Abstract
Background:
While phosphodiesterase type-5 inhibitors (PDE5Is) are highly effective for the treatment of erectile dysfunction (ED) and well tolerated, updated data on prescription patterns has been limited in real-world settings.
Aim:
To describe men in the United States who are prescribed PDE5Is for ED treatment and to evaluate patterns of initiation, switching, and treatment overlap.
Methods:
This retrospective claims study used MarketScan Commercial and Medicare Supplement Databases from January 1, 2010, to December 31, 2015 to identify initial PDE5I claims (index date) for sildenafil, tadalafil, and/or vardenafil. Adults aged ≥18 years with ED were identified between July 1, 2010, and December 31, 2014, allowing for a 6-month preindex and 12-month follow-up period from the index date.
Outcomes:
Outcomes included patient demographics and treatment-related patterns following treatment initiation.
Results:
A total of 106,206 identified patients met all inclusion criteria. Of these, 51,694, 40,193, and 14,319 had initial claims for sildenafil, tadalafil, and vardenafil, respectively. Mean age was 50.35 years and comorbidities included dyslipidemia (44.17%), hypertension (43.09%), diabetes (15.32%), and depression (10.61%). More patients (48.67%) initiated on sildenafil than tadalafil (37.85%) or vardenafil (13.48%). Rate of switching was lower in the 60 days following the end of days supply of the initial prescription in the sildenafil cohort (2.71%) compared with the tadalafil (2.81%) and vardenafil (3.88%) cohorts (P<0.001 for sildenafil vs tadalafil or vardenafil). Treatment overlap was lower in the sildenafil cohort (0.35%) compared with the tadalafil (0.75%) and vardenafil (0.62%) groups (P<0.001 for sildenafil vs tadalafil or vardenafil).
Clinical Implications:
These findings provide insight into updated patterns of PDE5I prescriptions in the United States and may aid in clinical decision-making.
Strengths & Limitations:
Strengths include the large sample size, long data coverage period, and the real-world nature of the study. Limitations include the retrospective study design, use of data collected with a primary focus of claims, and lack of further details regarding reasons that drive switching. Actual rates of ED and impact on prescription patterns may be underestimated because the claims database only captured patients electing to visit a healthcare provider.
Conclusion:
Among men with ED in the United States, rates of switching and treatment overlap were low for all PDE5Is but were found to be the lowest for sildenafil compared with tadalafil and vardenafil.
Keywords: erectile dysfunction, phosphodiesterase type-5 inhibitors, PDE5I, sildenafil, real-world evidence, treatment patterns
Twitter message:
Claims data from more than 100,000 US adult men prescribed PDE5Is for treatment of erectile dysfunction indicate that rates of switching and treatment overlap were generally low, but lowest for sildenafil compared with tadalafil and vardenafil.
Introduction
Erectile dysfunction (ED) affects more than 20 million men in the United States and over 150 million men worldwide [1-3]. The prevalence of ED is expected to increase substantially in coming years with an estimated global prevalence of nearly 322 million by 2025 [1,2]. ED can have a significant adverse impact on patient and partner quality of life and negatively affects physical and psychosocial health [4,5]. Risk factors for development of ED include increased age, smoking, diabetes mellitus, hypertension, dyslipidemia, depression, obesity, and sedentary lifestyle [2,4].
Phosphodiesterase type-5 inhibitors (PDE5Is) have been studied extensively for the treatment of ED and are a well-established first-line therapy [2,4]. These drugs are well tolerated, easy to use, highly effective, and they produce improvements in health-related quality of life [4]. In 1998, sildenafil citrate (Viagra®, Pfizer Inc, New York NY) became the first approved oral PDE5I [6]. Subsequently, 2 other oral PDE5I drugs, tadalafil (Cialis®, Lilly USA, Indianapolis, IN) and vardenafil (Levitra®, Bayer-GlaxoSmithKline) were introduced to the market in 2003 [7,8].
Although real-world PDE5I treatment patterns in the United States were described in 2005 [9], changes in these patterns in recent years have not been reported. Given that patient populations and PDE5I use continue to evolve, an update can offer critical perspectives on product selection and continued utilization. The objective of this study is therefore to inform a more contemporary understanding of PDE5I selection (ie, from the time of newly initiating PDE5I treatment) and continued use behavior, including prescription switch patterns and treatment overlaps in patients with ED in the United States.
Methods
Study Design
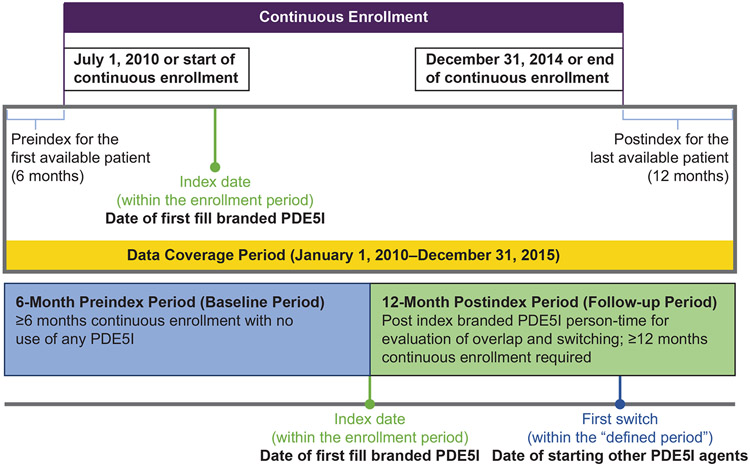
This retrospective claims study used data from the MarketScan Commercial and Medicare Supplement Databases during the time period January 1, 2010, to December 31, 2015 (Figure 1). The MarketScan database includes claims data from >30 million patients, including employees and their adult dependents from approximately 100 employers and 12 US health plans [10].
Figure 1.
Study period and patient observational period. Diagram indicates enrollment period, data coverage period, and dates for first prescription fill and first switch to a different PDE5I. PDE5I=phosphodiesterase type-5 inhibitor.
Inclusion/Exclusion Criteria
Male patients aged ≥18 years at the index date, which is the date of first fill for branded PDE5I, with ≥1 ED diagnosis within 6 months prior to or within 30 days after index PDE5I prescription and ≥1 branded PDE5I claim during the study period were eligible for inclusion in the study. Because patients were also required to have ≥6 months continuous enrollment prior to and ≥12 continuous enrollment following the index PDE5I prescription claim, all included index dates were between July 1, 2010 and December 31, 2014 to allow for appropriate pre- and post-index follow-up. Patients were excluded if they used any branded PDE5I within the preindex period, meaning they had exposure to PDE5I before the index date and were therefore not newly initiating PDE5I treatment. Patients were also excluded if they had received a guanylate cyclase stimulator for the treatment of pulmonary arterial hypertension (PAH) or nitrates in any form for the treatment of coronary artery disease during the study period, or if they had been diagnosed with PAH or benign prostatic hyperplasia (BPH) at any time 6 months pre- or postindex. Exclusion of patients with BPH ensured that the PDE5I was indicated primarily to treat ED.
Outcomes
Patient characteristics included age, gender, region, insurance plan, and presence of clinical comorbidities (hypertension, dyslipidemia, diabetes, depression, obesity). Treatment patterns were reviewed within 6 months of the postindex period. Outcomes reviewed included the proportion of patients initially prescribed each PDE5I of interest, switch rates between PDE5Is during the 6 months postindex, time to first switch, and any overlapping therapy. Because treatment frequency may be either on demand (tadalafil, sildenafil, and vardenafil) or once daily (tadalafil), depending on the frequency of sexual intercourse, a prescription switch was chosen as the threshold of continued use. Treatment switch was defined as a switch from the initial index PDE5I to another PDE5I within the 6-month postindex observation period. Time to switch was defined as the number of days from the index date to the date of the first prescription fill of a PDE5I other than the index PDE5I. Overlapping therapy refers to patients having ≥2 prescription fills for a different PDE5I brand without discontinuation of the index PDE5I. Outcomes were analyzed at 15, 30, 45, and 60 days.
Statistical Analysis
Frequencies and percentages were calculated and reported for categorical endpoints; statistical significance was assessed using a chi-square or Fisher exact test. For continuous endpoints, summary statistics of mean, standard deviation, median, interquartile range (IQR), and minimum and maximum were reported. Statistical significance for the comparison of 2 independent comparison groups (ie, switcher vs nonswitcher) was assessed using t tests. Multisample comparison groups were evaluated by 1-way ANOVA, whereas paired t tests were used for paired-sample comparisons.
Results
Patient Characteristics
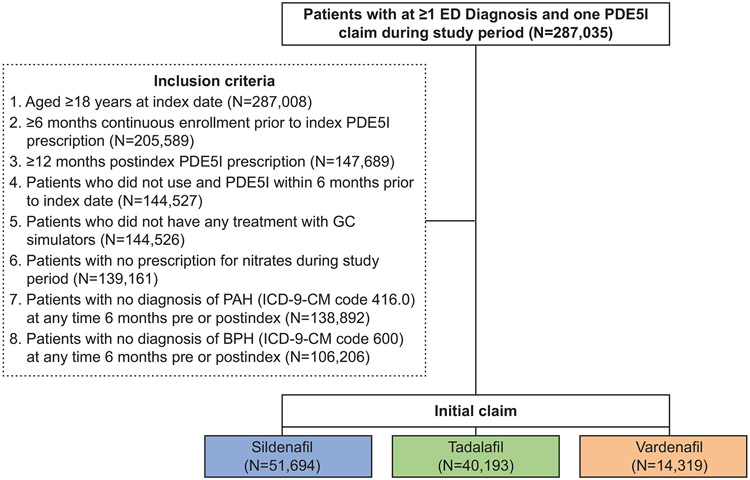
A total of 287,035 patients with ≥1 ED diagnosis and 1 PDE5I claim during the study period were identified. Of these, 106,206 met inclusion criteria and were included in the analysis; 51,694, 40,193, and 14,319 patients had initial claims for sildenafil, tadalafil, and vardenafil, respectively (Figure 2). Patient characteristics are summarized in Table 1. The overall mean age was 50.35 years (SD, 10.69; range 18–96) years, and the mean Charlson Comorbidity Score was 0.45 (SD, 1.01). Common comorbidities included dyslipidemia (44.17%), hypertension (43.09%), diabetes (15.32%), and depression (10.61%) in the overall group. The majority of patients (61.08%) were part of a preferred provider organization (PPO) health plan. Patients were most commonly from the South region (35.30%) of the United States, followed by the West (22.56%), North Central (20.96%), and Northeast (18.17%) regions (P<0.001).
Figure 2.
Cohort selection. Flow chart illustrates initial possible patient group, inclusion criteria and numbers of qualifying patients prescribed sildenafil, tadalafil, and vardenafil. BPH=benign prostatic hyperplasia; ED=erectile dysfunction; ICD-9-CM=International Classification of Diseases-9th Revision-Clinical Modification; PAH=pulmonary arterial hypertension; PDE5I=phosphodiesterase type-5 inhibitor.
Table 1.
Patient Characteristics
| Variable | Statistic or Category |
All PDE5I Users (N=106,206) |
Initial Claim of Sildenafil (n=51,694) |
Initial Claim of Tadalafil (n=40,193) |
Initial Claim of Vardenafil (n=14,319) |
P Value |
|---|---|---|---|---|---|---|
| Age, y | Mean (SD) | 50.35 (10.69) | 50.41 (10.67) | 49.74 (10.52) | 51.82 (11.08) | <0.001* |
| Age group, n (%), y | 18–34 | 8283 (7.80) | 3980 (7.70) | 3340 (8.31) | 963 (6.73) | <0.001† |
| 35–44 | 21,598 (20.34) | 10,358 (20.04) | 8676 (21.59) | 2564 (17.91) | ||
| 45–54 | 37,494 (35.30) | 18,398 (35.59) | 14,367 (35.75) | 4729 (33.03) | ||
| 55–64 | 31,944 (30.08) | 15,611 (30.20) | 11,674 (29.04) | 4659 (32.54) | ||
| ≥65 | 6887 (6.48) | 3347 (6.47) | 2136 (5.31) | 1404 (9.81) | ||
| Gender, n (%) | Male | 106,206 (100) | 51,694 (100) | 40,193 (100) | 14,319 (100) | <0.001† |
| Region, n (%) | North Central | 22,257 (20.96) | 11,491 (22.23) | 8940 (22.24) | 1826 (12.75) | <0.001† |
| Northeast | 19,298 (18.17) | 10,679 (20.66) | 7154 (17.80) | 1465 (10.23) | ||
| South | 37,487 (35.30) | 17,713 (34.27) | 16,009 (39.83) | 3765 (26.29) | ||
| Unknown | 3207 (3.02) | 1696 (3.28) | 1137 (2.83) | 374 (2.61) | ||
| West | 23,957 (22.56) | 10,115 (19.57) | 6953 (17.30) | 6889 (48.11) | ||
| Health plan type, n (%) | CDHP | 5062 (4.77) | 2477 (4.79) | 2177 (5.42) | 408 (2.85) | 0.011† |
| Comprehensive | 2703 (2.55) | 1422 (2.75) | 1013 (2.52) | 268 (1.87) | ||
| EPO | 1448 (1.36) | 725 (1.40) | 584 (1.45) | 139 (0.97) | ||
| HDHP | 3230 (3.04) | 1627 (3.15) | 1375 (3.42) | 228 (1.59) | ||
| HMO | 16,421 (15.46) | 6079 (11.76) | 4276 (10.64) | 6066 (42.36) | ||
| Missing/Unknown | 3966 (3.73) | 2155 (4.17) | 1453 (3.62) | 358 (2.50) | ||
| POS | 7926 (7.46) | 3998 (7.73) | 3134 (7.80) | 794 (5.55) | ||
| POS with capitation | 576 (0.54) | 350 (0.68) | 163 (0.41) | 63 (0.44) | ||
| PPO | 64,874 (61.08) | 32,861 (63.57) | 26,018 (64.73) | 5995 (41.87) | ||
| Charlson Comorbidity Score | Mean (SD) | 0.45 (1.01) | 0.45 (1.01) | 0.46 (1.02) | 0.45 (0.98) | <0.001* |
| Hypertension, n (%) | Yes | 45,394 (43.09) | 21,947 (42.80) | 17,511 (43.88) | 5936 (41.92) | <0.001† |
| Dyslipidemia, n (%) | Yes | 46,536 (44.17) | 22,361 (43.60) | 18,069 (45.28) | 6106 (43.12) | <0.001† |
| Diabetes, n (%) | Yes | 16,141 (15.32) | 7675 (14.97) | 6187 (15.50) | 2279 (16.10) | 0.001† |
| Depression, n (%) | Yes | 11,180 (10.61) | 5452 (10.63) | 4384 (10.99) | 1322 (9.49) | 0.008† |
One-way ANOVA test (continuous outcomes).
Chi-square/Fisher exact (categorical outcomes).
CDHP=consumer-driven health plan; EPO=exclusive provider organization; HDHP=high deductible health plan; HMO=health maintenance organization; PDE5I=phosphodiesterase type-5 inhibitor; POS=point of service; PPO=preferred provider organization.
Treatment Patterns
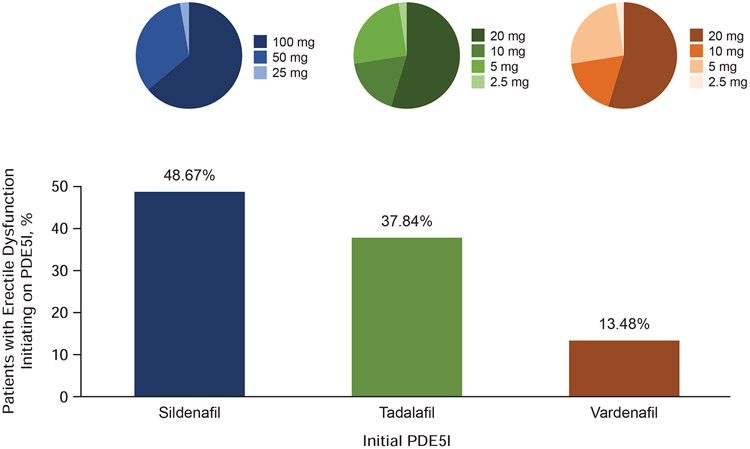
Of 106,206 patients, a higher proportion of patients initiated on sildenafil (51,694 patients, 48.67%) compared with either tadalafil (40,193 patients, 37.84%) or vardenafil (14,319 patients, 13.48%) (Figure 3). Patients tended to initiate on higher doses of PDE5I, with the majority of patients initiating on sildenafil receiving a 100-mg dose (63.37%) and most of those initiating on tadalafil or vardenafil receiving a 20-mg dose (56.05% and 73.82%, respectively).
Figure 3.
Treatment initiation pattern. Percentages of patients initiating on sildenafil, tadalafil, or vardenafil are shown; distribution of initiating dose levels are presented in as pie charts.
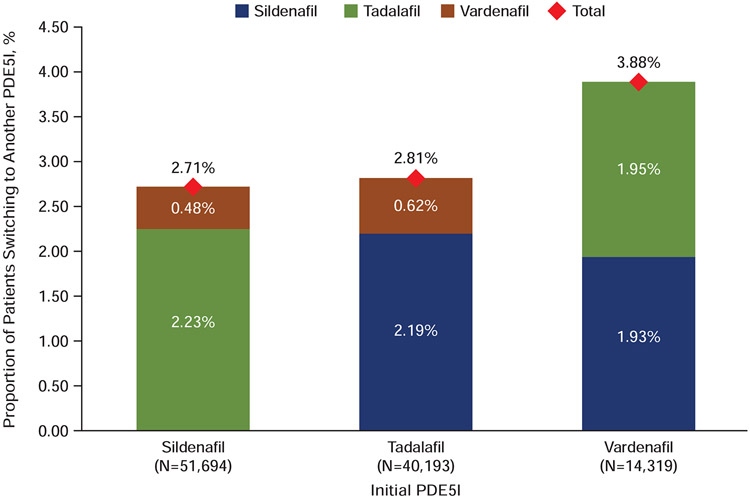
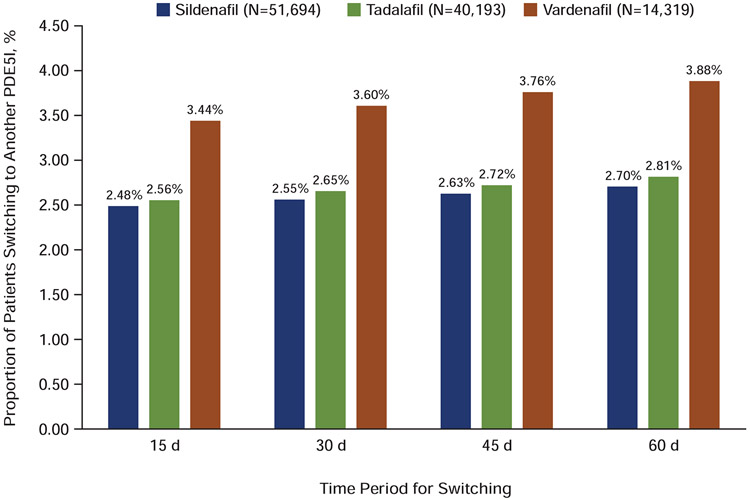
The treatment switching pattern within 60 days following the end of days supply of the initial prescription is illustrated in Figure 4. A significantly lower percentage of patients who initiated on sildenafil (2.71%) switched to another PDE5I during this time period compared with those initiated on tadalafil (2.81%) and vardenafil (3.88%) (P<0.001). Among tadalafil patients switching to another PDE5I, the majority switched to sildenafil; vardenafil patients switched in similar proportions to sildenafil or tadalafil. This switch pattern remained consistent even after varying the switch window to 15, 30, 45, and 60 days after the first PDE5I prescription fill (P<0.001), with a lower percentage of patients switching from sildenafil to a different PDE5I (Figure 5).
Figure 4.
Treatment switching pattern. Percentages of patients switching from the initial PDE5I (x-axis) to other PDE5Is; total percentages switching are shown at the top of each column. PDE5I=phosphodiesterase type-5 inhibitor.
Figure 5.
Switching pattern within different time periods. Percentages of patients switching from initiating PDE5I drug to another PDE5I are shown for 15, 30, 45, and 60 days after initial PDE5I prescription. PDE5I=phosphodiesterase type-5 inhibitor.
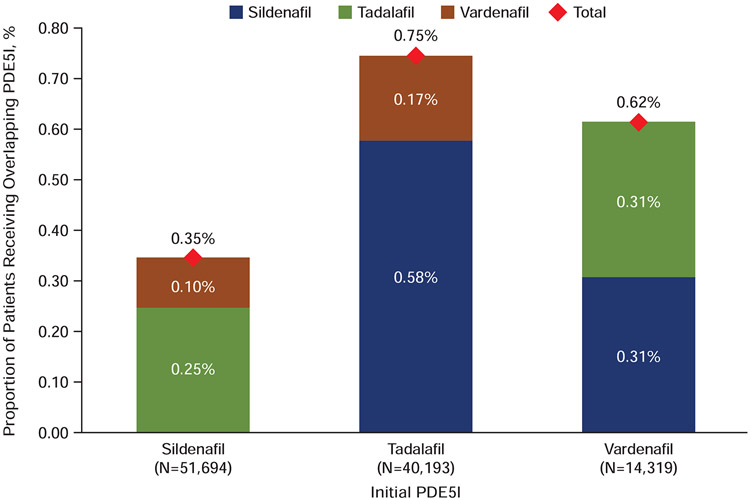
Treatment overlap was observed in 0.54% of the cohort (Figure 6). A lower percentage of patients who initiated on sildenafil had overlapping therapy with another PDE5I compared with tadalafil or vardenafil groups (P<0.001). For patients who initiated on tadalafil, overlapping therapy was primarily with sildenafil (0.58%); for those initiated on vardenafil, equal percentages had overlapping therapy with sildenafil and tadalafil (0.31%, each).
Figure 6.
Treatment overlapping pattern. Percentages of patients with 2 overlapping prescriptions for PDE5Is are shown by initial PDE5I prescribed. PDE5I= phosphodiesterase type-5 inhibitor.
Discussion
ED is a common condition in men and is associated with impairments in quality of life for both men and their partners [5]. The current study of data from >106,000 ED patients highlighted recent patterns of PDE5I adoption and continuation. Sildenafil was found to be the most frequently prescribed of the 3 PDE5Is reviewed, and patients who initiated on sildenafil were less likely to switch to or overlap with another PDE5I.
The results from this study are generally consistent with findings from a study we previously conducted in the United States using prescription claims for PDE5Is from NCDHealth’s Intelligent Health Repository [9]. In that study, 52% of patients receiving sildenafil refilled their prescription within 6 months of the initial prescription compared with 30% of patients initially receiving vardenafil and 29% of those initially receiving tadalafil (P<0.001) [9]. Furthermore, a significantly lower percentage of patients in the sildenafil cohort switched medications (6.4%) compared with those receiving vardenafil (10.4%) and tadalafil (9.0%)(P<0.001) [9].
Strengths of this study included the large sample size of recent ED patients and a data set that reflected real-world patient characteristics and treatment patterns over time in the United States. The study also had some limitations, including that adherence data were not assessed. However, as it is difficult to make meaningful comparisons between adherence rates to PDE5Is that are recommended for daily versus on-demand use, instead assessing the frequency of treatment switching provided a more direct approach for comparison. The reasons for PDE5I choice were also not evaluated. Based on available data, PDE5Is do not differ significantly in terms of efficacy and tolerability [11]; however, PDE5Is have differing pharmacokinetic profiles (eg, long- vs short-acting, drug-drug interactions, drug-food interactions) [12,13], which may impact patient preference [11].
Reasons for initial PDE5I choice and subsequent switching remain topics of continued exploration. Baseline characteristics, clinical experience, timing and frequency of intercourse, and cost all play important roles in treatment decisions and adherence [13-15]. However, as treatment success is determined by patient-reported outcomes, it has been suggested that patient preference should ultimately drive treatment choice, with different agents being trialed until a preferred treatment is found [15]. Previous studies investigating reasons for patient preference have generated contradictory results [15,16], and therefore additional studies are needed to provide further insights.
An additional limitation was that data were collected primarily for claims and may therefore have exhibited selection bias. Moreover, analyses of claims data are limited by the time in which they are collected, which may affect applicability of results. As such, several changes in treatment patterns of ED therapies may have occurred since 2010–2015, the study time period, such as novel treatments [17] (eg, low-intensity shockwave therapy [18]) and the spread of generics [19]. Updated studies are recommended to further contextualize other ED treatment dynamics since the study time period. The studied cohort was also limited to patients who see their healthcare provider for ED treatment, who may differ from the entire population of ED patients.
Conclusions
This study provides important updates on the details of treatment patterns among men with ED in a real-world setting in the United States. Sildenafil was the most frequently prescribed PDE5I, and patients initiating with sildenafil had the lowest rates of treatment switching and overlap. Further insights into the initial PDEI5 selection and reasons for switching between treatments may ultimately help to improve patient experience.
Acknowledgements
Pharmerit International conducted the study on behalf of Pfizer Inc. Editorial support was provided by Jill E. Kolesar, PhD, of Complete Healthcare Communications, LLC, (North Wales, PA), a CHC Group company, and was funded by Pfizer Inc. JM has no financial relationship with Pfizer Inc in relation to the present study. IC and DP were employees of Pharmerit International, who were paid consultants, at the time of study execution and conducted this study and analysis on behalf of Pfizer Inc in connection with the development of this manuscript. WYT and TAH are employees of Pfizer Inc and hold stock.
Funding Statement
This study was funded by Pfizer Inc. Pfizer Inc employee-authors participated in study design, data analysis and interpretation, initial writing of the report, and the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999;84:50–6. [DOI] [PubMed] [Google Scholar]
- [2].Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh J, Khera M, McVary KT, Miner MM, Nelson CJ, Sadeghi-Nejad H, Seftel AD, Shindel AW. Erectile dysfunction: AUA guideline. J Urol 2018. [DOI] [PubMed] [Google Scholar]
- [3].McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res 2000;12:S6–S11. [DOI] [PubMed] [Google Scholar]
- [4].Hatzimouratidis K, Giuliano F, I. M, Muneer A, Salonia A, Verze P. EAU guidelines on erectile dysfunction, premature ejaculation, penile curvature, and priapism. Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Sexual-Dysfunction-2016-3.pdf. Accessed June 29, 2018, 2018.
- [5].Wagner G, Fugl-Meyer KS, Fugl-Meyer AR. Impact of erectile dysfunction on quality of life: patient and partner perspectives. Int J Impot Res 2000;12:S144–6. [DOI] [PubMed] [Google Scholar]
- [6].Viagra® (sildenafil citrate) Full Prescribing Information, Pfizer Inc, New York, NY, 2015. [Google Scholar]
- [7].Cialis® (tadalafil) Full Prescribing Information, Eli Lilly and Company, Indianapolis, IN, 2016. [Google Scholar]
- [8].Levitra® (vardenafil HCl) Full Prescribing Information, Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, 2014. [Google Scholar]
- [9].Mulhall JP, McLaughlin TP, Harnett JP, Scott B, Burhani S, Russell D. Medication utilization behavior in patients receiving phosphodiesterase type 5 inhibitors for erectile dysfunction. J Sex Med 2005;2:848–55. [DOI] [PubMed] [Google Scholar]
- [10].Truven Health Analytics. MarketScan Databases. Available at: http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. Accessed July 18, 2017.
- [11].Giannitsas K, Konstantinopoulos A, Patsialas C, Perimenis P. Preference for and adherence to oral phosphodiesterase-5 inhibitors in the treatment of erectile dysfunction. Patient Prefer Adherence 2008;2:149–55. [PMC free article] [PubMed] [Google Scholar]
- [12].Bella AJ, Lee JC, Carrier S, Benard F, Brock GB. 2015 CUA Practice guidelines for erectile dysfunction. Can Urol Assoc J 2015;9:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang SA, Lie JD. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T 2013;38:407–19. [PMC free article] [PubMed] [Google Scholar]
- [14].Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, Fusco F. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med 2011;8:3418–32. [DOI] [PubMed] [Google Scholar]
- [15].Raheem AA, Kell P. Patient preference and satisfaction in erectile dysfunction therapy: a comparison of the three phosphodiesterase-5 inhibitors sildenafil, vardenafil and tadalafil. Patient Prefer Adherence 2009;3:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mulhall JP, Montorsi F. Evaluating preference trials of oral phosphodiesterase 5 inhibitors for erectile dysfunction. Eur Urol 2006;49:30–7. [DOI] [PubMed] [Google Scholar]
- [17].Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J 2017;93:679–85. [DOI] [PubMed] [Google Scholar]
- [18].Campbell JD, Trock BJ, Oppenheim AR, Anusionwu I, Gor RA, Burnett AL. Meta-analysis of randomized controlled trials that assess the efficacy of low-intensity shockwave therapy for the treatment of erectile dysfunction. Ther Adv Urol 2019;11:1756287219838364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Feng S, Zhou L, Liu Q, He Q, Liao B, Wei X, Li H, Wang K, Zhu Y. Are phosphodiesterase type 5 inhibitors associated with increased risk of melanoma?: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e9601. [DOI] [PMC free article] [PubMed] [Google Scholar]