Abstract
Deformed wing virus (DWV) is a persistent pathogen of European honey bees and the major contributor to overwintering colony losses. The prevalence of DWV in honey bees has led to significant concerns about spillover of the virus to other pollinating species. Bumble bees are both a major group of wild and commercially-reared pollinators. Several studies have reported pathogen spillover of DWV from honey bees to bumble bees, but evidence of a sustained viral infection characterized by virus replication and accumulation has yet to be demonstrated. Here we investigate the infectivity and transmission of DWV in bumble bees using the buff-tailed bumble bee Bombus terrestris as a model. We apply a reverse genetics approach combined with controlled laboratory conditions to detect and monitor DWV infection. A novel reverse genetics system for three representative DWV variants, including the two master variants of DWV—type A and B—was used. Our results directly confirm DWV replication in bumble bees but also demonstrate striking resistance to infection by certain transmission routes. Bumble bees may support DWV replication but it is not clear how infection could occur under natural environmental conditions.
Subject terms: Viral pathogenesis, Viral reservoirs, Viral transmission
Introduction
Deformed wing virus (DWV) is a widely established pathogen of the European honey bee, Apis mellifera. In synergistic action with its vector—the parasitic mite Varroa destructor—it has had a devastating impact on the health of honey bee colonies globally1,2. As the primary managed insect pollinator, honey bees are of high ecological and economic value and contribute an estimated 30–50% of mobile pollination activity. This is carried out by seasonal transportation of honey bee hives between agricultural areas requiring pollination services3,4. For example, two-thirds of all colonies in the USA (~ 1.6 million hives) are transported to California in February/March for almond pollination5. Inevitably, transporting bees also transports their pathogens. This, coupled with the local pathogen density associated with ~ 50 000 bees in a single hive, has raised concerns about pathogen spillover from managed honey bees to other pollinators6. DWV was found as a frequent component of pollen pellets7 and is also present in bee faeces8, suggesting honey bee foragers and colonies could facilitate horizontal virus transmission to the wider pollinator community. Significantly, DWV RNA has been detected in many insects sharing the environment with managed honey bees, including Asian bee species, solitary bees, bumble bees, wasps, cockroaches and ants6,7,9–21. Due to their extended activity at lower temperatures (compared to honey bees) bumble bees are considered particularly important pollinators in temperate and subarctic climates22,23 and are also reared and managed for commercial-scale pollination23. As a consequence, the potentially negative impact of extensive honey bee management and failing pathogen control on co-located Bombus species has received significant attention.
Following a report that DWV was detected in symptomatic Bombus terrestris and Bombus pascuorum individuals with deformed wings9 there have been several studies of DWV prevalence in a wide range of Bombus species6,7,13,14,24–27. In Varroa-infested honey bee colonies DWV levels can exceed 1011 genome copies per bee28, with considerable potential for environmental contamination. DWV-positive Bombus sp. have been shown to correlate to areas with high DWV prevalence in Apis6,25–27. The majority of screening studies have used end-point polymerase chain reaction (PCR) for detecting DWV RNA in environmental bumble bee samples (reviewed in29). However, the near ubiquitous presence of managed hives, the honey bee—and consequently pathogen—density around hives, and the foraging range of Apis mean that DWV is likely widespread7. Although detection of the negative strand intermediate of replication is regarded as a marker of DWV replication, quantitative analysis of virus amplification in the infected tissues is required to unequivocally demonstrate infection and replication.
The name DWV is currently attributed to an evolving complex of closely related viruses, which includes DWV-A30, Kakugo virus31, Varroa destructor virus-1 (VDV-1; also referred to as DWV-B1,32,33) and a range of recombinants between DWV-A and -B34–37. All viruses exhibit at least 84% identity at the nucleotide level and 95% identity at the protein level34,37,38. The sensitivity of current diagnostic methods means DWV detection in environmental samples is regularly reported, with different DWV variants identified in Bombus6. Far less frequent are studies investigating potential routes of transmission from Apis to Bombus, or the subsequent replication of DWV in bumble bees. Due to the absence of a suitable cell line for in vitro propagation, laboratory-based assays have been limited to the application of field-sourced virus. Infectivity of DWV obtained from field honey bee samples was tested via inoculations of adult Bombus terrestris6. It was reported that a DWV complex containing both DWV-A and -B is infectious when fed at high concentrations—109 GE (genome equivalents) of virus per bee.
We have used a reverse genetic (RG) approach to generate near-clonal populations of genetically tagged DWV-A, -B and a B/A recombinant after transfection of honey bees with in vitro transcribed RNA. A similar system has recently been reported for DWV-A39–41. Using RG-derived DWV inocula we address the question of DWV pathogenesis and likely transmission routes in Bombus terrestris at both the individual and colony level. Using this strategy we provide direct evidence of DWV replication in bumble bees via virus feeding and injection. Importantly, adult Bombus terrestris appear resistant to productive infection by DWV orally and do not exhibit the developmental defects characteristic of DWV infection and replication in honey bees.
Results
Infectivity of DWV RNA and virus in injected honey bee and bumble bee pupae
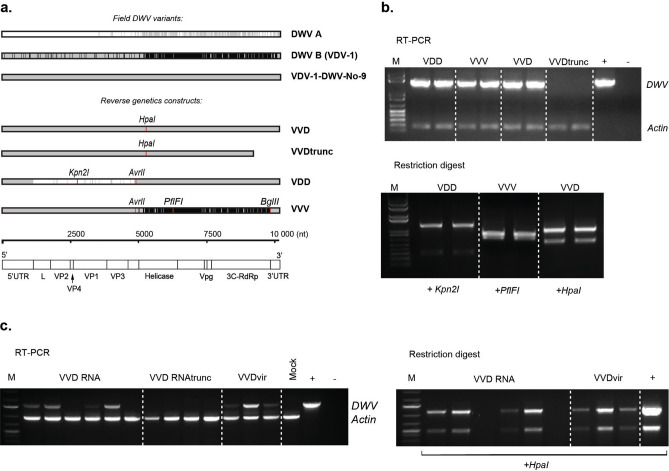
In order to test infectivity of DWV variants in controlled laboratory experiments we used RG systems for DWV-A and -B master variants and a recombinant B/A variant reported earlier by our group42. Full-length viral RNA was prepared in vitro and directly injected into honey bee pupae from which near-clonal infectious virus was recovered. Incorporation of synonymous mutations, which create new restriction sites in each RG DWV genome, allow unambiguous distinction from wild type virus genomes. The following nomenclature was used for the generated viruses: “VDD”—type A DWV (type A polyprotein-coding part only), “VVV”—type B, “VVD”—B/A recombinant (Fig. 1a). Full sequences of DWV cDNAs used in this study are available online (GenBank accession numbers: DWV-VDD—MT415949, DWV-VVD—MT415950, DWV-VVD_truncated—MT415951, DWV-VVV—MT415952)42.
Figure 1.
Reverse genetics (RG) system for three DWV variants. (a) Diagram showing identical regions between genomic RNA sequences of field DWV variants (DWV-A—NC_004830.2, DWV-B—GenBank AY251269.2, B/A recombinant VDV-1-DWV-No-9—GenBank HM067438.1) and three RG clones (VVV, VDD and VVD); DWV-A and DWV-B specific regions are shown in white and black respectively, regions identical to the VDV-1-DWV-No-9 recombinant are shaded in grey, location of restriction sites introduced into cDNA of each variant is indicated by red marks, DWV genomic RNA organization is presented below to help interpretation. (b) Detection of RG DWV RNA by end-point PCR in honey bee pupae injected with in vitro synthesized RNA transcripts: “VDD”, “VVV”, and “VVD”—PCR products from pupae injected with corresponding full-length RNA, “VVDtrunc”—PCR from pupae, injected with truncated VVD RNA, “+” and “−” —PCR controls, “M”—molecular weight DNA marker; restriction digest—verification of the RG origin of detected DWV by the digest of the PCR products at artificially introduced restriction sites. (c) Detection of RG DWV in bumble bee pupae injected with VVD RNA and with virus stock obtained from RNA-injected bumble bee pupae (“VVDvir”); “VVD RNAtrunc”—PCR from pupae injected with truncated RNA, “Mock”—PBS-injected pupae, “ + ”—positive PCR control for DWV, “M”—molecular weight DNA marker; RG origin of the PCR products for all DWV-positive samples was confirmed by digest with HpaI restriction enzyme, amplification of the actin mRNA product was used as an indicator of RNA integrity and loading control.
In temperate climates honey bee brood is only available for ~ 50% of the year and in vivo research is of necessity seasonal. Although the internal ribosome entry site of DWV retains partial activity in at least one cell line of non-honey bee origin (Lymanthria dispar LD652Y cells)43, attempted infection of those cells with DWV or transfection of in vitro-generated DWV RNA does not result in virus replication (Gusachenko, unpublished data). This impediment to DWV studies prompted us to investigate the recovery of clonal stocks of DWV in bumble bees (Bombus terrestris audax) injected with viral RNA. Commercially farmed bumble bee colonies are available year-round and, in our preliminary studies, are free of DWV RNA (data not shown). Four of six RNA-injected bumble bee pupae tested positive for DWV, while all honey bee pupae injected with full-length RNA were shown to be DWV-positive (Fig. 1b,c). The RG origin of DWV in injected samples was confirmed by restriction digest of PCR products using endonucleases specific for the introduced genetic tag (Fig. 1b,c—restriction digest). The remaining tissue from RNA-injected samples was used to prepare crude DWV stocks. Quantitative PCR (qPCR) analysis showed that the Apis-derived stocks contained between 107–109 GE of DWV/μl, while Bombus-derived inoculum contained 3.75 × 105 GE/μl. For comparison, DWV levels in mock-injected Apis pupae did not exceed 105 GE DWV/μg of RNA (representing the endogenous viral load) and Bombus pupae had no detectable DWV sequences in mock-inoculated samples.
DWV extracted from Bombus was infectious when re-inoculated by injection to Bombus and Apis pupae. We observed no differences in the infectivity of Apis- or Bombus-derived DWV (Fig. S1) and, at the genome level, no obvious signs of adaptation following comparison of the parental cDNA sequence with next generation sequencing (NGS) data from the second passage of Bombus-derived DWV (Fig. S2).
In honey bees, both DWV-A and -B are pathogenic when inoculated, though recent studies have produced contradictory results when comparing their relative virulence1,42,44–46. We therefore tested infectivity of VDD, VVV and VVD DWV in bumble bee brood and adults. As our primary interest was to investigate the potential for DWV spillover from infected honey bees we used DWV inocula prepared from RNA-injected honey bee pupae for all further experiments.
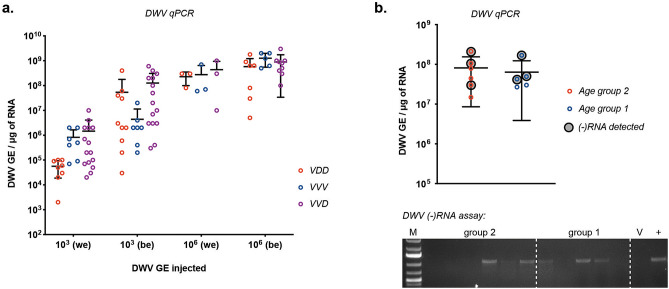
White-eyed (P0-P1 pupa stage according to the published classification on bumble bee pupae morphology47) or brown-eyed (P7-P8) bumble bee pupae were injected with 103 or 106 GE of each DWV variant, and virus levels quantified 48 h post-inoculation. In all cases we observed a 102–104 increase in total DWV load compared to the innocula, providing clear evidence for replication (Fig. 2a). More DWV accumulated in older pupae but this was only statistically significant for 106 GE of the VVV variant (Tukey’s multiple comparisons test, P = 0.03).
Figure 2.
Inoculation of bumble bee pupae and larvae with RG-DWV. (a) qPCR analysis of DWV accumulation in bumble bee pupae injected with VVV, VDD and VVD DWV. Pupae were injected at white-eyed (P0-P1)—“we”—and brown-eyed (P7-P8)—“be”—stages and analyzed 48 h post injection. Each value corresponds to an individual sample analyzed, black lines show mean ± SD, “GE” —genome equivalents. (b) Detection of DWV RNA in bumble bee larvae from two different age groups fed with 108 GE VVD DWV: qPCR analysis of DWV levels in individual larvae samples, black-circled values correspond to samples which produced a positive result in (−)RNA assay; each value corresponds to an individual sample analyzed, error bars show mean ± SD, “GE” —genome equivalents. DWV (-)RNA assay—PCR products obtained after strand-specific reverse transcription and run in 1% agarose gel, “ + ”—positive PCR control for DWV, “M”—molecular weight DNA marker, “V”—PCR from the DWV inoculum used for larvae feeding.
Bombus larvae can be infected with DWV per os
Since bumble bee pupae are not parasitised by Varroa naturally-infected pupae must acquire the virus by prior exposure of larvae. We therefore investigated virus infection after feeding DWV to 1st and 2nd instar Bombus larvae (age group 1 and 2 on Fig. 2b respectively). Each larva individually received a single dose of diet containing 108 GE of DWV on the first day of the experiment. DWV was detectable in all fed larvae on the 5th day post inoculation, although virus levels were comparable to the amount initially administered. When analysed ~ 50% of fed larvae had detectable levels of DWV negative strand RNA, absent from the inocula and indicative of virus replication (Fig. 2b).
Investigation of colony scale transmission of DWV and adult infection per os
In honey bees, horizontal transmission of DWV occurs per os in larvae or adults, or when Varroa feed on pupae and adults. Whilst we show here that DWV can replicate in Bombus pupae after direct injection, and in virus fed larvae, the route by which adult bumble bees could become infected remains unclear. We reasoned that two likely routes would be via direct oral exposure of adult Bombus to virus in the environment or indirectly following larval infection with virus carried by adult bees.
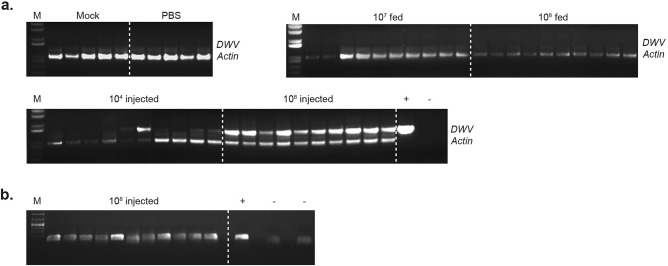
Groups of adult bumble bees received 107 or 108 DWV GE per bee via feeding with virus-supplemented sucrose solution. All control group bees remained viable during the experiment, and only one dead bee was found in each of the virus fed groups. Bees were analyzed 7 days after DWV feeding and none of the virus-fed samples tested positive for DWV in end-point PCR (Fig. 3). According to qPCR analysis of DWV-fed bees no sample contained greater than 105 GE DWV per 1 μg of total RNA. Average RNA yield per bee did not exceed 50 μg and therefore the level of virus detected was 100–200-fold lower than the amount administered. Hence, despite evidence that bumble bee larvae displayed susceptibility to DWV infection per os, feeding of adult bumble bees up to 108 GE DWV per bee did not lead to infection or detectable replication. Since bumble bees, like many host species, may exhibit differential age-related susceptibility to pathogens we additionally tested the oral infection of newly eclosed workers only, and a mixed group of randomly selected older bees collected from declining nests. However, in no cases were we able to demonstrate infection by the oral route (data not shown).
Figure 3.
Detection of DWV in adult bumble bees. (a) Detection of DWV RNA by end-point PCR in adult bumble bees after inoculation with VVD DWV via feeding or injections (10 samples from each group shown): “Mock” – non-treated bumble bees from the same colony, “PBS”—PBS-injected group (no virus), “107 and 108 fed”—bumble bees fed with sucrose solution containing 107 or 108 DWV GE of DWV per bee, “104 and 108 injected”—bumble bees injected with 104 or 108 DWV GE of DWV respectively. (b) Detection of the DWV (-)RNA strand in adult bumble bees injected with 108 DWV GE. “ + ” and “-”—positive and negative PCR controls, “M”—molecular weight DNA marker. Detection of the bumble bee actin RNA was used to assay the quality of extracted RNA. RG origin of the PCR products for all DWV-positive samples was confirmed by restriction enzyme digest.
We extended this study to investigate whole-nest inoculation with DWV. Three individual bumble bee nests were fed for 4–6 weeks with a sucrose solution supplemented with 2 × 108 GE/adult bee/day of VVD, VVV or VDD DWV. All brood (pooled egg samples, 30 larvae, 47 pupae) and 30 randomly selected adults from virus-exposed nests were screened for DWV using end-point PCR assay and showed no positive results (data not shown).
Direct inoculation of adult bumble bees
With no evidence that adult bumble bees could be infected when fed a DWV-supplemented diet, or that they were able to transfer virus to larvae, we performed direct virus injections of adult bumble bees to determine whether adults could support DWV replication. Two groups of adult bumble bees were intra-abdominally injected with 104 or 108 DWV GE per bee. Envisaging a possible impact of injection on viability, an additional group of bumble bees was injected with phosphate buffered saline (PBS) only. 20–30% of injected bees died before termination of the experiment in each group. The remaining bumble bees were screened for DWV RNA 8 days post-inoculation. End-point PCR analysis indicated that 40% and 100% were DWV positive in the 104-injected and 108-injected groups respectively (Fig. 3a), with qPCR analysis showing that the virus levels in these groups ranged between 2.7 × 104–1.4 × 107 and 4.8 × 106–2.1 × 108 GE/μg of total RNA respectively. Accumulation of DWV (-)RNA in DWV-positive samples was confirmed by strand-specific PCR assay (Fig. 3b). This demonstrates that DWV can replicate in adult Bombus terrestris after direct injection of 108 GE per bee. We therefore proceeded to investigate if there were any pathogenic consequences of virus infection and replication.
Pathogenicity of DWV in Bombus terrestris
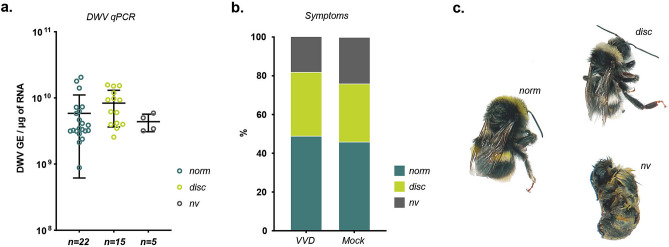
DWV produces characteristic pathogenicity in honey bees and similar symptoms have been reported in bumble bees9. We injected 43 white-eyed (P0–P1) bumble bee pupae with 106 GE of DWV and maintained them through development in an incubator. In parallel, a group of 37 similarly-aged pupae were injected with PBS. All fully-developed bumble bees could be classified into one of three groups: phenotypically normal, discolored with normal wings or non-viable, which did not eclose (Fig. 4). Analysis by qPCR demonstrated that all DWV-inoculated bumble bees contained high levels of virus (8.8 × 108–2.2 × 1010 GE/μg of RNA) with no significant differences in viral load between the three phenotypic groups (ANOVA, P = 0.21) (Fig. 4). DWV levels were 1–2 log10 greater than in pupae analysed 48 h post-inoculation (Fig. 2a) reflecting the additional time the virus had to replicate. This further supports the conclusion that pupae support productive infection with DWV following direct inoculation. Strikingly, none of the eclosed bumble bees showed any signs of the wing deformities that are characteristic of DWV infection of honey bees. Inspection of PBS-injected pupae showed that they could be separated into the same phenotypical groups; normal, discoloured and dead. There was no statistical difference between the proportions in each group of virus- or PBS-injected bumble bees (ANOVA, P > 0.999). Upon analysis, none of the mock inoculated group samples showed evidence of DWV infection. Injection of honey bee pupae with the same virus variant caused characteristic wing deformities in eclosed bees42.
Figure 4.
Morbidity of DWV in bumble bees. (a) qPCR analysis of DWV level in developed bumble bees injected with 106 GE of VVD DWV at white-eyed (P0-P1) pupa stage. Individual values for each sample are shown with dots, lines represent mean ± SD, “GE” —genome equivalents. (b) Percentage of visually normal (“norm”), discolored (“disc”) and non-viable (“nv”) bumble bees developed from pupae in VVD DWV-injected (n = 43) and PBS-injected (“Mock”, n = 37) groups. (c) Phenotype of bumble bees developed from pupae in the incubator.
Discussion
The term “pathogen spillover” describes the transmission of a pathogen from a reservoir host to a different species in a shared environment48. To achieve this, several conditions must be fulfilled; the reservoir host must be sufficiently abundant to guarantee exposure, pathogen prevalence must be high enough to ensure direct or environmental transmission, and the recipient host must be susceptible to infection via direct or indirect transmission49.
Managed honey bee colonies are near-ubiquitous in many environments, including both rural and urban locations. On agricultural crops that require commercial pollination very high colony densities are achieved through migratory beekeeping. The combination of colony movements following the introduction of Varroa destructor resulted in the near-global distribution of the ectoparasite, with the concomitant spread of a range of honey bee viruses that are detrimental to colony survival1. Most important of these viruses is DWV which likely accounts for the majority of overwintering colony losses50. DWV causes overt and lethal developmental defects after pupal inoculation and reduces the longevity of bees that do successfully emerge. Colonies that collapse due to high Varroa/DWV levels may be robbed-out by other insects including bumble bees, ants and wasps.
In addition to the cocktail of Varroa and DWV, honey bees are increasingly subject to stresses through a combination of limited dietary variation, repeated transportation to new pollination sites and exposure to agrochemicals, all of which are associated with increased susceptibility to pathogens and potential colony failure51. As a consequence, honey bees readily fulfill two of the requirements needed for pathogen spillover i.e. abundance in the environment and pathogen prevalence. In addition, due to the extensive foraging range of honey bees and the excretion of viable DWV in faeces8, a high density of hives ensures widespread environmental contamination.
Notwithstanding the likely exposure of other species to the pathogen-laden environment, spillover also requires susceptibility of the species exposed and pathogen infectivity via a relevant transmission route. We exploited commercially available colonies of Bombus terrestris to determine if and how they could support infection by DWV. The buff-tailed bumble bee is a relevant model system in which to explore pathogen spillover; it is naturally found in the same environment as honey bees6, and there are reports of DWV infection of wild-caught B. terrestris and several related species6,7,13,14,24–27.
We developed a RG system for the two prototype strains of DWV (type A and B) and a hybrid previously reported to predominate in Varroa-infested colonies42. The synthesized virus genomes contain silent restriction sites that unambiguously distinguish the three types and—since they are unique to the RG sequences—allow discrimination from endogenous DWV. Direct inoculation of honey bee pupae with in vitro generated RNA resulted in DWV replication (Fig. 2). Virus purified from these pupae caused symptomatic infection when reinoculated into honey bee pupae and accumulated to 1010 GE/μg of RNA42, a level similar to that observed in Varroa-exposed pupae28.
Using a similar strategy we demonstrated that bumble bee pupae could be infected when inoculated with in vitro generated DWV RNA. The resulting virus was purified, shown to be infectious for naïve bumble bee pupae when re-injected and deep sequencing of the virus population indicated no significant sequence changes from the originating cDNA (Fig. S2). Infected pupae reached up to ~ 109 GE of DWV/μg of RNA two days post-injection (Fig. 2a) and accumulation of the negative strand DWV RNA was confirmed by strand-specific qPCR (Fig. S3). Therefore DWV is infectious for both honey bee and bumble bee pupae and adaptive changes are unlikely to create a bottleneck in any potential transmission between the species.
Direct recovery and amplification of DWV in bumble bees offers advantages to virologists and entomologists attempting to determine the significance of the limited differences between the reported strains. Since essentially all honey bee pupae are infected with DWV, perhaps with the exclusion of those from Australia52, it is impossible to obtain truly clonal virus preparations. By recovering virus after RNA inoculation in bumble bees pure populations of DWV strains can be prepared for subsequent studies of virus pathogenesis and evolution.
Since Varroa does not parasitise bumble bee pupae it is difficult to rationalise direct injection as a potential transmission route. Therefore, considering robbing by bumble bees of collapsing honey bee colonies and the likely widespread DWV contamination in environments with large numbers of honey bees, we reasoned that oral transmission was a more likely route for virus acquisition. We therefore investigated oral susceptibility of larvae and adult bumble bees, fed directly or by extended exposure of bumble bee nests to virus-supplemented diet.
Direct feeding with 108 GE of DWV enabled the detection of the negative strand of DWV, absent from the input virus preparation and indicative of virus replication, in ~ 50% of larvae (Fig. 2). In contrast, DWV fed adult bumble bees, or larvae and pupae harvested from nests supplemented with diet containing 2 × 108 GE of DWV per bee, failed to provide any evidence for virus acquisition and transmission per os. Therefore, whilst larvae exhibit susceptibility to infection by orally acquired DWV, it is clear that the threshold for infection may be high and that it was not achieved with continuous feeding by adult bees with high levels of input virus. Further studies will be required to determine whether this was due to virus inactivation after ingestion by nurse bees, viable virus not being fed to the larvae, or some other undefined cause.
Although we found no evidence for oral infection of adult bumble bees with DWV, we were able to demonstrate the presence of DWV in adult Bombus after direct virus injections. Adult bumble bees are able to support replication of DWV though, as with pupal inoculation with RNA, there may be a threshold (exceeded at 108 GE) needed to achieve 100% infection.
In this study we allowed pupae to complete development and scored them phenotypically upon eclosion. Bumble bee pupae are susceptible to handling and survival rates (~ 50%) were similar in virus- or mock-inoculated samples (Fig. 4). Amongst the three phenotypically-distinct groups there was no difference in viral load in virus inoculated samples. Strikingly, the same three groups and the proportion of each were present in the mock-inoculated samples. No wing deformities were detected in eclosed bumble bees from either group. It has previously been reported that DWV-positive field isolates of both B. terrestris and B. pascuorum have been identified with wing deformities characteristic of those seen in DWV-infected honey bees9. However, these studies did not demonstrate DWV replication or reproduce symptoms by DWV inoculation. In honey bees apart from DWV infection, wing malformation can be caused during development by injury, hormonal disorders, intoxication or absence of cocoon39.
While laboratory inoculations represent the gold standard for virus infectivity assays this approach probably does not recapitulate an environmentally meaningful infection route. Compared to their independent effect a combination of stressors is suggested to introduce a greater threat to wild pollinators in their native environment53. For example, exposure to clothianidin—a neonicotinoid insecticide—was found to have a negative impact on honey bee immune status and promote DWV infection54. In Bombus terrestris condition mediated virulence of Slow bee paralysis virus upon starvation has been demonstrated55. Our study uses a reverse genetic approach to investigate pathogen spillover from honey bees to bumble bees. Whilst clear evidence is obtained to confirm DWV replication in bumble bee larvae, pupae and adults, we were unable to demonstrate a compelling route by which transmission would likely occur in the natural environment. The levels of virus required to orally infect bumble bee larvae are significantly higher than have been reported in environmental pollen samples56. Indeed, the levels required are likely higher than larvae are ever exposed to. In contrast, adult bumble bees may experience very high virus levels in collapsing honey bee colonies while robbing. However, adult bumble bees feeding on virus-supplemented syrup remain uninfected and—importantly—were unable to transmit infectious virus to the developing larvae. Further studies will be required to determine whether the gut environment of adult bumble bees is sufficiently hostile to DWV that the virus is inactivated e.g. by gut proteases, or if there are other factors that restrict infection and replication of DWV in bumble bees.
The results obtained from this study provide a strong indication that oral acquisition of virus from a contaminated environment does not represent an effective DWV transmission route from Apis to otherwise healthy Bombus individuals. Bumble bees are known to carry their own mite parasites, such as Locustacarus buchneri, which infest the air sacs of adult bumble bees and feed via piercing the tracheal wall57, however, these mites do not parasitise on honey bees. Other mites found on Bombus lack feeding behaviour similar to Varroa and hence the capacity for virus vectoring during feeding. Non-Acari parasites of bumble bees such as protozoans Apicystis bombi and Crithidia bombi were shown to be present in honey bee collected pollen58, but no evidence of their ability to transmit viruses between bee species has been reported. Therefore, a route for productive DWV spillover to bumble bees from infected honey bees remains to be determined. Previous reports have emphasised the haplotype identity of DWV between honey bee and bumble bee populations6,26,27. We propose that the detection of DWV RNA in geographically co-located bumble bees may reflect environmental contamination from the abundant honey bee population without necessarily supporting DWV replication in the bumble bee population. Further studies on defining the stressors that may account for DWV infection of bumble bees are required in order to estimate the actual impact of DWV on this important group of pollinators.
Materials and methods
Honey bees
All honey bee (Apis mellifera) brood were collected from the University of St Andrews research apiary. All hives used for sampling were routinely treated for Varroa with an appropriate miticide. Pupae were extracted from the comb and maintained in the incubator set at 34.5 °C with 90% relative humidity.
Bumble bees
Bumble bees (Bombus terrestris audax; Biobest, Belgium) were maintained in the laboratory in isolated nests supplemented with feeders containing 50% aqueous sucrose solution. Nests were regularly fed with bee pollen from a DWV-free region (Saxonbee, Australia). For adult inoculation experiments newly eclosed (harvested directly on emergence from sealed pupal cells) or mixed-age workers were selected at random from established nests in groups of 20–25 and maintained at RT in separate cages with ad libidum feeding on pollen/syrup. Prior to inoculation each group was left to recover for 24 h to account for any mortality caused by the handling. Pupae and larvae were extracted from the brood cells and transferred into the incubator set at 30.5 °C with 90% relative humidity. Larvae were fed a diet consisting of 25% (v:v) ground pollen and 25% sucrose solution (w:v) in H2O.
RG system and in vitro RNA synthesis
All RG constructs in this study were based on a cDNA clone of a recombinant DWV variant VDV-1-DWV-No-9 (GenBank HM067438.1) and described in42.
Viral RNA was generated in vitro from linearised plasmid templates, using the T7 RiboMAX Express Large Scale RNA Production System (Promega). Full length and truncated templates were linearised with Pme I (cuts at the end of poly-A tract) or Nru I (cuts at nucleotide position 9231 located within the sequence encoding the virus polymerase) respectively. Truncated RNA transcripts were polyadenylated using the poly(A) Tailing Kit (Thermo Fisher Scientific) to ensure their stability. After purification, RNA transcripts were eluted in RNAse free H2O, their integrity confirmed by gel electrophoresis, and stored at − 80 °C.
RNA and virus injections
RNA and virus injections of 5 and 10 μl were performed with insulin syringes (BD Micro Fine Plus, 1 ml, 30 G, Becton Dickinson) into honey bee and bumble bee pupae respectively. Up to 10 μg (equivalent to 1.7 × 1012 of DWV RNA copies) of in vitro transcribed RNA was injected individually into pupae. Truncated VVD transcript injections were used as a negative control. All RNA-injected pupae were analyzed at 72 h post injection. Injections of pupae or low temperature-immobilized adult bees with virus stocks diluted in PBS were performed using the same technique as for RNA transcripts.
Virus stocks
DWV stocks were prepared from pupae injected with in vitro transcribed RNA as described earlier42. Homogenized tissue was diluted with sterile PBS in 1:1 (w:v) ratio and centrifuged at 13 000 g, 4 °C for 10 min. Supernatant was filter sterilized with 0.22 μM filter (PES, Merck Millipore) and treated with RNase A to destroy all unencapsidated RNA. RNA was extracted from 100 μl of the virus stock using RNeasy kit (Qiagen) and analyzed by reverse transcription followed by qPCR. Apart from the virus inoculum used for testing the infectivity of the Bombus-derived DWV, all virus stocks were prepared from RNA-injected honey bee pupae.
Oral inoculation and bumble bee nest feeding
Individual bumble bee larvae were placed into 96 well plates containing 2 μl of pollen/syrup mixed with DWV inoculum. DWV feeding was delivered once on the first day of the experiment. Fresh diet without virus was added individually after virus-containing food had been consumed. On the fifth day larvae were snap-frozen in liquid nitrogen and stored at − 80 °C.
Adult bumble bees were fed with virus in groups of 20 on the first and on the second day of the experiment. Total amount of DWV supplied with each of the two virus feedings was 2.2 × 109 and 2.2 × 108 DWV GE for 108 and 107 groups respectively. Bumble bees were snap-frozen in liquid nitrogen and analyzed for the presence of DWV RNA 7 days after DWV feeding.
For the colony-scale inoculations three bumble bee nests each containing a mated queen and 120–125 adult bees were used. Prior to the start of the experiment all brood was removed from the nests. Virus feeding was delivered daily by replacing the nest feeder with a tube containing 2 ml of sucrose solution supplemented with DWV, previously confirmed to be infectious for bumble bee pupae by direct injection. Each nest received a daily dose of the virus corresponding to 2.4 × 1010 DWV GE. The virus-containing solution was fully consumed (< 3–4 h) before replacement with the regular sucrose feeder. DWV feeding continued for 4–6 weeks and stopped when the first group of larvae developed during the virus-feeding period approached pupation. Upon termination of the experiment all brood (eggs, larvae and pupae) and 10 adult workers were sampled from each nest and tested for the presence of DWV.
RNA extraction, reverse transcription and PCR
Samples were homogenized with a Precellys Evolution homogenizer (Bertin Instruments). Total RNA was extracted using the GeneJet RNA Purification Kit (Thermo Fisher Scientific). cDNA was prepared with qScript cDNA Synthesis Kit (Quanta Biosciences) from 1 μg of total RNA following the manufacturer’s protocol. Detection of DWV and actin mRNA, used as an internal RNA quality control, was carried out by end-point PCR with Taq DNA polymerase (New England Biolabs) and 2 μl of cDNA. DWV_RTPCR primers were designed to detect all three DWV variants under study (Table S1). To amplify the regions containing restriction site tags in VDD and VVV Kpn2I_F/R and PflFI_F/R primer pairs were used respectively. PCR cycling conditions were 30 cycles of 95 °C (15 s), 55 °C (15 s), 68 °C (2 min) with an initial 95 °C step (30 s) and a final extension at 68 °C (5 min). PCR samples were analyzed on a 1% agarose gel and when required, PCR products were subjected to restriction digest prior to loading on the gel.
The quantification of DWV genome copies was performed by SYBR-Green Real-Time Quantitative PCR using Luna Universal qPCR master mix (New England Biolabs), 0.25 μM forward and reverse DWV_qPCR primers and 2 μl of cDNA. The following thermal profile was used: 1 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C with a final post-amplification melting curve analysis step. DWV titres were calculated by comparison of the resulting Ct value to the standard curve generated from a serial dilution of the VVD cDNA qPCR standard prepared by reverse transcribing the RNA transcript.
Negative strand assay
Strand-specific detection of DWV RNA was performed as described earlier. Briefly, 1 µg of total RNA was used in reverse transcription reaction carried out with Superscript III reverse transcriptase (Invitrogen) and the adapter extended primer designed to anneal to the negative strand RNA of DWV. The PCR step was carried out by Taq DNA polymerase (New England Biolabs) using forward primer 388 identical to the adapter sequence (Table S1) and DWV_RTPCR_R primer. PCR was run for 35 cycles in the same conditions as described above.
Next generation sequencing sample preparation and analysis
Gene specific cDNA was generated from selected samples using SSIII reverse transcriptase (Invitrogen) and DWV FG RP1 primer (Table S1). The cDNAs were amplified using LongAmp Taq polymerase (New England Biolabs) following standard protocols and DWV FG RP1 and FP4 primers to produce a single ~ 10 Kb PCR fragment spanning the DWV genome. Following PCR purification (Wizard PCR cleanup kit, Promega) samples were sequenced using an Illumina Hi-seq. Paired-end reads were processed at the University of St Andrews and sequences were analyzed for genetic diversity using ShoRAH59,60. Phylogenetic analysis was performed using Geneious Prime 2019.1.3.
Supplementary information
Acknowledgements
This work was supported by Grant funding from BBSRC BB/M00337X/2 and BB/I000828/1. This research was also supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) Grant 2017-06481 (EVR).
Author contributions
D.J.E. and O.N.G. designed the study. O.N.G., L.W. and K.B.-C. performed and analyzed experiments. E.V.R. developed a reverse genetics system for VVD DWV. D.J.E. supervised the study. O.N.G. wrote the manuscript. D.J.E. and L.W. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olesya N. Gusachenko, Email: olesya.gusachenko@gmail.com
David J. Evans, Email: d.j.evans@st-andrews.ac.uk
Supplementary information
is available for this paper at 10.1038/s41598-020-73809-3.
References
- 1.Martin SJ, et al. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 2.Wilfert L, et al. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science. 2016;351:594–597. doi: 10.1126/science.aac9976. [DOI] [PubMed] [Google Scholar]
- 3.Gallai N, Salles JM, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econom. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- 4.Potts SG, et al. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–229. doi: 10.1038/nature20588. [DOI] [PubMed] [Google Scholar]
- 5.Cavigli I, et al. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie. 2016;47:251–266. doi: 10.1007/s13592-015-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature. 2014;506:364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R, et al. RNA viruses in hymenopteran pollinators: Evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS ONE. 2010 doi: 10.1371/journal.pone.0014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YP, Pettis JS, Collins A, Feldlaufer MF. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 2006;72:606–611. doi: 10.1128/AEM.72.1.606-611.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genersch E, Yue C, Fries I, De Miranda JR. Detection of Deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. J. Invertebr. Pathol. 2006;91:61–63. doi: 10.1016/j.jip.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Loope KJ, Baty JW, Lester PJ, Wilson Rankin EE. Pathogen shifts in a honeybee predator following the arrival of the Varroa mite. Proc. Biol. Sci. 2019;286:20182499. doi: 10.1098/rspb.2018.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, et al. New evidence that deformed wing virus and black queen cell virus are multi-host pathogens. J. Invertebr. Pathol. 2012;109:156–159. doi: 10.1016/j.jip.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Forsgren E, et al. Preliminary observations on possible pathogen spill-over from Apis mellifera to Apis cerana. Apidologie. 2015;46:265–275. doi: 10.1007/s13592-014-0320-3. [DOI] [Google Scholar]
- 13.Levitt AL, et al. Cross-species transmission of honey bee viruses in associated arthropods. Virus Res. 2013;176:232–240. doi: 10.1016/j.virusres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Reynaldi FJ, Sguazza GH, Albicoro FJ, Pecoraro MR, Galosi CM. First molecular detection of co-infection of honey bee viruses in asymptomatic Bombus atratus in South America. Braz. J. Biol. 2013;73:797–800. doi: 10.1590/s1519-69842013000400016. [DOI] [PubMed] [Google Scholar]
- 15.Gamboa V, et al. Bee pathogens found in Bombus atratus from Colombia: A case study. J. Invertebr. Pathol. 2015;129:36–39. doi: 10.1016/j.jip.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Graystock P, Meeus I, Smagghe G, Goulson D, Hughes WOH. The effects of single and mixed infections of Apicystis bombi and deformed wing virus in Bombus terrestris. Parasitology. 2016;143:358–365. doi: 10.1017/s0031182015001614. [DOI] [PubMed] [Google Scholar]
- 17.Sachman-Ruiz B, Narváez-Padilla V, Reynaud E. Commercial Bombus impatiens as reservoirs of emerging infectious diseases in central México. Biol. Invasions. 2015;17:2043–2053. doi: 10.1007/s10530-015-0859-6. [DOI] [Google Scholar]
- 18.Sébastien A, et al. Invasive ants carry novel viruses in their new range and form reservoirs for a honeybee pathogen. Biol. Lett. 2015;11(9):20150610. doi: 10.1098/rsbl.2015.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman-Novoa E, et al. First detection of honey bee viruses in stingless bees in North America. J. of Apicultural Res. 2015;54:93–95. doi: 10.1080/00218839.2015.1100154. [DOI] [Google Scholar]
- 20.Ravoet J, et al. Widespread occurrence of honey bee pathogens in solitary bees. J. Invertebr. Pathol. 2014;122:55–58. doi: 10.1016/j.jip.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Lucia M, Reynaldi FJ, Sguazza GH, Abrahamovich AH. First detection of deformed wing virus in Xylocopa augusti larvae (Hymenoptera: Apidae) in Argentina. J. Apic. Res. 2014;53:466–468. doi: 10.3896/IBRA.1.53.4.11. [DOI] [Google Scholar]
- 22.Bingham RA, Orthner AR. Efficient pollination of alpine plants. Nature. 1998;391:238–239. doi: 10.1038/34564. [DOI] [Google Scholar]
- 23.Velthuis HHW, van Doorn A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie. 2006;37:421–451. doi: 10.1051/apido:2006019. [DOI] [Google Scholar]
- 24.Li J, et al. Cross-species infection of deformed wing virus poses a new threat to pollinator conservation. J. Econ. Entomol. 2011;104:732–739. doi: 10.1603/ec10355. [DOI] [PubMed] [Google Scholar]
- 25.Radzevičiūtė R, et al. Replication of honey bee-associated RNA viruses across multiple bee species in apple orchards of Georgia Germany and Kyrgyzstan. J. Invertebr. Pathol. 2017;146:14–23. doi: 10.1016/j.jip.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Alger SA, Burnham PA, Boncristiani HF, Brody AK. RNA virus spillover from managed honeybees (Apis mellifera) to wild bumblebees (Bombus spp.) PLoS ONE. 2019;14:e0217822. doi: 10.1371/journal.pone.0217822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manley R, et al. Knock-on community impacts of a novel vector: spillover of emerging DWV-B from Varroa-infested honeybees to wild bumblebees. Ecol. Lett. 2019;22:1306–1315. doi: 10.1111/ele.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryabov EV, et al. A Virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Path. 2014;10(6):e1004230. doi: 10.1371/journal.ppat.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tehel A, Brown MJF, Paxton RJ. Impact of managed honey bee viruses on wild bees. Curr. Opin. Virol. 2016;19:16–22. doi: 10.1016/j.coviro.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Lanzi G, et al. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.) J. Virol. 2006;80:4998–5009. doi: 10.1128/jvi.80.10.4998-5009.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiyuki T, et al. Prevalence and phylogeny of Kakugo virus, a novel insect picorna-like virus that infects the honeybee (Apis mellifera L.), under various colony conditions. J. Virol. 2006;80:11528–11538. doi: 10.1128/jvi.00754-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mordecai GJ, Wilfert L, Martin SJ, Jones IM, Schroeder DC. Diversity in a honey bee pathogen: First report of a third master variant of the Deformed Wing Virus quasispecies. ISME J. 2015;10:1–10. doi: 10.1038/ismej.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ongus JR, et al. Complete sequence of a picorna-like virus of the genus Iflavirus replicating in the mite Varroa destructor. J. Gen. Virol. 2004;85:3747–3755. doi: 10.1099/vir.0.80470-0. [DOI] [PubMed] [Google Scholar]
- 34.Moore J, et al. Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. J. Gen. Virol. 2011;92:156–161. doi: 10.1099/vir.0.025965-0. [DOI] [PubMed] [Google Scholar]
- 35.Zioni N, Soroker V, Chejanovsky N. Replication of varroa destructor virus 1 (VDV-1) and a varroa destructor virus 1-deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology. 2011;417:106–112. doi: 10.1016/j.virol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Ryabov EV, et al. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 2017;7:17447. doi: 10.1038/s41598-017-17802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalmon A, et al. Evidence for positive selection and recombination hotspots in Deformed wing virus (DWV) Sci. Rep. 2017;7:41045. doi: 10.1038/srep41045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker AC, Schroeder DC. The use of RNA-dependent RNA polymerase for the taxonomic assignment of Picorna-like viruses (order Picornavirales) infecting Apis mellifera L. populations. Virol. J. 2008;5:10. doi: 10.1186/1743-422x-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamp B, et al. Construction and rescue of a molecular clone of deformed wing virus (DWV) PLoS ONE. 2016;11(11):e0164639. doi: 10.1371/journal.pone.0164639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posada-Florez F, et al. Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci. Rep. 2019;9:12445. doi: 10.1038/s41598-019-47447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryabov EV, et al. Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biol. 2019;17:e3000502. doi: 10.1371/journal.pbio.3000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gusachenko ON, et al. Green Bees: reverse genetic analysis of deformed wing virus transmission, replication, and tropism. Viruses. 2020;12:532. doi: 10.3390/v12050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ongus JR, Roode EC, Pleij CWA, Vlak JM, van Oers MM. The 5’ non-translated region of Varroa destructor virus 1 (genus Iflavirus): Structure prediction and IRES activity in Lymantria dispar cells. J. Gen. Virol. 2006;87:3397–3407. doi: 10.1099/vir.0.82122-0. [DOI] [PubMed] [Google Scholar]
- 44.McMahon DP, et al. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. Biol. Sci. 2016;283:443–449. doi: 10.1098/rspb.2016.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mordecai GJ, et al. Superinfection exclusion and the long-term survival of honey bees in Varroa-infested colonies. ISME J. 2015;10:1–10. doi: 10.1038/ismej.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tehel A, et al. The two prevalent genotypes of an emerging infectious disease cause equally low pupal mortality and equally high wing deformities in host honey bees. Viruses. 2019;11:114. doi: 10.3390/v11020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian L, Hines HM. Morphological characterization and staging of bumble bee pupae. PeerJ. 2018;6:e6089. doi: 10.7717/peerj.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker DJ, et al. Dynamic and integrative approaches to understanding pathogen spillover. Trans. R. Soc. Lond. B Biol. Sci Philos. 2019 doi: 10.1098/rstb.2019.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plowright RK, et al. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dainat B, Evans JD, Chen YP, Gauthier L, Neumanna P. Dead or alive: Deformed wing virus and varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2012;78:981–987. doi: 10.1128/aem.06537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 52.Roberts JMK, Anderson DL, Durr PA. Absence of deformed wing virus and Varroa destructor in Australia provides unique perspectives on honeybee viral landscapes and colony losses. Sci. Rep. 2017;7:6925. doi: 10.1038/s41598-017-07290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belsky J, Joshi NK. Impact of biotic and abiotic stressors on managed and feral bees. Insects. 2019;10:233. doi: 10.3390/insects10080233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Prisco G, et al. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Nat. Acad. Sci. 2013;110:18466–18471. doi: 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manley R, Boots M, Wilfert L. Condition-dependent virulence of slow bee paralysis virus in Bombus terrestris: are the impacts of honeybee viruses in wild pollinators underestimated? Oecologia. 2017;184:305–315. doi: 10.1007/s00442-017-3851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzei M, et al. Infectivity of DWV associated to flower pollen: Experimental evidence of a horizontal transmission route. PLoS ONE. 2014;9:e113448. doi: 10.1371/journal.pone.0113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoneda M, Furuta H, Tsuchida K, Okabe K, Goka K. Commercial colonies of Bombus terrestris (Hymenoptera: Apidae) are reservoirs of the tracheal mite Locustacarus buchneri (Acari: Podapolipidae) Appl. Entomol. and Zool. 2008;43:73–76. doi: 10.1303/aez.2008.73. [DOI] [Google Scholar]
- 58.de Pereira S, Meeus I, Smagghe G. Honey bee-collected pollen is a potential source of Ascosphaera apis infection in managed bumble bees. Sci. Rep. 2019;9:4241. doi: 10.1038/s41598-019-40804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zagordi O, Bhattacharya A, Eriksson N, Beerenwinkel N. ShoRAH: Estimating the genetic diversity of a mixed sample from next-generation sequencing data. BMC Bioinform. 2011;12:119. doi: 10.1186/1471-2105-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McElroy K, Zagordi O, Bull R, Luciani F, Beerenwinkel N. Accurate single nucleotide variant detection in viral populations by combining probabilistic clustering with a statistical test of strand bias. BMC Genom. 2013;14:501. doi: 10.1186/1471-2164-14-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.