Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has reached pandemic levels. Cardiovascular complications in COVID-19 have been reported frequently, however evidence for a causal relationship has not been established. This report describes the detection of SARS-CoV-2 viral genomes in a patient with symptoms of heart failure, in whom endomyocardial biopsy was investigated following a latency period of 4 weeks after the onset of pulmonary symptoms. The viral infection was accompanied by myocardial inflammation indicating an infection of the heart muscle.
Keywords: SARS-CoV-2, COVID-19, Viral myocarditis, Endomyocardial biopsy
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly became a worldwide health emergency and has reached pandemic levels (Zhu et al., 2020). The disease caused by SARS-CoV-2, coronavirus disease 2019 (COVID-19), which manifests mainly as a respiratory disease, has alarmed not only pulmonologists, but also clinicians of other disciplines. Clinical reports have confirmed cardiac involvement during COVID-19 (Inciardi et al., 2020, Madjid et al., 2020). SARS-CoV-2 is an enveloped RNA virus that binds to its target cell via a viral envelope-anchored spike protein and the putative host receptor angiotensin-converting enzyme 2 (ACE2). Besides other tissues and cell types, ACE2 is highly expressed in vascular endothelial cells and in heart muscle cells (Liu et al., 2020).
Previous work from our group identified SARS-CoV-2 genomes by PCR in the myocardium of COVID-19 patients affected by intramyocardial inflammation (Escher et al., 2020). The presence of viral genomes in the heart of COVID-19 patients has also been verified in autopsy studies, without an association with myocarditis in fulminant stages (Lindner et al., 2020). The cardiotropism of SARS-CoV-2 has been confirmed in vitro, but might occur after a latency period following acute infection of the respiratory tract in vivo (Sharma et al., 2020).
To investigate the presence of SARS-CoV-2 as a potentially cardiotropic virus with a possible latency until detection in the myocardium, a real-time reverse transcription PCR (RT-qPCR) assay was performed on endomyocardial biopsies (EMBs). EMBs were analysed in the Institute for Cardiac Diagnostics and Therapy, Berlin, Germany by histology, immunohistology, and molecular biology (Schultheiss et al., 2019).
Methods and results
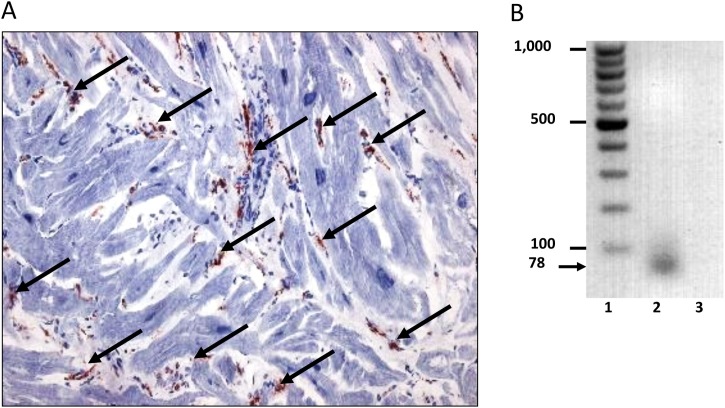
SARS-CoV-2 RNA was detected in RNA extracted from EMB tissue from a COVID-19 affected patient by RT-qPCR, indicating an endomyocardial infection (SARS-CoV-2 E-gene-specific RT-qPCR; sample cycle threshold (Ct) value = 33.4 ± 1.9; positive control Ct value = 35.9 ± 0.5; Figure 1 ) (TIB Molbiol, Berlin, Germany). This was an otherwise healthy 59-year-old woman of Caucasian race, admitted to the intensive care unit with a severe acute respiratory syndrome. Dyspnoea, without fever or cough, was the only symptom corresponding to a typical clinical picture of acute heart failure. The first clinical contact was on February 17, 2020. At that time, clinical experience of COVID-19 in Germany was scarce and therefore COVID-19 was not suspected in the differential diagnosis. Throat swab specimens tested negative for respiratory syncytial virus and influenza A and B virus. Blood tests revealed elevated levels of markers of myocyte injury (high-sensitivity troponin T level of 83.6 pg/ml, creatine kinase (CK) level of 125 U/l, and creatine kinase myocardial band (CK-MB) level of 43 U/l), which remained positive during the first days of her hospitalization. Transthoracic echocardiography showed a severe diastolic dysfunction III with an increased wall thickness (interventricular septum, 14 mm) and minimal pericardial effusion. After respiratory improvement, coronary artery disease could be excluded, and due to symptoms of progressive heart failure, an EMB was performed by left ventricular catheterization 4 weeks after the onset of pulmonary symptoms. In addition to positive proof of SARS-CoV-2 RNA, immunohistochemical EMB analysis in accordance with digital image quantification confirmed pronounced intramyocardial inflammation in the absence of signs of necrosis. Increased numbers of CD45R0+ T memory cells (96.15 cells/mm2), CD3+ cells (20.54 cells/mm2), CD11a+ cells (lymphocyte function-associated antigen 1) (24.36 cells/mm2), CD11b+ cells (macrophage-1 antigen) (91.56 cells/mm2), and CD54+ cells (intercellular adhesion molecule 19) (area fraction 1.91%) (Dako, Glostrup, Denmark) were observed (Figure 1). Histological evaluations were performed on paraffin-embedded EMB sections with haematoxylin and eosin staining and revealed hypertrophied myocytes (diameter 31 μm). Active myocarditis according to the Dallas criteria could not be confirmed. A search for other common cardiotropic infectious agents yielded negative results. Following EMB analysis, nasopharyngeal swab specimens tested negative for SARS-CoV-2 RNA. A follow-up EMB, 3 weeks after the first EMB, revealed a reduction of inflammatory cell infiltration (CD45R0+ 81.43 cells/mm2, CD3+ 13.6 cells/mm2, CD11a+ 12.49 cells/mm2, CD11b+ 28.43 cells/mm2) and tested negative for SARS-CoV-2 RNA in accordance with the patient's clinical improvement.
Figure 1.
Intramyocardial inflammation and SARS-CoV-2 RNA in endomyocardial biopsy. Representative image of immunohistochemical staining for the assessment of inflammation in a SARS-CoV-2-positive endomyocardial biopsy specimen and qualitative gel blot image of a SARS-CoV-2 genome PCR amplicon. (A) Arrows indicate increased CD45R0+ T memory cells (96.15 cells/mm2) (Dako, Glostrup, Denmark). Immunohistochemical staining was quantified by digital image analysis. Magnification 200×. (B) Representative agarose gel electrophoresis gel blot image of a SARS-CoV-2 amplicon (78 bp) from quantitative SARS-CoV-2 E-gene specific RT-qPCR (TIB Molbiol, Berlin, Germany). The dimensions of the marker bands in base pairs are shown in lane 1. Lane 1 = molecular weight standard, lane 2 = patient, lane 3 = negative control.
Discussion
Although subgenomic SARS-CoV-2 RNA indicating a replicative infection has, to date, only been detected in the respiratory tract, viral genomes have been detectable in other organs of infected individuals, such as the kidneys, liver, skin, eyes, and nervous system (Wang et al., 2020, Wolfel et al., 2020). The presence of SARS-CoV-2 genomes in EMBs indicates that SARS-CoV-2 is potentially able to infect the heart muscle. A recently published electron microscopy study identified viral particles in interstitial cytopathic macrophages in an EMB from a COVID-19 patient with cardiogenic shock (Tavazzi et al., 2020). In the present study, direct cardiac involvement associated with intramyocardial inflammation as a complication in a patient affected by COVID-19 was observed. In this study, it was found that virus positivity in the heart was still present after a latency period of 4 weeks after the onset of pulmonary symptoms.
During this study, it was not possible to determine the exact cell type that was susceptible to SARS-CoV-2 infection within the myocardium. The elevated histopathological and systemic parameters of myocardial inflammation in the absence of signs of necrosis indicate that cardiac dysfunction and myocyte hypertrophy might be caused by ischemic conditions resulting from damage to the intramural vessels and small capillaries (Varga et al., 2020). Limitations in EMB sample size did allow a conclusive histopathological evaluation of capillary damage using specific endothelial cell markers in the present study. However, CD11b+ macrophage infiltration was more pronounced in the areas surrounding capillaries.
A follow-up EMB revealed a significant reduction of inflammatory cell infiltration and tested negative for SARS-CoV-2 RNA, indicating viral clearance from the myocardium. The results of this study suggest that the successful detection of SARS-CoV-2 genomes in the heart during the course of infection might only be possible during a temporally restricted phase. The long-term effect of COVID-19 in the heart muscle needs to be investigated and one may speculate that further cardiac complications in the progression of COVID-19 following viral clearance may result from an abundant inflammatory reaction or the induction of autoimmune processes (Galeotti and Bayry, 2020). This EMB-based finding should prompt the clinician to continue cardiac surveillance after the pulmonary infection, as SARS-CoV-2 is a potential new causative agent in the development of heart failure.
Limitations of the study
Since COVID-19 was not suspected initially, preserved blood samples corresponding to the date of catheterization were not available, which would have allowed systemic molecular testing for SARS-CoV-2 genomes. The sample size of an EMB is limited, thus the amount of RNA isolated was not sufficient for an in-depth analysis of the SARS-CoV-2 genome targeting different regions or for sequencing of the viral genome. However, the high sensitivity and specificity of the PCR systems used to detect SARS-CoV-2 genomes have been demonstrated recently (Corman et al., 2020).
Funding source
This study did not receive any funding.
Ethical approval
The patient gave permission and informed consent for the publication of this case report.
Conflict of interest
HP, FE, GA, CB, LM, and HPS report personal fees from IKDT Berlin outside the submitted work. There are no other relationships, conditions, or circumstances that present a potential conflict of interest relevant to the work.
References
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus, (2019-nCoV) by real-time RT-PCR. Euro Surveill Bull Eur sur les Mal Transm = Eur Commun Dis Bull. 2020;25(3 (January)) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher F., Pietsch H., Aleshcheva G., Bock T., Baumeier C., Elsaesser A. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Hear Fail [Internet] 2020 doi: 10.1002/ehf2.12805. Available from: https://pubmed.ncbi.nlm.nih.gov/32529795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8 (August)):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Gai S., Wang X., Zeng J., Sun C., Zhao Y. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc Res. 2020;116(10 (August)):1733–1741. doi: 10.1093/cvr/cvaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- Schultheiss H.P., Fairweather D.L., Caforio A.L.P., Escher F., Hershberger R.E., Lipshultz S.E. Dilated cardiomyopathy. Nat Rev Dis Prim [Internet] 2019;5(1):32. doi: 10.1038/s41572-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Garcia G.J., Wang Y., Plummer J.T., Morizono K., Arumugaswami V. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1(4 (July)):100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA [Internet] 2020;323(18 (March)):1843–1844. doi: 10.1001/jama.2020.3786. Available from: https://pubmed.ncbi.nlm.nih.gov/32159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8 (February)):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]