Abstract
Background:
The systemic effect of intracavernosal liposomal bupivacaine (Exparel) injection during inflatable penile prosthesis (IPP) implantation on systemic hemodynamics has not been clarified.
Aim:
To evaluate whether intraoperative intracavernosal Exparel injection affects systemic hemodynamics.
Methods:
We studied 56 consecutive patients who underwent IPP implantation under general anesthesia using the transverse scrotal approach. Exparel [10 ml of 1.3% (13.3 mg/ml)] was instilled bilaterally intracavernosally via a 25-gauge needle around 30 min after starting the surgery. All patients graded their postoperative pain using a standard 10-point scale.
Outcomes:
Perioperative vital signs at defined time intervals and postoperative pain were monitored. The presence and degree of postoperative pain using a standard 10-point scale.
Results:
Medians and interquartile ranges (IQR) of the patients’ age [64 years (57.3–69.3)], operation time [85 min (78–96)], and estimated blood loss were [75 ml (29–100)] were recorded. Although the median preoperative systolic noninvasively measured blood pressure of 131 mm/Hg (IQR 122–139) fell 25% to 98 mm/Hg (IQR 90–100) (P < .001) after anesthesia started, there was no significant decrease in blood pressure between before and after Exparel injection. The perioperative pulse rate and pulse oximetry oxygen saturation were stable. Neither arrhythmia nor convulsion occurred. Patients reported postoperative pain as 0 (IQR 0–2.25) on a scale of 0–10.
Clinical Implications:
Intraoperative intracavernosal Exparel injection is safe and effective in patients undergoing IPP implantation surgery.
Strength and Limitations:
This is the first report to describe systemic hemodynamics of intracavernosal injection of Exparel. Limitations are lack of blood concentration of Exparel at various time points and long-term follow-up for pain assessment.
Conclusion:
Although limited by a lack of the blood concentration of Exparel and short follow-up for pain assessment, we concluded that Intraoperative intracavernosal Exparel injection does not affect systemic hemodynamics.
INTRODUCTION
Penile implant (PI) surgery is a well-recognized treatment strategy for patients with medication-refractory erectile dysfunction (ED).1 Several studies have addressed the issue of patient satisfaction following PI surgery and found excellent satisfaction rates for both patients and their partners.2, 3 However, perioperative pain management is one of concerns for patients undergoing PI surgery and can affect the patient's short-term satisfaction. Hagedorn et al. reported that 2–5% of patients complained dissatisfaction after PI surgery because of pain.4
A novel liposomal formulation of bupivacaine (Exparel) was approved by the FDA in the USA in 2011.5, 6 This agent is intended for single-dose infiltration into a surgical site, and its effect lasts 72-96 hours, compared to <10 hours with traditional bupivacaine5 The efficacy of Exparel for pain management was first demonstrated after bunionectomy and hemorrhoidectomy.6, 7
Recently, Cotta et al. reported a small study of patients undergoing PI demonstrating that patients given Exparel required significantly less narcotic medication 24 hours postoperatively compared to those not receiving Exparel.8 This report, however, did not record the systemic hemodynamics after Exparel administration, despite the fact that local anestghetic systemic symptoms (LAST) has been raised as a concern for Exparel.9 Thus, the aim of the present study was to evaluate whether the intracavernosal injection of Exparel during PI surgery has any adverse effects on systemic hemodynamics.
METHODS
Patient Population:
Consecutive patients who had first time PI surgery at our institution had their medical records reviewed in a retrospective fashion. Patients who had undergone revision PI surgery were excluded. The database was registered with the institutional ethics committee.
Exparel Injection:
PI surgery was performed using a transverse scrotal approach. Reservoir placement was performed after making the scrotal incision and identification of the tunica albuginea bilaterally. All reservoirs were placed either in the traditional retropubic space (space of Retzius), in a submuscular space using the transverse scrotal incision or in an extraperitoneal space behind the rectus muscle using a left sided McBurney type incision. Following this and prior to making corporotomies usually around 30 mins after the procedure commenced, 10 ml of 1.3% (13.3 mg/ml) Exparel* (Pacira Pharmaceuticals, Inc., Parsippany, NJ, USA) mixed 1:1 with normal saline was instilled intracavernosally using a 25-gauge needle. In total, 20 ml (266 mg) of Exparel was administered. Corporotomies were made commencing 6 cm from the crus and carries distally for approximately 5 cm. At the end of the procedure Exparel 5 ml was instilled in the area around the incision was given. A single surgeon performed all operations.
Data Acquisition:
We recorded the following measurements from the anesthesiology chart review: perioperative vital signs (blood pressure, pulse rate, oxygen saturation, electrocardiograms). All blood pressure (BP) was measured non-invasively from the arm. All operations were performed as a same-day procedure. Just before discharge from the post-anesthesia care unit, the patient recorded his postoperative pain on a standard 10-point scale.
Statistics:
Any changes in blood pressure before and after Exparel injection were assessed using a Wilcoxon signed-rank test. Data are presented as medians and interquartile range (IQR) and a p value < 0.05 was considered to indicate statistical significance.
RESULTS
Patient Population:
The median (IQR) age of the 56 patients included was 64 (57.3-69.3) years. All patients underwent inflatable penile implant surgery. 48 patients had a three-piece device placed (36 post-radical prostatectomy, 4 post-prostate radiation, 8 due to vasculogenic ED, 4 of whom had diabetes), and 8 patients had a two-piece device placed (all post-radical cystectomy). Median operation time and estimated blood loss were 85 (IQR 78–96) min and 75 (IQR 29–100) ml, respectively. The American Society of Anesthesiologists (ASA) score10 was 3 in 42 patients and 2 in 16 patients. ASA 2 and 3 are assigned for patients with mild systemic disease and those with severe systemic disease, respectively.
Vital Sign Data:
The flowsheet of the perioperative vital signs is shown in Figure 1. The preoperative median (IQR) systolic blood pressure was 131 (122–139) mmHg, which decreased 25% to 98 (90–100) mmHg after anesthesia induction prior to any incision being made (P < .001, ES: d = 1.85, (1- ·) = 0.96) Following this, before and after Exparel injection there was no significant BP decrease. Comparison of systolic and diastolic blood pressures at 5 minute intervals after surgery started did not show any significant decrease (Table 1). The pulse rate and pulse oximetry oxygen saturation (SpO2) during the immediate preoperative and operative periods were also stable (Figure 1). No arrhythmia or convulsions occurred. The median 10-point scale of postoperative pain just before discharge from the post-anesthesia care unit was 0 (IQR 0–2.25). No difference in hemodynamics were seen between patients who underwent different implant types or reservoir locations and there were no complications related to Exparel injection such as penile hematoma, decreased sensation in the penis.
Figure 1. Flowsheet of perioperative vital signs.
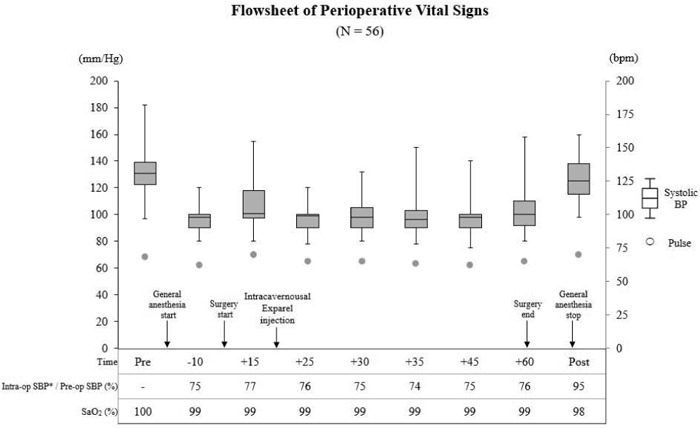
Systolic blood pressure, pulse rate, systolic blood pressure ratio, and saturation of pulse oximetry oxygen (SpO2) from preoperatively to postoperatively were evaluated. Systolic blood pressure is represented by box-and-whisker plots, pulse rate by dots. SpO2 is presented as median values. All patients were administered general anesthesia, and Exparel was instilled intracavernosally about 20 min after starting the anesthesia.
Table 1.
Systolic and diastolic blood pressure at defined time intervals
| Time before and after surgery started (min.) |
Systolic blood pressure | Diastolic blood pressure | ||||
|---|---|---|---|---|---|---|
| (mm/Hg) | p Value vs 25 min.† |
p Value vs 30 min. |
(mm/Hg) | p value vs 25 min. |
p Value vs 30 min. |
|
| Pre surgery | 131 (122 - 139)* | - | - | 79 (73 - 86) | - | - |
| 25 | 99 (90 - 100) | - | - | 55 (52 - 60) | - | - |
| 30 | 98 (90 - 105) | 0.74 | - | 55 (52 - 60) | 0.88 | - |
| 35 | 97 (90 - 103) | 0.39 | 0.41 | 55 (52 - 60) | 0.53 | 0.56 |
| 45 | 98 (90 - 101) | 0.77 | 0.69 | 57 (52 - 62) | 0.29 | 0.16 |
| 60 | 100 (92 - 110) | 0.06 | 0.04 | 58 (52 - 62) | 0.13 | 0.16 |
| Post surgery | 125 (118 - 138) | < .0001 | < .0001 | 70 (60 - 80) | < .0001 | < .0001 |
median (interquartile ranges)
Wilcoxon signed-rank test
DISCUSSION
Penile implant (PI) surgery is a mainstay in the management of medication-refractory ED, and annual sale of implant have recently reached around 28,000 worldwide.11 In contrast, a recommendation from the International Consultation on Sexual Medicine and some other studies described that certain patients are at higher risk for postoperative dissatisfaction, such as uncontrolled diabetes, Peyronie’s disease, post-radical prostatectomy and body mass index >30 kg/m2. 12-14 Hence, thorough informed consent is an essential component.14 Postoperative pain is a considerable factor in early patient satisfaction and acceptance of PI surgery.8 Another potential benefit of optimal postoperative pain control is patient mobility with the potential for lower rates of thromboembolic complications.15 PI while historically performed as an inpatient procedure, is being increasingly performed as an out-patient procedure further amplifying the importance of excellent early postoperative pain management.16 Recently, the Prospective Registry of Outcome with Penile Prosthesis for Erectile Restoration (PROPPER) study showed that, in terms of hospital length of stay among 1018 patients, 52% were had 24 hours in-hospital observation, 43% underwent same-day surgery with hospital discharge, and only 5% were admitted to the hospital for >24 hours.17
It is recognized that postoperative pain arises from the somatosensory peripheral nerve irritation in the penis (corposa cavernosa), area of incision and the location of the reservoir. Hsu et al. reported the efficacy of a proximal dorsal nerve block with peripenile infiltration and penile crural block using lidocaine (n = 137) compared to a group who underwent pudendal nerve block (n = 21) in men who underwent PI surgery. The crural block was done in order to block the cavernous nerve with a 23-gauge 1.5″ (3.81 cm) needle with an average of 277.9 ± 23.6 mg of 0.8% lidocaine injected from the point crossing the penopubic fold and one fingerbreadth bilaterally. Pudendal nerve block was done using 30-gauge x 3.5″ (8.89 cm) needle with an average of 264.6 ± 10.3 mg of 0.8% lidocaine solution targeted to the pudendal nerve housed in Alcock’s canal. 18 Their study found that 43% of the patients treated with pudendal nerve block experienced severe aching pain over the perineum for a couple of weeks postoperatively, whereas the group with both proximal dorsal nerve block with peripenile infiltration and penile crural block saw markedly fewer of these events (12.4%). They also reported no significant complications related to the crural block.18
Several local anesthetics have been used for pain management in patients undergoing PPI. The duration of action varies from very short-acting chloroprocaine (15–30 min; maximum dose 800 mg for 70-kg adult), to medium-acting lidocaine (30–120 min; maximum dose 300 mg for 70-kg adult) to long-acting bupivacaine (120–240 min; maximum dose 175 mg for 70-kg adult).19 A long-acting local anesthetic, Exparel (liposomal bupivacaine) injection received approval from the U.S. Food and Drug Administration (FDA) in October 2011.5 This novel extended-release formulation of a bupivacaine delivery system consists of biocompatible, biodegradable, spherical, lipid-based particles ranging in size from 10-30 μm and containing encapsulated drug designed to allow diffusion over an extended an duration of 72-96 h.15
With regard to Exparel use for PI surgery, there has been a single other report including the present one. Cotta et al. studied 37 patients who underwent PI surgery: 13 were given Exparel, and 24 were not. Though the dose was not described, Exparel (mixed with normal saline to produce a total volume of 20 ml) was utilized for peri-incisional block and was also instilled into the spermatic cords, corporal bodies, reservoir space, and the area of the scrotal pump. The non-Exparel group (n=24) received standard bupivacaine or no anesthetic. The morphine equivalent utilization during 23 hour-observation period after PI surgery was 3.2-fold higher in the non-Exparel group that the Exparel group (18.0 vs 5.6 respectively; p<0.05).8
Though our patients were in relatively anesthesiologic high-risk group (72% of patients were ASA 3 before the operation), the analysis has shown that no change occurs in vital signs before and after intracavernosal Exparel injection, irrespective of ASA grouping. Blood pressure decreased after induction of general anesthesia and remained stable during surgery. No arrhythmia or convulsions occurred. Furthermore, immediate postoperative pain control was acceptable. The median 10-point scale for postoperative pain was 0.
Although these studies indicated the efficacy and safety of intracavernosal Exparel injection, Aggarwal has described local anesthetic systemic toxicity (LAST) with Exparel.9 LAST presents as central nervous symptoms/signs (seizures, convulsions, agitation, dizziness, tinnitus) or cardiovascular symptoms/signs (bradycardia, hypotension). The author analyzed 130 LAST cases reported to be associated with Exparel as the primary suspected drug in the FDA Adverse Event Reporting System (FAERS) between January 2012 and June 2016 (4.5-year period) and among them, 44 cases were serious events. There was 1 death due to seizures, cardiac arrest was reported in 7 cases and cardiopulmonary arrest was reported in 6 cases. Their report suggested that severe systemic toxicity due to Exparel (seizures with or without cardiac arrest) could possibly occur after peripheral nerve block using Exparel, similar to those occurring with traditional bupivacaine.9 The FAERS obviously fails to denote the number of Exparel exposures over this 4.5 year period and thus the incidence of serious adverse events is unknown.
The main limitation of this analysis is the absence of Exparel blood level data.20 Saying that, we have shown excellent pain control and no systemic effects thus, we now use this approach in all of our PI surgery patients. In the future, perhaps a prospective, randomized controlled study with a long follow-up that includes measuring the blood concentration of Exparel and further pain assessment can be performed but many authorities in the field have suggested that this might be unethical given the obvious benefit of Exparel use in this population.
CONCLUSION
Intraoperative hemodynamics during intracavernosal Exparel injection did not affect systemic penile implant surgery. We believe these data suggest that intracavernosal Exparel injection is safe in such patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. The Journal of urology. 2005;174: 230–9. [DOI] [PubMed] [Google Scholar]
- [2].Garber BB. Mentor Alpha 1 inflatable penile prosthesis: patient satisfaction and device reliability. Urology. 1994;43: 214–7. [DOI] [PubMed] [Google Scholar]
- [3].Goldstein I, Newman L, Baum N, et al. Safety and efficacy outcome of mentor alpha-1 inflatable penile prosthesis implantation for impotence treatment. The Journal of urology. 1997;157: 833–9. [PubMed] [Google Scholar]
- [4].Hagedorn J, Osbun N, Lundy S. Inflatable penile prosthesis failure and complications: review of a national database. Presented at: American Urological Association; 2015. [Google Scholar]
- [5].Administration. UFaD FDA Label Approved on 10/28/2011 (PDF) for EXPAREL. US Silver Spring, MD: US Food and Drug Administration; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022496s000lbl.pdf. Accessed May 01, 2012. . 2012. [Google Scholar]
- [6].Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam(R) bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Advances in therapy. 2011;28: 776–88. [DOI] [PubMed] [Google Scholar]
- [7].Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Diseases of the colon and rectum. 2011;54: 1552–9. [DOI] [PubMed] [Google Scholar]
- [8].Cotta BH, Welliver C, Brahmamdam A, Bednarchik CL, Dynda D, Kohler TS. Long-acting liposomal bupivacaine decreases inpatient narcotic requirements in men undergoing penile prosthesis implantation. Turkish journal of urology. 2016;42: 230–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aggarwal N Local anesthetics systemic toxicity association with exparel (bupivacaine liposome)- a pharmacovigilance evaluation. Expert opinion on drug safety. 2017: 1–7. [DOI] [PubMed] [Google Scholar]
- [10].Smetana GW. Preoperative pulmonary evaluation. The New England journal of medicine. 1999;340: 937–44. [DOI] [PubMed] [Google Scholar]
- [11].Mulcahy JJ. The Development of Modern Penile Implants. Sexual medicine reviews. 2016;4: 177–89. [DOI] [PubMed] [Google Scholar]
- [12].Akin-Olugbade O, Parker M, Guhring P, Mulhall J. Determinants of patient satisfaction following penile prosthesis surgery. The journal of sexual medicine. 2006;3: 743–48. [DOI] [PubMed] [Google Scholar]
- [13].Trost LW, Baum N, Hellstrom WJ. Managing the difficult penile prosthesis patient. The journal of sexual medicine. 2013;10: 893–906; quiz 07. [DOI] [PubMed] [Google Scholar]
- [14].Levine LA, Becher E, Bella A, et al. Penile Prosthesis Surgery: Current Recommendations From the International Consultation on Sexual Medicine. The journal of sexual medicine. 2016;13: 489–518. [DOI] [PubMed] [Google Scholar]
- [15].Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. Journal of pain research. 2012;5: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alwaal A, Harris CR, Hussein AA, et al. The Decline of Inpatient Penile Prosthesis over the 10-Year Period, 2000-2010. Sexual medicine. 2015;3: 280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Henry GD, Karpman E, Brant W, et al. The Who, How and What of Real-World Penile Implantation in 2015: The PROPPER Registry Baseline Data. The Journal of urology. 2016;195: 427–33. [DOI] [PubMed] [Google Scholar]
- [18].Hsu GL, Hsieh CH, Wen HS, et al. Outpatient penile implantation with the patient under a novel method of crural block. International journal of andrology. 2004;27: 147–51. [DOI] [PubMed] [Google Scholar]
- [19].Reinstatler L, Shee K, Gross MS. Pain Management in Penile Prosthetic Surgery: A Review of the Literature. Sexual medicine reviews. 2017. [DOI] [PubMed] [Google Scholar]
- [20].Tucker G, Mather L. Absorption and disposition of local anesthetics: Pharmacokinetics, Neural Blockade in Clinical Anesthesia & Management of Pain. Edited by Cousins MJ and Bridenbaugh PO. JB Lippincott, Philadelphia: 1980: 45–85. [Google Scholar]


