Abstract
Research priorities are best determined by the most pressing scientific questions, in the context of current knowledge. However, definitive research studies take time, while real-world experience accumulates. Adoption of new practices before adequate comparison with current treatments threatens successful study conduct and may expose patients to what ultimately turns out to be inferior treatment. We conducted a survey to understand the hematopoietic cell transplantation (HCT) community's predictions about future practice trends in the HCT field and results of ongoing Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trials to gauge how the HCT community views the treatments being studied. The survey was distributed between February and March 2019 to an electronic mailing list of HCT clinicians practicing in the United States maintained by the Center for International Blood and Marrow Transplant Research (CIBMTR). Of 986 clinicians surveyed, 315 responded (32%). They predicted an increase in the number of HCTs performed for malignant hematologic diseases and benign diseases such as sickle cell, autoimmune, and genetic disorders. The majority (63%) predicted that matched related donors will remain the preferred donor source for adult HCT recipients in 2023, but 21% predicted haploidentical (haplo) donors and 17% predicted matched unrelated donors would be the preferred source. Most respondents (65%) predicted a decrease in the use of umbilical cord blood (UCB) as a graft source for HCT. Most respondents also predicted that calcineurin-based graft-versus-host disease (GVHD) prophylaxis would be replaced by post-transplantation cyclophosphamide (PTCy) (55%), biomarker use would become standard practice to guide GVHD therapy (73%), and steroids would be combined with other agents as first-line therapy for newly diagnosed acute (53%) and chronic GVHD (54%). In ongoing BMT CTN trials in which outcomes are not yet known, 60% to 92% of respondents had an opinion about which arm they thought would be superior. However, not all respondents predicted the same outcome, with 44% to 88% choosing the same arm. There was no clear relationship between the proportion predicting the same arm would win and accrual to the trial. Survey respondents were optimistic about an increasing volume of transplantation procedures, and they also expected significant changes in HCT practice over the next few years, including wider adoption of PTCy GVHD prophylaxis, increased use of biomarkers to guide GVHD therapy, and decreased use of UCB HCT. The degree of equipoise in the community about the relative efficacy of therapies being studied did not seem to affect accrual to current BMT CTN trials, but this is an area that needs further investigation.
Key Words: Future, Hematopoietic cell transplantation, Clinical trial network
INTRODUCTION
The field of hematopoietic cell transplantation (HCT) is characterized by substantial practice variations in therapeutic approaches including preferred donor, graft source, conditioning regimens, and graft-versus-host disease (GVHD) prophylaxis. Many factors influence practice, but a major one is the lack of definitive data about best practices. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducts clinical trials that aim to define best practices and improve the outcomes of patients undergoing HCT. Since its formation, the BMT CTN's research priorities have been guided by a series of State of the Science Symposia (SOSS), conducted to determine the most important and clinically relevant questions to be addressed by cooperative research activities [1,2]. The last SOSS was held in 2014, and another is planned for 2021. For each, 15 or more expert committees are charged with identifying the most important clinical questions in their area that should be addressed in large multicenter trials in the next few years. Final decisions about the highest-priority studies to pursue are made by BMT CTN Steering Committee members considering the most pressing scientific questions, in the context of current knowledge and perceived advances.
Enrollment of patients on prospective, randomized clinical trials assumes community equipoise about the approaches being studied, even if individual practitioners may have beliefs about which arm is superior. However, definitive research studies take time while real-world experience accumulates and new approaches are introduced. Adoption of new practices before they are adequately compared with current treatments threatens successful completion of studies and may expose patients to treatments that are ultimately determined to be inferior or worse are actually inferior but definitive evidence to determine this is never obtained. Accordingly, we conducted a survey to solicit HCT practitioners’ predictions about future practice trends in the HCT field. In addition, by studying their predictions of the results of ongoing BMT CTN trials, we hoped to gauge how the HCT community views the treatments being studied and to gain insight into how that might affect accrual to these studies.
METHODS
Study Population
A description of the survey and an invitation to participate were emailed to HCT physicians who were actively practicing in the United States and had an email address on file with the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR email listserv was reviewed by an investigator (N.F.) to remove participants who were not identified as HCT physicians.
Survey Development
The survey consisted of 3 sections. In the first section, 14 questions collected information about participant and practice characteristics, including gender, age, experience, center geographic region, center transplantation volume, and center participation in BMT CTN trials. The second section of 19 questions asked participants to describe their predictions about the future of cellular therapy and HCT. Examples of topics explored included predictions about use of graft and donor types, autologous transplantation for autoimmune diseases, acute GVHD prophylaxis, and therapy. A third section consisting of 11 questions asked participants to predict results of current BMT CTN clinical trials. Based on the type of transplantation performed by the center and the patient population cared for (pediatric versus adult), skip patterns were developed to determine whether a respondent should be asked certain subsequent questions. The survey was pilot-tested by 2 of the investigators (N.F and S.J.L.), as well as by 2 additional HCT physicians, and then revised for clarity. The distribution of the finalized survey was approved by the Institutional Review Board at the University of Florida and the National Marrow Donor Program (NMDP). The survey is reproduced in the Supplementary Material.
Survey Distribution and Data Collection
The survey was distributed to an electronic mailing list maintained by the CIBMTR, using Survey Gizmo (www.surveygizmo.com). The survey was open from February 6, 2019, to March 9, 2019. Four weekly email reminders were sent to all potential participants. Two drawings ($750 and $500 prizes) for respondents were held to incentivize participation.
Statistical Analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Descriptive statistics, including frequency and percentage for categorical variables and mean ± SD for continuous variables, were used to examine participants’ responses to survey questions. Exploratory analyses were conducted using Fisher's exact test, with Monte Carlo estimates of the exact P values to compare responses across characteristic categories; unadjusted P values were reported.
In addition, based on the planned accrual timeline of the BMT CTN trials determined at the launch of each trial, we were able to determine in which BMT CTN trials enrollment lagged behind expectations. We then looked for an association between strong community opinion about one arm or another and trial accrual.
RESULTS
Participants
The survey was sent to 1189 potentially eligible subjects, of whom 203 (17%) were not eligible to respond owing to undeliverable or “out of office” e-mail (n = 5) or confirmation that the recipient was ineligible (ie, not a transplantation physician) (n = 198). Of the 986 potentially eligible participants, 367 (37%) responded to the survey; 619 did not respond to email reminders, and we were unable to further classify this group into passive nonrespondents versus inactive email addresses. Of these 367 email recipients, 43 actively declined to participate in the study and 9 did not provide information beyond the “general information” section of the survey. Thus, the response rate was 32% of all potentially eligible invitations (315 of 986). Participant characteristics are summarized in Table 1 . One hundred ninety-nine (63%) considered themselves adult transplantation physicians, and 116 (37%) considered themselves pediatric transplantation physicians.
Table 1.
. Characteristics of Survey Respondents (N = 315)
| Characteristic | Value |
|---|---|
| Age, yr, n (%) | |
| <30 | 1 (.3) |
| 30-40 | 71 (22.5) |
| 41-50 | 104 (33.0) |
| 51-60 | 75 (23.8) |
| 61-70 | 53 (16.8) |
| >70 | 11 (3.5) |
| Gender, n (%) | |
| Female | 116 (36.8) |
| Male | 195 (61.9) |
| Practice setting, n (%) | |
| Teaching hospital, academic | 284 (90.2) |
| Teaching hospital, not affiliated with academic center | 13 (4.1) |
| Nonteaching hospital | 12 (3.8) |
| Office/not affiliated with hospital | 1 (.3) |
| Duration of practice as HCT and cellular therapy physician, yr, n (%) | |
| <5 | 40 (12.7) |
| 5-10 | 66 (20.9) |
| 11-14 | 56 (17.8) |
| 15-20 | 41 (13.0) |
| >20 | 112 (35.6) |
| BMT CTN center status, n (%) | |
| Core center | 211 (67.0) |
| Affiliate center | 76 (24.1) |
| Not a BMT CTN center | 28 (8.9) |
| Center autologous HCT experience per year, n (%) | |
| <25 | 62 (20.1) |
| 25-49 | 43 (13.9) |
| 50-100 | 59 (19.1) |
| >100 | 145 (46.9) |
| Center allogeneic HCT experience per year, n (%) | |
| <5 | 3 (1.0) |
| 5-10 | 14 (4.5) |
| 11-49 | 91 (21.3) |
| 50-100 | 99 (31.8) |
| >100 | 104 (33.4) |
The majority of participants (66%) had at least 10 years of experience as transplantation and cellular therapy physicians. A majority (67%) practiced at a BMT CTN core center. Nearly one-half (47%) were practicing at centers performing a high volume (>100 annually) of autologous HCTs. One-third of the participants (33%) were at centers performing a high annual volume (>100) of allogeneic HCTs. Owing to the anonymity of responses, the characteristics of nonresponders are not available.
Predictions of HCT Activities in the Year 2023
Participants were asked to provide predictions about the trends in HCT for malignant and non-malignant hematologic diseases in the year 2023 as compared to 2018. Nearly one-half of all the participants predicted an overall increase in the number of HCTs performed for hematologic malignancies (Figure 1 ). Clinicians providing care for adults versus those providing care for children (62% versus 28%; P < .0001), those practicing in high-volume versus lower-volume allogeneic HCT centers (65% versus 41%; P = .0003) and autologous HCT centers (64% versus 37%; P < .0001), and those practicing in BMT CTN core centers versus those who do not (57% versus 54%; P = .001) were more likely to predict an increase in the numbers of HCT.
Figure 1.
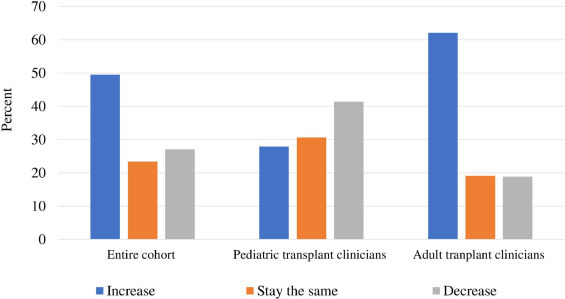
Predictions of the number of HCTs to be performed in 2023 for hematologic malignancies.
In the setting of hematologic malignancies, nearly 59% of respondents predicted that cellular therapy, including chimeric antigen receptor (CAR) T cell therapy will lead to a decreased number of HCTs. Clinicians providing care for pediatrics compared with those providing care for adults (60% versus 55%; P = .04) were more likely to predict that a decrease in the number of HCTs will occur owing to the availability of new cellular or genetic therapies in the future.
Regarding nonmalignant diseases, the majority predicted an increase in the number of transplantations for sickle cell disease (70%) and autoimmune diseases (64%). Clinicians providing care for pediatric patients (83% versus 62%; P < .001) and practicing in non-BMT CTN core centers (77% versus 66%; P = .017) were more likely to predict increases in HCTs performed for sickle cell disease. Approximately one-half of the respondents predicted increases in the number of HCTs performed for genetic disorders (51%), immunodeficiencies (49%), and regenerative medicine (42%). Approximately one-third anticipated that HCT would be a standard of care for systemic sclerosis (37%) or multiple sclerosis (35%), followed by systemic lupus erythematosus (12%), Crohn's disease (10%), and rheumatoid arthritis (6%).
Predictions of Changes in the Distribution of Donor and Graft Sources
The majority of respondents (63%) predicted that matched related donors (MRDs) will remain the preferred donor source in adults (age ≥18 years) with acute myelogenous leukemia (AML) undergoing allogeneic HCT in 2023 (Figure 2 ), 21% and 17% predicting that haploidentical (haplo) donors or matched unrelated donors (MUDs), respectively, would be the preferred donor source. Few respondents predicted that umbilical cord blood (UCB) would be the preferred donor source. Mismatched unrelated donors (MMUDs) were predicted to be preferred over UCB in recipients who lack an MRD, MUD, or haplo donor by 53% of respondents. The majority of respondents (65%), irrespective of whether they care for children or adults, predicted a decrease in the number of UCB HCTs.
Figure 2.
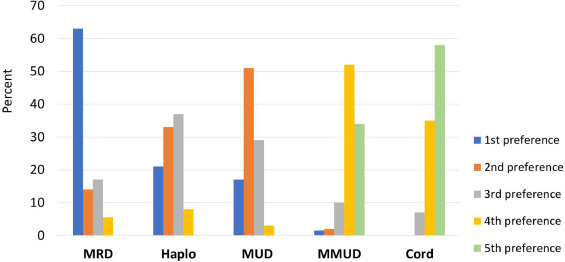
Predicted preferred donor sources for adults (age ≥18 years) with acute myelogenous leukemia undergoing allogeneic HCT in 2023.
Survey respondents were asked to choose the most common donor source in 2023 for children (age <18 years) with primary immune deficiency who lack an MRD (Figure 3 ). MUD was predicted to be the preferred donor source by 43% of participants, followed by haplo TCRαβ+/CD19+-depleted grafts (33%), haplo with post-transplantation cyclophosphamide (PTCy) (22%), UCB (11%), and MMUD (1%).
Figure 3.
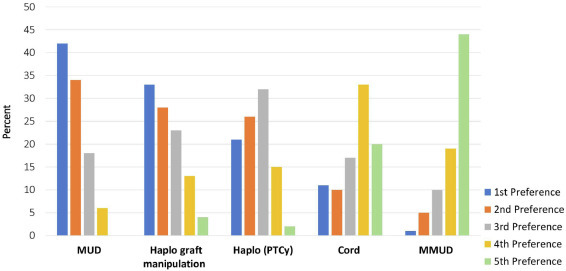
Predicted preferred donor sources for pediatric patients (age <18 years) with primary immune deficiency who lack a matched related donor and undergo allogeneic HCT in 2023.
Changes in GVHD Prophylaxis and Treatment
Respondents were queried about the use of newer transplantation strategies and treatments for the prophylaxis and initial management of acute and chronic GVHD. Clinicians caring for adults predicted a wider adoption of PTCy GVHD prophylaxis in 2023 (Figure 4 ). Two-thirds of adult transplant physicians predicted that PTCy would be the most common GVHD prophylaxis strategy for MUD HCT, followed by calcineurin-based prophylaxis (20%) and graft manipulation (10%). These results reflect a belief that the increased utilization of PTCy GVHD prophylaxis observed over the past decade would continue [3]. Physicians practicing in high-volume allogeneic HCT centers were more likely than those practicing in low-volume centers to predict greater use of PTCy (75% versus 45%; P < .0001). Among clinicians caring for children, the use of graft manipulation strategies (35%) was predicted to be the most common GVHD prophylaxis, followed by PTCy (32%) and calcineurin inhibitor-based (30%) prophylaxis (Figure 4).
Figure 4.
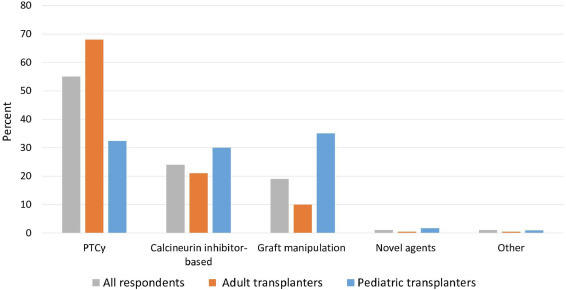
Predicted GVHD prophylaxis used for matched unrelated donor transplantation in 2023.
The majority of respondents (73%) predicted that biomarkers, in addition to clinical features, would be used for both the diagnosis and risk stratification of acute and chronic GVHD. Although these respondents predicted that steroids will remain the front-line treatment of newly diagnosed acute GVHD, most predicted that steroids would be used in combination with other agents (55%) rather than as a single agent (28%). Similarly, the majority of respondents (53%) predicted that steroids would be used in combination with other agents for newly diagnosed chronic GVHD. In contrast, nearly 20% of survey respondents predicted that newer agents would be used for initial therapy of GVHD, including infusions of regulatory cells, immunomodulatory molecules, or antibodies, and this prediction was associated with practicing in a high-volume transplantation center (35% versus 19%; P= .01).
Predictions of the Results of Ongoing Clinical Trials
Finally, participants were asked to predict the results of ongoing BMT CTN clinical trials. In 8 ongoing BMT CTN trials, 60% to 92% of respondents indicated that they thought a particular arm would be found to be superior at the end of the trial (Table 2 ). Predictions for a specific intervention ranged from 44% to 88% in favor of the experimental arm being superior to conventional care. There was no clear relationship between the proportion predicting superiority of the experimental arm and accrual to the trial. Between 5% and 12% of the respondents indicated that they were not aware of specific clinical trials. Lack of awareness about the trials was more prevalent among HCT physicians practicing in centers that are not BMT CTN core centers. Between 8% and 31% of the respondents practicing in non-BMT CTN core centers and between 5% and 13% of the respondents practicing in BMT CTN core centers reported lack of awareness for the trials listed in Table 2.
Table 2.
. Predictions of Outcomes of Ongoing BMT CTN Clinical Trials
| Clinical Trial | Predictions of Clinical Trials Outcomes | |
|---|---|---|
| BMT CTN 1101 | Better outcomes with haplo HCT | 44% |
| Double umbilical cord versus haplo HCT for hematologic malignancy | No difference in outcomes | 36% |
| Better outcomes with umbilical cord HCT | 15% | |
| Not aware of the study | 5% | |
| BMT CTN 1102 | Improved OS with HCT | 78% |
| Biological assignment allogeneic HCT versus supportive care in patient age 50-75 yr with MDS | Comparable OS | 1% |
| Reduced OS with HCT | 14% | |
| Not aware of the study | 7% | |
| BMT CTN 1301 | PTCy | 44% |
| GVHD prophylaxis regimens with the lowest rate of CRFS | T cell-depleted PBSC | 23% |
| Comparable outcomes | 15% | |
| Tacrolimus/methotrexate | 6% | |
| Not aware of the study | 12% | |
| BMT CTN 1503 | Improved OS with HCT | 48% |
| Standard of care versus allogeneic HCT in adolescents and young adults with severe sickle cell disease | Comparable OS | 29% |
| Improved OS with standard of care | 11% | |
| Not aware of the study | 12% | |
| BMT CTN 1506 | Improved outcomes with gilteritinib (PFS/OS) | 88% |
| Maintenance FLT-3 inhibitor, gilteritinib, after allogeneic HCT | No impact on outcomes | 7% |
| Not aware of the study | 5% | |
| ECOG-ACRIN EA4151/BMT CTN 1601 | Improved outcomes with HCT | 72% |
| Auto-HCT followed by maintenance rituximab versus rituximab alone in mantle cell lymphoma | Comparable outcomes | 16% |
| Worse outcomes with HCT | 1% | |
| Not aware of the study | 11% | |
| BMT CTN 1702 | Better OS with matched unrelated donor | 21% |
| For patients without an HLA-identical sibling, is it better to proceed quickly to an alternative donor graft or to find a matched unrelated donor | Better OS with alternative donor | 68% |
| Not aware of the study | 11% | |
| BMT CTN 1703 | PTCy/tacrolimus/MMF | 63% |
| GVHD prophylaxis with the best rate of GRFS | Comparable rate of GRFS | 22% |
| Tacrolimus/methotrexate | 7% | |
| Not aware of the study | 8% | |
CRFS indicates chronic GVHD-free, relapse-free survival; GRFS, GVHD-free, relapse-free survival; MMF, mycophenolate mofetil; OS, overall survival; PBSC, peripheral blood stem cell.
Among the clinical trials listed in Table 2, the results of only a single trial (BMT CTN 1101, a multicenter phase II trial of reduced-intensity conditioning followed by transplantation of either a double unrelated UCB graft or a haplo bone marrow graft for patients with hematologic malignancies) was available at the time of survey data analysis (but not known at the time of the survey). Accrual to this trial lagged behind initial projections, and eventually the accrual was closed at 94% of the desired enrollment. This trial found no significant difference in 2-year progression-free survival (PFS) between the double UCB and haplo bone marrow arms [4]. Approximately one-third of the respondents (36%) predicted similar PFS between the 2 arms of the study, whereas 44% and 15% of the survey participants predicted a better PFS with haplo donors and UCB, respectively. Nearly 5% reported lack of awareness of this clinical trial.
DISCUSSION
We surveyed a large group of HCT clinicians in the United States about the future of transplantation practices and predictions for the results of ongoing clinical trials through the BMT CTN. Our survey respondents represent a broadly representative sample of individuals from centers of different sizes of varying ages, and with varying levels of experience. Based on the results, survey respondents are optimistic about growth in the volume of transplantation procedures, and they also expect significant changes in the practice of HCT over the next several years. These changes included the use of new emerging transplantation techniques, such as a wider adoption of PTCy GVHD prophylaxis, increased use of biomarkers to both diagnose and guide GVHD therapy, and the incorporation of newer medications in addition to steroids in the initial management of acute and chronic GVHD. In general, it appears that the predicted use of newer transplantation strategies was most prevalent in survey respondents who practiced in large-volume transplantation centers and academic centers, perhaps because they were more likely to have clinical trials testing these interventions. These results are in accordance with the previous survey study reported by Wood et al [5] that revealed an association of center size with greater diffusion of newer transplantation techniques into clinical practice.
Other predictions included a decrease in the numbers of UCB transplantations, which is in agreement with the ongoing trend of a declining number of UCB transplantations over the past decade [6]. Nearly one-fourth of the adult HCT physicians predicted haplo donors would be the preferred donor source in the future. This reflects a belief that the increased use of haplo HCTs with use of PTCy GVHD prophylaxis observed over the past decade would continue [5]. In addition, a majority of adult HCT physicians predicted that MMUDs would be the preferred donor source over UCB, which is surprising given the lack of clinical trials comparing the 2 donor sources.
We also found considerable variation in predictions among adult and pediatric HCT physicians including the anticipated numbers of HCTs and preferences for graft and donor sources and GVHD prophylaxis strategies. A wide practice variation among adult and pediatric HCT physicians has been noted in previous survey studies [7].
We also found that a substantial minority of survey respondents (5% to 12%) were unaware of ongoing BMT CTN trials, although this was predominantly reported by clinicians who are not in BMT CTN core centers. Only a small percentage of patients with cancer participate in clinical trials, owing to numerous issues, including a lack of awareness that clinical trials are a treatment option. Health care providers play a major role in raising awareness about the option of clinical trial participation [8]. We believe that this finding should encourage increased efforts to promote awareness of clinical trials in community/non-BMT CTN centers, because participation in BMT CTN trials is not restricted to core centers.
We acknowledge several limitations of our study. Because of difficulties in defining the denominator of eligible physicians, the true estimate of actual survey response rate is not clear (between 32% and 85%), which is in line with previous physician-based survey studies [9,10]. In addition, to ensure responders’ anonymity, we cannot explore many of our findings further. For example, we cannot comment on any differences between respondents and nonrespondents, and we did not collect data on institutional affiliations to explore whether predictions about the future of transplantation were consistent within practice groups, whether center characteristics were associated with predictions, and whether predictions of trial results influenced local accrual. We had hoped to gain insights into attitudes and accrual to trials, but the data are too limited with only the trials that have reached completion of enrollment. This would be of interest to explore in future studies. Finally, although we have surveyed a large group of HCT clinicians, the majority of respondents practiced at a BMT CTN core or affiliate centers. Thus, the survey results may largely represent the opinion of clinicians practicing in large academic centers.
In conclusion, transplantation physicians see a bright future in the field but anticipate that many changes will occur in the 5 years after the survey. We plan to return to these predictions in 2023 to see how closely they match reality. Of note, this survey was conducted before the novel coronavirus pandemic, and HCT practices have had to adapt to COVID-19 restrictions, including temporarily ceasing clinical trial enrollment and restricting certain transplantation procedures. Although no one anticipated the COVID-19 crisis, 2023 is still a long time from now. It will be interesting to see whether COVID-19 just caused a pause in HCT or if it will lead to more profound changes that no one could have anticipated.
ACKNOWLEDGMENTS
Support for this study was provided by grants #U10HL069294 and #U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The views expressed in this article are those of the authors and do not reflect the views or the official policy or position of the National Heart, Lung, and Blood Institute, the National Cancer Institute, or the National Marrow Donor Program.
The CIBMTR registry is supported primarily by the U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases and from HHSH234200637015C (HRSA/DHHS) to the Center for International Blood and Marrow Transplant Research.
Financial disclosure: The authors declare no competing financial interests.
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement:
N.F. and S.J.L designed the study, developed the protocol, interpreted the data, and wrote the manuscript; L.J.B and T.M. designed the study, analyzed and interpreted the data, and generated the figures; B.E.S, C.M.B, S.M.D, M.M.H, R.J.J, H.S.M and J.R.W participated in the design of the study and edited the final manuscript; and the final manuscript was reviewed and approved by all authors.
Footnotes
Financial disclosure: See Acknowledgments on page 183.e6.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2020.10.006.
Appendix. Supplementary materials
REFERENCES
- 1.Appelbaum FR, Anasetti C, Antin JH, et al. Blood and Marrow Transplant Clinical Trials Network State of the Science Symposium 2014. Biol Blood Marrow Transplant. 2015;21:202–224. doi: 10.1016/j.bbmt.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JLM, Anasetti C, Zhu X, Stadtmauer E. Blood and marrow transplantclinical trials network state of the science symposium 2007. Biol Blood Marrow Transplant. 2007;13:1268–1285. doi: 10.1016/j.bbmt.2007.08.005. PMID:17950914. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza A, Lee S, Zhu X, Pasquini M. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2017;23:1417–1421. doi: 10.1016/j.bbmt.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs EJ, O'Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood versus HLA-haploidentical bone marrow transplantation (BMT CTN 1101) [e-pub ahead of print]. Blood. doi:10.1182/blood.2020007535, accessed August 31, 2020.
- 5.Wood WA, McGinn MK, Wilson D, et al. Practice patterns and preferences among hematopoietic cell transplantation clinicians. Biol Blood Marrow Transplant. 2016;22:2092–2099. doi: 10.1016/j.bbmt.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–e182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26:2162–2170. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 8.Comis RL, Miller JD, Aldigé CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol. 2015;15:32. doi: 10.1186/s12874-015-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Jawahri A, LeBlanc TW, Burns LJ, et al. What do transplant physicians think about palliative care? A national survey study. Cancer. 2018;124:4556–4566. doi: 10.1002/cncr.31709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


