Abstract
Objective
To examined outcomes for infants born with congenital diaphragmatic hernias (CDH), according to specific treatment center volume indicators.
Study design
A population-based retrospective cohort study was conducted involving neonatal intensive care units in California. Multivariable analysis was used to examine the outcomes of infants with CDH including mortality, total days on ventilation, and respiratory support at discharge. Significant covariables of interest included treatment center surgical and overall neonatal intensive care unit volumes.
Results
There were 728 infants in the overall CDH cohort, and 541 infants (74%) in the lower risk subcohort according to a severity-weighted congenital malformation score and never requiring extracorporeal membrane oxygenation. The overall cohort mortality was 28.3% (n = 206), and 19.8% (n = 107) for the subcohort. For the lower risk subcohort, the adjusted odds of mortality were significantly lower at treatment centers with higher CDH repair volume (OR, 0.41; 95% CI, 0.23–0.75; P = .003), ventilator days were significantly lower at centers with higher thoracic surgery volume (OR, 0.56; 9 5% CI, 0.33–0.95; P = .03), and respiratory support at discharge trended lower at centers with higher neonatal intensive care unit admission volumes (OR, 0.51; 9 5% CI, 0.26–1.02; P = .06).
Conclusions
Overall and surgery-specific institutional experience significantly contribute to optimized outcomes for infants with CDH. These data and follow-on studies may help inform the ongoing debate over the optimal care setting and relevant quality indicators for newborn infants with major surgical anomalies.
There is a well-documented trend toward decentralization of high-acuity neonatal care in the US through an increase in low- and mid-level neonatal intensive care unit (NICU) capacity and accreditation.1–3 As a result, more infants with congenital anomalies requiring neonatal surgical management are being treated at lower volume centers with less experience managing newborns with complex birth defects.3
Distinguishing which institutional factors at the hospital of birth and treatment center impact mortality and morbidity is critical to optimize care for infants with surgical congenital anomalies and acquired surgical disease. A recent pertinent study on necrotizing enterocolitis demonstrated that infant mortality was affected by both hospital level-of-care and annual volume of very low birth weight (VLBW) infants, which represent well-defined proxies for proficiency in handling complicated neonates.4,5 A follow-up study on gastroschisis by the authors corroborated the importance of birth into a higher level of care facility.6
Congenital diaphragmatic hernia (CDH) is associated with significant neonatal mortality (40%–60%).7,8 However, there is a sense of improving outcomes for CDH associated with significant pulmonary hypoplasia given the increased use of specialized perinatal care and ventilator management guidelines, judicious use of extracorporeal membrane oxygenation (ECMO), and optimized timing of surgery.9,10 Prenatal diagnosis of CDH can guide perinatal decisions, including location of birth or postnatal transfer to specialized centers; however, existing hospital performance data are confined to institutional studies demonstrating improved CDH outcomes.
Care for infants with CDH continues to occur outside expert centers, and the features that differentiate centers of excellence have not been sufficiently understood or quantified.9 There is a need to determine the hospital level factors associated with optimal CDH outcomes. The objective of this study was to examine the impact of hospital experience on CDH-related mortality and morbidity in California as a function of relevant volume indicators at treating centers.
Methods
After obtaining institutional review board approval, a population-based retrospective cohort study was performed using data collected by the California Perinatal Quality Care Collaborative (CPQCC) from 2008 to 2014. More than 90% of all NICUs in California submit detailed clinical data to the CPQCC, enabling high-integrity data capture for infants that meet eligibility criteria. The CPQCC data are collected in a prospective fashion for infants by use of an expanded version of the Vermont Oxford Network dataset, on infants that have any of the following: (1) surgery requiring anesthesia, (2) mechanical ventilation for more than 4 hours, (3) death, (4) acute transfer, and (5) birth weight <1500 g or gestational age of <32 weeks, as well as other criteria.11,12
Based on the criterion of mechanical ventilation, nearly every infant with CDH admitted to the NICU at participating hospitals would be included. The study cohort included all infants with CDH born into a CPQCC-enrolled facility or a co-located facility (ie, born into a separate hospital with an onsite satellite NICU) during the study period. The CPQCC birth defect code for CDH was used to locate the study cohort, and the CPQCC surgical code for CDH repair to identify the treatment hospital.11 The CPQCC infant mortality code was used to assign the treatment hospital for those who did not survive until surgical repair.
To account for hospital level of care, each NICU’s self-reported level of care was assessed on an annual basis, with designations corresponding to the period-appropriate American Academy of Pediatrics policy statement (2004).13 For surgical volumes, hospital-specific annual volumes were tal-lied for neonatal cardiac, thoracic, and abdominal surgeries, including a combination category for all complex neonatal surgeries. CDH repair volume was assigned using the relevant CPQCC surgical code. Treatment hospital total NICU, VLBW, and ECMO volumes were assigned according to total admissions for the associated calendar year. Each infant was associated with the American Academy of Pediatrics level of care and volumes at the treatment hospital.13
The dataset was indexed for each infant’s treatment hospital admission and transposed to include information on all previous hospital admissions and transfers. Infant mortality was determined as death at any NICU throughout the clinical course. Total days on ventilation were calculated by adding ventilation days across all hospitalizations if the initial hospitalization included more than one hospital. Ventilation was converted into a low and high category based on the cohort median, after infant deaths were excluded. To represent each infant’s respiratory function at discharge, the need for respiratory monitoring or oxygen support at discharge was assessed using data recorded by CPQCC.
Infants were listed in order by increasing volume metric at their treatment hospital, separating the cohort into low-volume and high-volume groups that were roughly equal in size. This allowed the following: (1) examination of institutional volumes of different relative magnitudes (eg, a hospital’s total NICU volume significantly outnumbers its annual ECMO volume), (2) avoidance of potential selection bias in selecting arbitrary cutoff points, and (3) categorical comparison that most closely reflected the linear relationships. Linear regression was not used because results would be difficult to interpret and report and would need to incorporate nonparametric analysis because hospital volumes were non-normally distributed.
Infant and maternal characteristics were compared according to the annual volume measurements. The Fisher exact test was used for binary variables, the χ2 test for categorical variables, and the Wilcoxon rank-sum test for continuous variables with a non-normal distribution. Univariable and multivariable logistic regression were performed using hospital repair volumes as the independent variable and modeled for the following outcome variables: in-hospital mortality, total days on ventilation, and respiratory support at discharge. Covariates included for risk adjustment included sex, race, birth weight, estimated gestational age, and Apgar scores.
A series of covariables were considered using clinical judgment and 2 variable-selection algorithms to establish the best predictive model. These included mode of delivery, neonatal respiratory distress, delivery room intubation, use of ECMO, postnatal steroid use, surfactant use, and the presence of maternal or obstetric perinatal complications. When clinically relevant or statistically significant, the model included variables for outborn vs inborn, transfer from hospital of birth within 48 hours, and level of care at the treatment hospital. For example, infants transferred within 48 hours had a 1.75 times higher unadjusted odds of mortality, which was corrected for in the associated multivariable analysis.
To account for varying illness severities for infants with CDH, a subanalysis was performed for a standardized lower risk subcohort. Infants who received ECMO were removed, because the use of ECMO was found to be a statistical effect modifier requiring stratification into subgroups. The ECMO group was not large enough to power its own logistic regression, and these infants were likely more critically ill, requiring complex care and difficult to risk stratify. Additionally, infants were removed who had coinciding birth defects or genetic syndromes that would increase mortality risk. To do this, investigators used a severity-weighted congenital malformation score built by the CPQCC and Vermont Oxford Network using internal data on anomaly-specific mortality. This proprietary categorization assigns an higher value to diseases with increasing average mortality. For infants with genetic syndromes or multiple anomalies, the score of the most severe anomaly is assigned.4,6
Both cohort and subcohort predictor models were optimized for area under the curve and validated with the Homer and Lemeshow goodness-of-fit test for logistic regression. All statistical analyses were performed using SAS Enterprise Guide, version 7.1 (SAS Institute Inc, Cary, NC).
Results
Between 2008 and 2014, 728 infants with CDH were born and treated at 49 CPQCC NICUs in California. Infants with CDH were predominantly born full term (gestational age of >37 weeks; 78%) and normal birth weight (birth weight of >2500 g; 78%), and almost one-half were of Hispanic race (49%), reflecting the overall population demographics in the state of California. In total 429 infants (60%) were out-born; 313 infants (43%) were transferred acutely within 48 hours of birth, 463 infants (63%) were intubated in the delivery room, and 152 infants (21%) required ECMO during their hospitalization (Table I).
Table I.
Demographic information for infants with CDH for the entire cohort and the lower risk subcohort
| Patient characteristics | Entire CDH infant cohort (n = 728) | Lower risk CDH subcohort (n = 541) | P value* |
|---|---|---|---|
| Sex | .61 | ||
| Female | 303 (41.7) | 233 (43.1) | |
| Male | 424 (58.3) | 307 (56.9) | |
| Birth weight, g | .26 | ||
| <2000 | 64 (8.8) | 51 (9.4) | |
| 2000–2499 | 97 (13.3) | 75 (13.9) | |
| >2500 | 567 (77.9) | 415 (76.7) | |
| Gestational age, wk | .33 | ||
| <32 | 21 (2.9) | 18 (3.3) | |
| 32–36 | 139 (19.1) | 107 (19.8) | |
| >37 | 567 (78.0) | 415 (76.9) | |
| Delivery mode | .53 | ||
| Cesarean | 369 (50.7) | 264 (48.8) | |
| Vaginal | 359 (49.3) | 277 (51.2) | |
| Infant condition at birth | |||
| Apgar <7 at 1 min | 478 (67.4) | 326 (62.2) | .06 |
| Apgar <7 at 5 min | 287 (42.0) | 176 (35.1) | .01 |
| Respiratory distress | 74 (10.2) | 57 (10.6) | .85 |
| Maternal race | .34 | ||
| Black | 31 (4.3) | 22 (4.1) | |
| Hispanic | 354 (48.8) | 259 (48.0) | |
| White | 244 (33.6) | 185 (34.3) | |
| Asian | 61 (8.4) | 49 (9.1) | |
| Other | 36 (5.0) | 25 (4.6) | |
| Maternal age, y | .70 | ||
| <25 | 206 (28.5) | 147 (27.4) | |
| ≥25 | 518 (71.5) | 390 (72.6) | |
| Perinatal complications | |||
| Maternal hypertension | 56 (7.7) | 48 (8.9) | .92 |
| Maternal diabetes | 72 (9.9) | 57 (10.5) | .71 |
| Maternal chorioamnionitis | 13 (1.8) | 11 (2.0) | .84 |
| Any maternal complication | 343 (47.3) | 273 (50.6) | .26 |
| Any obstetric complications | 283 (39.0) | 217 (40.2) | .41 |
| Transfer information | |||
| Acute transfer <48 h from birth | 313 (43.0) | 219 (40.5) | .39 |
| Inborn | 299 (41.1) | 238 (44.0) | .30 |
| Outborn | 429 (58.9) | 303 (56.0) | |
| Born at a level IIIC center | 384 (52.8) | 295 (54.5) | .53 |
| Treated at a level IIIC center | 638 (87.6) | 465 (86.0) | .40 |
| Treatment information | |||
| Antenatal steroids | 99 (13.8) | 78 (14.6) | .68 |
| Postnatal steroids | 274 (37.6) | 159 (29.4) | c |
| Surfactant | 182 (25.0) | 117 (21.6) | .18 |
| Delivery room intubation | 463 (63.6) | 321 (59.3) | .13 |
| ECMO | 152 (20.9) | N/A | |
| Outcomes | |||
| Death before CDH repair | 120 (16.5) | 76 (14.0) | .24 |
| Death after acute transfer <48 h | 109 (15.0) | 69 (12.8) | .29 |
| Overall mortality | 206 (28.3) | 107 (19.8) | <.001 |
| Time on ventilation, d | 11 (6–21) | 10 (5.5–16) | |
| Total length of stay, d | 36 (22–70) | 31 (20–57.5) | |
| Pulse oximetry at discharge | 239 (33.1) | 134 (25.0) | <.01 |
| Supplemental O2 at discharge | 256 (35.4) | 146 (27.2) | <.01 |
Values are number (%) or median (IQR).
Fisher exact test was used for binary variables, the χ2 test for categorical variables, and the Wilcoxon rank-sum test for continuous variables with non-normal distributions.
Approximately one-half of the cohort (n = 384 [52.8%]) were born at level IIIC centers and most (n = 638 [87.6%]) were ultimately treated at level IIIC centers (using the 2004 American Academy of Pediatrics designation).13 Between 2008 and 2014, the majority of hospitals (29/49 [59.2%]) had treated ≤5 infants within the study period. Among all hospitals, the median annual volumes were 130 for all primary complex surgery, 37 for cardiac surgery, 23 for thoracic surgery, 55 for abdominal surgery, 7 for CDH repairs, 410 for CPQCC-eligible NICU admissions, 85 for VLBW admissions, and 8 for admissions for infants on ECMO.
Outcomes for the overall and standardized lower risk subcohort are shown in Table I. The overall CDH mortality was 28.3% (n = 206), 47% (n = 99) for the high-risk group, and 19.8% (n = 107) for the standardized lower risk subcohort (n = 541, 74.3%). Among the infant deaths, 120 (58.2%) overall and 76 (71.0%) lower risk subcohort infants never survived to surgery; 109 (52.9%) overall and 69 (64.5%) in the lower risk subcohort died after acute transfer within 48 hours of birth; and 82 (39.8%) overall received ECMO before death (82.8% of the high-risk cohort deaths). Outcomes are further broken down by high-volume and low-volume measurements in Table II. Descriptive information on deaths and survivals can be seen in Table III (available at www.jpeds.com).
Table II.
Unadjusted outcomes for infants with CDH, 2008–2014, by 8 different annual hospital volume indicators
| CDH (entire infant cohort; n = 728) | CDH (lower risk subcohort; n = 541) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Volume (median, IQR)* | Outcome | Low volume | High volume | P value | Volume (median, IQR)* | Outcome | Low volume | High volume | P value |
| All surgical volume (130, 79–155) | Mortality | 107 (28.7) | 99 (27.9) | .81 | All surgical volume (123, 63–146) | Mortality | 61 (22.3) | 46 (17.2) | .14 |
| Ventilation days | 115 (44.1) | 140 (56.0) | .007 | Ventilation days | 90 (43.3) | 111 (51.4) | .09 | ||
| O2 at discharge† | 110 (29.6) | 146 (41.5) | .001 | O2 at discharge | 67 (24.6) | 79 (29.8) | .18 | ||
| Cardiac surgery volume (37, 18–61) | Mortality | 109 (29.5) | 97 (27.0) | .45 | Cardiac surgery volume (38, 17–56) | Mortality | 55 (20.4) | 52 (19.2) | .73 |
| Ventilation days | 127 (50.0) | 128 (49.8) | .96 | Ventilation days | 100 (47.8) | 101 (47.0) | .86 | ||
| O2 at discharge | 123 (33.6) | 133 (37.3) | .31 | O2 at discharge | 63 (23.6) | 83 (30.7) | .06 | ||
| Thoracic surgery volume (23, 11.5–32) | Mortality | 112 (28.7) | 94 (27.8) | .79 | Thoracic surgery volume (22, 11–32) | Mortality | 62 (22.5) | 45 (16.9) | .10 |
| Ventilation days | 136 (50.0) | 119 (49.8) | .96 | Ventilation days | 98 (47.1) | 103 (47.7) | .91 | ||
| O2 at discharge | 112 (28.9) | 144 (42.9) | <.001 | O2 at discharge | 60 (21.9) | 86 (32.7) | .005 | ||
| Abdominal surgery volume (55, 37.5–73) | Mortality | 111 (29.4) | 95 (27.1) | .48 | Abdominal surgery volume (52, 35–71) | Mortality | 64 (23.3) | 43 (16.2) | .038 |
| Ventilation days | 116 (45.0) | 139 (54.9) | .024 | Ventilation days | 90 (44.3) | 111 (50.2) | .22 | ||
| O2 at discharge | 111 (29.6) | 145 (41.7) | .001 | O2 at discharge | 71 (26.0) | 75 (28.4) | .53 | ||
| CDH repair volume (7, 3–13) | Mortality | 122 (31.5) | 84 (24.6) | .039 | CDH repair volume (6, 2–13) | Mortality | 68 (25.1) | 39 (14.4) | .002 |
| Ventilation days | 122 (47.5) | 133 (52.4) | .27 | Ventilation days | 84 (42.9) | 117 (51.3) | .08 | ||
| O2 at discharge | 110 (28.6) | 146 (43.1) | <.001 | O2 at discharge | 59 (21.9) | 87 (32.6) | .005 | ||
| NICU volume (410, 277.5–556) | Mortality | 108 (29.5) | 98 (27.1) | .47 | NICU volume (413, 268–559) | Mortality | 56 (20.0) | 51 (19.5) | .89 |
| Ventilation days | 129 (51.2) | 126 (48.6) | .57 | Ventilation days | 104 (47.7) | 97 (47.1) | .89 | ||
| O2 at discharge | 124 (34.1) | 132 (36.8) | .45 | O2 at discharge | 72 (25.9) | 74 (28.6) | .49 | ||
| VLBW volume (85, 56–120) | Mortality | 102 (27.6) | 104 (29.0) | .69 | VLBW volume (87, 60–120) | Mortality | 51 (18.4) | 56 (21.2) | .41 |
| Ventilation days | 135 (51.3) | 120 (48.4) | .51 | Ventilation days | 109 (49.3) | 92 (45.3) | .41 | ||
| O2 at discharge | 127 (34.8) | 129 (36.0) | .73 | O2 at discharge | 66 (24.1) | 80 (30.4) | .10 | ||
| ECMO volume (8, 3–13) | Mortality | 104 (26.4) | 102 (30.5) | .22 | ECMO volume (8, 2–11) | Mortality | 68 (20.9) | 39 (18.1) | .44 |
| Ventilation days | 124 (43.8) | 131 (57.5) | .002 | Ventilation days | 108 (43.0) | 93 (53.8) | .030 | ||
| O2 at discharge | 114 (29.1) | 142 (42.9) | <.001 | O2 at discharge | 81 (24.9) | 65 (30.7) | .14 | ||
Infants with CDH were split into low and high-volume groups in relation to each median volume measurements (creating equal infant counts/bins).
O2 at discharge designates the outcome respiratory support at discharge, which includes home oxygen or home pulse oximetry (or other) monitoring.
Table III.
Descriptive information on infants who died and survivors among infants with CDH in California, 2008–2014
| Total CDH cohort (n = 728) | ||||
|---|---|---|---|---|
| Deaths (n = 206) | Survivors (n = 522) | |||
| Patient outcomes | Acutely transferred* | Note acutely transferred | Acutely transferred | Not acutely transferred |
| ECMO used† | 29 (26.6) | 53 (54.6) | 39 (7.5) | 31 (5.9) |
| ECMO never used | 80 (38.8) | 44 (21.4) | 165 (31.6) | 287 (55.0) |
| Survived to surgery | 30 (14.6) | 56 (27.2) | 194 (38.3) | 312 (61.7) |
| Died before surgery | 79 (38.3) | 41 (19.9) | N/A | N/A |
| Time survived from birth until death | ||||
| <48 h | 68 (33.0) | N/A | ||
| <3 wk | 73 (35.4) | N/A | ||
| >3 wk | 65 (31.6) | N/A | ||
| Birth episode length of stay, wk | ||||
| <4 | N/A | 205 (39.3) | ||
| <8 | N/A | 140 (26.8) | ||
| >8 | N/A | 177 (33.9) | ||
N/A, not applicable.
Values are number (%).
Acute transfer designates interhospital transfer within 48 hours of birth.
ECMO use at any time during the birth episode before death or discharge.
Figure 1 shows aOR and 95% CIs from the multivariable regression analysis for the entire cohort for mortality, longer time on ventilation, and discharge on home oxygen. The adjusted odds of mortality trended lower (P = .08) at treatment centers with higher CDH repair volumes. The overall odds of having longer time on ventilation were significantly lower (P = .02) at centers with higher thoracic surgery volumes. The odds of being discharged on oxygen were significantly lower at higher VLBW admission volumes (P = .006). Many of the higher volumes (thoracic, abdominal, CDH repair, ECMO volumes) were significantly associated with higher rates of being discharged on oxygen (P < .05 for all).
Figure 1.
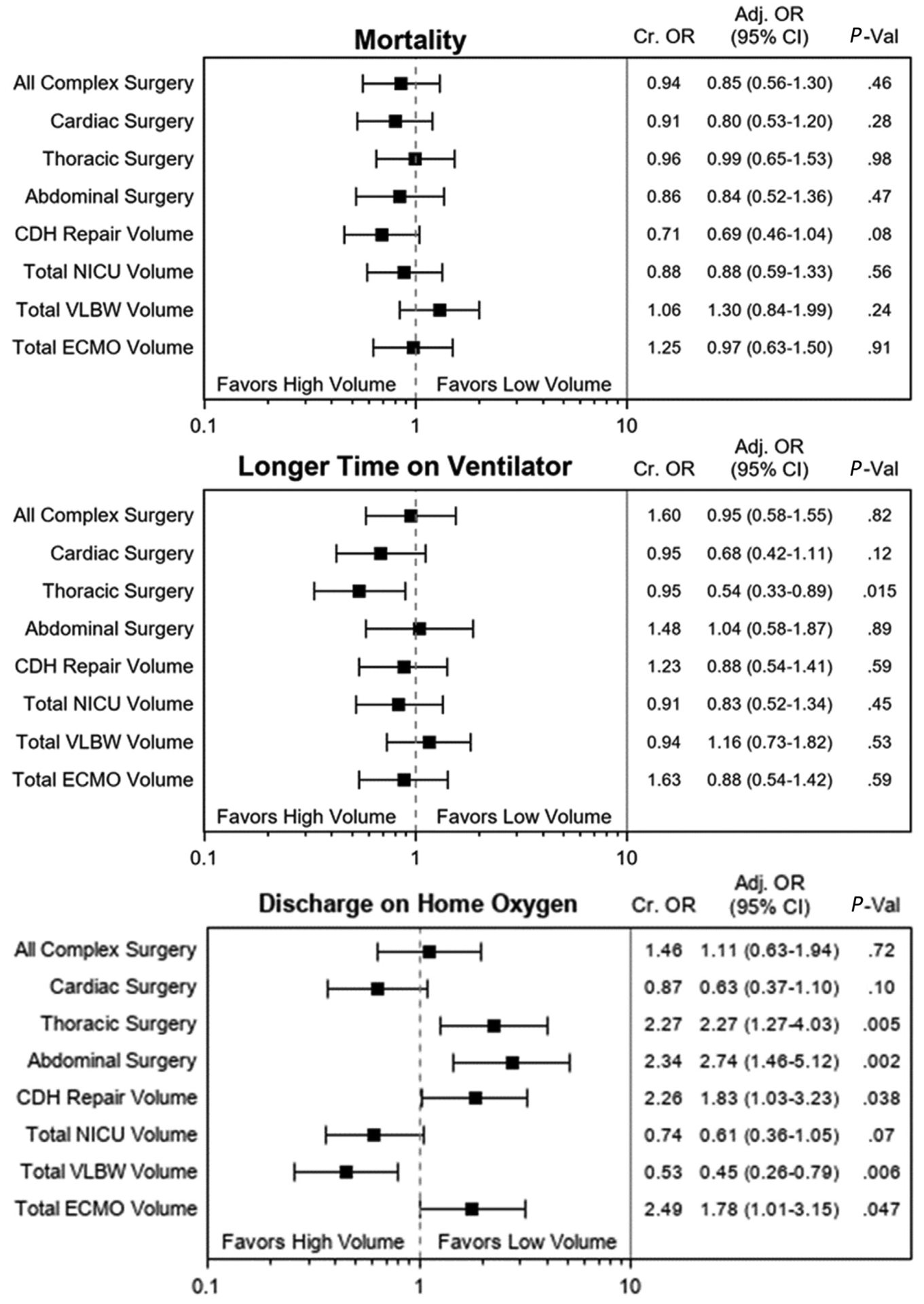
Multivariable logistic regression testing volume-outcome relationships for the entire cohort of infants with CDH. *High-volume and low-volume categories are compared with the median annual volumes including for all complex neonatal surgery (n = 130), cardiac surgery (n = 37), thoracic surgery (n = 23), abdominal surgery (n = 55), CDH repair volume (n = 7), overall NICU volume (n = 410), VLBW volume (n = 85), and ECMO volume (n = 8).
Figure 2 shows aORs and 95% CIs from the multivariable regression for the lower risk subcohort. The adjusted odds of mortality were significantly lower at treatment centers with higher CDH repair volumes (P = .003). Increased cardiac surgical volumes were significantly associated with decreased ventilation time for the subcohort (P = .04). Several treatment center volumes (thoracic, CDH repair, ECMO volumes) were significantly associated with higher rates of being discharged on oxygen (P < .05 for all).
Figure 2.
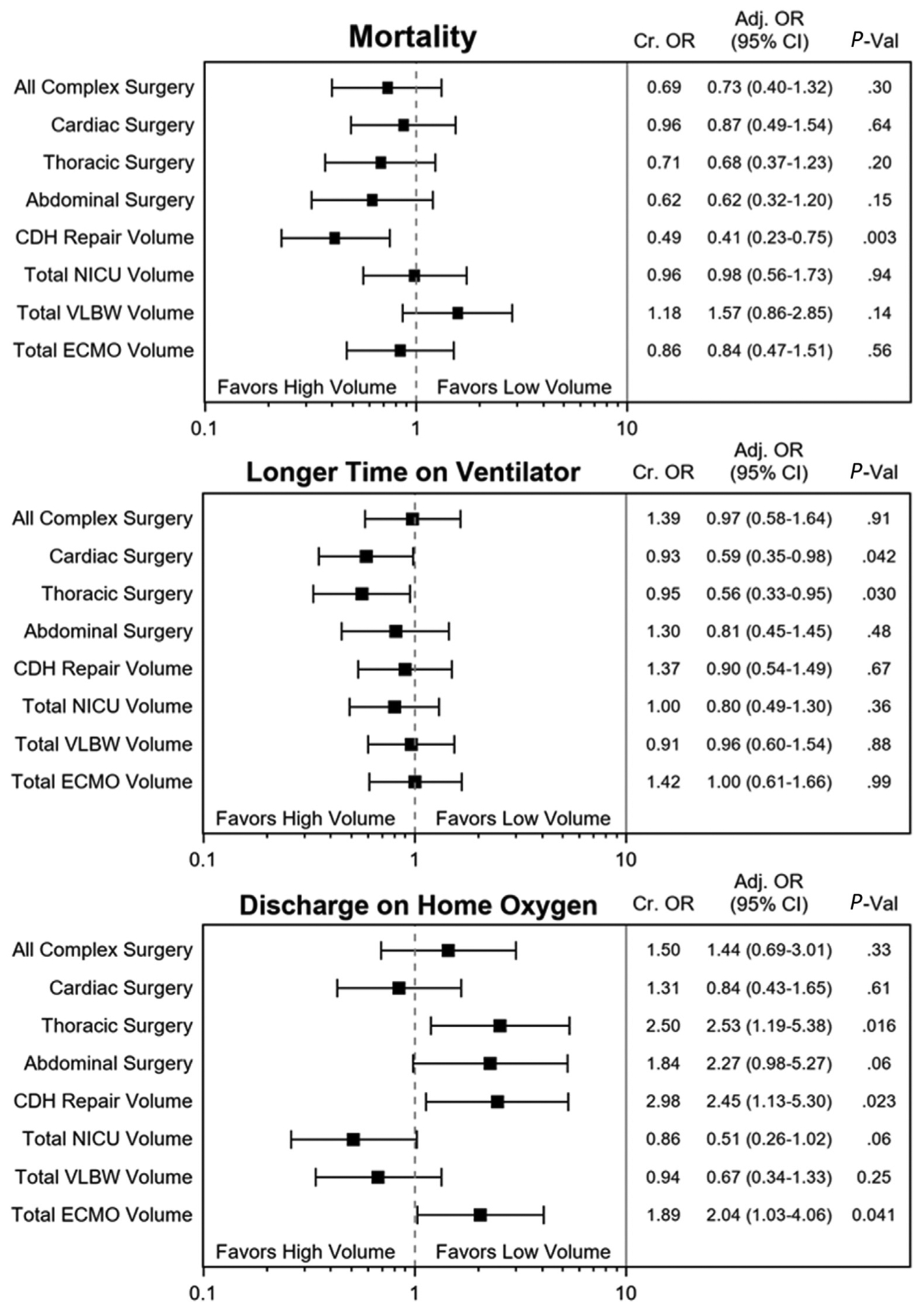
Multivariable logistic regression testing volume-outcome relationships for the risk-standardized subcohort of infants with CDH. *High-volume and low-volumes categories are compared with the median annual volume including for all complex neonatal surgery (n = 123), cardiac surgery (n = 38), thoracic surgery (n = 22), abdominal surgery (n = 52), CDH repair volume (n = 6), overall NICU volume (n = 413), VLBW volume (n = 87), and ECMO volume (n = 8).
We also examined volumes as continuous variables, and the relationship between CDH repair volume and decreased mortality was corroborated in both cohorts (+1 case = −3.7% mortality [P = .04] in the entire cohort; +1 case = −4.9%[C0]mortality [P = .03] in the subcohort), with increased repair volume trending toward association with lower ventilation times in the risk-standardized cohort (P = .14). For the subcohort, increasing ECMO volumes were significantly associated with decreased ventilation time (P = .05).
Discussion
The relationship between increased volume of patients treated and superior short-term outcomes is well-established in neonatal intensive care, as well as in complex adult surgery.14–20 However, these relationships are less well-understood for neonatal birth defects of moderate to high complexity, and the analysis of these is further complicated by their relative rarity. Previous studies have documented an increase in NICU capacity and deregionalization of complex neonatal care in California in the last decade, which has been accompanied by increased mortality and morbidity at these centers.21–26 This study sought to determine the impact of hospital and neonatal surgery-specific experience on the outcomes of complex birth defects requiring newborn surgery. Investigators examined patterns of care along with clinical outcomes for infants with CDH in California, categorized by volume indicators at the hospital of definitive treatment.
For specific outcomes, including mortality, time on mechanical ventilation, and respiratory support at discharge (which suggests more severe lung disease), significant volume-outcome relationships are reported. Most important, the unadjusted and adjusted odds of mortality were significantly decreased for infants treated at centers with higher CDH repair volumes, with infants >2 times less likely to die among the risk-standardized subcohort of patients with lower birth anomaly severity. Our observations provide new insights regarding (1) the current care patterns of less-complicated CDH repairs occurring at low volume neonatal surgery and anomaly centers, (2) data demonstrating the majority of infants with CDH are transferred for definitive treatment in an era of ubiquitous prenatal diagnosis, and (3) infants frequently die prior to surgery (n = 120 [16.5%]), even when controlling for degree of overall illness and complexity (n = 76 [14.0%]).
Annual thoracic surgical volume proved the most highly associated with decreased need for mechanical ventilation, in addition to showing an association with decreased mortality in the standardized lower risk subcohort. This perhaps reflects improved center experience in NICU pulmonary critical care, disease-specific cardiopulmonary physiology, and perioperative anesthesia for thoracic surgery. Cardiac surgery volume was also associated with shorter ventilation times and was the only surgical volume indicator with a favorable inverse association with needing respiratory support at discharge. It is plausible that expert centers offering high-volume neonatal thoracic and congenital heart surgery possess the most comprehensive neonatal physiologic management (ie, care pathways and packages, multidisciplinary care, disease-specific training, and high volume of complexity) that yields improved outcomes in newborns with CDH.
In the overall cohort, VLBW volume had the strongest relationship toward lower rates of discharge on oxygen, supporting previous studies’ findings that VLBW volume can serve as a proxy for experience in managing complex neonates.15,17 Overall NICU volume trended similarly, and this relationship remained in the subgroup analysis after the removal of the high-risk cohort. In contrast, the VLBW volume-outcome relationship diminished after removing the most complex infants that would benefit from greater proficiency in providing complex care. There is no obvious reason why the most complex infants with the worst lung function would be treated at centers with low overall NICU and VLBW volumes. Therefore, our best explanation for these volume-outcome relationship points to an increased overall experience in managing high-acuity infants through the birth episode until hospital discharge.
In California, although high-volume CDH centers treated more complex infants with improved survival, they did not have improved outcomes related to time on ventilation or discharge on oxygen. That high surgical volume centers did not perform better in these secondary outcomes likely reflects a need for better NICU support, but also imperfect correction for severity of pulmonary illness of those infants who survived. Taken as a whole, these relationships suggest that infants with complex birth defects benefit from disease-specific experience as well as a broader capture of body system-specific (ie, thoracic) surgical disease management.
Previous literature has described stark variability in perinatal healthcare quality among regionalized hospital systems treating VLBW infants; however, research is sparse describing such notable trends for noncardiac birth defects.27–32 In examining volume-outcome relationships for the care of a major neonatal surgical anomalies, CDH may be an ideal candidate given its anatomic and physiologic complexity, because optimal care would require expert care before, during, and after surgery. This study provides evidence that hospital experiences differentially impact particular healthcare objectives for neonatal surgical diseases. These data provide evidence against labeling 1 volume metric as representing comprehensive care surrounding a life-threatening anomaly like CDH. Our data suggest the need to develop improved performance indicators that reflect combined management, including medical and surgical expertise that may not be best captured by hospital volume alone.
Study limitations included difficulty in correcting for clinical severity of illness within a retrospective population-level analysis; thus, we included a subcohort analysis to remove the statistical effect modification seen for infants on ECMO or with multiple congenital anomalies. Findings in the risk-standardized subcohort featured similar but often strengthened associations, possibly owing to better outcomes after the removal of the sickest infants from higher volume centers. Additionally, volumes were evaluated according to a binary (high vs low) category to rigorously test the volume-outcome relationship without selection bias, after tertile analysis revealed no additional volume-outcome trends and quartile analysis was statistically underpowered. Any further characterization would require examining individual volume categories on their own, without the benefit of the more comprehensive survey that this study provides.
A strength of this study is the capture of nearly all infants cared for with CDH, because CPQCC collects data on 90% of NICUs in California. However, it is possible that patients with CDH may have been transferred to centers in which ECMO was performed in a pediatric or cardiac intensive care unit. Although the authors are not aware of such situations, this is a theoretical possibility. Finally, it has been pointed out that the ECMO rates (20.9%) reported in this study are lower than previously published rates and, considering this, that the mortality rate (28.3%) seems relatively high. Of note, 120 of our reported deaths (58.3 %) occurred before attempted surgical repair, and a majority (n = 76 [36.9% of deaths]) of those never received ECMO, potentially explaining the higher than commensurate mortality for relatively lower ECMO rates. Related concerns include hospitals potentially not providing timely access to ECMO (internal or external), or determining that an infant was not an ECMO candidate owing to severity of disease. Observing that 97 infants (47.1%) who died were not transferred within 48 hours after birth, these findings deserve prompt further investigation.
Previous studies on the role of the birthing center documented an ongoing trend toward deregionalized newborn care in California and the significant detrimental effect of this increased NICU capacity on the outcome of infants with necrotizing enterocolitis and gastroschisis.4,6 This study demonstrated that hospital and surgical volumes differentially impacted outcomes for infants with CDH. Most important, increased CDH surgical repair volume significantly improved infant survival. As this study suggests, and as ongoing verification/accreditation processes for Pediatric Surgical Centers continue, there continues to be an unmet need to define institutional factors that are associated with optimized outcomes for newborn surgical anomalies.33
Acknowledgments
Funded by the Stanford Medical Scholar Research Program. The authors declare no conflicts of interest.
Glossary
- CDH
Congenital diaphragmatic hernia
- CPQCC
California Perinatal Quality Care Collaborative
- ECMO
Extracorporeal membrane oxygenation
- NICU
Neonatal intensive care unit
- VLBW
Very low birth weight
Footnotes
This study was presented at the American Academy of Pediatrics National Conference & Exhibition, September 15, 2017, Chicago, IL.
References
- 1.Goodman DC, Fisher ES, Little GA, Stukel TA, Chang CH. Are neonatal intensive care resources located according to need? Regional variation in neonatologists, beds, and low birth weight newborns. Pediatrics 2001;108:426–31. [DOI] [PubMed] [Google Scholar]
- 2.Howell EM, Richardson D, Ginsburg P, Foot B. Deregionalization of neonatal intensive care in urban areas. Am J Public Health 2002;92:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberland CA, Phibbs CS, Baker LC. Effect of opening midlevel neonatal intensive care units on the location of low birth weight births in California. Pediatrics 2006;118:e1667–79. [DOI] [PubMed] [Google Scholar]
- 4.Kastenberg ZJ, Lee HC, Profit J, Gould JB, Sylvester KG. Effect of deregionalized care on mortality in very low-birth-weight infants with necrotizing enterocolitis. JAMA Pediatr 2015;169:26. [DOI] [PubMed] [Google Scholar]
- 5.Chung JH, Phibbs CS, Boscardin WJ, Kominski GF, Ortega AN, Gregory KD, et al. Examining the effect of hospital-level factors on mortality of very low birth weight infants using multilevel modeling. J Perinatol 2011;31:770–5. [DOI] [PubMed] [Google Scholar]
- 6.Apfeld JC, Kastenberg ZJ, Sylvester KG, Lee HC. The effect of level of care on gastroschisis outcomes. J Pediatr 2017;190:79–84.e1. [DOI] [PubMed] [Google Scholar]
- 7.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 2003;112:532–5. [DOI] [PubMed] [Google Scholar]
- 8.Zalla JM, Stoddard GJ, Yoder BA. Improved mortality rate for congenital diaphragmatic hernia in the modern era of management: 15year experience in a single institution. J Pediatr Surg 2015;50:524–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg 2003;38:729–32. [DOI] [PubMed] [Google Scholar]
- 10.Harting MT, Hollinger L, Tsao K, Putnam LR, Wilson JM, Hirschl RB, et al. Aggressive Surgical Management of Congenital Diaphragmatic Hernia: worth the effort? A multicenter, prospective, cohort study. Ann Surg 2018;267:977–82. [DOI] [PubMed] [Google Scholar]
- 11.California Perinatal Quality Care Collaborative (CPQCC). CPQCC Network Database Manual of Definitions For Infants Born in 2015, Version 14.0 September 23 Stanford, CA: California Perinatal Quality Care Collaborative; 2014. [Google Scholar]
- 12.Vermont Oxford Network. Vermont Oxford Network. www.vtoxford.org/about/about.aspx. Accessed January 1, 2018. [Google Scholar]
- 13.Stark AR, on behalf of the American Academy of Pediatric Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics 2004;114:1341–7. [DOI] [PubMed] [Google Scholar]
- 14.Phibbs CS. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA 1996;276:1054. [PubMed] [Google Scholar]
- 15.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med 2007;356:2165–75. [DOI] [PubMed] [Google Scholar]
- 16.Chung JH, Phibbs CS, Boscardin WJ, Kominski GF, Ortega AN, Needleman J. The effect of neonatal intensive care level and hospital volume on mortality of very low birth weight infants. Med Care 2010;48:635–44. [DOI] [PubMed] [Google Scholar]
- 17.Jensen EA, Lorch SA. Effects of a birth hospital’s neonatal intensive care unit level and annual volume of very low-birth-weight infant deliveries on morbidity and mortality. JAMA Pediatr 2015;169:e151906. [DOI] [PubMed] [Google Scholar]
- 18.Birkmeyer JD, Siewers AE, Finlayson EVA, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
- 19.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery. JAMA Surg 2014;149:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee on Perinatal Health. Toward improving the outcome of pregnancy: recommendations for the regional development of maternal and perinatal health services. White Plains, NY: March of Dimes Foundation; 1976. [Google Scholar]
- 22.Warner B, Musial MJ, Chenier T, Donovan E. The effect of birth hospital type on the outcome of very low birth weight infants. Pediatrics 2004;113:35–41. [DOI] [PubMed] [Google Scholar]
- 23.Cifuentes J, Bronstein J, Phibbs CS, Phibbs RH, Schmitt SK, Carlo WA. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics 2002;109:745–51. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Perinatal Health. Toward improving the outcome of pregnancy III: enhancing perinatal health through quality, safety and performance initiatives. White Plains, NY: March of Dimes Foundation; 2011. [Google Scholar]
- 25.Lorch SA, Myers S, Carr B. The regionalization of pediatric health care. Pediatrics 2010;126:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants. JAMA 2010;304:992. [DOI] [PubMed] [Google Scholar]
- 27.Blackmon LR, Barfield WD, Stark AR. Hospital neonatal services in the United States: variation in definitions, criteria, and regulatory status, 2008. J Perinatol 2009;29:788–94. [DOI] [PubMed] [Google Scholar]
- 28.Little GA. Variation, perinatal regionalization and total cohort accountability. J Perinatol 2009;29:777–8. [DOI] [PubMed] [Google Scholar]
- 29.Safavi A, Synnes AR, O’Brien K, Chiang M, Skarsgard ED, Chiu PPL. Multi-institutional follow-up of patients with congenital diaphragmatic hernia reveals severe disability and variations in practice. J Pediatr Surg 2012;47:836–41. [DOI] [PubMed] [Google Scholar]
- 30.Brennan A, Gauvreau K, Connor J, Almodovar M, DiNardo J, Banka P, et al. A method to account for variation in congenital heart surgery length of stay. Pediatr Crit Care Med 2017;18:550–60. [DOI] [PubMed] [Google Scholar]
- 31.Stagg H, Cameron BH, Ahmed N, Butler A, Jimenez-Rivera C, Yanchar NL, et al. Variability of diagnostic approach, surgical technique, and medical management for children with biliary atresia in Canada—is it time for standardization? J Pediatr Surg 2017;52:802–6. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez DO, Cooper JN, St Peter SD, Minneci PC, Deans KJ. Variability in outcomes after gastroschisis closure across U.S. children’s hospitals. J Pediatr Surg 2018;53:513–20. [DOI] [PubMed] [Google Scholar]
- 33.Optimal Resources for Children’s Surgical Care 2015 Optimal Resources for Children’s. Chicago, IL: Am Coll Surg Optim Resour Child Surg Care; 2015. p. 1–92. [Google Scholar]


