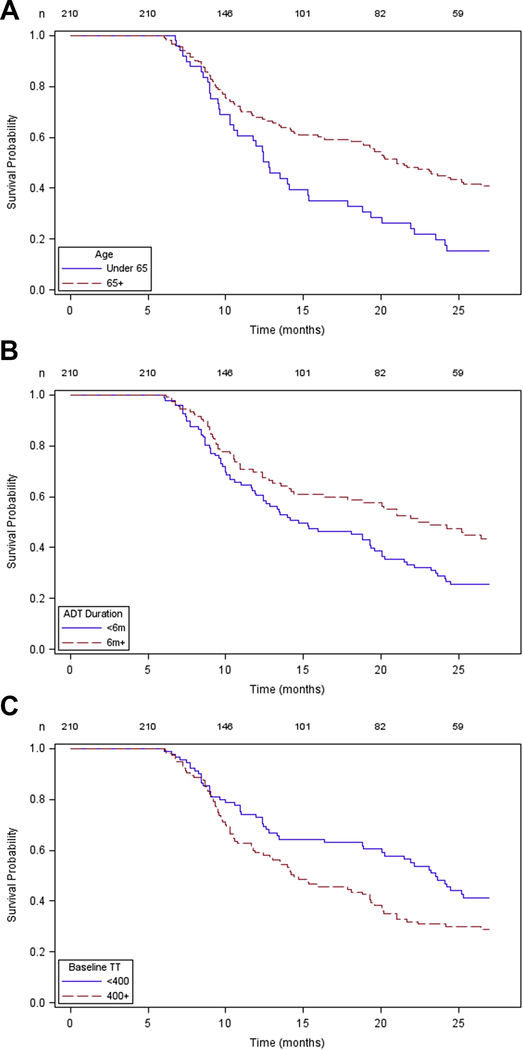
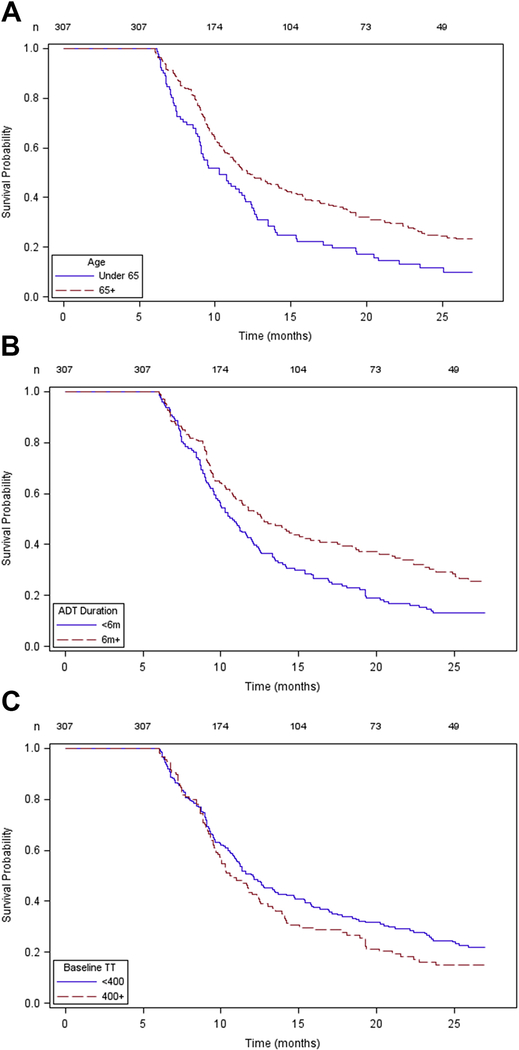
Kaplan-Meier curves for recovery of TT to normal levels (>300ng/dl) assessed the impact of patient age (Figure 1A), duration of ADT exposure (Figure 1B), and baseline T (Figure 1C) on time to recovery. In the curves a shorter ‘survival’ is the desirable situation as this represents faster time to recovery. A similar analysis for TT recovery above castrate level (>50ng/dl) is also available (Figure 2). Plots of the Schoenfeld residuals over time did not show strong trends and none of the time-predictor interaction terms were significant at the 0.10 level
Figure 1.
Kaplan Meier plot generated for time-to-event (T normalization) considering patient age (1A), ADT duration (1B), and baseline T level (1C). In this analysis, a shorter survival probability is the desirable situation because this represents faster time to recovery.
Figure 2.
Kaplan Meier plot generate for time-to-event (T return above castrate level) considering patient age (2A), ADT duration (2B), and baseline T level (2C). In this analysis, a shorter survival probability is the desirable situation because this represents faster time to recovery.
At a time point 24 months after ADT cessation, 8% of patients remained at castrate level, 76% had normalized T (T>300ng/dl) and 51% reached baseline TT levels. Analyzing TT normalization rates at ≥24m, patients with ADT duration ≥6 month and those with baseline TT <400ng/dl had less chance of normalizing TT (Table 2). The chance of remaining at castrate level ≥24m was almost 4 times higher in patients exposed to ADT longer than 6 months, and more than 3 times higher in patients with baseline TT<400 ng/dl (Table 3). The chances of recovery back to baseline TT level was significantly lower in patients older than 65 years, in patients with baseline TT level ≥400 ng/dl, and in patients with Diabetes (Table 4).
Table 2.
Probability of Normalization of Testosterone Levels (>300 ng/dl) 24 months after ADT Cessation
| Percentage (CI 95%) | Adjusted P value | ||
|---|---|---|---|
| ADT Duration | <6 months | 83.3 (75.2–89.6) | 0.019 |
| ≥6 months | 67.9 (58.2–76.6) | ||
| Patient Age | <65 years | 83.6 (73.8–90.8) | 0.132 |
| ≥65 years | 71.3 (63.0–78.6) | ||
| Baseline TT > 400ng/dl | <400 ng/dl | 66.7 (56.4–75.8) | 0.009 |
| ≥400 ng/dl | 83.3 (75.5–89.4) | ||
| Diabetes | Yes | 68.8 (45.2–86.8) | 0.103 |
| No | 76.7 (70.2–82.4) | ||
| Sleep Apnea | Yes | 100 (77.9–100) | 0.974 |
| No | 74.0 (67.5–79.8) | ||
| Overall | 75.9 (69.8–81.4) | ||
Table 3.
Probability of Remaining at Castrate Testosterone Level (<50 ng/dl) 24 Months after ADT Cessation
| Percentage (CI 95%) | Adjusted P value | ||
|---|---|---|---|
| Duration | <6 months | 3.1 (1.1–7.0) | 0.011 |
| ≥6 months | 12.4 (7.8–18.5) | ||
| Age | <65 years | 6.4 (3.1–11.7) | 0.488 |
| ≥65 years | 8.6 (5.0–13.5) | ||
| Baseline T | <400 ng/dl | 10.4 (6.6–15.4) | 0.049 |
| ≥400 ng/dl | 3.2 (0.9–8.0) | ||
| Diabetes | Yes | 9.1 (2.5–21.9) | 0.833 |
| No | 7.4 (4.7–11.0) | ||
| Sleep Apnea | Yes | 0 (NA) | 0.966 |
| No | 8.3 (5.5–11.9) | ||
| Overall | 7.6 (5.3–10.9) | ||
Table 4.
Probability of Testosterone Level Returning to Baseline Level 24 months after ADT Cessation
| Percentage (CI 95%) | Adjusted P value | ||
|---|---|---|---|
| Duration | <6 months | 52.5 (44.6–60.4) | 0.402 |
| ≥6 months | 50.0 (41.5–58.5) | ||
| Age | <65 years | 61.2 (52.4–69.5) | 0.012 |
| ≥65 years | 43.5 (36.0–51.3) | ||
| Baseline T | <400 ng/dl | 59.4 (52.1–66.5) | 0.003 |
| ≥400 ng/dl | 38.1 (29.2–47.6) | ||
| Diabetes | Yes | 31.0 (17.3–47.9) | 0.010 |
| No | 54.4 (48.2–60.5) | ||
| Sleep Apnea | Yes | 62.5 (44.5–56.4) | 0.814 |
| No | 50.5 (44.5–56.4) | ||
| Overall | 51.4 (45.9 – 56.8) | ||
Unadjusted and adjusted models indicated that patients younger than 65 years and exposed to ADT for less than 6 months recovered to normal TT (>300 ng/dl) faster than their counterparts (Table 5A). An unadjusted model also showed that men with sleep apnea normalized faster than those without, though this association was not sustained after adjustment for other predictors. Analyzing recovery above castrate level, unadjusted and adjusted models showed that younger patients had faster recovery. Patients who received ADT for less than 6 months had significantly faster recovery in unadjusted model and a trend toward significance on adjusted model (p=0.05). Baseline TT ≥400ng/dl showed no difference in speed of recovery to T ≥50ng/dl (Table 5B). Also, both in adjusted and unadjusted time-to-event analysis, recovery back to baseline was faster in younger patients and significantly slower in patients with baseline TT ≥400ng/dl and those with diabetes (Table 5C).
Table 5.
Adjusted Cox Proportional Hazards Model for Testosterone Recovery after ADT Cessation
| 5A - Normalization of Testosterone (T>300ng/dl) | |||
| Parameter | HR | 95% CI | P value |
| ADT Duration <6m | 1.47 | 1.06–2.04 | 0.02 |
| Patient Age <65y | 1.72 | 1.20–2.47 | <0.01 |
| Baseline TT >400 ng/dl | 1.28 | 0.93–1.77 | 0.13 |
| Diabetes | 0.60 | 0.33–1.07 | 0.08 |
| Sleep Apnea | 1.53 | 0.84–2.79 | 0.16 |
| 5B - Recovery above Castrate Level (T>50ng/dl) | |||
| Parameter | HR | 95% CI | P value |
| ADT duration <6m | 1.28 | 1.00 – 1.64 | 0.05 |
| Patient Age <65y | 1.46 | 1.12 – 1.92 | 0.006 |
| Baseline TT >400 ng/dl | 1.11 | 0.87 – 1.42 | 0.41 |
| Diabetes | 0.83 | 0.57–1.20 | 0.32 |
| Sleep Apnea | 1.33 | 0.84–2.11 | 0.22 |
| 5C - Testosterone Recovery to Baseline Levels | |||
| Parameter | HR | 95% CI | P value |
| ADT duration < 6m | 1.35 | 0.98 – 1.85 | 0.07 |
| Patient Age < 65y | 2.08 | 1.45 – 2.98 | <0.001 |
| Baseline T > 400 ng/dl | 0.51 | 0.36 – 0.72 | <0.001 |
| Diabetes | 0.42 | 0.24–0.73 | 0.002 |
| Sleep Apnea | 1.11 | 0.60–2.03 | 0.75 |
DISCUSSION
Prostate cancer (PC) is the most common non-skin malignancy diagnosed in men and ADT plays an important therapeutic role in certain PC populations.13 Some analyses have shown that more than one third of PC patients will receive ADT at some time-point in their treatment journey. 14 In addition, while ADT has historically been used primarily for metastatic disease, data also supports its role in the neoadjuvant treatment of patients undergoing radiation therapy. 15 Given that, by 2030, 1.7 million new PC cases are expected worldwide it is reasonable to estimate that hundreds of thousands of new patients will be exposed to ADT in the future.
Persistently low T levels are associated with a variety of adverse events including bone density loss,16 abnormalities in glycemic control,17 lipid abnormalities,18 cardiovascular events and cognitive dysfunction.19 With regard to glycemic control, ADT has shown to reduce insulin sensitivity within a few months of initiation of treatment17, 18 and to increase the risk of diabetes by up to 60%.20 ADT use is linked to coronary heart disease, myocardial infarction and sudden cardiac death.14 Osteoporosis has also been linked to this therapy, with studies demonstrating bone density loss of 7.6% after 2 years and an increased rate of osteoporotic fractures21–23. Thus, persistently low T levels places a man at significant risk to a reduction in his overall health and quality of life.
With regard to sexual dysfunction, ADT is known to reduce sex drive, impair ability to achieve orgasm, and alter cavernosal smooth muscle structure with resultant ED5. In a study comparing ED in men receiving ADT, RP or RT, ADT was associated with the highest ED rate (86%).24 With regard to sex drive and activity, the same study showed that 73% of men ceased any form of sexual activity after ADT therapy started.24
Thus, the duration to which a man is exposed to ADT and more specifically, the duration to which he is exposed to castrate or very low T levels is an important consideration when it comes to the impact on QOL and health parameters. In this regard, studies have shown that the duration of use of ADT remains highly variable, ranging from a single dose of short-term preparations25 to longer than 48 months of continuous therapy. 10 Also, studies analyzing T recovery are problematic to interpret because they are heterogeneous in nature and, as reviewed elsewhere,26 literature on testosterone has several relevant methodological challenges that can compromise results interpretation. Particularly in the literature exploring T recovery after ADT cessation it is noteworthy how studies use variable definitions of T recovery (variable cutoff values for return above castrate level and T normalization), ADT duration (short term vs long term), ADT modality (primary, neoadjuvant, adjuvant to radiotherapy or radical prostatectomy), population age and follow up durations. In an attempt to present clinically meaningful data, we used guideline-based definitions of TT normalization (>300 ng/dl) 27, defined castrate level as <50 ng/dl and performed subset analysis for the major factors relating to T recovery, specifically, patient age, ADT duration, baseline TT level, presence of DM and Sleep Apnea.
In general, studies have shown that longer ADT duration is associated with delayed and lower recovery rates. Analyzing short-term ADT effects, Murthy et al9 studied men (n=59) with mean duration of ADT of 116 days. They showed that 35% of patients met their extremely low cut-off for TT normalization (>6 nmol/L, >174 ng/dl) within 12 weeks and 96% after 1 year. Also, Kaku et al7 evaluated T recovery after longer ADT duration. In this study, in a very small cohort (n=32), after more than 2 years of ADT, they showed that 21% had TT in the normal range (>10 nmol/L, >290ng/dl) after 6 months and 52% after 2 years of ADT cessation. However, 10.5% of patients remained at castrate level (defined by them as <100ng/dl) 2 years after ADT cessation. For even longer ADT duration, Bong et al10 showed in a tiny group of patients (n=15) with a mean ADT duration of 73 months and 31 months of follow up, that 53% remained at castrate level (T<50ng/dl), 40% had sub-normal levels (50–240ng/dl) and only 1 patient (7%) met their definition of normal TT (>240ng/dl). Other studies also point towards this association in which T recovery is impaired by longer ADT duration.11, 12, 25, 28
This association is supported by our study. In our study, mean ADT duration was 17 months with variability in ADT duration (47% <6 months and 20% >24 months). Recovery above castrate level and normalization rates were significantly higher for patients with ADT duration <6 months. ADT duration, however, was not associated with the time period to or the ability to recover back to baseline TT level. On multivariable time-to-event analysis, after adjustment, short-term ADT was associated with a faster recovery to normal T (HR 1.47, p=0.02) and was marginally significant for faster recovery out of castrate range (HR 1.28, p=0.05).
Patient age is a commonly cited factor influencing T recovery after ADT.7, 11, 29 Kaku et al7, for instance, showed that delayed T recovery time was associated with age greater than 65 years. Kobayashi and colleagues prospectively investigated T recovery after long-term (>30 months) ADT11. In their small cohort (n=10), patients with rapid recovery (less the 100 days) tended to be younger than those with delayed recovery. On the other hand, Planas et al28 in an older population (n=40, mean age 71.5 years) after long term ADT (mean duration 74.6 months) showed that 38% remained at castrate level 18 months after ADT cessation and age was not correlated with the chance of recovery, albeit likely resulting from the extended duration of ADT and the high level of persistent castrate levels in this study. In our study, after adjusted analysis, patients younger than 65 years showed a greater chance to recover back to baseline TT levels (61.2% vs 43.5%, p=0.012), as well as faster recovery to normal TT values (HR 1.47, p=0.02), back from castrate level (HR 1.46, p=0.006) and back to baseline TT levels (HR 2.08, p<0.001).
It is relatively uncommon in the literature to see baseline T levels analyzed as a predictor, at least in part due to the failure of investigators to assess baseline T levels prior to commencing ADT. Indeed, in our dataset, 81% of all ADT patients had no baseline TT level checked. Ofelein et al 8 showed that 18 months after a single 3-month dose of LHRH agonist, all patients (n=13) had TT levels recovered from castrate level (>20ng/dl) but the TT level never reached baseline levels. Padula et al. 30 reported in a study (n=88) after short-term adjuvant therapy that only 26% of patients normalized (T>270ng/dl) at 6 months, 38% at 12 months and 59% at 2 years. On multivariable analysis in this study, baseline TT in the lower normal range was the only predictor of delayed TT normalization. Supporting this idea, our results confirmed that higher baseline TT level (≥400 ng/dl) was associated with significantly higher TT normalization rates (83% vs 67%, p=0.009). Interestingly, this factor was associated with lower and slower recovery to baseline TT levels which is likely a consequence of the greater T drop experienced by those patients while on ADT. Also, baseline TT level greater than 400 ng/dl was associated with a greater chance of recovery from castrate level, with only 3% of patients with such higher baseline TT level remaining at castrate level after 24 months of follow-up. Therefore, assessing baseline T levels prior to starting ADT can only improve the discussion regarding the chances of T recovery in an individualized way.
Furthermore, we were able to capture in this database some other clinical factors known to be associated with low T and investigate whether they have any impact on T recovery after ADT, such as DM 31 and Obstructive Sleep Apnea 32, 33. There are even fewer reports in the literature analyzing such factors, but Tsumura et al. 12 included in their analysis of T recovery and found a non-significant correlation. In contrast, our results showed that the presence of DM was associated with a lower chance of T recovery BTB and slower recover back from castrate level (Table 4 and Table 5C respectively), showing a possible impairing role of DM on this process. In regards to O.S.A., we found no significant association on multivariable analysis, and, in fact, T deficiency and O.S.A. link is much more controversial. 34
It is important to emphasize the implications of the data presented here for the clinical setting. Overall, our study showed that 2 years after ADT cessation, roughly one quarter of men (24%) never regain normal TT levels (>300 ng/dl), about half (49%) never got back to baseline T levels and about one tenth of men (8%) are left castrate long-term. In the clinician-patient discussion prior to starting ADT this fact should be addressed, as it is clear that the presumed temporary effect on T production is not always so. However, even after our study, it is still difficult to estimate individualized chances of recovery. The next step will be the development of nomogram in which each parameter would be appropriately weighted and personalized figures could be generated for return to normal TT levels (T>300 ng/dl), return above castrate levels and return to baseline TT levels.
Limitations in this analysis include: its retrospective design, using a proprietary TT cut-off of 400 ng/dl, and loss of so many patients due to failure to check a baseline TT level. Despite these shortcomings, this work represents one of the largest analyses on this topic in the literature. Furthermore, we believe we have conducted a comprehensive analysis of the data with clinically meaningful endpoints. Finally, analysis of 24-month recovery rates using probability analysis combined with a time-to-recovery analysis add strength to this study.
CONCLUSION
Testosterone recovery rates after ADT cessation vary according to patient age, ADT duration and baseline T levels. Approximately a quarter of patients undergoing ADT for prostate cancer at our center failed to normalize their TT level and one tenth of men remained at castrate levels 24 months after ADT cessation.
Acknowledgments
COMPLIANCE WITH ETHICAL STANDARDS
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors declare no conflict of interest.
Footnotes
Conflict of Interest and Disclosure Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66: 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. European urology. 2012;61: 1079–92. [DOI] [PubMed] [Google Scholar]
- [3].Bandini M, Pompe RS, Marchioni M, et al. Improved cancer-specific free survival and overall free survival in contemporary metastatic prostate cancer patients: a population-based study. International urology and nephrology. 2017. [DOI] [PubMed] [Google Scholar]
- [4].Mossanen M, Krasnow RE, Nguyen PL, Trinh QD, Preston M, Kibel AS. Approach to the Patient with High-Risk Prostate Cancer. The Urologic clinics of North America. 2017;44: 635–45. [DOI] [PubMed] [Google Scholar]
- [5].Mazzola CR, Mulhall JP. Impact of androgen deprivation therapy on sexual function. Asian journal of andrology. 2012;14: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Traish AM. Benefits and Health Implications of Testosterone Therapy in Men With Testosterone Deficiency. Sexual medicine reviews. 2017. [DOI] [PubMed] [Google Scholar]
- [7].Kaku H, Saika T, Tsushima T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. The Prostate. 2006;66: 439–44. [DOI] [PubMed] [Google Scholar]
- [8].Oefelein MG. Time to normalization of serum testosterone after 3-month luteinizing hormone-releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. The Journal of urology. 1998;160: 1685–8. [PubMed] [Google Scholar]
- [9].Murthy V, Norman AR, Shahidi M, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU international. 2006;97: 476–9. [DOI] [PubMed] [Google Scholar]
- [10].Bong GW, Clarke HS Jr., Hancock WC, Keane TE. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology. 2008;71: 1177–80. [DOI] [PubMed] [Google Scholar]
- [11].Kobayashi T, Nishizawa K, Mitsumori K. Individual variation of hormonal recovery after cessation of luteinizing hormone-releasing hormone agonist therapy in men receiving long-term medical castration therapy for prostate cancer. Scandinavian journal of urology and nephrology. 2006;40: 198–203. [DOI] [PubMed] [Google Scholar]
- [12].Tsumura H, Satoh T, Ishiyama H, et al. Recovery of serum testosterone following neoadjuvant and adjuvant androgen deprivation therapy in men treated with prostate brachytherapy. World journal of radiology. 2015;7: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocrine-related cancer. 2010;17: R305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24: 4448–56. [DOI] [PubMed] [Google Scholar]
- [15].Connolly RM, Carducci MA, Antonarakis ES. Use of androgen deprivation therapy in prostate cancer: indications and prevalence. Asian journal of andrology. 2012;14: 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. The Journal of urology. 2002;167: 1952–6. [PubMed] [Google Scholar]
- [17].Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. The Journal of clinical endocrinology and metabolism. 2008;93: 2042–9. [DOI] [PubMed] [Google Scholar]
- [18].Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. The Journal of urology. 2013;189: S34–42; discussion S43–4. [DOI] [PubMed] [Google Scholar]
- [19].Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. European urology. 2015;67: 825–36. [DOI] [PubMed] [Google Scholar]
- [20].Tsai HT, Keating NL, Van Den Eeden SK, et al. Risk of diabetes among patients receiving primary androgen deprivation therapy for clinically localized prostate cancer. The Journal of urology. 2015;193: 1956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Daniell HW. Osteoporosis after orchiectomy for prostate cancer. The Journal of urology. 1997;157: 439–44. [PubMed] [Google Scholar]
- [22].Jackson JA, Kleerekoper M. Osteoporosis in men: diagnosis, pathophysiology, and prevention. Medicine. 1990;69: 137–52. [DOI] [PubMed] [Google Scholar]
- [23].Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. The Journal of urology. 2000;163: 181–6. [PubMed] [Google Scholar]
- [24].Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19: 3750–7. [DOI] [PubMed] [Google Scholar]
- [25].Pai HH, Pickles T, Keyes M, et al. Randomized study evaluating testosterone recovery using short-versus long-acting luteinizing hormone releasing hormone agonists. Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2011;5: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Trost LW, Mulhall JP. Challenges in Testosterone Measurement, Data Interpretation, and Methodological Appraisal of Interventional Trials. The journal of sexual medicine. 2016;13: 1029–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010;95: 2536–59. [DOI] [PubMed] [Google Scholar]
- [28].Planas J, Celma A, Placer J, et al. Hormonal response recovery after long-term androgen deprivation therapy in patients with prostate cancer. Scandinavian journal of urology. 2016;50: 425–28. [DOI] [PubMed] [Google Scholar]
- [29].Oefelein MG. Serum testosterone-based luteinizing hormone-releasing hormone agonist redosing schedule for chronic androgen ablation: a phase I assessment. Urology. 1999;54: 694–9. [DOI] [PubMed] [Google Scholar]
- [30].Padula GD, Zelefsky MJ, Venkatraman ES, et al. Normalization of serum testosterone levels in patients treated with neoadjuvant hormonal therapy and three-dimensional conformal radiotherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2002;52: 439–43. [DOI] [PubMed] [Google Scholar]
- [31].Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. The Journal of clinical endocrinology and metabolism. 2004;89: 5462–8. [DOI] [PubMed] [Google Scholar]
- [32].Bercea RM, Mihaescu T, Cojocaru C, Bjorvatn B. Fatigue and serum testosterone in obstructive sleep apnea patients. Clin Respir J. 2015;9: 342–9. [DOI] [PubMed] [Google Scholar]
- [33].Luboshitzky R, Lavie L, Shen-Orr Z, Lavie P. Pituitary-gonadal function in men with obstructive sleep apnea. The effect of continuous positive airways pressure treatment. Neuro endocrinology letters. 2003;24: 463–7. [PubMed] [Google Scholar]
- [34].Wittert G The relationship between sleep disorders and testosterone in men. Asian journal of andrology. 2014;16: 262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]