Abstract
Shear-thinning, self-healing hydrogels are promising vehicles for therapeutic cargo delivery due to their ability to be injected using minimally invasive surgical procedures. We present an injectable hydrogel using a novel combination of dynamic covalent crosslinking with thermoresponsive engineered proteins. Ex situ at room temperature, rapid gelation occurs through dynamic covalent hydrazone bonds by simply mixing two components: hydrazine-modified elastin-like protein (ELP) and aldehyde-modified hyaluronic acid. This hydrogel provides significant mechanical protection to encapsulated human mesenchymal stem cells during syringe needle injection and rapidly recovers after injection to retain the cells homogeneously within a 3D environment. In situ, the ELP undergoes a thermal phase transition, as confirmed by Coherent anti-Stokes Raman scattering microscopy observation of dense ELP thermal aggregates. The formation of the secondary network reinforces the hydrogel and results in a 10-fold slower erosion rate compared to a control hydrogel without secondary thermal crosslinking. This improved structural integrity enables cell culture for three weeks post injection, and encapsulated cells maintain their ability to differentiate into multiple lineages, including chondrogenic, adipogenic, and osteogenic cell types. Together, these data demonstrate the promising potential of ELP-HA hydrogels for injectable stem cell transplantation and tissue regeneration.
Keywords: injectable hydrogel, dynamic covalent chemistry, elastin-like protein (ELP), secondary crosslinking, mesenchymal stem cell
Graphical Abstract
Shear-thinning and self-healing hydrogels containing protein-engineered ELP and hyaluronic acid are fabricated through dynamic covalent crosslinking, followed by thermoresponsive physical crosslinking for reinforcement and enhanced stability. These hydrogels have highly tunable stiffness, provide delivered stem cells significant mechanical protection, and maintain the cells after delivery in a three-dimensional environment that supports further differentiation.

1. Introduction
Hydrogels are water-swollen, insoluble networks of crosslinked polymers. These materials are characterized by facile diffusion of biomolecules and tissue-like elasticity, making them attractive candidates to serve as carriers for the controlled delivery of cells, drugs, and other bioactive molecules. [1-4] In particular, injectable hydrogels as cell-delivery vehicles have been attracting increasing interest in recent years due to their ability to homogeneously encapsulate cells, to be surgically delivered in a minimally invasive way, and to localize the delivered cargo at the desired site of repair. [5-7]
Injectable hydrogels for cell delivery can be designed using either physical or chemical crosslinking mechanisms. Physically crosslinked gels are commonly formed using self-assembly that is controlled by external stimuli, [8-10] which may expose cells to non-physiological conditions, such as high ionic strength or low pH. Chemically crosslinked gels are commonly formed through the use of crosslinking agents or externally-initiated polymerization, [11, 12] which may expose cells to cytotoxic chemicals, photosensitizers, or free radicals.[13] Therefore, a potential limitation of many injectable hydrogels is the use of physical or chemical environments that may interfere with the sensitive, cellular cargo.[14] Another potential limitation of many injectable hydrogels is a narrow window of acceptable gelation time. [15, 16] If crosslinking occurs very rapidly, the needle may become clogged due to premature gelation, resulting in surgical delivery failure. On the other hand, a slow crosslinking process may lead to delivery of an incompletely-gelled sample, resulting in their drainage away from the target site and an undesirable loss of cargo. [17, 18]
An appealing strategy to address these limitations is to design adaptable hydrogels with shear-thinning and self-healing properties.[11, 19] At higher shear rates, the material has a lower viscosity to facilitate injection. After injection, the material rapidly recovers to localize the cellular cargo at the target site. However, many shear-thinning hydrogels are mechanically soft and subject to fast erosion. [15, 20-22] Recently, dynamic covalent crosslinking has emerged as a promising approach to address these challenges. Dynamic covalent chemistry (DCC) is the study of covalent bonds that can form, break, and re-form reversibly under equilibrium control.[23] While these interactions have been widely explored to build acellular self-healing materials,[24-26] only more recently have researchers begun to apply dynamic covalent chemistry to the tissue engineering field due to the challenges in identifying dynamic chemistry that is both non-cytotoxic and reversible at physiological conditions. To date, this strategy has been applied to crosslink a variety of synthetic and natural polymers, including polyethylene glycol (PEG), chitosan, hyaluronic acid (HA), and gelatin. [27-31] However, the use of DCC in the design of shear-thinning hydrogels for injectable applications has been lacking, which can be attributed to the rapid erosion of gels formed with reversible, dynamic crosslinks. [57-59] To overcome this limitation, here we demonstrate the combined use of dynamic covalent chemistry and thermoresponsive engineered proteins to form a double network hydrogel. This novel strategy enables both easy injectability due to reversible DCC crosslinking as well as maintenance of long-term gel integrity with slower erosion rates due to secondary thermal crosslinking.
To achieve this thermal crosslinking, we have selected to use a thermo-responsive protein that is recombinantly engineered. Protein engineering offers both precise sequence control and predictable biofunctionality, properties that are lacking in many natural or synthetic polymeric materials. [33, 34] For example, engineered proteins have been designed to exhibit a variety of functions, including thermo-responsiveness, enzyme-responsiveness, and cell-adhesion, for potential medical applications. [35-39] Cell-mediated proteolytic degradation of the matrix is a desirable property in the biomedical field. [6, 40] Cell-adhesion functionality is particularly important for adherence-dependent cell types, such as mesenchymal stem cells (MSCs), to promote attachment to the matrix in order to maintain their survival and function. [41, 42]
In this contribution, we have designed a family of double network, injectable hydrogels that includes a protein-engineered, multifunctional elastin-like protein (ELP) that is cell-adhesive, thermo-responsive, and enzymatically degradable (Figure 1). The hydrogels undergo a first gelation through the formation of dynamic covalent hydrazone bonds by mixing hydrazine-modified elastin-like protein (ELP-HYD) and aldehyde-modified hyaluronic acid (HA-ALD) without any additional chemical crosslinkers. Upon heating (e.g. to physiological temperatures after injection), a secondary physical crosslinking occurs via thermoresponsive phase segregation of ELP to form a reinforced network. Compared with hydrogels formed solely via dynamic hydrazone linkages, these materials have significantly slower erosion rates. Hence, these hydrogels are injectable due to the reversible, shear-thinning nature of the dynamic covalent chemistry and self-healing and thermoresponsive after injection to form a cell-adhesive scaffold with long-term stability (Figure 1c, Movie S1), which is required for many regenerative cell delivery therapies.
Figure 1.
Injectable ELP-HA hydrogels. a) ELP-HA is composed of hydrazine-modified elastin-like protein (ELP-HYD) and aldehyde-modified hyaluronic acid (HA-ALD). b) Schematic of ELP-HA hydrogel formation. c) Photographs demonstrating the injectability and rapid self-healing of ELP-HA hydrogels.
2. Results and Discussion
2.1. Design and Synthesis of ELP-HA Components
Elastin-like proteins (ELPs), a class of genetically engineered polypeptides inspired by tropoelastin, are composed of the pentapeptide repeat Val-Pro-Gly-Xaa-Gly, where the guest residue Xaa can be any amino acid except proline (Pro).[43] ELP sequences have been used in the modular design of a wide array of multifunctional biomaterials. [44-47] Incorporation of the lysine residue (Lys) at the Xaa position of ELP biomaterials enables site-specific, covalent crosslinking through reaction with the primary amine.[48-50] In this study, we utilized this moiety to functionalize ELP with hydrazine groups. The degree of hydrazine modification, ~ 79.4%, was determined by 1H-NMR (Figure S1, Supporting Information). ELPs undergo a lower critical solution temperature (LCST) phase transition, i.e., they become less soluble when the temperature is raised above the transition temperature (Tt). [51] Our unmodified ELP has a Tt of 33.9 °C, while ELP-HYD has a slightly lower Tt of 25.6 °C (Figure S1d-e, Supporting Information). This can be attributed to the slightly lower pKa of the hydrazine group compared with the lysine side chain (8.10 vs. 10.54, respectively). Therefore, at physiological pH, ELP-HYD has fewer polar residues compared with ELP, resulting in a lower Tt. This is consistent with previous work demonstrating that less hydrophilic amino acids in the Xaa position results in ELP with lower Tt. [34, 52]
Hyaluronic acid (HA) is a linear polysaccharide found in native extracellular matrix (ECM) throughout the body and is widely used in tissue engineering and regenerative medicine due to its role in angiogenesis, cell migration, and cartilage regeneration.[53, 54] Aldehyde groups were introduced to HA through reaction with sodium periodate, as previously reported. [29, 30] Fourier transform infrared spectroscopy (FTIR) was used to confirm the reaction, with a newly formed peak at 1733 cm−1 corresponding to the stretching vibration of the C=O bond in HA-ALD (Figure S2a, Supporting Information). HA with two different degrees of oxidation were prepared, 6% and 26% (Figure S2b, Supporting Information).
2.2. Dual-Crosslinked ELP-HA Hydrogels with Slower Erosion Rates
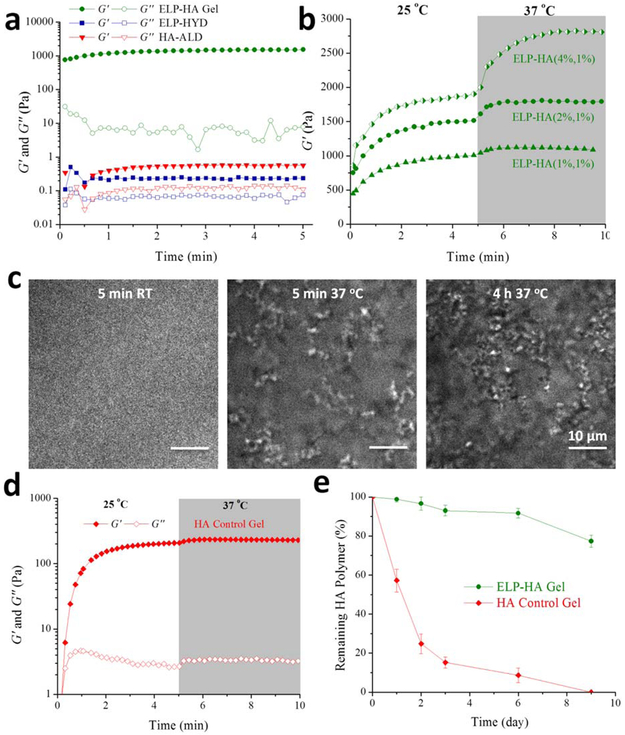
Upon mixing of ELP-HYD and HA-ALD at room temperature, an ELP-HA hydrogel is rapidly formed via dynamic covalent hydrazone linkages between the hydrazine and aldehyde groups (Figure 2a), which have recently been proven to be reversible and cytocompatible at physiological conditions. [28] While the individual components have very low storage moduli (G′), less than 1 Pa, the ELP-HA hydrogel reaches a G′ of ~ 1000 Pa less than 10 seconds after mixing. Since ELP-HYD has a Tt of 25.6 °C, which is very close to room temperature (Figure S1e, Supporting Information), to further confirm that the system forms a crosslinked network through DCC in the absence of ELP thermal aggregation, we conducted a gelation time sweep at a much lower temperature of 5 °C. As expected, the gelation occurs at a slightly slower rate at this temperature due to slower DCC kinetics, however the gel reaches a similar G’ plateau value of ~ 1 kPa as compared to gels crosslinked at room temperature (Figure S3a, Supporting Information). After heating to physiological temperature, the hydrogel is further strengthened by the thermal phase segregation of ELP (Figure 2b). As the concentration of ELP-HYD in the final hydrogel is increased from 1 wt% to 4 wt%, the thermoresponsive stiffening effect is enhanced (Figure 2b). The formation of a secondary physical network was further confirmed using Coherent anti-Stokes Raman scattering (CARS) microscopy (Figure 2c). For ELP-HA gels containing 4 wt% ELP-HYD and 1 wt% HA-ALD, no obvious protein-rich domains were observed at room temperature. Upon heating to 37 °C for 5 min, ELP thermal aggregates on the order of 1 μm in size were observed. After 4 hrs of incubation at 37 °C, the number of ELP aggregates increased. These large ELP-rich domains serve as crosslinking points to significantly stiffen the ELP-HA network measured as a ~ 50% increase in the storage modulus (Figure 2b). In contrast, for ELP-HA gels containing 2 wt% or 1 wt% ELP-HYD, no evidence of ELP thermal aggregation was detected within the spatial resolution of CARS microscopy (Figure S4, Supporting Information). This is consistent with the mechanical characterization data for these two gels that show only modest changes in hydrogel stiffness after increasing the temperature to 37 °C, ~ 17% and 7%, respectively (Figure 2b). We next performed an oscillatory rheometry measurement on a preformed ELP-HA hydrogel during a heating and cooling cycle to investigate if the material exhibits any hysteresis associated with ELP thermal crosslinking (Figure S3b, Supporting Information). When increasing the temperature from 5 °C to 60 °C, G’ increased more than 8-fold, indicating the presence of physical crosslinks through the formation of ELP aggregates. During the reverse cooling process, hysteresis was initially observed, and the thermal aggregates required lower temperatures to become disassembled. Interestingly, after the entire heating-cooling cycle, the G’ was reduced to about half of its initial value, potentially due to the breakage of hydrazone crosslinks during the thermal aggregate disassembly process that had not yet had sufficient time to be reformed.
Figure 2.
Characterization of double network ELP-HA rheology, network structure, and erosion kinetics. a) Oscillatory time sweep of ELP-HYD (4 wt%) and HA-ALD (2 wt%) before mixing and after mixing to form the ELP-HA (2 wt%, 1 wt%) hydrogel. Storage modulus (G’) shown with filled symbols and loss modulus (G’’) shown with empty symbols; tested at 25 °C. b) Oscillatory time sweep of ELP-HA hydrogels for 5 min at 25 °C and 5 min at 37 °C. c) Structure of protein-rich ELP thermal aggregates observed by CARS microscopy at various temperatures. d). Oscillatory time sweep of a control hydrazone-crosslinked HA hydrogel (3 wt%) without thermal crosslinking. e) Hydrogel erosion profiles for ELP-HA (2 wt%, 1wt%) and control HA hydrogel (3 wt%) without thermal crosslinking.
While hydrogels formed through hydrazone crosslinking have been previously reported, [57, 58] the application of hydrazone-crosslinked hydrogels for injectable applications has been lacking. This is partially attributed to the fast hydrogel degradation that typically occurs when using this crosslinking mechanism,[59, 60] due to the reversible nature of hydrazone linkages. [61] Injectable hydrogels formed via purely dynamic crosslinks tend to have fast degradation rates that can lead to low localized cargo retention and decreased treatment efficiency. [15, 20, 21] To investigate whether the formation of secondary thermal crosslinking could increase the long-term integrity of hydrazone-crosslinked hydrogels, we compared the erosion rate of the double network ELP-HA gel to that of a control gel with only hydrazone crosslinking (Figure 2d-e). The control HA gel was formulated to consist of the same overall polymer concentration and the same stoichiometric ratio of aldehyde and hydrazine groups as the ELP-HA gel. Fitting of the erosion data to a zero-order kinetic model yielded an initial erosion rate for ELP-HA of 2.3% per day (over the full nine days of the experiment), while the erosion rate for the control HA gel was more than 10-fold greater at 28.7% per day (over the first three days of the experiment). By day 3, more than 80% of the control HA hydrogel without thermal crosslinking had eroded, while less than 5% of the double network ELP-HA had degraded. By day 9, the control HA hydrogel had completely disappeared, while more than 80% of the double network ELP-HA gel remained (Figure 2e).
While preventing uncontrolled erosion, the incorporation of protein-engineered ELP also allows for enzymatic degradation of the hydrogel, which is desirable for materials designed for implantation. [62] Oscillatory time sweep rheology of the gel in the presence of the protease elastase demonstrated that the material is degradable in a concentration-dependent manner, with G' at 10 minutes being 1600, 600, and 150 Pa in the presence of 0, 0.2, and 1 mg/ml elastase, respectively (Figure 2b and S5, Supporting Information). As described above, the mechanical stiffness of ELP-HA hydrogels can be tuned by adjusting the ELP concentration from 4 to 1 wt% (Figure 2b). In addition to ELP concentration, the extent of HA oxidation can also be used to easily manipulate the hydrogel stiffness by controlling the ratio of aldehyde to hydrazine reactive groups. While maintaining the ELP-HA hydrogel at a concentration of 1wt% ELP and 1 wt% HA, a modest 4-fold increase in HA oxidation resulted in a 20-fold decrease in G’ (Figure S6a, Supporting Information). This is due to an excess of aldehyde groups relative to hydrazines for the 26% HA-oxidized gel (estimated to be about 4:1) compared to an estimated 1:1 stoichiometry for the 6% HA-oxidized gel. When an excess of one reactive group is present, this leads to the formation of a gel network with multiple dangling ends and loops that do not effectively contribute to the network mechanics. [63] The reactive group stoichiometry can be explicitly controlled for each ELP-HA formulation. As a further example, for the gels with 4 wt% ELP-HYD and 1 wt% HA-ALD, increasing HA oxidation from 6% to 26% led to a stiffening of the gel network, as the aldehyde: hydrazine ratio changed from 1:4 to 1:1, respectively (Figure S6a, Supporting Information). Again, gels formed with an equimolar ratio of reactive groups are stiffer.
2.3. ELP-HA Injectability and Cell Protection
For all three ELP-HA formulations tested, G’ was stable across two decades of frequency range at 37 °C and 5% strain (Figure S6b, Supporting Information). We next evaluated the rheological behavior in response to variable strain at a constant frequency of 1 Hz. As expected, compared with the linear viscoelastic region (LVR) of ELP-PEG hydrogels formed through permanent covalent crosslinks, [64] the LVR of the adaptable ELP-HA hydrogel is much larger (8% vs. 100%, respectively) (Figure S6c, Supporting Information). The larger LVR is due to the dynamic nature of the hydrazone linkage, which allows the network to adapt to the applied strain. These data are consistent with work by Lu et al. on Dock-and-Lock hydrogels, which demonstrated that gels with adaptable crosslinks had much higher yield strains than gels with permanent covalent crosslinks.[20] In addition, ELP-HA hydrogels displayed shear-thinning properties at 37 °C (Figure 3a). The hydrogels exhibited a sharp yielding transition upon exposure to high shear rates (10 s−1) at physiological temperature, resulting in a low viscosity (~ 10 Pa·s) (Figure 3b). At low shear rates (0.1 s−1), the networks reversibly and rapidly (~ 6 s) recovered due to reformation of the dynamic covalent bonds. Rapid shear-thinning and self-healing kinetics are desirable for injectable applications to facilitate easy transplantation and good localization of cellular cargo at the desired site. [11]
Figure 3.
Shear-thinning and self-healing ELP-HA hydrogel can protect cells during syringe needle flow. a) ELP-HA hydrogel viscosity as a function of shear rate at 37 °C under continuous flow. b) Shear-thinning and self-healing behavior of ELP-HA hydrogel under alternating shear rates of 0.1 and 10 s −1 at 37 °C. c) Live/dead (green/red) confocal projection images of MSCs exposed to syringe needle flow (28-gauge, 0.5 inch, 4 mL/min) within PBS or ELP-HA hydrogel. d) Quantification of cell viability immediately post injection for multiple flow rates. Error bars represent mean ± SD. * p < 0.05, n=5. e & f) Confocal 3D reconstructions of live/dead MSCs (green/red) post injection (28-gauge, 0.5 inch, 4 mL/min) within PBS (e) and ELP-HA hydrogel (f).
We then tested the potential of the ELP-HA hydrogel to be used as an injectable cell carrier using human mesenchymal stem cells (MSCs) as a model cell type. Several studies have shown that MSC viability is reduced after exposure to syringe needle flow. [65, 66] Our previous work has demonstrated that some shear-thinning and self-healing hydrogels can provide cell protection from the damaging mechanical stresses experienced during injection.[67, 68] MSCs were encapsulated in the hydrogel and ejected through a 28-gauge needle at multiple flow rates of 0.5, 1, 2, and 4 mL/min. As flow rate increased, a larger percentage of cells delivered in phosphate-buffered saline (PBS) were found to have damaged cell membranes (Figure 3c, d). In contrast, all samples delivered with ELP-HA had similar cell viability (~ 90%) regardless of flow rate. Compared to delivery in PBS, the ELP-HA hydrogel provided significant protection to the stem cells at all flow rates (Figure 3d). Immediately after ejection at the highest rate (4 mL/min), 94.5% ± 3.4% of the cells within the hydrogel had intact membranes, while cells delivered with saline had a statistically lower cell viability of 79.7% ± 2.6% (Figure 3c, d). Post injection, cells delivered with ELP-HA were homogeneously distributed throughout the gel, while cells delivered with PBS settled to form a dense cell layer at the bottom (Figure 3e, f). These data indicate that the fast gel recovery rate of ELP-HA is sufficient to maintain cells in a 3D dispersion at the target site.
2.4. Maintenance of MSC Differentiation Potential
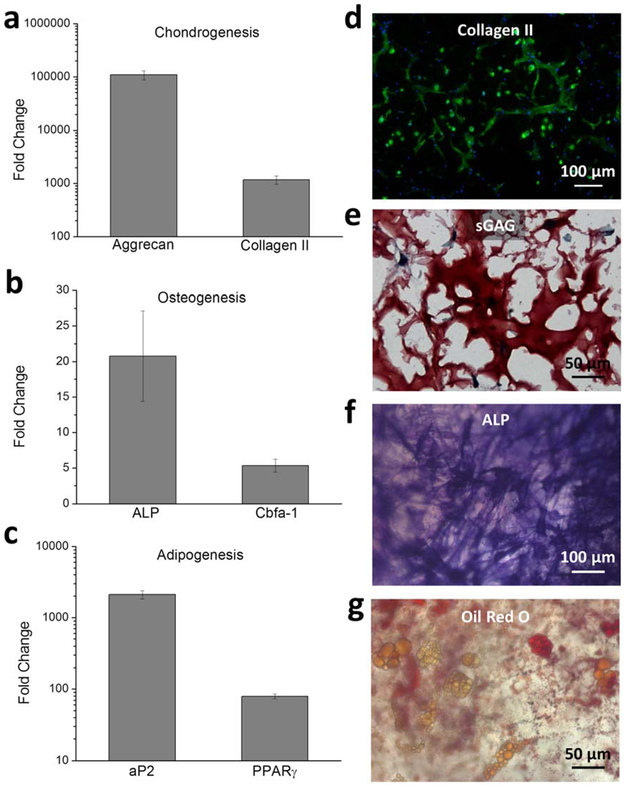
While it has been demonstrated that MSCs can have a reduced viability when exposed to syringe needle flow, previous work has also suggested no changes in MSC differentiation potential after injection. [66, 69] To evaluate the ability of the ELP-HA hydrogel to maintain long-term cell viability and differentiation, MSCs were encapsulated in ELP-HA, injected through a 28-gauge needle, and cultured for up to 3 weeks in chondrogenic, osteogenic, or adipogenic media. At day 7, cell viability was measured and gene expression was analyzed to quantify early markers of differentiation (Figure 4a-c). No significant difference in cell viability was found among the three culture conditions, and all were quantified to be > 90% after 1 week of in vitro culture after injection (Figure S7, Supporting Information). Aggrecan and collagen II are the major extracellular components of cartilage and were used as indicators of chondrogenesis;[70] alkaline phosphatase (ALP) and the osteoblast-specific transcription factor core binding factor α1 (Cbfa-1) were selected as makers of osteogenesis;[71] and fatty acid-binding protein (aP2) and its up-stream transcription factor peroxisome proliferator activated receptor-γ (PPARγ) were chosen as markers for adipogenesis.[71] All of the analyzed genes increased expression at least 5 fold over that at day 1, indicating that the stem cells maintained their potential to differentiate toward multiple lineages (Figure 4a-c). Furthermore, immunofluorescence staining within chondrogenic cultures of collagen showed that collagen II nodules were well distributed throughout the hydrogel network (Figure 4d) and importantly showed a higher density and quantity than collagen X (Figure S8, Supporting Information). In addition, the MSCs had produced their own cartilage-like ECM within the ELP-HA gel, with sulfated glycosaminoglycans (sGAG) forming intercellular, connected neocartilage structures (Figure 4e). These findings strongly suggest that the cells are able to go through chondrogenic differentiation after injection within ELP-HA hydrogels. For osteogenic and adipogenic cultures, positive staining for ALP activity and lipid accumulation, respectively, further proved that MSCs were able to follow osteogenic and adipogenic differentiation, respectively, after injection (Figure 4f & 4g). Taken together, these data demonstrate that our shear-thinning and self-healing ELP-HA hydrogels can protect stem cells during injection and maintain their differentiation capability into multiple lineages.
Figure 4.
MSCs maintain differentiation potential for multiple lineages after syringe needle delivery within ELP-HA hydrogel. a-c) Quantitative gene expression of chondrogenic markers aggrecan and collagen II (a), osteogenic markers ALP and Cbfa-1 (b), and adipogenic markers aP2 and PPARϒ (c) at day 7. Values are normalized to gene expression level at day 0. Error bars represent mean ± SD, n=3. d) Immunofluorescence staining for chondrogenic marker collagen II (green) and cell nuclei (blue) after 3-week culture in chondrogenic differentiation medium. e) Safranin-O staining for chondrogenic marker sGAG after 3-week culture in chondrogenic differentiation medium. f) Fast blue staining for osteogenic marker ALP after 2-week culture in osteogenic differentiation medium. g) Oil Red O staining for adipogenic marker of neutral lipid accumulation after 2-week culture in adipogenic differentiation medium.
3. Conclusion
In conclusion, we have developed a hydrogel system that harnesses the beneficial features of both dynamic covalent crosslinking to enable easy injectability and thermal crosslinking to enable prolonged scaffold stability for cell culture. Namely, the utilization of dynamic covalent crosslinking enables the hydrogel to be shear-thinning and to protect cells from the mechanically disruptive forces experienced during injection. Following this, hydrogel reinforcement is achieved through the thermal phase segregation of the multifunctional, engineered elastin-like protein. We further demonstrate that mechanical stiffness of the network can be tuned from 50 to 5000 Pa by simply altering the component concentration and the degree of functional group modification. Additionally, stem cells encapsulated within the hydrogel maintain high viability and differentiation potential post injection. The approach of combining dynamic covalent crosslinking and secondary crosslinking may have great potential for many materials applications, including the injectable delivery of regenerative stem cells.
4. Experimental Section
Elastin-like protein (ELP) expression and purification:
The design and synthesis of a modular recombinant ELP, containing bioactive domains and lysine residues to act as amine-reactive crosslinking sites, was previously reported. [72, 73] The amino acid sequences of ELP used in these experiments are shown in the Supporting Information, Figure S1a. ELP was expressed and purified using standard recombinant protein technology. Briefly, protein sequences were cloned into pET15b plasmids, expressed in Escherichia coli, strain BL21(DE3), and induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at an OD600 of 0.8 for ∼6 h. The harvested cell pellets were suspended, lysed by three freeze–thaw cycles, and purified by iterative inverse temperature-cycling as previously reported. [73] Protein molecular weight and purity were confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Purified ELP was dialyzed three times (10,000 MWCO, 36 h, 4 °C, deionized water) to desalt. The ELP was then lyophilized and stored at 4 °C until use.
Synthesis and characterization of ELP-HYD:
Hydrazine-modified ELP (ELP-HYD) was synthesized using a slightly modified procedure from the previously reported method. [56] HATU, 4-methylmorpholine, tri-Boc hydrazinoacetic acid, trifluoroacetic acid (TFA), anhydrous dimethylformamide (DMF), and dichloromethane (DCM) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tri-Boc hydrazinoacetic acid (0.149 g, 0.37 mmol, 2 equiv. per amine) was dissolved in anhydrous DMF (5 mL) and activated with HATU (0.145 g, 0.37 mmol, 2.0 equiv.) and 4-Methylmorpholine (102.2 μL, 0.925 mmol, 4.5 equiv.). The reaction was stirred for 5 minutes, and then ELP (MW: 37840 Da; 0.5 g, 0.185 mmol amine, dissolved in DMF) was added, and the reaction was allowed to proceed overnight at room temperature. The product was precipitated in ice-cold diethyl ether, dried, treated with a solution of 50:50 DCM:TFA ( with 2.5% tri-isopropylsilane added) for 4 hours to remove the Boc group. The resulting compound was precipitated in ether, dissolved in deionized water, dialyzed (10,000 MWCO) against deionized water for 2 days, and lyophilized.
To quantify the modification efficiency, samples were saved before the removal of Boc groups for 1H NMR spectroscopy. The spectrum was acquired on a Varian Inova 500 MHz NMR spectrometer using deuterated dimethyl sulfoxide as a solvent. The modification efficiency was determined by comparing the integration of methyl protons of the Boc group with the aromatic protons of tyrosine. Each ELP molecule has 14 amine groups for modification and 4 tyrosine groups.
Synthesis and characterization of HA-ALD:
Aldehyde-modified HA (HA-ALD) was synthesized using a slightly modified procedure from the previously reported method. [29] HA (0.4g, sodium salt, MW: 1500 −1800 kDa; Sigma-Aldrich) was dissolved in H2O (4 mg/mL). An aqueous solution of sodium periodate (Sigma-Aldrich; 216 mg and 648 mg for sample 1 and sample 2, respectively) was added dropwise, and the reaction was stirred overnight at room temperature in the dark to achieve differing levels of oxidation. Ethylene glycol (500 μL) was then added to inactivate any unreacted periodate for 1 h at room temperature. The product was dialyzed (10,000 MWCO) against deionized water for 2 days and lyophilized. An FTIR spectrometer (Vertex 70, Bruker Optics) was used to identify the aldehyde functional groups and confirm the successful oxidization of HA. Air was used as a background control, and a single measurement consisted of 32 scans with a resolution of 4 cm−1. The molecular weight of HA-ALD was characterized using the Nano-Series Zeta Sizer (Nano-ZS ZEN3600, Malvern Instruments, Worcestershire, United Kingdom) by dynamic light scattering (DLS) method as previously reported. [74] A refractive index increment (dn/dc) for hyaluronic acid of 0.165 mL/g was used in the measurement. Sample 1 and sample 2 were characterized to have molecular weights of 22.8 ± 4.52 kDa and 23.3 ± 7.16 kDa, respectively.
TNBS assay:
The degree of HA oxidation was quantified using a slightly modified procedure that was previously described. [30] HA-ALD (25 μL, 0.6 wt%) was mixed with tert-butyl carbazate (t-BC; 25 μL, 30 mM; Sigma-Aldrich) in 1% aqueous trichloroacetic acid to react for 24 h at room temperature. Aqueous 2, 4, 6 - trinitrobenzene sulfonic acid (TNBS; Thermo Scientific Inc.) solution (0.5 ml, 2%, 0.1 M sodium bicarbonate buffer, pH 8.5) was transferred into the tube to react with the excess t-BC for 2 h at 37 ˚C. The final mixture was diluted with 0.5 N hydrochloric acid and transferred into a 96-well plate. The absorbance at 340 nm was measured using a SpectraMax M2 microplate reader. A standard calibration curve from the aqueous t-BC solutions (5 −15 mM) was used to determine the amount of unreacted t-BC and further, to convert the result into dialdehyde content. All measurements were in triplicate.
Formation of ELP-HA Hydrogels:
Lyophilized ELP-HYD, and HA-ALD were solubilized in chilled phosphate buffered saline (PBS) (1X, pH 7.4) at a stock concentration of 4 wt% and 2 wt%, respectively. Air bubbles were removed by centrifugation, and the final solutions were kept on ice until use. By mixing ELP-HYD and HA-ALD stock solutions at a volume ratio of 1:1 at room temperature, the crosslinked hybrid ELP-HA (2 wt%, 1 wt%) hydrogel was formed. Stock concentrations were adjusted to make gels with different formulations.
Hydrogel Mechanical Characterization:
Mechanical testing was performed on a stress-controlled ARG2 rheometer (TA Instruments) using a 20-mm diameter, cone-on-plate geometry. Samples were allowed to gel in situ on the rheometer, and a humidity chamber was secured in place to prevent dehydration. To prove the formation of a first network in the absence of ELP thermal aggregation, time sweep was performed at an oscillatory stress of 4.74 Pa at 5 °C for 20 min. To explore the hysteresis behavior of the material during the assembly and disassembly of ELP thermal crosslinking, the hydrogel was left to gel at 5 °C for 4 h to ensure full gel formation followed by an oscillatory temperature ramp experiment at a heating/cooling rate of 0.5 °C/min for a temperature change between 5 °C and 60 °C. To enable full gel formation, time sweeps were performed at an oscillatory stress of 4.74 Pa at 25 °C for 5 min, followed by a second-round time sweep at 37 °C for 5 min. For time sweep measurement in the presence of elastase, elastase from porcine pancreas (Sigma-Aldrich) was first dissolved in HA-ALD, then mixed with ELP-HYD and gelled in situ on the rheometer. All further measurements were made at 37 °C. A strain sweep from 0.1 to 1000% was performed at an angular frequency of 1 Hz to test the linear viscoelastic region (LVR). Angular frequency sweeps were conducted from 0.1 to 10 Hz with constant 5% strain amplitude. Storage (G’) and loss (G’’) moduli at 1 Hz were selected from the frequency sweep. Shear-thinning and self-healing properties of the gel samples were characterized by measuring linear viscosity (η) under a time sweep mode at alternating low and high shear rates of 0.1 s−1 and 10 s−1, respectively, for 1 min each and a total of 6 min. All measurements were in triplicate.
Characterization with Coherent Anti-Stokes Raman Scattering (CARS) Microscopy:
In order to visualize the ELP aggregate formation within the ELP-HA (4 wt%, 1 wt%) hydrogel, maps of the carbon–hydrogen vibration at 2930 cm−1,[75] characteristic for proteins, were collected by CARS microscopy. The CARS microscope is described in detail elsewhere.[76] Briefly, a Nd:Vanadate laser (Picotrain, HighQ Lasers GmbH, Hohenems, Austria) generated two ps pulsed laser beams (532 and 1064 nm, 7 ps, 76 MHz), the 532 nm beam of which pumped an optical parametric oscillator (Levante Emerald OPO, Angewandte Physik & Elektronik GmbH, Berlin, Germany, 690–900 nm). The OPO was tuned to 811 nm in order to drive the carbon–hydrogen vibration at 2930 cm−1 by overlapping the pulses in time and space with those of the fundamental 1064 nm beam of the pump laser in the sample. The two excitation beams were focused onto the sample plane by an oil immersion objective (Nikon Plan Fluor, 40× NA 1.30) mounted in an inverted optical microscope (Eclipse TE2000-E with a C2 Confocal Microscope scanning head, Nikon). The near-infrared excitation beams assured deep penetration depth, and the label-free approach of CARS microscopy ascertained imaging of the true ELP aggregate distribution, unbiased by photodegradation and 3D diffusion properties of labeling molecules. A spatial resolution of ∼300 nm was achieved, as the emission of the CARS signal is limited to the high-intensity region of the focal volume. A single-photon counting detector (Becker & Hickl GmbH) was used to detect the CARS signal by simultaneously scanning the two excitation beams over the sample pixel-by-pixel. Dichroic mirrors and high optical-density filters were used to separate the CARS signal from the excitation beams before the detector.
Hydrogel erosion study:
ELP-HA (2wt%, 1 wt%) hydrogel was prepared as described in the previous section. A control hydrogel, consisting of 3 wt% HA-ALD, formed at the same stoichiometric ratio of hydrazine to aldehyde groups as the ELP-HA gel, was crosslinked using a small molecule crosslinker, adipic acid dihydrazide (ADH, Sigma-Aldrich). The control gel was formed by mixing the 4.5 wt% HA-ALD stock solution with 4.665 mg/mL ADH in PBS at a 2:1 volume ratio. 30 μL of the desired hydrogels were prepared in separate centrifuge tubes for the erosion test. Four replicates were prepared in each group. The gels were immersed in 1 mL PBS and incubated at 37 °C. 200 μL of the buffer was collected and replaced by fresh PBS at days 1, 2, 3, 6, and 9. Data collection was stopped on day 9 since the control hydrogel was completely degraded. The remaining ELP-HA hydrogels were degraded in 2 mg/mL hyaluronidase to allow determination of remaining hydrogel content for data normalization. HA erosion was quantified by uronic acid assay as previously reported. [77]
Cell culture:
Human adult mesenchymal stem cells (MSCs) were purchased (Lonza) at passage 2 and expanded for 4 passages in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, Carlsbad, CA) supplemented with 5 ng/mL basic fibroblast growth factor (bFGF), 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin (Gibco), and 0.1 mg/mL streptomycin (Gibco) at 37 °C in 5% CO2. Hydrogel-encapsulated cells were exposed to either chondrogenic, osteogenic, or adipogenic differentiation medium and were cultured for 21 days post inductions, and medium was changed three times per week. Chondrogenic medium consists of high-glucose DMEM, 100 nM dexamethasone (Sigma-Aldrich), 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich), 40 μg/mL proline (Sigma-Aldrich), 100 μg/mL sodium pyruvate (Gibco), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and ITS Premix (5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenious acid; BD Biosciences) supplemented with 10 ng/mL transforming growth factor beta-3 (TGF-β3; PeproTech, Rocky Hill, NJ). Osteogenic medium consists of high-glucose DMEM, 100 nM dexamethasone, 50 mg/mL ascorbic acid 2-phosphate (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Adipogenic medium consists of high-glucose DMEM, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μM dexamethasone, 10 μM indomethacin (Sigma-Aldrich), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 1 μg/mL insulin (Sigma-Aldrich).
In vitro cell injection and quantification of viability:
For in vitro cell injection studies, cells were first mixed with stock HA-ALD solutions at a concentration of 20,000 per μL before further mixing with ELP-HYD stock solution. The final mixing step was performed in the barrel of a 1-mL insulin syringe fitted with a 28-gauge needle. The mixture was allowed to gel for 5 min before injecting into a circular silicone mold (diameter = 4 mm, height = 2.5 mm) within a 24-well plate using a syringe pump (SP220I; World Precision Instruments) at a flow rate of 0.5 mL/min, 1 mL/min, 2 mL/min and 4 mL/min. Cell viabilities were determined using live/dead cytotoxicity kit (Invitrogen) on days 0and 7 post-injection (n = 5). Images were collected using a Leica confocal microscope by creating z-stacks of around 250-μm depth with 3.9-μm intervals between slices in the middle of the hydrogel and then compressing into a maximum projection image. Cell numbers were quantified using ImageJ at each time point.
Gene expression RT-PCR:
Total RNA was extracted from cell-hydrogel constructs (n = 3/group) after 7 days of culture using TRIzol (Invitrogen) and the RNeasy mini kit (Qiagen, Valencia, CA) as previously described. [78] Real-time polymerase chain reaction (PCR) was performed on an Applied Biosystems 7900 Real-Time PCR system using SYBR green master mix (Applied Biosystems, Carlsbad, CA) and primers listed in Supplementary Table S1. The relative expression levels of the genes of interest were determined using the comparative CT method, in which target gene expression was first normalized to that of the housekeeping gene encoding glyceraldehyde-3-phosphate dehydrogenase, then normalized by gene expression measured in the control group (day 1 MSCs).
Histological analysis and immunofluorescence staining:
To visualize the chondrogenesis extracellular matrix (ECM) production and distribution, immunofluorescence staining of collagen I, II, and X was performed after 21 days of culture. The cell-hydrogel samples were harvested and fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich) for 1 h at room temperature and then transferred to 30% sucrose solution (Sigma-Aldrich) overnight at 4 ˚C before snap freezing in optimal cutting temperature (O.C.T) according to standard cryopreservation techniques. Sections were incubated for 15 min at 37 ˚C for enzymatic antigen retrieval, and then incubated with blocking buffer containing 2% goat serum and 3% bovine serum albumin (BSA). Rabbit polyclonal antibody to collagen type I, II, and X (Abcam, Cambridge, MA) were diluted at 1:100 and added to the samples separately and incubated overnight at 4 ˚C. Secondary antibody was diluted at 1:200 (Alexa Fluor 488 goat anti-rabbit; Invitrogen) with Hoechst (2 μg/ml; Invitrogen), and sections were incubated with secondary antibody for 1 h at room temperature. Samples were mounted (Vectashield;Vector Laboratories, Burlingame, CA) and images were taken with a Zeiss fluorescence microscope.
sGAG histological staining and imaging:
Sections were washed and hydrated in distilled water to remove O.C.T and then stained with hematoxylin solution for 5 min and washed in distilled water. Sections were destained quickly in acid alcohol (1% glacial acetic acid in 70% ethanol, 2-3 dips) and washed with distilled water. Fast green solution was used to stain the sections for 3 min and rinsed quickly with 1% acetic acid solution within 10 s. Samples were then stained in 0.1% safranin O solution for 5 min before dehydrating and mounting for imaging.
Alkaline Phosphatase (ALP) staining:
Diazonium salt solution was prepared by dissolving 14mg of Fast Blue salt (Sigma-Aldrich) in 48 mL DI water at room temperature. Naphthol AS-MX Phosphate Alkaline solution (2 mL, Sigma-Aldrich) was added to the pre-made diazonium salt solution. Samples were fixed with 4% PFA for 3 min, and then rinsed with DI water before incubating in Alkaline-Dye mixture for 1 hr. Before imaging under brightfield microscope, samples were thoroughly rinsed with DI water to remove background color.
Oil Red O staining:
Oil Red O staining stock solution was made by weighing 300 mg of oil red O powder and adding to 100 mL of 99% isopropanol. The working solution was made on the same day of staining by mixing 3 parts (30 mL) of oil red O stock solution with 2 parts (20 mL) DI water and allowing to sit at room temperature for 10 min. Samples were harvested and incubated in 4% PFA for 30 min and rinsed in Dulbecco’s phosphate-buffered saline (DPBS) three times. Samples were rinsed with sterile water before adding 60% isopropanol for 5 min incubation. Then the oil red O working solution was added to the samples and incubated for 30 min before imaging under a brightfield microscope.
Statistical Analysis:
All data (modulus and cell viability) are represented as mean ± standard deviation. Statistical difference between samples was analyzed by one way ANOVA and Tukey post test. For all statistical tests, a threshold value of α = 0.05 was chosen, and a p-value at or below 0.05 indicated significance.
Supplementary Material
Acknowledgements
The authors acknowledge funding provided by National Institutes of Health (U19 AI116484-01 and R21 EB018407-02 to S.C.H. and R01 DE024772 to F.Y.), National Science Foundation (DMR 1508006 to S.C.H. and Career Award CBET-1351289 to F.Y.), California Institute for Regenerative Medicine (RT3-07948 to S.C.H. and RT3-07804 to F.Y.), Kodak Fellowship (H.W.), EU FP7 Marie Curie Innovative Training Network Grant 607842 (A.P. and A.E.) and VINNOVA VINNMER grant (A.E.), Stanford ChEM-H Institute (F.Y. and S.C.H.), Stanford Graduate Fellowship (D.Z.) and Stanford Interdisciplinary Graduate Fellowship (D.Z.). The authors acknowledge Dr. Christopher B. Rodell and Prof. Jason A. Burdick for generously sharing the uronic acid assay protocol. The authors acknowledge Brad Krajina for assistance in characterization of polymer molecular weight.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- [1].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Adv Mater 2006, 18, 1345. [Google Scholar]
- [2].Tibbitt MW, Anseth KS, Biotechnol Bioeng 2009, 103, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA, Adv Mater 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Drury JL, Mooney DJ, Biomaterials 2003, 24, 4337. [DOI] [PubMed] [Google Scholar]
- [5].Kretlow JD, Klouda L, Mikos AG, Adv Drug Deliver Rev 2007, 59, 263. [DOI] [PubMed] [Google Scholar]
- [6].Li YL, Rodrigues J, Tomas H, Chem Soc Rev 2012, 41, 2193. [DOI] [PubMed] [Google Scholar]
- [7].Yu L, Ding JD, Chem Soc Rev 2008, 37, 1473. [DOI] [PubMed] [Google Scholar]
- [8].Jeong B, Bae YH, Lee DS, Kim SW, Nature 1997, 388, 860. [DOI] [PubMed] [Google Scholar]
- [9].Mano JF, Adv Eng Mater 2008, 10, 515. [Google Scholar]
- [10].Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA, Science 1998, 281, 389. [DOI] [PubMed] [Google Scholar]
- [11].Guvendiren M, Lu HD, Burdick JA, Soft Matter 2012, 8, 260. [Google Scholar]
- [12].Minh KN, Lee DS, Macromol Biosci 2010, 10, 563. [DOI] [PubMed] [Google Scholar]
- [13].Tan HP, Marra KG, Materials 2010, 3, 1746. [Google Scholar]
- [14].Foo CTSWP, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC, P Natl Acad Sci USA 2009, 106, 22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, Burdick JA, Adv Funct Mater 2015, 25, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tseng TC, Tao L, Hsieh FY, Wei Y, Chiu IM, Hsu SH, Adv Mater 2015, 27, 3518. [DOI] [PubMed] [Google Scholar]
- [17].Lee F, Chung JE, Kurisawa M, Journal of Controlled Release 2009, 134, 186. [DOI] [PubMed] [Google Scholar]
- [18].Gupta D, Tator CH, Shoichet MS, Biomaterials 2006, 27, 2370. [DOI] [PubMed] [Google Scholar]
- [19].Wang HY, Heilshorn SC, Adv Mater 2015, 27, 3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu HD, Soranno DE, Rodell CB, Kim IL, Burdick JA, Advanced healthcare materials 2013, 2, 1028. [DOI] [PubMed] [Google Scholar]
- [21].Cai L, Dewi RE, Heilshorn SC, Adv Funct Mater 2015, 25, 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Glassman MJ, Chan J, Olsen BD, Adv Funct Mater 2013, 23, 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rowan SJ, Cantrill SJ, Cousins GR, Sanders JK, Stoddart JF, Angewandte Chemie 2002, 41, 898. [DOI] [PubMed] [Google Scholar]
- [24].Deng GH, Tang CM, Li FY, Jiang HF, Chen YM, Macromolecules 2010, 43, 1191. [Google Scholar]
- [25].Imato K, Nishihara M, Kanehara T, Amamoto Y, Takahara A, Otsuka H, Angew Chem Int Edit 2012, 51, 1138. [DOI] [PubMed] [Google Scholar]
- [26].Ying HZ, Zhang YF, Cheng JJ, Nat Commun 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wei Z, Yang JH, Liu ZQ, Xu F, Zhou JX, Zrinyi M, Osada Y, Chen YM, Adv Funct Mater 2015, 25, 1352. [Google Scholar]
- [28].McKinnon DD, Domaille DW, Cha JN, Anseth KS, Adv Mater 2014, 26, 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tan HP, Chu CR, Payne KA, Marra KG, Biomaterials 2009, 30, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen YC, Su WY, Yang SH, Gefen A, Lin FH, Acta biomaterialia 2013, 9, 5181. [DOI] [PubMed] [Google Scholar]
- [31].Tseng TC, Tao L, Hsieh FY, Wei Y, Chiu IM, Hsu SH, Adv Mater 2015. [DOI] [PubMed] [Google Scholar]
- [32].Lu HD, Charati MB, Kim IL, Burdick JA, Biomaterials 2012, 33, 2145. [DOI] [PubMed] [Google Scholar]
- [33].Cai L, Heilshorn SC, Acta biomaterialia 2014, 10, 1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].DiMarco RL, Heilshorn SC, Adv Mater 2012, 24, 3923. [DOI] [PubMed] [Google Scholar]
- [35].Straley KS, Heilshorn SC, Adv Mater 2009, 21, 4148. [Google Scholar]
- [36].Sinclair SM, Bhattacharyya J, McDaniel JR, Gooden DM, Gopalaswamy R, Chilkoti A, Setton LA, Journal of Controlled Release 2013, 171, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li LQ, Tong ZX, Jia XQ, Kiick KL, Soft Matter 2013, 9, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim Y, Renner JN, Liu JC, Biomater Sci-Uk 2014, 2, 1110. [DOI] [PubMed] [Google Scholar]
- [39].Gil ES, Mandal BB, Park SH, Marchant JK, Omenetto FG, Kaplan DL, Biomaterials 2010, 31, 8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lutolf MP, Hubbell JA, Nature biotechnology 2005, 23, 47. [DOI] [PubMed] [Google Scholar]
- [41].Burdick JA, Anseth KS, Biomaterials 2002, 23, 4315. [DOI] [PubMed] [Google Scholar]
- [42].Salinas CN, Anseth KS, Journal of tissue engineering and regenerative medicine 2008, 2, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Betre H, Setton LA, Meyer DE, Chilkoti A, Biomacromolecules 2002, 3, 910. [DOI] [PubMed] [Google Scholar]
- [44].Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A, J Am Chem Soc 2008, 130, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang Q, Xia XX, Huang WW, Lin YN, Xu QB, Kaplan DL, Adv Funct Mater 2014, 24, 4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Raucher D, Massodi I, Bidwell GL, Expert Opin Drug Del 2008, 5, 353. [DOI] [PubMed] [Google Scholar]
- [47].Liu JC, Heilshorn SC, Tirrell DA, Biomacromolecules 2004, 5, 497. [DOI] [PubMed] [Google Scholar]
- [48].Heilshorn SC, DiZio KA, Welsh ER, Tirrell DA, Biomaterials 2003, 24, 4245. [DOI] [PubMed] [Google Scholar]
- [49].Lim DW, Nettles DL, Setton LA, Chilkoti A, Biomacromolecules 2007, 8, 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chung C, Lampe KJ, Heilshorn SC, Biomacromolecules 2012, 13, 3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chilkoti A, Dreher MR, Meyer DE, Raucher D, Adv Drug Deliver Rev 2002, 54, 613. [DOI] [PubMed] [Google Scholar]
- [52].Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, Safavy A, J Am Chem Soc 1991, 113, 4346. [Google Scholar]
- [53].Burdick JA, Prestwich GD, Adv Mater 2011, 23, H41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia XQ, Soft Matter 2012, 8, 3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McKinnon DD, Domaille DW, Cha JN, Anseth KS, Chem Mater 2014, 26, 2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McKinnon DD, Domaille DW, Brown TE, Kyburz KA, Kiyotake E, Cha JN, Anseth KS, Soft Matter 2014, 10, 9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dahlmann J, Krause A, Moller L, Kensah G, Mowes M, Diekmann A, Martin U, Kirschning A, Gruh I, Drager G, Biomaterials 2013, 34, 940. [DOI] [PubMed] [Google Scholar]
- [58].Gurski LA, Jha AK, Zhang C, Jia XQ, Farach-Carson MC, Biomaterials 2010, 31, 4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hahn SK, Park JK, Tomimatsu T, Shimoboji T, Int J Biol Macromol 2007, 40, 374. [DOI] [PubMed] [Google Scholar]
- [60].Domingues RMA, Silva M, Gershovich P, Betta S, Babo P, Caridade SG, Mano JF, Motta A, Reis RL, Gomes ME, Bioconjugate Chem 2015, 26, 1571. [DOI] [PubMed] [Google Scholar]
- [61].Oommen OP, Wang SJ, Kisiel M, Sloff M, Hilborn J, Varghese OP, Adv Funct Mater 2013, 23, 1273. [Google Scholar]
- [62].Chilkoti A, Christensen T, MacKay JA, Curr Opin Chem Biol 2006, 10, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou HX, Woo J, Cok AM, Wang MZ, Olsen BD, Johnson JA, P Natl Acad Sci USA 2012, 109, 19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang H, Cai L, Paul A, Enejder A, Heilshorn SC, Biomacromolecules 2014, 15, 3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Agashi K, Chau DY, Shakesheff KM, Regenerative medicine 2009, 4, 49. [DOI] [PubMed] [Google Scholar]
- [66].Walker PA, Jimenez F, Gerber MH, Aroom KR, Shah SK, Harting MT, Gill BS, Savitz SI, Cox CS Jr., Tissue engineering. Part C, Methods 2010, 16, 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC, Tissue Eng Pt A 2012, 18, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cai L, Dewi RE, Heilshorn SC, Adv Funct Mater 2015, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Garvican ER, Cree S, Bull L, Smith RKW, Dudhia J, Stem Cell Res Ther 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Benoit DS, Schwartz MP, Durney AR, Anseth KS, Nat Mater 2008, 7, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Frith J, Genever P, Transfus Med Hemoth 2008, 35, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liu JC, Tirrell DA, Biomacromolecules 2008, 9, 2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Straley KS, Heilshorn SC, Soft Matter 2009, 5, 114. [Google Scholar]
- [74].Blumberg BS, Oster G, Science 1954, 120, 432. [DOI] [PubMed] [Google Scholar]
- [75].Benitez PL, Sweet JA, Fink H, Chennazhi KP, Nair SV, Enejder A, Heilshorn SC, Advanced healthcare materials 2013, 2, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Enejder A, Brackmann C, Svedberg F, Ieee J Sel Top Quant 2010, 16, 506. [Google Scholar]
- [77].Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R, Biomacromolecules 2005, 6, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F, Sci Rep-Uk 2013, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.