Abstract
Currently, the emergence of a novel coronavirus, referred to as SARS-CoV-2, has become a global health concern which cause severe respiratory tract infections in humans. Person-to-person transmission of SARS-CoV-2 has occurred across the globe, within a short period of SARS-CoV-2 emergence. The goal of this analysis is to summarize in various inanimate surfaces and environments information about the frequency, persistence, potential dissemination, and infectivity of SARS-CoV-2. Most respiratory viruses, including coronaviruses, SARS-CoVs, or influenza may persist for a few days on the surfaces or objects. The length of tenacity on various inanimate surfaces depends on the environmental and growth conditions and overall survival rate could range from minutes to month time. The SARS-CoV-2 may survive and maintain infectivity in the air in unventilated buses for 30 min. As no specific vaccines or therapeutic drugs are available for this contagious virus, timely prevention measures would be crucial to control the future outbreak of this infectious disease. Precautionary strategies such as wearing masks and frequent washing hands are effective to mitigate COVID-19. Following careful consideration of the above-mentioned scenarios, the short review spotlights the pressing environmental issues regarding the persistence, transmission, and infectivity of SARS-CoV-2 in different environmental matrices. Aiming to address this issue with further and deeper insight into the SARS-CoV-2 emergence, a list of most concerned questions is given that should be carefully considered and answered in future studies.
Keywords: Coronavirus, SARS-CoV-2, Persistence, Inanimate surfaces, Temperature, Humidity, Disinfection
1. Introduction
The recent emergence of a novel coronavirus (SARS-CoV-2) has greatly threatened human health and society. Together with two earlier epidemics including Severe Acute Respiratory Syndrome (SARS) coronavirus and Middle East Respiratory Syndrome (MERS) coronavirus [1], SARS-CoV-2 is the 3rd extremely infectious and transmissible human coronavirus, which sprouted in the past twenty years. Due to the rapid emergence and an alarming number of infections and mortalities by this novel coronavirus, the World Health Organization (WHO) declared this outbreak a worldwide pandemic. Person-to-person transmission of SARS-CoV-2 has occurred across the wider community, hospitals, and family settings [[2], [3], [4]]. Therefore, it is supremely important to limit any further spread in healthcare and public environments. Near touch, viral bio-aerosols, respiratory fomites or droplets, and self-inoculation of the mouth, nose or eye, are postulated for the transfer of SARS-CoV-2 to contaminated surfaces or objects [[5], [6], [7]]. However, many controversial aspects of airborne spreading and coronavirus infection on inanimate surfaces remain (Table 1 ) [5]. This study, therefore, aims to provide information on SARS-CoV-2 in different inanimate areas and habitats, persistence, potential dissemination, and infectivity.
Table 1.
Persistence of coronaviruses on different types of inanimate surfaces. Reference to the studies are available in the original article Ref. Kampf et al. [5]. Reprinted from Ref. Kampf et al. [5] with permission from Elsevier. © 2020 The Healthcare Infection Society. Published by Elsevier Ltd. (License Number: 4911580724359).
| Type of surface | Virus | Strain/isolate | Inoculum (viral titer) | Temperature | Persistence |
|---|---|---|---|---|---|
| Steel | MERS-CoV | Isolate HCoV-EMC/2012 | 105 | 20 °C | 48 h |
| MERS-CoV | Isolate HCoV-EMC/2012 | 105 | 30 °C | 8–24 h | |
| TGEV | Unknown | 106 | 4 °C | ≥28 d | |
| TGEV | Unknown | 106 | 20 °C | 3–28 d | |
| TGEV | Unknown | 106 | 40 °C | 4–96 h | |
| MHV | Unknown | 106 | 4 °C | ≥28 d | |
| MHV | Unknown | 106 | 20 °C | 4–28 d | |
| MHV | Unknown | 106 | 40 °C | 4–96 h | |
| HCoV | Strain 229E | 103 | 21 °C | 5 d | |
| Aluminium | HCoV | Strains 229E and OC43 | 5 × 103 | 21 °C | 2–8 h |
| Metal | SARS-CoV | Strain P9 | 105 | RT | 5 d |
| Wood | SARS-CoV | Strain P9 | 105 | RT | 4 d |
| Paper | SARS-CoV | Strain P9 | 105 | RT | 4–5 d |
| SARS-CoV | Strain GVU6109 | 106 | RT | 24 h | |
| SARS-CoV | Strain GVU6109 | 105 | RT | 3 h | |
| SARS-CoV | Strain GVU6109 | 104 | RT | <5 min | |
| Glass | SARS-CoV | Strain P9 | 105 | RT | 4 d |
| HCoV | Strain 229E | 103 | 21 °C | 5 d | |
| Plastic | SARS-CoV | Strain HKU39849 | 105 | 22°-25 °C | ≤5 d |
| MERS-CoV | Isolate HCoV-EMC/2012 | 105 | 20 °C | 48 h | |
| MERS-CoV | Isolate HCoV-EMC/2012 | 105 | 30 °C | 8–24 h | |
| SARS-CoV | Strain P9 | 105 | RT | 4 d | |
| SARS-CoV | Strain FFM1 | 107 | RT | 6–9 d | |
| HCoV | Strain 229E | 107 | RT | 2–6 d | |
| PVC | HCoV | Strain 229E | 103 | 21 °C | 5 d |
| Silicon rubber | HCoV | Strain 229E | 103 | 21 °C | 5 d |
| Surgical glove (latex) | HCoV | Strains 229E and OC43 | 5 × 103 | 21 °C | ≤8 h |
| Disposable gown | SARS-CoV | Strain GVU6109 | 106 | RT | 2 d |
| SARS-CoV | Strain GVU6109 | 105 | RT | 24 h | |
| SARS-CoV | Strain GVU6109 | 104 | RT | 1 h | |
| Ceramic | HCoV | Strain 229E | 103 | 21 °C | 5 d |
| Teflon | HCoV | Strain 229E | 103 | 21 °C | 5 d |
MERS = Middle East Respiratory Syndrome; HCoV = human coronavirus; TGEV = transmissible gastroenteritis virus; MHV = mouse hepatitis virus; SARS = Severe Acute Respiratory Syndrome; RT = room temperature.
2. Persistence and transmission of SARS-CoV-2 in the air
Under regular circumstances, the air matrices are generally considered safe and should be free from pathogenic microbes, including viruses, without an epidemic environment. In conditions of the SARS-CoV-2 epidemic, COVID-19 infected people will sneeze, cough, or even speak to SARS-CoV-2. Transmission by air can occur in close contact with COVID-19 patients of approximately <1 m. Thus, the likelihood of air-flowing SARS-CoV-2 is a worrying public concern [8]. In Singapore, Ong et al. [9] collected surface and air samples, before and after the routine cleaning practice, from the patient’s (infected with COVID-19) bedrooms. Until cleaning, around 87% of 15 room sites (including fans of the aerial outlet) and 60% of 5 toilet sites (door handle, drain, and toilet bowl) have been identified positive. The collected data signifying that the environment has been heavily polluted. More specifically, the shedding of SARS-CoV-2 into stools can act as a prospective viral transmission path. Corridor and anteroom samples were recorded negative. Reverse transcription polymerase chain reaction (RT-PCR) results from two stool specimens of one COVID-19 infected patient without diarrhea and/or pneumonia were also positive. The negative results of post-cleaning samples raise the possibility that current sanitization and decontamination strategies were adequate. Despite the high environmental contamination, all the air collected samples were tested negative. However, positive results of swabs collected from the air exhaust outlets suggest that virus-carrying bio-aerosols may be evacuated by airflows resulting in the deposition on equipment like vents. Jiang et al. [10] investigated 158 viral samples (130 surface and 28 air samples) from isolation wards, nurse stations, and intensive care units guiding service, and outpatient fever clinics by using RT-PCR. Tests show that only one surface sample from nurseries was detected as positive for SARS-CoV-2 and one air sample from intensive care units. Tracheal intubation among health workers was intensively related to the highest risk of SARS-CoV transmission [11]. In many hospitalized COVID-19 patients, a persistence shedding of the virus was observed from the nasopharynx, which was continued to 7 d or for extended periods. The virus has also been detected in the blood (1/12, 8%) and stool (4/8, 50%) and stool (4/8, 50%) of patients by using RT-PCR analysis [12]. Inevitable environmental contamination by SARS-CoV dispersion via respiratory droplets and fecal shedding highlighted the environment as a potential transmission medium and thus necessitate the importance of strict adherence to environmental and hand cleanliness to combat vial contamination [9,12].
3. Persistence of SARS-CoV-2 in liquid environments
SARS-CoV survival time is affected by solutions, temperature, and viral titers. According to a recent study, Coronavirus could survive up to 14 d in dechlorinated tap water or domestic sewage at 4 °C, 2 d in tap water at 20 °C and 14 d in BPS at 20 °C [8,13∗]]. A SARS pharyngeal swab was isolated from the P9 SARS-CoV strain. SARS-CoV can persist in the urine for at least 96 h and 72 h under testing conditions in plasma, diluted sputum, and feces. SARS-CoV P9 infection continued at room temperature for 60 h then decreased subsequently in 72 h and became virtually invisible after 120 h. However, it remained persistent at 4 °C, 20 °C and 37 °C with cell infection for at least 2 h. Sarg-CoV conditions for non-infection should be 90 min for 56 °C, 67 °C for 60 min and 75 °C for 30 min [14], to prevent infection with the SARS-CoV. At 25 °C, a 99% reduction in reagent water grade was required in 22 d for a transmissible gastroenteritis virus (TGEV) and 57 d for a mouse hepatitis virus (MHV). In decontaminated settled sewage, a shorter time (9 d for TGEV and 7 d for MHV) was necessary to decrease 99%. Such facts show that polluted water is a likely source of human exposure [8]. Various earlier reports have demonstrated that the survival and persistence of SARS-CoV-2 in wastewater profoundly depends on the wastewater characteristics [[15], [16], [17], [18], [19]]. Possible SARS-CoV-2 routes to water supplies/streams are schematically shown in Fig. 1 .
Fig. 1.
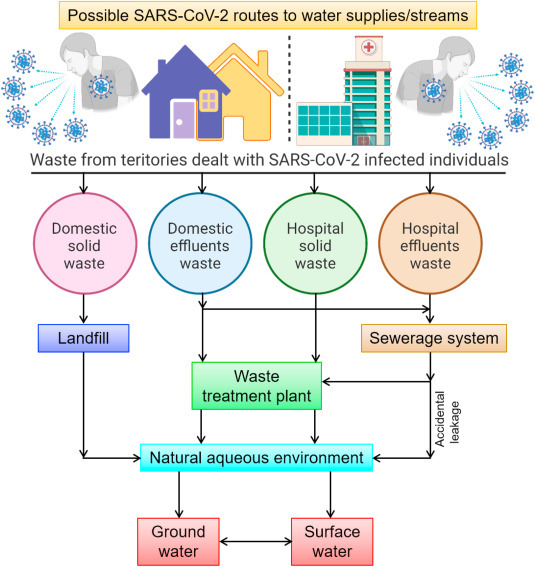
A schematic illustration of various possible SARS-CoV-2 routes to water supplies/streams.
4. Persistence of SARS-CoV-2 in cold (domestic) environments
Equine coronavirus is an eternally circulating pneumonic disease that infects animals like the intestines and airway of wild ruminants. A Bovine coronavirus strain 88 (BCoV-88) infection could last at least 14 d on Roman lettuce surface under household cooling conditions [13∗]. BCoV-88 infectivity declined more rapidly at 10% of bovine fecal suspension than at least a vital FBS suspension of 2%, although the reason was not clear. In bovine intestines and bovine fecal suspensions, bovine enzymes such as proteases or lipases are abundant, cleavage vulnerable virion surface spike glycoproteins, and lipase-sensitive cover [5]. The absence of a responsive protein kinase spike on the surface of the virus or damage of a virus envelope can transmit Bovine coronavirus strain infections. The strains (HuCoV 229E and OC43) induce 1/3 of chronic colds as well as HuCoV infection and live in the respiratory tract saline solution. The decrease in prednisone of <1 log10 was recorded after 4 weeks in TGEV and MHV at 4 °C. The time needed to reduce interactivity to 4 log10 titers at 4 °C was around 98 d for TGEV and 139 d for MHV. A 4 log10 reduction in the time of TGEV and MHV infection was predicted to be roughly one year for reagent-grade water [20].
5. Influence of humidity and temperature on the persistence of SARS-CoV-2
The existence of most fungi and bacteria on surfaces (such as SARS-CoV) depends on conditions such as air and relative moisture, inoculation, and the material on which they have been maintained [9]. Low temperature, high inoculation, and sufficient humidity are related to longer longevity for most viruses. The climate change role in air virus survival can be more challenging and substantial. Relative humidity has a notable impact on the virus’s survival time and the association between humidity and temperature inactivation was not tedium [21]. The temperature has a greater impact on the survival of the virus than that of humidity level. The titer of the transmittable virus decreases swiftly at 25 °C as compared to at 4 °C in all water forms evaluated (i.e. reactant-grade soil, lake water, and settled sewage). The extreme heat of >95% at 28 °C or 33 °C did not expressively disturb SARS CoV HKU 39849 infectivity. An increase in the temperature to 38 °C is not conducive to the virus, where there is a gradual loss of viability at 38 °C and an Hour >95%. Owing to high temperatures (e.g., 30–40 °C), the long-life of extremely pathogenic MERS-CoV, TGEV, and MHV has reduced. The survival of low inoculate SARS-CoV was extended [22]. HCoV-229E at room temperature persists longer at relative humidity (RH) 50% than at RH 30%. Airborne survival HuCoV 229E has been tested under different temperature changes and HR climates. The best survival for Aerosolized HuCoV-229E was with a half-life of 67.33 ± 8.24 h at 20 °C and 50% RH, with a virus detectable at nearly 20% infectious at 6 d, while a half-life of 30% RH was 26.76 h. Half-life at a high 80% HR was just around 3 h, and no virus could be found in aerosols after 24 h. HuCoV-229E’s survival at 6 °C was considerably improved in either 50% or 30% RH conditions and the decay pattern was basically comparable to the scene at 20 °C [8]. However, the half-life of HuCoV 229E increased to 86.01 h at 6 °C, 80% RH, closely 30 times that of 80% RH and 20 °C [8].
6. Survival and persistence of SARS-CoV-2 on inorganic exteriors
A less humid or dry environment is incompatible with the survival of the virus. Under numbering the paper money, paper records, and mail-wrapping papers during the SARS-CoV-2 epidemic is a significant problem for people familiar with suck records in their daily jobs [8]. After returning home from hospitals, the healthcare-related people might carry fomites containing SARS-CoV-2 through their cotton clothes or impervious gowns. On inanimate surfaces, human coronavirus strain 229E has been reported to remain contagious at room temperature from 2 h to 9 d on many various kinds of resources [23]. At a temperature of 21 °C and relative humidity of 30–40%, HuCoV-229E remained infective for at least 5 d on glass, ceramic tiles, polyvinyl chloride, stainless steel, and poly flu or tetraethylene, whereas persisted for 3 d on silicon rubber. However, this virus survived 1 h or less following drying on sterile cotton gauze, aluminum, and sterile latex gloves at room temperature for 3 h with unnoticeable infectivity [24]. Persistence of fomites or droplets on disposable gloves or gown may present a contamination risk to the environment. Mostly during the 2003 SARS outbreak, the SARS-CoV strain GVU6109 infection, obtained from the lungs of an infected person with SARS, died within 5 min after drying in cotton or paper at ambient temperature [25]. Likewise, no viral contamination was detected within 5 min when a piece of paper with an elevated concentration of viral suspension was dried at room temperature. Thus, the possibility of risk infection by exposure to the droplet-laden paper is comparatively little, and hand sanitization and washing after touching contaminated materials are proven effective against the spread of SARS-CoV-2.
7. Inactivation of SARS-CoV-2 by exposure to metal-containing copper surfaces
Copper harboring metals may display broad-spectrum antiviral activities. Two metal-based chemical catalysts, Cu/Al2O3, and Ag/Al2O3 have demonstrated the potential to impede the transmission and spreading of SARS and other respiratory transmittable disorders [26]. Around 100 μL SARS-CoV (106 TCID50/mL), E. coli (106) and 100 μL of recombinant baculovirus (106 PFU/mL) carrying hamster’s prion protein were slowly dropped on the surfaces of the two metal catalysts-based wafers (Cu/Al2O3 and Ag/Al2O3), and allowed to expose 20 and for 5 min, respectively. The infection rate of baculovirus in Sf9 cell lines and SARS-CoV in Vero cells was significantly reduced to an undetectable level. The hamster’s prion protein expression was dropped down to only 21.8% in the recombinant baculovirus-infected Sf9 cells within a contact time of 5 min and was vanished after exposure to a period of 20 min. It was observed that contact with Cu/Al2O3 and Ag/Al2O3 surfaces for 5–20 min could inhibit the baculo virus, SARS-CoV, and E’s proliferation and replication capabilities. Similarly, exposures to copper and copper alloy surfaces causing the irreparability of the viral genome, as demonstrated by morphological changes in the TEM analysis, inactivated the HuCoV-229E by releasing copper ion and ROS. Coronavirus was inactivated in 40 and 120 min on exposure to brass and copper nickels (including < 70% copper). Brass carrying about 70% or more of copper will easily inactivate HuCoV-229E replication in 20 min. The SARS-CoV strain P9 also disappeared for 1 h in culture medium and became imperceptible following UV treatment [27].
8. Concluding remarks and future recommendations
In conclusion, the survival and persistence of the coronaviruses and SARS-CoV-2 in various environmental matrices such as water, airborne particulate matter, tap water, dust, and domestic sewage under diverse environmental settings call for immediate systematic investigations. Low levels of this pathogenic virus in environmental samples warrants the development of highly sensitive technologies for accurate detection and quantification of SARS-CoV-2. In the future, COVID-19 is likely to become a seasonal infectious disease. In order to determine the survival, existence, and behavior of SARS-CoV-2 in environmental matrices, the development of an automatic and high throughput strategy is of great importance. Meanwhile, to reduce the chance of infection, it is noteworthy to execute large-scale disinfectant treatment of COVID-19 in all kinds of environmental compartments. A sustainable and strong international data sharing and the collaborative network is needed to limit the spread and threat of novel COVID-19 infectious diseases. In this context, apart from knowledge proficiency and expertise in the fields of public health, and medicine, the dedicated involvement of environmental engineers would be prolific to combat the threat of this infectious disease globally.
9. SARS-CoV-2 – most concerned questions [28∗]
-
✓
How much do we know about the human-to-human transmission of SARS-CoV-2?
-
✓
Does the SARS-CoV-2 appear to be significantly contagious in a human-to-human context?
-
✓
What makes SARS-CoV-2 strain significantly concerning, regardless of its resembling symptoms from the common cold to as severe as SARS-CoV?
-
✓
How concerned should we be about the SARS-CoV-2 origin, although there are more confirmed cases around the globe and the infected cases are keep growing?
-
✓
What are the main symptoms of SARS-CoV-2, and how are they distinguishable compared to the typical common cold and seasonal flu?
-
✓
What do people need to know to take preventive measures to protect themselves and avoid getting sick?
-
✓
Are the currently available viral vaccine development approaches good enough to tackle SARS-CoV-2, effectively?
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The listed author(s) appreciatively acknowledge the literature services provided by representative universities.
References
Papers of particular interest, published within the period of review, have been highlighted as:
- ∗ of special interest
- ∗∗ of outstanding interest
- 1.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016 Aug;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. Journal of Environmental Chemical Engineering. 2020 May 5;8:104006. doi: 10.1016/j.jece.2020.104006. ∗∗, ∗∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study summarizes and integrate environmental and human health aspects related to the monitoring, fate and treatment solutions for COVID-19. A summary of recent findings is given to better understand the environmental and health problems associated with COVID-19.
- 3.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 Feb 15;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal H.M., Romero-Castillo K.D., Bilal M., Parra-Saldivar R. The emergence of novel-coronavirus and its replication cycle-an overview. J. Pure Appl. Microbiol. 2020;14(1):13–16. Article: 6146. [Google Scholar]
- 5.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020 Mar 1;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahbaz M., Bilal M., Moiz A., Zubair S., Iqbal H.M. Food safety and COVID-19: precautionary measures to limit the spread of coronavirus at food service and retail sector. J. Pure Appl. Microbiol. 2020 May [Google Scholar]
- 7.Bilal M., Iqbal H.M. Human Vaccines & Immunotherapeutics; 2020. Recent Advances in Therapeutic Modalities and Vaccines to Counter COVID-19/SARS-CoV-2; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren S.Y., Wang W.B., Hao Y.G., Zhang H.R., Wang Z.C., Chen Y.L., Gao R.D. Stability and infectivity of coronaviruses in inanimate environments. World Journal of Clinical Cases. 2020 Apr 26;8(8):1391. doi: 10.12998/wjcc.v8.i8.1391. ∗∗, ∗∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study summarizes data on the persistence of different coronaviruses on inanimate environment surfaces.
- 9.Ong S.W., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020 Apr 28;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y., Wang H., Chen Y., He J., Chen L., Liu Y., Hu X., Li A., Liu S., Zhang P., Zou H. medRxiv; 2020 Jan 1. Clinical Data on Hospital Environmental Hygiene Monitoring and Medical Staff Protection during the Coronavirus Disease 2019 Outbreak. [Google Scholar]
- 11.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young B.E., Ong S.W., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama. 2020 Apr 21;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-A scoping review. Water Res. 2020 Apr 28;179:115899. doi: 10.1016/j.watres.2020.115899. ∗, ∗. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This work summarizes research data on CoV in water environments. It comprehend relevant studies investigating three main areas: 1) CoV persistence/survival in waters; 2) CoV occurrence in water environments; 3) methods for recovery of CoV from waters.
- 14.Bestle D., Heindl M.R., Limburg H., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Becker S. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020 Jan 1 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annalaura C., Ileana F., Dasheng L., Marco V. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020 May 5;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M. Water matrices as potential source of SARS-CoV-2 transmission–An overview from environmental perspective. Case Studies in Chemical and Environmental Engineering. 2020:100023. doi: 10.1016/j.cscee.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F.…La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J.…Gikas P. Frontier review on the propensity and repercussionof SARS-CoV-2 migration to aquatic environment. Journal of Hazardous Materials Letters. 2020:100001. doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar M., Mohapatra S., Mazumder P., Singh A., Honda R., Lin C.…Kuroda K. Making waves perspectives of modelling and monitoring of SARS-CoV-2 in aquatic environment for COVID-19 pandemic. Current Pollution Reports. 2020:1–12. doi: 10.1007/s40726-020-00161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denis M., Vandeweerd V., Verbeke R., Laudisoit A., Wynants L., Van der Vliet D. Information available to support the development of medical countermeasures and interventions against COVID-19. 2020. (Version 2020-05-19). Transdisciplinary Insights. [DOI]
- 21.Zhou J., Kroll A.V., Holay M., Fang R.H., Zhang L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020 Apr;32(13):1901255. doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorillo L., Cervino G., Matarese M., D’Amico C., Surace G., Paduano V., Fiorillo M.T., Moschella A., La Bruna A., Romano G.L., Laudicella R. COVID-19 surface persistence: a recent data summary and its importance for medical and dental settings. Int. J. Environ. Res. Publ. Health. 2020 Jan;17(9):3132. doi: 10.3390/ijerph17093132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015 Dec 31;6(6) doi: 10.1128/mBio.01697-15. e01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sizun J., Yu M.W., Talbot P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: a possible source ofhospital-acquired infections. J. Hosp. Infect. 2000 Sep 1;46(1):55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005 Oct 1;41(7):e67–71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J., Chen L., Duan S.M., Yang Q.X., Yang M., Gao C., Zhang B.Y., He H., Dong X.P. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomedical and environmental sciences: BES (Biomed. Environ. Sci.) 2005 Jun;18(3):176–180. [PubMed] [Google Scholar]
- 27.Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and environmental sciences: BES (Biomed. Environ. Sci.) 2003 Sep;16(3):246–255. [PubMed] [Google Scholar]
- 28.Bilal M., Nazir M.S., Parra-Saldivar R., Iqbal H.M. 2019-nCoV/COVID-19 - approaches to viral vaccine development and preventive measures. J. Pure Appl. Microbiol. 2020;14(1):25–29. ∗, ∗. Article: 6168. [Google Scholar]
- This work reports that several epidemiological measures against COVID-19 severity, the case fatality risk (CFR) assessment is an important measure to track record the overall proportion/ratio of the cumulative number of infected patients with the known outcome (confirmed, recovered, or deceased).


