ABSTRACT
Periplasmic flagella are complex nanomachines responsible for distinctive morphology and motility of spirochetes. Although bacterial flagella have been extensively studied for several decades in the model systems Escherichia coli and Salmonella enterica, our understanding of periplasmic flagella in many disease-causing spirochetes remains incomplete. Recent advances, including molecular genetics, biochemistry, structural biology, and cryo-electron tomography, have greatly increased our understanding of structure and function of periplasmic flagella. In this chapter, we summarize some of the recent findings that provide new insights into the structure, assembly, and function of periplasmic flagella.
The flagellum is a major organelle for motility in many bacterial species. It confers locomotion and is often associated with virulence of bacterial pathogens. Flagella from different species share a conserved core but also exhibit profound variations in flagellar structure, flagellar number, and placement (1, 2), resulting in distinct flagella that appear to be adapted to the specific environments that the bacteria encounter. While many bacteria possess multiple peritrichous flagella, such as those found in Escherichia coli and Salmonella enterica, other bacteria, such as Vibrio spp. and Pseudomonas aeruginosa, normally have a single flagellum at one cell pole (Fig. 1). Spirochetes uniquely assemble flagella that are embedded in periplasmic space between their inner and outer membranes, thus called periplasmic flagella (3). Although the flagella of E. coli and Salmonella have been extensively studied for several decades, periplasmic flagella are less understood, despite their profound impact on the distinctive morphology and motility of spirochetes. In this chapter, many aspects of periplasmic flagella are discussed, with particular focus on their structure and assembly.
FIGURE 1.
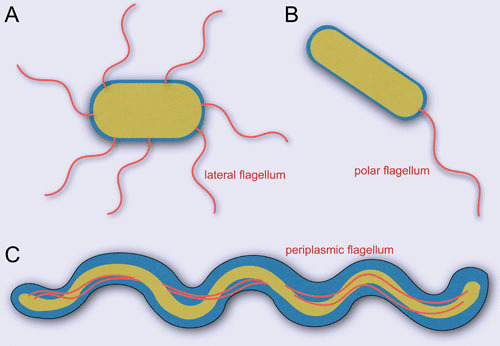
Distinctive placement of bacterial flagellum. (A) Bacteria with flagella distributed all over the cell (e.g., Escherichia coli) are peritrichous. (B) Monotrichous bacteria, such as Vibrio cholerae, Pseudomonas aeruginosa, and Caulobacter crescentus, have a single flagellum present at one end of the cell. (C) Spirochetes, including species of Borrelia, Treponema, and Leptospira, possess specialized flagella located within the periplasmic space. The rotation of the periplasmic flagella allows the bacterium to swim forward in a corkscrew-like motion.
SPIROCHETES ARE A DISTINCTIVE GROUP OF BACTERIA OF SIGNIFICANT IMPORTANCE IN HUMAN HEALTH
Spirochetes cause several major diseases in humans, such as Lyme disease (Borrelia burgdorferi), syphilis (Treponema pallidum), leptospirosis (Leptospira interrogans), and periodontitis (Treponema spp.). Lyme disease is the most commonly reported tick-borne illness in the United States, and the incidence is growing rapidly. The number of patients diagnosed with Lyme disease each year in the United States is approximately 300,000. The disease is caused by B. burgdorferi and related organisms, and it is transmitted to humans through the bite of infected Ixodes ticks (4). Syphilis is a common sexually transmitted disease in many areas of the world. Leptospirosis is the most common waterborne zoonosis worldwide.
MOTILITY IS ESSENTIAL FOR SPIROCHETES TO INFECT AND DISSEMINATE IN MAMMALIAN HOSTS
Spirochetal motility is unique, as the entire bacterium is involved in translocation without the involvement of external appendages. The motility is driven by periplasmic flagella, and rotation of the flagella causes a serpentine movement, allowing the organism to very efficiently bore its way through viscous media or tissue (3). To complete the host-vector life cycle, B. burgdorferi is able to adapt to divergent host environments and also evade the defense of its mammalian reservoir (4). Several studies provide direct evidence that the unique motility and chemotaxis of B. burgdorferi are essential for the establishment of infection in mammals and the completion of its enzootic cycle (5–8).
PERIPLASMIC FLAGELLA ARE NECESSARY FOR THE FLAT-WAVE MORPHOLOGY AND DISTINCTIVE MOTILITY OF B. BURGDORFERI
B. burgdorferi possesses 7 to 11 periplasmic flagella that are inserted at each cell pole and wrap around the cell cylinder to produce the spirochete’s distinctive flat-wave morphology. Periplasmic flagella are crucial not only for motility but also for the overall shape of B. burgdorferi, as mutant cells lacking flagella are nonmotile and exhibit a rod-shaped morphology (9). Similar to the external flagella found in the model organisms E. coli and S. enterica, periplasmic flagella are composed of the flagellar motor, the hook, and the filament. The flagellar motor is a rotary motor that anchors the flagellum to the inner membrane. The motors of the periplasmic flagella are noticeably larger than those of external flagella (Fig. 2). Importantly, the periplasmic flagellar motor possesses a spirochete-specific “collar” (Fig. 2) (10–13). The motor can be further divided into two parts: the rotor and the stator. The rotation of the motor is driven by the torque generated by the stator-rotor interaction, utilizing energy generated by the flow of protons through the stator channel. The rotor is composed of the MS ring, the C ring, and the rod. The MS ring is the base of the rotor, and it is formed by multiple copies of FliF. The C ring is located in the cytoplasm and is also known as the switch complex. It consists of the proteins FliG, FliM, and FliN and controls the direction of flagellar rotation. The rod serves as a drive shaft and consists of multiple different proteins (FlgB, FlgC, FlgF, and FlgG). The hook of the periplasmic flagellum is located in periplasmic space, in contrast to the externally localized hook in E. coli and S. enterica. A recent study indicates that the hook proteins are cross-linked by a covalent bond, an unusual property necessary for transmission of high rotational torque from the motor to the filament (14). The filament is the longest component of the periplasmic flagella. Multiple filaments arising from both poles form flat ribbons that wrap around the spirochete cell body in a right-handed fashion (15). The flagellar type III secretion system (fT3SS), which is embedded in the flagellar motor, is responsible for the transport and assembly of the protein components of the rod, the hook, and the filament (16).
FIGURE 2.
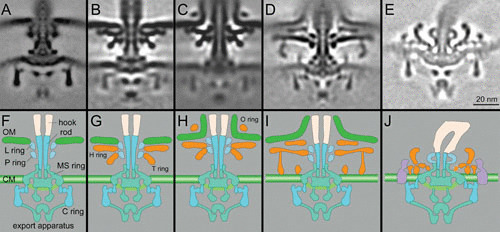
Comparison of motor structures from E. coli, Vibrio, H. pylori, and Borrelia. (A) A central section of an E. coli flagellar motor. (B) A central section from a nonsheathed Vibrio flagellar motor. (C) A central section from a sheathed Vibrio flagellar motor. (D) A central section from a sheathed flagellar motor of H. pylori. (E) A central section from a Borrelia flagellar motor. (F to J) Schematic models derived from the central sections shown in panels A to E, respectively. Adapted from prior publications (16, 50, 51), with permission.
CHARACTERIZATION OF THE UNIQUE PERIPLASMIC STRUCTURE OF SPIROCHETAL FLAGELLA
The periplasmic flagella possess a unique spirochete-specific collar that has not been found in other bacterial flagella reported to date (1, 2). The collar in the B. burgdorferi periplasmic flagellar motor is ∼71 nm in diameter and ∼24 nm in height, presumably composed of many different proteins. However, there is limited information regarding its structure, function, and protein components. Recently, a hypothetical membrane protein, FlbB, was identified as a candidate for involvement in collar assembly (10). In addition, the novel tetratricopeptide repeat protein BB0236 was also proposed to contribute to collar assembly (17). Mutants deficient in either FlbB or BB0236 are nonmotile, and their periplasmic flagella lack the collar, its associated proteins (including FliL), and the stator (Fig. 3). These findings provide direct evidence that the collar is indeed an important (as well as unique) component of periplasmic flagella (Fig. 2). Although additional unknown proteins are likely involved in collar assembly, it is evident that the periplasmic collar provides a static framework promoting the recruitment and stable association of stator units, which could, in turn, facilitate the higher torques to rotate the periplasmic flagellum. The rotation of the flagella within the confinements of the periplasm enables the spirochete to bore its way through complex, viscous environments in vertebrate and tick tissues.
FIGURE 3.
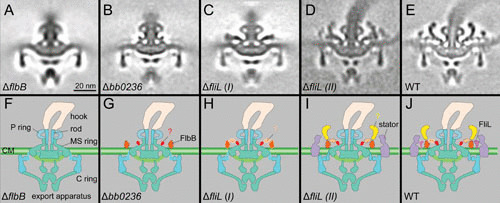
Characterization of the unique features in periplasmic flagella, as examined through mutational analysis. (A) Central section from a mutant lacking FlbB. (B) Central section from a mutant lacking BB0236. (C) Central section from a class average of a mutant lacking FliL. (D) Central section from another class average of a mutant lacking FliL. (E) A central section from wild-type flagellar motor. (F to J) Schematic models derived from panels A to E, respectively. Adapted from a prior publication (17), with permission.
STATOR-ROTOR INTERACTION
Powered by the electrochemical gradient across the cytoplasmic membrane, the flagellar motor can rotate the filament at high speed. It is believed that the flagellar rotation is mediated by the interaction between the cytoplasmic loop region of MotA and the C-terminal domain of FliG in the C ring. However, there is limited structural information on stator-rotor interaction in model systems E. coli and S. enterica, largely because the stator is poorly resolved due to its dynamic nature and low occupancy (18–20). In contrast, the in situ flagellar motor structures of B. burgdorferi and other spirochetes determined by cryo-electron tomography (cryo-ET) reveal more detailed information regarding the stators and their interactions with the C ring (1, 12, 13, 21–23). The presence of the collar in spirochetes is likely essential for the better visualization of the stator and its interaction with the rotor, because the collar provides a stable framework to recruit and stabilize the stators.
CheY-P BINDING AND FLAGELLAR SWITCHING OF ROTATIONAL DIRECTION
The flagellar motor in many bacterial species can rotate in both counterclockwise (CCW) and clockwise (CW) directions to achieve swimming towards attractants or away from repellents. The rotation direction is controlled by a sophisticated chemotactic system. In the signaling pathway, CheY is phosphorylated by CheA kinase; then the phosphorylated CheY binds to the FliM protein in the C ring and induces conformational changes that alter the stator-rotation interaction and cause switching (24). Studies with E. coli of the correlation between the CW rotation and the intracellular level of phosphorylated CheY in individual cells indicated that binding and switching are highly cooperative (25). The switching spreads from one or more nucleation points on the C ring, a phenomenon referred to as conformational spread (26). Recent experiments revealed that the flagellar motor can adapt to varied levels of phosphorylated CheY by increasing the content of FliM (27). Additional experiments suggested that it is not CheY-P binding but rather the direction of motor rotation that has the largest effect on remodeling of the FliM (28). It was suggested that there are ∼34 molecules of FliM in a motor with exclusively CW rotation and ∼44 molecules in a motor with CCW rotation. These E. coli studies also indicate that motors with even more FliM molecules may exist. It is unclear how the C ring can accommodate such a large change and if similar C-ring modifications also occur in spirochetes.
Because periplasmic flagellar motors are located at the two cell poles, it was hypothesized that spirochetal motors rotate asymmetrically at one end relative to the other during a run (29, 30). CheX is the only CheY-P phosphatase identified in the B. burgdorferi genome. A cheX mutant constantly flexes and is not able to run or reverse (31), while both cheA2 and cheY3 mutants constantly run in one direction (30, 32). A comparison of the motor structures from two different motions (flex and run) will likely shed new light upon the mechanisms underlying CheY-P binding and the switching of rotational direction.
FLAGELLAR ASSEMBLY
The bacterial flagellum is built from the inside out, from proximal to distal structures, in a temporally and spatially regulated fashion. Detailed insights into the flagellar assembly have been well established for E. coli and S. enterica (33, 34). In these organisms, multiple copies of FliF form the MS ring (35), which serves as the initial base for flagellar assembly, structural maturation, and function. The MS ring also serves as a scaffold for the assembly of the C ring. FliG proteins directly associate with the cytoplasmic face of the MS ring and form the FliG ring (36, 37). FliM and FliN proteins form a stable complex with a stoichiometry of 1:4 (38, 39). The FliM-FliN4 complex binds to the FliG ring to form the completed C ring. The export apparatus, which is assembled inside the MS ring and the C ring, is responsible for exporting flagellar axial protein components from the cytoplasm to the distal end of the nascent flagellar apparatus. FliE proteins are likely assembled first and form a junction between the MS ring and the rod to overcome their symmetry mismatch. Then multiple copies of FlgB, -C, -F, and -G form the rod, FlgI proteins form the P ring, and FliH proteins form the L ring. The FlgD cap assembles at the rod tip to support the assembly of the hook. After the hook assembly, the filament cap (FliD) is formed to support the assembly of the filament (40). In B. burgdorferi, a series of genetic mutations were introduced to arrest its assembly, and the assembling process was then imaged by cryo-ET (16).
FLAGELLAR EXPORT APPARATUS AND ITS EVOLUTIONARILY RELATED INJECTISOME
The fT3SS consists of five integral membrane proteins (FlhA, FlhB, FliP, FliQ, and FliR) and three soluble proteins (FliH, FliI, and FliJ) and is located at the center of the cytoplasmic face of the MS ring. The ATP complex promotes the export process by binding and delivering substrates to the export apparatus (41, 42). FliI is an ATPase and shows structural similarity with the α and β subunits of the F0F1-ATP synthase (43); it exhibits its full ATPase activity when it self-assembles into a homohexamer (44). FliH, FliI, and FliJ coordinately deliver a chaperone-substrate complex to the export gate by binding to the docking platform of the fT3SS for substrate export. FliP, FliQ, and FliR form an export gate complex with helical symmetry (45).
fT3SSs in different bacterial species are highly conserved. In addition, they are evolutionally related to virulence T3SSs (vT3SSs). The evolutionary relationship between the flagellum and the injectisome has garnered significant debate. The latest phylogenomic and comparative analyses of fT3SSs and vT3SSs suggest that the vT3SS derived from a flagellar ancestor. The loss of flagellum-specific genes led to an eventual loss in the motility function, but this system presumably kept the ability to secrete proteins (46).
The overall organization of the fT3SS machine in periplasmic flagella shares many features similar to those observed in the vT3SS machine (47–49) (Fig. 4). However, the ATPase complex of the periplasmic flagella is noticeably different from those observed in the injectisome (Fig. 4). There are 23 spokes and one hub in the ATPase complex of the B. burgdorferi periplasmic flagella. Only 6 spokes and one hub were observed in Salmonella injectisome, presumably optimizing for substrate recruitment and export. In contrast, the ATPase complex in the periplasmic flagella not only facilitates substrate recruitment and secretion but also supports the integrity of the C ring, which undergoes rotation and switches rotational direction between CW and CCW.
FIGURE 4.
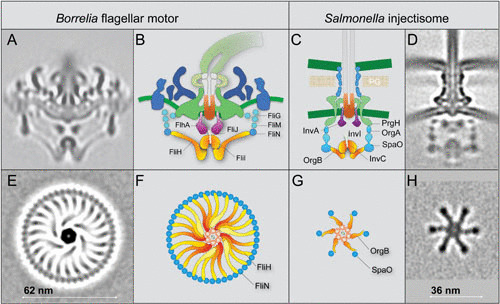
Comparison of the fT3SS from B. burgdorferi and the vT3SS from Salmonella. (A) A central section from the B. burgdorferi motor. (B) The fT3SS in the spirochete motor includes the ATPase complex (orange) and the export apparatus (purple) underneath the MS ring. (C and D) The vT3SS from the Salmonella injectisome is modeled in a similar color scheme. The difference between the two T3SSs is striking in a comparison of the cross sections of their ATPase complexes. Note that the C ring from the B. burgdorferi motor is a continuous ring with ∼46 copies of FliN tetramer. There are 23 visible FliH spokes (E and F). There are six pods in the Salmonella injectisome. Only six spokes of the FliH homolog OrgB connect the ATPase complex to the SpaO molecules that compose the pod of the injectisome. Adapted from a prior publication (52), with permission.
OUTLOOK AND PERSPECTIVE
Although the structure and functions of the bacterial flagellum have been studied for several decades, many important questions remain to be addressed. For example, how does the stator couple proton gradient to generate the torque? How does the C ring change its conformation to generate rotation or switch rotational direction? How does the proton gradient power the export apparatus and facilitate protein transport across inner membrane? Periplasmic flagella inspire additional questions: how do the periplasmic flagella coordinate their rotation from two cell poles, and how does the spirochete-specific collar assemble? Given that periplasmic flagella play critical roles in many bacterial pathogens, it will be important to understand not only the conserved structure and function among bacterial flagella but also the specific features that distinguish periplasmic flagella from others. Emerging techniques such as cryo-ET will be increasingly valuable to address many of these fundamental questions in periplasmic flagellum.
ACKNOWLEDGMENTS
The work in the Liu laboratory was supported by grants GM107629 and R01AI087946 from the National Institutes of Health.
Contributor Information
Yunjie Chang, Department of Microbial Pathogenesis, Yale University School of Medicine, New Haven, CT 06536; Microbial Sciences Institute University, West Haven, CT 06516.
Jun Liu, Department of Microbial Pathogenesis, Yale University School of Medicine, New Haven, CT 06536; Microbial Sciences Institute University, West Haven, CT 06516.
REFERENCES
- 1.Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Müller A, Dobro MJ, Jensen GJ. 2011. Structural diversity of bacterial flagellar motors. EMBO J 30:2972–2981. 10.1038/emboj.2011.186. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao X, Norris SJ, Liu J. 2014. Molecular architecture of the bacterial flagellar motor in cells. Biochemistry 53:4323–4333. 10.1021/bi500059y. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charon NW, Cockburn A, Li C, Liu J, Miller KA, Miller MR, Motaleb MA, Wolgemuth CW. 2012. The unique paradigm of spirochete motility and chemotaxis. Annu Rev Microbiol 66:349–370. 10.1146/annurev-micro-092611-150145. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. 10.1038/nrmicro2714. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan SZ, Sekar P, Zhao X, Manne A, Liu J, Wooten RM, Motaleb MA. 2015. Motor rotation is essential for the formation of the periplasmic flagellar ribbon, cellular morphology, and Borrelia burgdorferi persistence within Ixodes scapularis tick and murine hosts. Infect Immun 83:1765–1777. 10.1128/IAI.03097-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motaleb MA, Liu J, Wooten RM. 2015. Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Curr Opin Microbiol 28:106–113. 10.1016/j.mib.2015.09.006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Xu H, Zhang K, Liang FT. 2010. Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Mol Microbiol 75:1563–1576. 10.1111/j.1365-2958.2010.07078.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, Motaleb MA. 2013. Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun 81:2012–2021. 10.1128/IAI.01228-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, Charon NW. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A 97:10899–10904. 10.1073/pnas.200221797. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon KH, Zhao X, Manne A, Wang J, Yu Z, Liu J, Motaleb MA. 2016. Spirochetes flagellar collar protein FlbB has astounding effects in orientation of periplasmic flagella, bacterial shape, motility, and assembly of motors in Borrelia burgdorferi. Mol Microbiol 102:336–348. 10.1111/mmi.13463. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudryashev M, Cyrklaff M, Baumeister W, Simon MM, Wallich R, Frischknecht F. 2009. Comparative cryo-electron tomography of pathogenic Lyme disease spirochetes. Mol Microbiol 71:1415–1434. 10.1111/j.1365-2958.2009.06613.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, Norris SJ. 2009. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J Bacteriol 191:5026–5036. 10.1128/JB.00340-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy GE, Leadbetter JR, Jensen GJ. 2006. In situ structure of the complete Treponema primitia flagellar motor. Nature 442:1062–1064. 10.1038/nature05015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Miller KA, Bian J, James ME, Zhang S, Lynch MJ, Callery PS, Hettick JM, Cockburn A, Liu J, Li C, Crane BR, Charon NW. 2016. Spirochaete flagella hook proteins self-catalyse a lysinoalanine covalent crosslink for motility. Nat Microbiol 1:16134. 10.1038/nmicrobiol.2016.134. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, Rowe N. 2009. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol 191:600–607. 10.1128/JB.01288-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, James ME, Charon NW, Manson MD, Norris SJ, Li C, Liu J. 2013. Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc Natl Acad Sci U S A 110:14390–14395. 10.1073/pnas.1308306110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon KH, Zhao X, Xu H, Liu J, Motaleb MA. 2018. A tetratricopeptide repeat domain protein has profound effects on assembly of periplasmic flagella, morphology and motility of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 110:634–647. 10.1111/mmi.14121. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leake MC, Chandler JH, Wadhams GH, Bai F, Berry RM, Armitage JP. 2006. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature 443:355–358. 10.1038/nature05135. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka H, Wada T, Kojima S, Ishijima A, Homma M. 2009. Sodium-dependent dynamic assembly of membrane complexes in sodium-driven flagellar motors. Mol Microbiol 71:825–835. 10.1111/j.1365-2958.2008.06569.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Paulick A, Koerdt A, Lassak J, Huntley S, Wilms I, Narberhaus F, Thormann KM. 2009. Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol Microbiol 71:836–850. 10.1111/j.1365-2958.2008.06570.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, Norris SJ. 2010. Cellular architecture of Treponema pallidum: novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J Mol Biol 403:546–561. 10.1016/j.jmb.2010.09.020. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raddi G, Morado DR, Yan J, Haake DA, Yang XF, Liu J. 2012. Three-dimensional structures of pathogenic and saprophytic Leptospira species revealed by cryo-electron tomography. J Bacteriol 194:1299–1306. 10.1128/JB.06474-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudryashev M, Cyrklaff M, Wallich R, Baumeister W, Frischknecht F. 2010. Distinct in situ structures of the Borrelia flagellar motor. J Struct Biol 169:54–61. 10.1016/j.jsb.2009.08.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Welch M, Oosawa K, Aizawa S, Eisenbach M. 1993. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci U S A 90:8787–8791. 10.1073/pnas.90.19.8787. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cluzel P, Surette M, Leibler S. 2000. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science 287:1652–1655. 10.1126/science.287.5458.1652. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Bai F, Branch RW, Nicolau DV Jr, Pilizota T, Steel BC, Maini PK, Berry RM. 2010. Conformational spread as a mechanism for cooperativity in the bacterial flagellar switch. Science 327:685–689. 10.1126/science.1182105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Branch RW, Hosu BG, Berg HC. 2012. Adaptation at the output of the chemotaxis signalling pathway. Nature 484:233–236. 10.1038/nature10964. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lele PP, Branch RW, Nathan VSJ, Berg HC. 2012. Mechanism for adaptive remodeling of the bacterial flagellar switch. Proc Natl Acad Sci U S A 109:20018–20022. 10.1073/pnas.1212327109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charon NW, Goldstein SF. 2002. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu Rev Genet 36:47–73. 10.1146/annurev.genet.36.041602.134359. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Li C, Bakker RG, Motaleb MA, Sartakova ML, Cabello FC, Charon NW. 2002. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc Natl Acad Sci U S A 99:6169–6174. 10.1073/pnas.092010499. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motaleb MA, Miller MR, Li C, Bakker RG, Goldstein SF, Silversmith RE, Bourret RB, Charon NW. 2005. CheX is a phosphorylated CheY phosphatase essential for Borrelia burgdorferi chemotaxis. J Bacteriol 187:7963–7969. 10.1128/JB.187.23.7963-7969.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motaleb MA, Sultan SZ, Miller MR, Li C, Charon NW. 2011. CheY3 of Borrelia burgdorferi is the key response regulator essential for chemotaxis and forms a long-lived phosphorylated intermediate. J Bacteriol 193:3332–3341. 10.1128/JB.00362-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. 10.1038/nrmicro1887. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macnab RM. 2003. How bacteria assemble flagella. Annu Rev Microbiol 57:77–100. 10.1146/annurev.micro.57.030502.090832. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Yonekura K, Namba K. 2004. Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single-particle image analysis. J Mol Biol 337:105–113. 10.1016/j.jmb.2004.01.034. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Minamino T, Imada K, Kinoshita M, Nakamura S, Morimoto YV, Namba K. 2011. Structural insight into the rotational switching mechanism of the bacterial flagellar motor. PLoS Biol 9:e1000616. 10.1371/journal.pbio.1000616. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee LK, Ginsburg MA, Crovace C, Donohoe M, Stock D. 2010. Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching. Nature 466:996–1000. 10.1038/nature09300. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delalez NJ, Berry RM, Armitage JP. 2014. Stoichiometry and turnover of the bacterial flagellar switch protein FliN. mBio 5:e01216-14. 10.1128/mBio.01216-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown PN, Mathews MAA, Joss LA, Hill CP, Blair DF. 2005. Crystal structure of the flagellar rotor protein FliN from Thermotoga maritima. J Bacteriol 187:2890–2902. 10.1128/JB.187.8.2890-2902.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K, Qin Z, Chang Y, Liu J, Malkowski MG, Shipa S, Li L, Qiu W, Zhang J-R, Li C. 2019. Analysis of a flagellar filament cap mutant reveals that HtrA serine protease degrades unfolded flagellin protein in the periplasm of Borrelia burgdorferi. Mol Microbiol 111:1652–1670. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser GM, González-Pedrajo B, Tame JR, Macnab RM. 2003. Interactions of FliJ with the Salmonella type III flagellar export apparatus. J Bacteriol 185:5546–5554. 10.1128/JB.185.18.5546-5554.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamino T, Imada K. 2015. The bacterial flagellar motor and its structural diversity. Trends Microbiol 23:267–274. 10.1016/j.tim.2014.12.011. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Ibuki T, Imada K, Minamino T, Kato T, Miyata T, Namba K. 2011. Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases. Nat Struct Mol Biol 18:277–282. 10.1038/nsmb.1977. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Imada K, Minamino T, Tahara A, Namba K. 2007. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc Natl Acad Sci U S A 104:485–490. 10.1073/pnas.0608090104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhlen L, Abrusci P, Johnson S, Gault J, Deme J, Caesar J, Dietsche T, Mebrhatu MT, Ganief T, Macek B, Wagner S, Robinson CV, Lea SM. 2018. Structure of the core of the type III secretion system export apparatus. Nat Struct Mol Biol 25:583–590. 10.1038/s41594-018-0086-9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abby SS, Rocha EP. 2012. The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLoS Genet 8:e1002983. 10.1371/journal.pgen.1002983. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu B, Lara-Tejero M, Kong Q, Galan JE, Liu J. 2017. In situ molecular architecture of the Salmonella type III secretion machine. Cell 168:1065–1074.e1010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu B, Morado DR, Margolin W, Rohde JR, Arizmendi O, Picking WL, Picking WD, Liu J. 2015. Visualization of the type III secretion sorting platform of Shigella flexneri. Proc Natl Acad Sci U S A 112:1047–1052. 10.1073/pnas.1411610112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto A, Morimoto YV, Miyata T, Minamino T, Hughes KT, Kato T, Namba K. 2013. Common and distinct structural features of Salmonella injectisome and flagellar basal body. Sci Rep 3:3369. 10.1038/srep03369. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu S, Nishikino T, Hu B, Kojima S, Homma M, Liu J. 2017. Molecular architecture of the sheathed polar flagellum in Vibrio alginolyticus. Proc Natl Acad Sci U S A 114:10966–10971. 10.1073/pnas.1712489114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin Z, Lin WT, Zhu S, Franco AT, Liu J. 2017. Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo-electron tomography. J Bacteriol 199:e00695-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin Z, Tu J, Lin T, Norris SJ, Li C, Motaleb MA, Liu J. 2018. Cryo-electron tomography of periplasmic flagella in Borrelia burgdorferi reveals a distinct cytoplasmic ATPase complex. PLoS Biol 16:e3000050. 10.1371/journal.pbio.3000050. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


